Introduction
Toll-like receptors (TLRs) belong to a class of
innate immune receptors that detect and clear invading microbial
pathogens (1). Thirteen TLRs have
been identified in mammals since 1997. TLR1, TLR2, TLR4, TLR5,
TLR6, TLR10 and TLR11 are expressed on the cell surface, whereas
TLR3, TLR7, TLR8 and TLR9 are expressed intracellularly on
endosomal membranes (2). TLR11–13
are present in mice but lost from the human genome. The role of
TLRs in tumor angiogenesis is diverse. It has been demonstrated
that Helicobacter pylori, associated with gastric cancer,
acts through TLR2 and TLR9 contributing to cancer cell invasion and
angiogenesis (3). An association
between TLR3, TLR4 and TLR9 expression and tumor aggressiveness and
poor prognosis was identified in hepatocellular carcinoma (4). Findings of a meta-analysis showed
that polymorphisms in TLR9 may play a role in cancer development
(5). Human lung cancer cells have
been shown to express functional TLR9 molecules (6,7).
TLR9 expression in mononuclear cells was associated with an
angiogenic phenotype and promoted lung cancer progression (8). TLR9 agonist also promoted the growth
of human lung cancer cells (9).
However, the mechanism involved concerning the association of TLR9
with lung cancer has yet to be elucidated.
MicroRNAs (miRNAs or miRs) are endogenous small
non-coding RNAs of 21–25 nt in length (10), first discovered in
Caenorhabditis elegans (11), that exert biological functions by
post-transcriptional regulation of gene expression in a
sequence-specific manner (12).
Over 1,000 miRNAs have been identified in the human genome, and
over one-third of all human protein coding genes are potentially
regulated by miRNAs (13). miRNAs
regulate the expression of genes involved in development,
proliferation and growth (14–16). However, whether miRNAs are
involved in the effects of TLR9 signaling on lung cancer cells
remains to be elucidated.
Findings of recent studies have shown that miR-26a,
a unique member of miRNAs, is involved in the progression of
cancer. Expression of miR-26a in glioma cells significantly
increased the growth rate and colony formation in vitro and
tumor growth and angiogenesis in vivo, while a reduced
expression of miR-26a played the opposite roles (17). Overexpression of miR-26a increased
the proliferation of cholangiocarcinoma cells and colony formation
in vitro, whereas miR-26 depletion reduced these parameters.
In severe combined immune-deficient mice, overexpression of miR-26a
in cholangiocarcinoma cells increased tumor growth (18). However, the possible role of
miR-26a in TLR9 cancer growth and progression remains largely
unknown.
The present study was designed to determine the
roles of TLR9 on lung cancer and whether miR-26a is involved in the
TLR9-mediated lung cancer growth and migration and the downstream
signaling pathway.
Materials and methods
Tissue samples
Fresh lung cancer and corresponding normal lung
tissue samples (>10 cm away from the edge of the lung cancer)
were obtained from lung cancer patients, and then snap-frozen in
liquid nitrogen immediately after resection and kept at −80°C until
use. No patients had received chemotherapy or radiotherapy prior to
surgery.
Animals and xenograft model
Animal experiments were performed using female nude
mice (6- to 7-week-old) purchased from the Chinese Academy of
Medical Sciences Laboratory Animal Center. The animals were housed
in a temperature- and humidity-controlled room with a 12-h on-off
light cycle and given free access to food and water. To establish
the xenograft murine model, nude mice were anesthetized by
intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10
mg/kg) prior to intracardiac injections and were placed in the
supine position. With a 25-gauge needle, H460 cells
(2–3×105) were injected into the left ventricle (0.1 ml)
after visualization of arterial blood flow into the syringe. After
injection, the mice were placed on heating cages to recover from
anesthesia. Tumor size was calculated as length × width × depth
×0.5236 (19).
Cell line and culture
H460 human lung cancer cell line was purchased from
American Type Culture Collection (Manassas, VA, USA) and cultured
in RPMI-1640 with 10% fetal bovine serum (FBS), penicillin (100
IU/ml)/streptomycin (100 μg/ml) at 37°C in a humidified 5%
CO2 incubator. The cells were subcultured every 3–5 days
to maintain logarithmic growth until a sufficient number of cells
(5×107 cells/ml) was obtained for transfer to nude
mice.
Adenovirus infection
Cells were plated in DMEM/Ham F12 with 10% FCS, at a
density of 0.5–1×105 cells/cm2. Twenty-four
hours after plating, serum was removed and the cells were infected
with recombinant adenoviruses at a multiplicity of infection (MOI)
of 50.
Western blotting
The tissues were lysed in modified RIPA buffer or
lysed directly in 1× sodium dodecyl sulfate (SDS) loading buffer.
After the process of electrophoresis and transmembrane, proteins on
the nitrocellulose membrane were probed with the TLR9,
phosphatidylionositol 3 kinase (PI3K), protein kinase B (Akt),
phosphorylated-Akt (1:500; Cell Signaling Technology, Danvers, MA,
USA) and GAPDH (1:5,000; Bioworld Technology Inc., St. Louis Park,
MN, USA) primary antibody followed by incubation with the secondary
antibodies (1:5,000; Immunology Consultants Laboratory, Portland,
OR, USA). The bands were visualized by enhanced chemiluminescence
using ECL (Pierce Chemical) and captured on X-ray film. The total
TLR9 or PI3K protein level was normalized to the GAPDH protein
level, and the phosphorylated-Akt level was normalized to the Akt
protein level.
Northern blot analysis
Total RNA (20 μg), extracted using TRIzol reagent
according to the manufacturer’s instructions (Invitrogen), was
separated on 1% agarose gel with 3% formaldehyde and 10% 10×
4-morpholinepropanesulfonic acid. The RNA was transferred to an
uncharged nylon membrane and UV cross-linked. The membrane was
prehybridized at 42°C for 2 h with 1 ml/cm2 QuikHyb
Hybridization solution (Stratagene, La Jolla, CA, USA). DNA
oligonucleotides, complementary to the mature microRNAs, were
obtained from Integrated DNA Technologies (Coralville, IA, USA).
The probes were 5′-end labeled with Redivue adenosine
5′-[γ-32P] triphosphate, triethylammonium salt (Amersham
Biosciences) using a microRNA probe and marker kit (Ambion) and
used for hybridization (105/cm2). The blot
was hybridized overnight and then washed with 2× sodium chloride
sodium citrate buffer/0.1% SDS and exposed to X-ray film. Blots
were stripped using 0.5% SDS and reprobed after
prehybridization.
Cell proliferation assay
Cell proliferation was assessed by bromodeoxyuridine
(BrdUrd) incorporation using a BrdUrd ELISA colorimetric assay
(Roche). To determine the proliferation of H460 cells, the cells
were initially plated at a density of 2×105/60 mm dish.
After the cells had been incubated, they were counted using a
hemocytometer (Neubauer, Horsham, Germany) and then plotted.
Cell migration assay
H460 cells (105 cells/well) were
suspended in 0.5 ml of 1% FBS MEM and placed in the top chamber of
the well, while 0.750 ml of 10% FBS MEM were added to the bottom
compartment. Following a 48-h incubation, non-migrating cells were
scraped from the membrane of the top compartment, and cells that
had migrated through the membrane were fixed and stained using the
Protocol Diff-Quik stain set (Siemens, Munich, Germany). The
membranes were excised and mounted on a standard microscope slide
(Curtin Matheson Scientific, Inc., Houston, TX, USA). The number of
cells that migrated were determined from five random high-power
fields (HPFs).
Chemicals
TLR9 ligand CpG-oligodeoxynucleotides (CpG-ODN) were
purchased from InvivoGen (San Diego, CA, USA). Wortmannin (WM), the
inhibitor of PI3K, was purchased from Calbiochem (San Diego, CA,
USA). Triciribine hydrate (TCN), the inhibitor of Akt, was
purchased from Sigma Chemical Co. (St. Louis, MO, USA). The
chemicals were dissolved in phosphate-buffered saline (PBS). The
dose of CpG was 5 μg/ml, and the doses of WM and TCN were 25
μM.
Statistical analysis
Comparisons between two observations were assessed
by the Student’s paired t-test. One- or two-way ANOVA was used
followed by the Bonferroni test for post hoc analysis when multiple
comparisons were made. Data were expressed as the mean ± standard
error (SE). P<0.05 was considered statistically significant.
Results
Expression of TLR9 in lung cancer
tissue
The expression of TLR9 in lung cancer tissues
obtained from lung cancer patients was increased compared with the
controls (Fig. 1A).
Effects of TLR9 ligand on the tumor mass
weight and size
TLR9 ligand CpG-ODN caused an increase in the mean
tumor weight after 3 weeks compared with that treated with PBS. The
ligand of TLR9 CpG-ODN also increased the size of the tumor mass
from 11 days after treatment (Fig. 1B
and C).
Effects of TLR9 ligand on proliferation
and migration
H460 cells treated with TLR9 ligand CpG-ODN promoted
the proliferation as compared to that with PBS after 48 h. In the
Boyden chamber migration assays, CpG-ODN induced an increase in the
migration of H460 human lung cancer cells (Fig. 2).
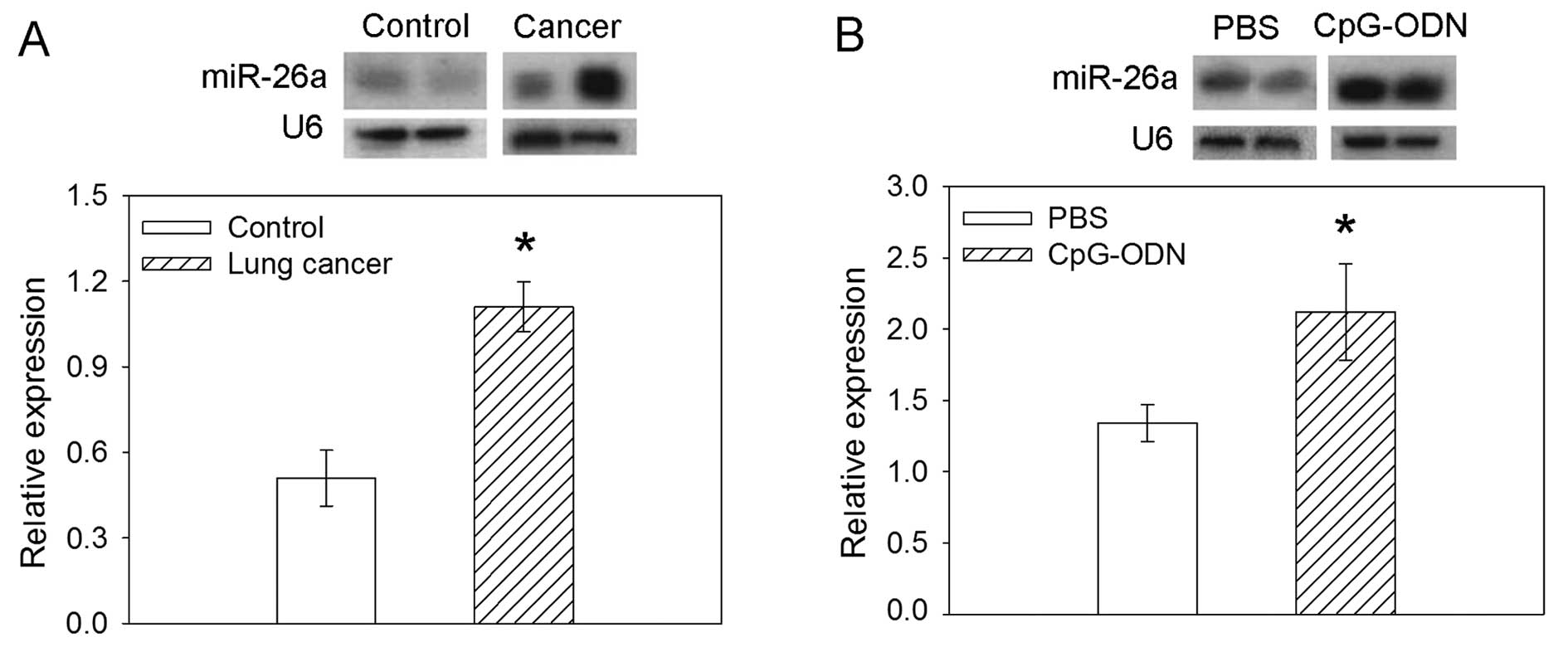
Expression of miR-26a
miR-26a expression was increased in the lung cancer
tissues obtained from lung cancer patients compared with the
controls. In H460 cells, TLR9 ligand CpG-ODN induced an increase in
the expression of miR-26a compared with PBS (Fig. 3).
Effects of miR-26a overexpression on the
tumor in mice and H460 human lung cancer cell line
Northern blot analysis in H460 cells cultured in
serum-free (SF) medium or infected at a MOI of 50 or 100 showed
efficient overexpression of the mature miR-26a. The overexpression
of miR-26a increased the tumor mass weight and size in the nude
mice. The proliferation and migration were promoted after
overexpression of miR-26a in the H460 human lung cancer cell line
(Fig. 4).
Effects of miR-26a overexpression on the
expression of PI3K and phosphorylated Akt
In the H460 human lung cancer cell line, the
overexpression of miR-26a increased the expression of the PI3K
protein level. The overexpression of miR-26a also significantly
induced an increase in the level of phosphorylation of Akt in H460
cells (Fig. 5).
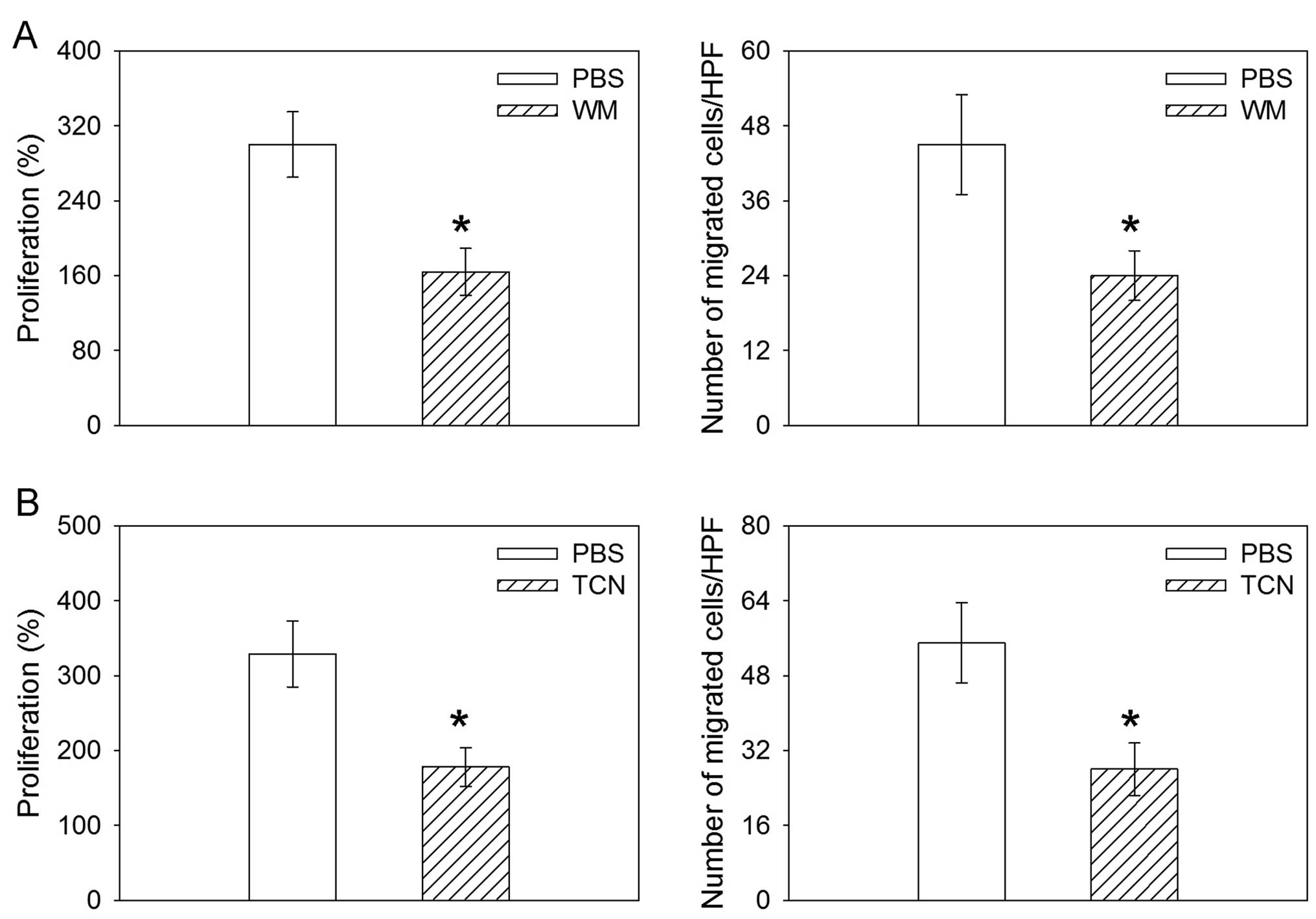
Effects of PI3K or Akt inhibitor on the
proliferation and migration induced by miR-26a overexpression
The inhibitor of PI3K WM abolished the increase in
the proliferation and migration induced by the overexpression of
miR-26a in the human lung cancer cell line H460. TCN, the inhibitor
of Akt, also abolished the increase in the proliferation and
migration induced by the overexpression of miR-26a in H460 cells
(Fig. 6).
Discussion
TLRs are widely expressed on various tumor cells,
including lung cancer cells (7,20).
TLR agonist alters the biological character of lung cancer cells,
promoting proliferation and enhancing the metastatic potential of
tumor cells in vitro and in vivo (21,22). miRNAs are differentially expressed
in various types of cancer and play important roles in cancer
progression (23). Additionally,
they are putative markers for improving cancer classification,
diagnosis and clinical prognostic information (24,25). Accumulating evidence have
demonstrated that miRNAs are involved in regulating the biological
effects of TLR on various cells (26,27). In the present study, we have
demonstrated that miR-26a is involved in the TLR9-mediated growth
and migration of lung cancer through the PI3K-Akt signaling
pathway.
TLRs pathways are key regulators in cancer
progression as well as chemoresistance. TLRs serve as cell surface
sensors that can initiate pathways leading to proliferation and
chemoresistance; as well as mediators that are able to regulate the
infiltrating immune cells to provide further support for cancer
progression (28). In the present
study, TLR9 ligand CpG-ODN caused an increase in the weight and the
size of tumor mass in the nude mice, and promoted the proliferation
and migration of H460 human lung cancer cells. In addition, the
expression of TLR9 in lung cancer tissues obtained from lung cancer
patients was increased. These results indicate that the activation
of TLR9 in lung cancer cells contributed to the growth and
metastasis of tumor cells and is involved in tumor progression,
which is supported by the previous finding that TLR9 agonist
CpG-ODN promotes the growth and metastatic potential of human lung
cancer cells (29).
It has been shown that miRNAs play critical roles in
regulating the biological effects of TLRs signaling pathways on
various types of cells (26,30). miR-26a promotes glioma progression
in vitro and in vivo and is associated with glioma
development (17). Human
cholangiocarcinoma tissues and cell lines had increased levels of
miR-26a compared with the non-cancerous biliary epithelial cells,
and miR-26a promotes growth of cholangiocarcinoma (18). In the present study, we show that
the expression of miR-26a was increased in the lung cancer tissues
obtained from lung cancer patients, and TLR9 ligand CpG-ODN induced
an increase in miR-26a expression in H460 human lung cancer cells.
Furthermore, miR-26a overexpression increased the weight and size
of the tumor mass in the nude mice, and promoted the proliferation
and migration in the human lung cancer cell line H460. These
results indicated that miR-26a contributes to the growth and
metastasis of tumor cells and is involved in the TLR9-mediated
growth and migration of lung cancer.
It is well known that the PI3K/Akt pathway plays a
critical role in tumor biology (31). TLR9 agonist may promote the
metastasis of human lung cancer cells via CXCR4/SDF-1/Akt pathway
(29). However, whether miR-26a
regulated the growth and migration of lung cancer through the
PI3K/Akt pathway is not understood. In the present study, we show
that miR-26a overexpression increased the expression of PI3K
protein level and phosphorylation of the Akt level in the H460
human lung cancer line. The inhibitor of PI3K WM or inhibitor of
Akt TCN abolished the increase in the proliferation and migration
induced by the overexpression of miR-26a in the H460 human lung
cancer cell line. These results demonstrate that miR-26a mediates
the growth and migration of lung cancer through the PI3K-Akt
signaling pathway.
In conclusion, TLR9 promoted the growth and
migration of lung cancer and miR-26a is involved in the
TLR9-mediated growth and migration of lung cancer through the
PI3K-Akt signaling pathway.
References
|
1
|
Gosu V, Basith S, Kwon OP and Choi S:
Therapeutic applications of nucleic acids and their analogues in
Toll-like receptor signaling. Molecules. 17:13503–13529. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takagi M: Toll-like receptor - a potent
driving force behind rheumatoid arthritis. J Clin Exp Hematop.
51:77–92. 2011.PubMed/NCBI
|
|
3
|
Chang YJ, Wu MS, Lin JT and Chen CC:
Helicobacter pylori-Induced invasion and angiogenesis of
gastric cells is mediated by cyclooxygenase-2 induction through
TLR2/TLR9 and promoter regulation. J Immunol. 175:8242–8252. 2005.
View Article : Google Scholar
|
|
4
|
Eiró N1, Altadill A, Juárez LM, et al:
Toll-like receptors 3, 4 and 9 in hepatocellular carcinoma:
Relationship with clinicopathological characteristics and
prognosis. Hepatol Res. Jun 6–2013.(Epub ahead of print).
|
|
5
|
Zhang L, Qin H, Guan X, Zhang K and Liu Z:
The TLR9 gene polymorphisms and the risk of cancer: evidence from a
meta-analysis. PLoS One. 8:e717852013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorrentino R, Morello S, Giordano MG, et
al: CpG-ODN increases the release of VEGF in a mouse model of lung
carcinoma. Int J Cancer. 128:2815–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Droemann D, Albrecht D, Gerdes J, et al:
Human lung cancer cells express functionally active Toll-like
receptor 9. Respir Res. 6:12005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Belmont L, Rabbe N, Antoine M, et al:
Expression of TLR9 in tumor-infiltrating mononuclear cells enhances
angiogenesis and is associated with a worse survival in lung
cancer. Int J Cancer. 134:765–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren T, Wen ZK, Liu ZM, Liang YJ, Guo ZL
and Xu L: Functional expression of TLR9 is associated to the
metastatic potential of human lung cancer cell: functional active
role of TLR9 on tumor metastasis. Cancer Biol Ther. 6:1704–1709.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
12
|
Cuellar TL and McManus MT: MicroRNAs and
endocrine biology. J Endocrinol. 187:327–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
14
|
Miller AM, Gilchrist DS, Nijjar J, et al:
MiR-155 has a protective role in the development of non-alcoholic
hepatosteatosis in mice. PLoS One. 8:e723242013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Y, Bao-Han W, Lv X, et al: MicroRNA-34a
mediates the autocrine signaling of PAR2-activating proteinase and
its role in colonic cancer cell proliferation. PLoS One.
8:e723832013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang X, Du WW, Li H, et al: Both mature
miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce
prostate tumor growth and invasion. Nucleic Acids Res.
41:9688–9704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian X, Zhao P, Li W, et al: MicroRNA-26a
promotes tumor growth and angiogenesis in glioma by directly
targeting prohibitin. CNS Neurosci Ther. 19:804–812.
2013.PubMed/NCBI
|
|
18
|
Zhang J, Han C and Wu T: MicroRNA-26a
promotes cholangiocarcinoma growth by activating β-catenin.
Gastroenterology. 143:246–256. e2482012.PubMed/NCBI
|
|
19
|
Savry A, Carre M, Berges R, et al:
Bcl-2-enhanced efficacy of microtubule-targeting chemotherapy
through Bim overexpression: implications for cancer treatment.
Neoplasia. 15:49–60. 2013.
|
|
20
|
Bhattacharya D and Yusuf N: Expression of
Toll-like receptors on breast tumors: taking a toll on tumor
microenvironment. Int J Breast Cancer. 2012:7165642012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He W, Liu Q, Wang L, Chen W, Li N and Cao
X: TLR4 signaling promotes immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance.
Mol Immunol. 44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Min R, Zun Z, Siyi L, et al: Increased
expression of Toll-like receptor-9 has close relation with tumour
cell proliferation in oral squamous cell carcinoma. Arch Oral Biol.
56:877–884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
24
|
Majumder S and Jacob ST: Emerging role of
microRNAs in drug-resistant breast cancer. Gene Expr. 15:141–151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minguez B and Lachenmayer A: Diagnostic
and prognostic molecular markers in hepatocellular carcinoma. Dis
Markers. 31:181–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen R, Alvero AB, Silasi DA, et al:
Regulation of IKKbeta by miR-199a affects NF-kappaB activity in
ovarian cancer cells. Oncogene. 27:4712–4723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wendlandt EB, Graff JW, Gioannini TL,
McCaffrey AP and Wilson ME: The role of microRNAs miR-200b and
miR-200c in TLR4 signaling and NF-κB activation. Innate Immun.
18:846–855. 2012.PubMed/NCBI
|
|
28
|
Chen R, Alvero AB, Silasi DA, Steffensen
KD and Mor G: Cancers take their Toll - the function and regulation
of Toll-like receptors in cancer cells. Oncogene. 27:225–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L, Zhou Y, Liu Q, et al: CXCR4/SDF-1
pathway is crucial for TLR9 agonist enhanced metastasis of human
lung cancer cell. Biochem Biophys Res Commun. 382:571–576. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tserel L, Runnel T, Kisand K, et al:
MicroRNA expression profiles of human blood monocyte-derived
dendritic cells and macrophages reveal miR-511 as putative positive
regulator of Toll-like receptor 4. J Biol Chem. 286:26487–26495.
2011. View Article : Google Scholar
|
|
31
|
Chen X and Zhou JY, Zhao J, Chen JJ, Ma SN
and Zhou JY: Crizotinib overcomes hepatocyte growth factor-mediated
resistance to gefitinib in EGFR-mutant non-small-cell lung cancer
cells. Anticancer Drugs. 24:1039–1046. 2013. View Article : Google Scholar : PubMed/NCBI
|