Introduction
Sonic hedgehog (Shh) is a secretory glycoprotein and
acts as an autocrine and paracrine factor (1). Shh binds to its receptor, patched 1
(Ptch1). In conditions in which Shh is inactivated, Ptch1 inhibits
the action of the receptor, Smoothened. When Shh binds to Ptch1,
this suppression of Smoothened is reversed, which in turn activates
the transcription factor, Gli1 (1,2).
Gli1 upregulates several downstream signaling genes, including
Ptch1 and Gli1 (3,4). Shh plays an important role in the
developmental process, which includes the regulation of axonal
guidance, cell differentiation and the proliferation of neural
progenitor cells (NPCs) (5–7).
In adults, Shh, is involved in hypoxia-induced neural progenitor
proliferation in the brain injured by stroke (8), controls stem cell maintenance in
post-natal and adult brain neurogenesis (9), regulates angiogenesis and
vasculogenesis (10,11) and strengthens vascular tightness
(12).
Angiopoietin (Ang)-1 and Ang-2 belong to the
angiopoietin family, and play an important role in blood vessel
formation both in normal development and pathological conditions
(13,14). The balance between Ang-1 and Ang-2
is important for the maintenance of the vasculature network
(15). Ang-1 upregulates vascular
integrity by modulating tight junction proteins (16,17), enhances endothelial regeneration
in diabetic mice (18) and
recovers ischemic limb injury through the recruitment of bone
marrow-derived progenitor cells (19). In addition to the role of Ang-1
and Ang-2 in angiogenesis, previous studies have shown that they
have a broad target cell spectrum. Ang-1 blocks cell death and
improves the survival of fibroblasts, skeletal myocytes and
cardiomyocytes (20–22). It also induces neurite outgrowth
(23), as well as the
proliferation and differentiation of NPCs (24). Ang-2 also regulates NPC
differentiation and migration (25). These reports indicate the
importance of angiopoietin as a broad spectrum regulatory
factor.
We have previously reported that Shh affects Ang-1
and Ang-2 mRNA expression only in fibroblasts (26). In the present study, we
demonstrate that Shh is produced by neurons, and that the mRNA
expression of Ang-1 and Ang-2 in fibroblasts is modulated by
co-culture with neurons, without exogenous Shh treatment. Moreover,
Shh-neutralizing antibody significantly blocked the regulation of
Ang-1 and Ang-2 in fibroblasts which was induced by co-culture with
neurons. Our data suggest that fibroblasts and neurons communicate
with each other through Shh signaling. Thus, we propose the concept
of fibroblast/neuron cross-talk.
Materials and methods
Animals, cell isolation and culture of
neural progenitors and neurons
All the animal experiments were performed under the
approval of the Institutional Animal Care and Use Subcommittee of
Inha University Hospital (Incheon, Korea). For the culture of fresh
NPCs, the cortices of fetuses obtained from CD1 mice [embryonic day
(E)14–16] were isolated, minced and incubated in a PBS solution
with 0.25% trypsin and 0.01% DNase I for 20 min as previously
described (26). The cells were
resuspended in DMEM/F12 medium (Gibco/Invitrogen, Carlsbad, CA,
USA) containing 1% FBS, N2 supplement (Gibco/Invitrogen), 0.6%
glucose, HEPES (50 mmol/l), bFGF (20 ng/ml), EGF (20 ng/ml),
heparin (5 μg/ml) and penicillin/streptomycin/amphotericin B (1:100
dilution; Gibco/Invitrogen). The cells were plated at
2×105 cells/well into 24-well plates coated with
poly-D-lysine and laminin (PDL/L plates; Biocoat Inc., Horsham, PA,
USA; BD Biosciences, Franklin Lakes, NJ, USA). After 24 h, 1% FBS
was removed. For the neuron cultures, mouse cortices (E15–17) were
harvested as described in our previous study (26). The cells were seeded at
3×105 cells/well into PDL/L plates (Biocoat Inc.) and
cultured in defined neuron culture medium; neurobasal medium
(Gibco, Invitrogen) containing 2% B27, glutamine (2 mmol/l) and 1%
penicillin/streptomycin. On days 1–3, glutamate (25 μg/ml) and
β-mercaptoethanol (10 μmol/l) were added. On day 3, Ara-C (10
μmol/l) was added for 24 h, and the medium was then exchanged with
fresh defined neuron culture medium.
Cell line and reagents
NIH3T3 mouse embryonic fibroblasts were purchased
[American Type Culture Collection (ATCC), Manassas, VA, USA] and
cultured in DMEM containing 10% FBS (Gibco/Invitrogen) and 1%
penicillin/streptomycin. Mouse recombinant Shh (mrShh) was
purchased from GenScript (Piscataway, NJ, USA; Cat. no. Z03050) and
the cells were incubated with 10 or 50 nM rShh for 16 h. MAB4641, a
neutralizing antibody against Shh [10 μg/m; R&D Systems
(Minneapolis, MN, USA)] was used for the specific blocking of Shh
in the culture medium.
Quantitative (real-time) reverse
transcription PCR (RT-qPCR)
Total RNA was extracted using the QIAshredder and
the RNeasy Mini kit (Qiagen, Hilden, Germany). Total RNA (1–2 μg)
was converted into cDNA using the PrimeScriptTM 1st
strand cDNA Synthesis kit (Takara Bio, Inc., Shiga, Japan). For
real-time PCR assay, mouse Ang-1, Ang-2, Ptch1, Shh and Gli1 were
analyzed with an ABI TaqMan Gene Expression Assay primer and FAM
probe sets (Applied Biosystems, Foster City, CA, USA). Transcript
levels were normalized to the 18S rRNA. Real-time samples were run
on an ABI PRISM-7500 sequence detection system (Applied
Biosystems). In addition, end-point PCR for Ang-1 and Ang-2 was
performed using following primers and GAPDH was used for
normalization: Ang-1 forward, 5′-aaacagcaaatgggaacagg-3′ and
reverse, 5′-gggcaggtgaactccactaa-3′ (melting temperature, 60°C; 35
cycles); Ang-2 forward, 5′-caaggcactgagagacacca-3′ and reverse,
5′-ctgaactcccacggaacatt-3′ (melting temperature, 60°C; 35 cycles);
and GAPDH forward, 5′-ccactggcgtcttcaccac-3′ and reverse,
5′-cctgcttcaccaccttcttg-3′ (melting temperature, 60°C; 27
cycles).
Western blot analysis
The cells were harvested and lysed in lysis buffer
(40 mM Tris pH 7.4, 10 mM EDTA, 120 mM NaCl, 0.1% NP-40) containing
protease inhibitors (Roche Diagnostics GmbH, Mannheim, Germany).
Total protein (10–30 μg) was fractionated in SDS-PAGE and
immunoblotted with specific antibodies against Ang-1 (Novus
Biologicals, Littleton, CO, USA) and Ang-2 (Novus Biologicals).
Actin (Calbiochem, La Jolla, CA, USA) was used as an internal
control. We used the Kodak Image Station 400R to detect the bands
and Kodak Molecular Imaging Software version 4.0. The
quantification of band intensity was analysed using TINA 2.0
(Raytest, Straubenhardt, Germany) and normalized to the intensity
of actin.
Co-culture assay
Neurons and NIH3T3 fibroblasts were co-cultured as
previously described with certain modifications (16). Fibroblasts were seeded at the
bottom of a 12-well plate. Neurons were plated into a Transwell
chamber at 2×105 cells/well (pore size, 0.4 μm; Corning
Life Science, Tewksbury, MA, USA). The Transwell chamber with the
confluent neurons was then placed in the 12-well plate and was
further incubated for 16 h in the defined neuron culture medium.
During co-culture, a neutralizing antibody against Shh (MAB4641; 10
μg/ml; R&D Systems) was added to the bottom well. Total RNA was
extracted from the fibroblasts to evaluate the effects of Shh
secreted from the neurons.
Statistical analysis
All the results are expressed as the means ±
standard deviation. The differences between the groups were
compared by an unpaired t-test or one-way analysis of variance
(ANOVA). P-values ≤0.05 were considered to indicate statistically
significant differences. All statistical analyses were performed
using SPSS 17.0 software (SPSS Inc., Chicago, USA).
Results
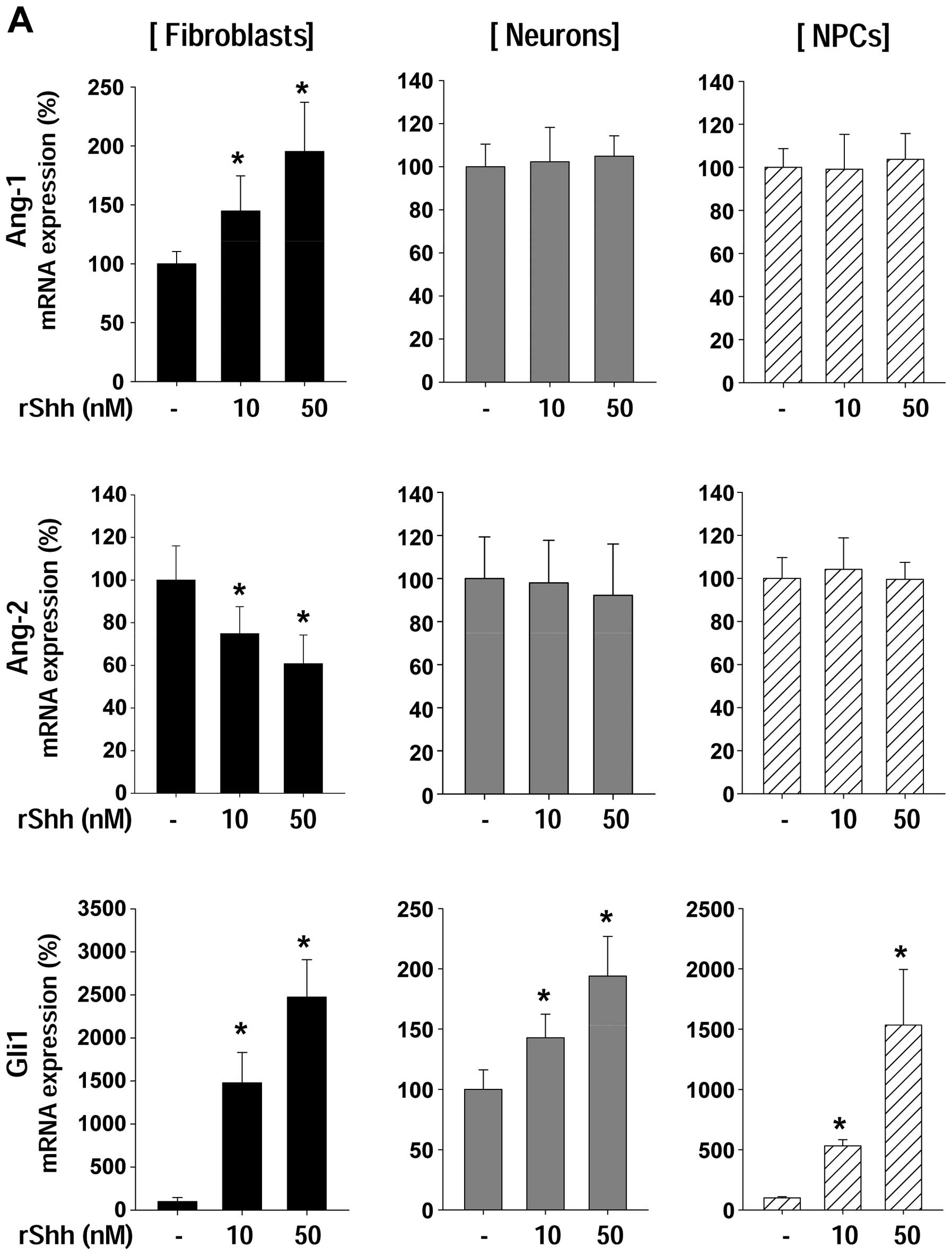
rShh regulates the expression of Ang-1
and Ang-2 in fibroblasts, but not in neurons and NPCs
We determined the expression of Ang-1 and Ang-2
following treatment with recombinant sonic hedgehog (rShh)
(Fig. 1A). Ang-1 mRNA expression
was increased in fibroblasts only, whereas Ang-2 mRNA expression
was decreased by rShh treatment. Ang-1 and Ang-2 mRNA expression
showed no change in either the neurons or the NPCs. Glil mRNA
levels significantly increased following treatment with rShh in all
cell types, indicating that neurons and NPCs responded to rShh
normally; however, rShh did not affect Ang-1 and Ang-2 mRNA
expression in neurons and NPCs (Fig.
1A), which is in accordance with the results of our previous
study (26). To confirm the
effects of rShh on angiopoietin at the protein level we performed
western blot anlaysis (Fig. 1B).
Ang-1 protein expression showed a marked increase in a
dose-dependent manner, while Ang-2 expression decreased in a
dose-dependent manner.
Shh-expressing cells and Shh-responsive
cells differ
We then investigated Shh mRNA expression in 3 types
of cells, fibroblasts, neurons and NPCs, in order to determine Shh
basal levels (Fig. 2A). Shh was
not expressed in fibroblasts, but it was expressed in both neurons
and NPCs. Of note, Shh expression levels in the neurons were much
higher than those in the NPCs (Fig.
2A). It is known that Shh acts in an autocrine and paracrine
manner (1). Shh binds to its
receptor, Ptch1, and activates its signaling with the upregulation
of Ptch1 and Gli1 expression (3).
Thus, we examined the mRNA expression of Ptch1 and Gli1 to clarify
which cells are influenced by rShh (Fig. 2B and C). We hypothesized that rShh
mainly affects neurons even though all 3 types of cells were
affected by rShh, as endogenous Shh was highly synthesized in
neurons. It should be noted however, that Shh downstream signaling,
Ptch1 and Gli1, was upregulated in fibroblasts, but not in neurons
(Fig. 2B and C). These data
indicate that although neurons are one of the major cell sources
for Shh expression, fibroblasts are the most responsive cells to
Shh. Therefore, we suggest that Shh-expressing cells and
Shh-responsive cells differ, and we suggest the existence of a cell
cross-talk in Shh-mediated angiopoietin regulation.
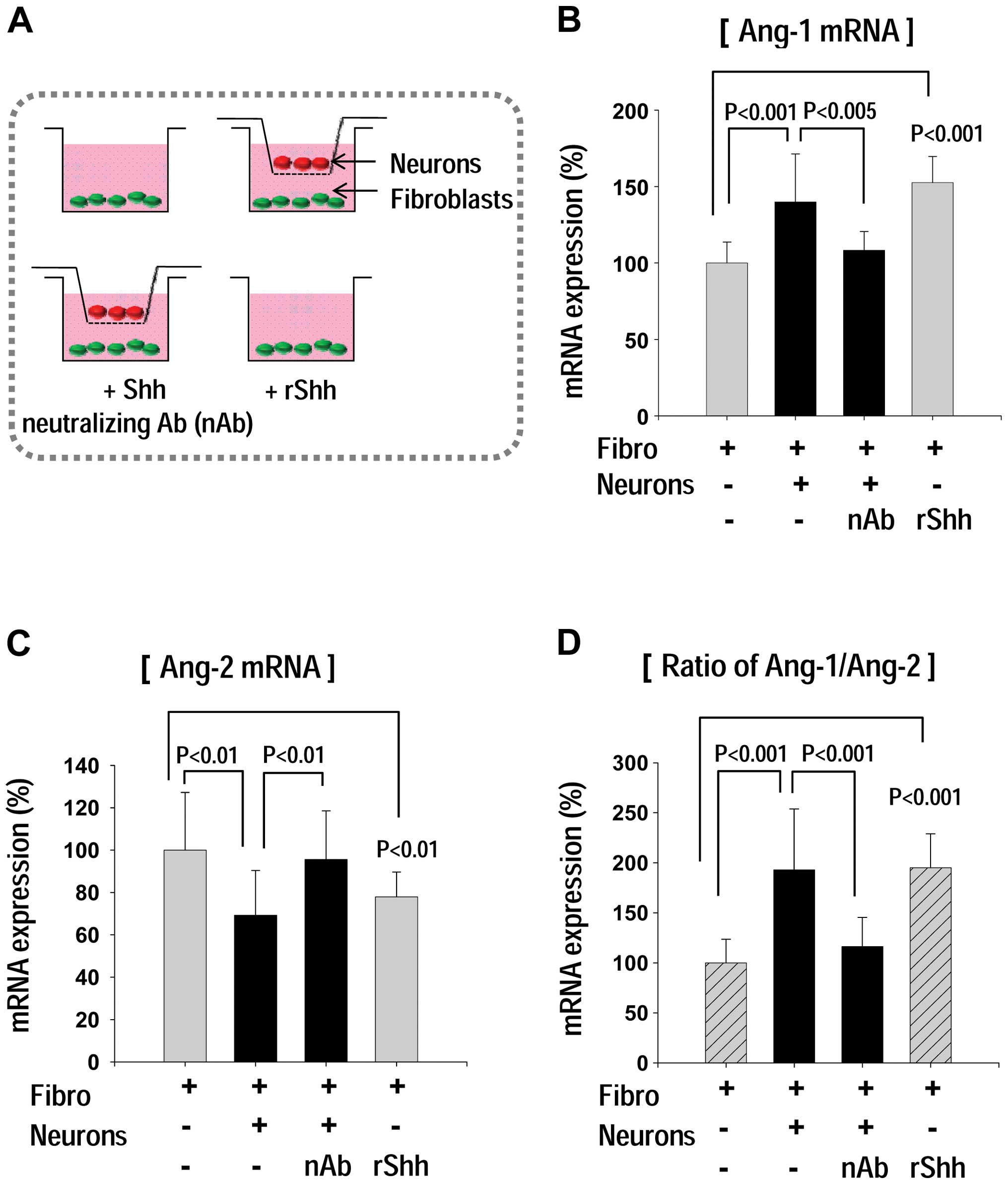
Shh secreted by neurons regulates Ang-1
and Ang-2 gene expression in fibroblasts in a co-culture
system
As shown in Fig.
2, we found that Shh expression was markedly increased in
neurons, whereas Shh mainly affects fibroblasts as opposed to
neurons. We suggested the existence of a cross-talk between neurons
and fibroblasts. To confirm our hypothesis, we performed co-culture
assay (Fig. 3). Fibroblasts were
seeded at the bottom well of 12-well plate, and then a Transwell
chamber with confluent neurons was placed in the 12-well plate
(Fig. 3A). Following further
incubation, total RNA was extracted from the fibroblasts and the
Ang-1 and Ang-2 mRNA expression was determined (Fig. 3B–D). It is of interest to note
that Ang-1 expression was markedly increased only when the neuron
co-culture system was used (Fig.
3B). Moreover, the co-culture-induced Ang-1 upregulation was
markedly inhibited by the addition of a Shh-neutralizing antibody
(MAB4641; Fig. 3B). On the other
hand, Ang-2 mRNA expression in the fibroblasts decreased in the
co-culture group; however, this reduction in Ang-2 expression
following co-culture was reversed by the addition of
Shh-neutralizing antibody (Fig.
3C). Our results suggest that Shh released from neurons, which
may influence neighboring fibroblasts, thus regulating Ang-1 and
Ang-2 expression (Fig. 3E).
Discussion
In this study, we found that neurons and fibroblasts
communicate with each other and that the main source of Shh
expression is from neurons, not fibroblasts or NPCs. In addition,
the regulation of angiopoietin expression in fibroblasts was
affected by neurons in the co-culture system, suggesting the
existence of a cross-talk between neurons and fibroblasts. Lately,
the importance of gaining an understanding of the communication
pathway between different cell types has been emphasized in the
research field of drug development. In the research area of
neurovascular and oligovascular units, there is increasing evidence
that there is a communication between blood vessels and brain cells
(27,28). Cerebral endothelial cells may
secrete trophic factors that affect neighboring cells (27,28). Likewise, astrocytes/endothelial
coupling regulate the development of the cerebral vasculature,
i.e., the blood-brain barrier, through Ang-1 (16). Nerves serve as a template for
arteries and determine the arterial differentiation through the
vascular endothelial growth factor (29,30). Osteoblast-secreted Ang-1 promotes
the survival and maintenance of hematopoietic stem cells (31). Endothelial cells stimulate
fibroblast differentiation following ischemic injury and therefore
increase tissue fibrosis following myocardial infarction (32). These studies have shown that the
complex interplay between types of cells is extensively regulated
in normal physiology.
As this cell cross-talk between cells may involve
common growth factors and may be regulated in an interconnected
manner, molecules, such as Shh hold great therapeutic potential.
Shh upregulates vascularization and osteoblastic differentiation
(33), and can also regulate both
angiogenesis and myogenesis (34). Ang-1 decreases both vascular
damage and cardiomyocyte death, thus improving cardiac function
following myocardial infarction (17). Ang-1 also stimulates muscle
regeneration after muscle injury in mice by promoting muscle
satellite cell self-renewal (35).
Moreover, previous studies have suggested that the
combined transplantation of different cell types improves
therapeutic potential. Vascular regeneration in hindlimb ischemia
was shown to be enhanced by the transplantation of a combination of
embryonic stem cell-derived endothelial and mural cells, compared
with single-cell transplantation (36). The combination of Shh gene
transfer and bone marrow-derived progenitor cells has also been
shown to improve angiogenesis and muscle regeneration in limb
ischemia to a greater degree than with each treatment individually
(37). Therefore, an improved
understanding of cell communication and the functions of regulatory
cytokines, such as Shh may provide a novel strategy for the
regeneration of damaged tissue.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(2013R1A1A3012024, awarded to Sae-Won Lee), and by the Basic
Science Research Program through the NRF grant funded by the
Ministry of Education (NRF-2009-0076309, NRF-2011-0025506, awarded
to Woo Jean Kim).
References
|
1
|
Rowitch DH, BSJS, Jacques B, Lee SM, Flax
JD, Snyder EY and McMahon AP: Sonic hedgehog regulates
proliferation and inhibits differentiation of CNS precursor cells.
J Neurosci. 19:8954–8965. 1999.PubMed/NCBI
|
|
2
|
Taipale J, Cooper MK, Maiti T and Beachy
PA: Patched acts catalytically to suppress the activity of
Smoothened. Nature. 418:892–897. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zedan W, Robinson PA, Markham AF and High
AS: Expression of the Sonic Hedgehog receptor “PATCHED” in basal
cell carcinomas and odontogenic keratocysts. J Pathol. 194:473–477.
2001.
|
|
4
|
Osterlund T and Kogerman P: Hedgehog
signalling: how to get from Smo to Ci and Gli. Trends Cell Biol.
16:176–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu G, Mehler MF, Zhao J, Yu Yung S and
Kessler JA: Sonic hedgehog and BMP2 exert opposing actions on
proliferation and differentiation of embryonic neural progenitor
cells. Dev Biol. 215:118–129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YP, Dakubo G, Howley P, et al:
Development of normal retinal organization depends on Sonic
hedgehog signaling from ganglion cells. Nat Neurosci. 5:831–832.
2002.PubMed/NCBI
|
|
7
|
Charron F, Stein E, Jeong J, McMahon AP
and Tessier-Lavigne M: The morphogen sonic hedgehog is an axonal
chemoattractant that collaborates with netrin-1 in midline axon
guidance. Cell. 113:11–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sims JR, Lee SW, Topalkara K, et al: Sonic
hedgehog regulates ischemia/hypoxia-induced neural progenitor
proliferation. Stroke. 40:3618–3626. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palma V, Lim DA, Dahmane N, et al: Sonic
hedgehog controls stem cell behavior in the postnatal and adult
brain. Development. 132:335–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pola R, Ling LE, Silver M, et al: The
morphogen Sonic hedgehog is an indirect angiogenic agent
upregulating two families of angiogenic growth factors. Nat Med.
7:706–711. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Surace EM, Balaggan KS, Tessitore A, et
al: Inhibition of ocular neovascularization by hedgehog blockade.
Mol Ther. 13:573–579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia YP, He QW, Li YN, et al: Recombinant
human sonic hedgehog protein regulates the expression of ZO-1 and
occludin by activating angiopoietin-1 in stroke damage. PLoS One.
8:e688912013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davis S, Aldrich TH, Jones PF, et al:
Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by
secretion-trap expression cloning. Cell. 87:1161–1169. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SW, Kim WJ, Choi YK, et al: SSeCKS
regulates angiogenesis and tight junction formation in blood-brain
barrier. Nat Med. 9:900–906. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SW, Kim WJ, Jun HO, Choi YK and Kim
KW: Angiopoietin-1 reduces vascular endothelial growth
factor-induced brain endothelial permeability via upregulation of
ZO-2. Int J Mol Med. 23:279–284. 2009.PubMed/NCBI
|
|
18
|
Jin HR, Kim WJ, Song JS, et al:
Intracavernous delivery of a designed angiopoietin-1 variant
rescues erectile function by enhancing endothelial regeneration in
the streptozotocin-induced diabetic mouse. Diabetes. 60:969–980.
2011. View Article : Google Scholar
|
|
19
|
Youn SW, Lee SW, Lee J, et al: COMP-Ang1
stimulates HIF-1alpha-mediated SDF-1 overexpression and recovers
ischemic injury through BM-derived progenitor cell recruitment.
Blood. 117:4376–4386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carlson TR, Feng Y, Maisonpierre PC,
Mrksich M and Morla AO: Direct cell adhesion to the angiopoietins
mediated by integrins. J Biol Chem. 276:26516–26525. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dallabrida SM, Ismail N, Oberle JR, Himes
BE and Rupnick MA: Angiopoietin-1 promotes cardiac and skeletal
myocyte survival through integrins. Circ Res. 96:e8–e24. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SW, Won JY, Lee HY, et al:
Angiopoietin-1 protects heart against ischemia/reperfusion injury
through VE-cadherin dephosphorylation and myocardiac
integrin-beta1/ERK/caspase-9 phosphorylation cascade. Mol Med.
17:1095–1106. 2011.
|
|
23
|
Chen X, Fu W, Tung CE and Ward NL:
Angiopoietin-1 induces neurite outgrowth of PC12 cells in a
Tie2-independent, beta1-integrin-dependent manner. Neurosci Res.
64:348–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosa AI, Goncalves J, Cortes L, Bernardino
L, Malva JO and Agasse F: The angiogenic factor angiopoietin-1 is a
proneurogenic peptide on subventricular zone stem/progenitor cells.
J Neurosci. 30:4573–4584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XS, Chopp M, Zhang RL, et al:
Angiopoietin 2 mediates the differentiation and migration of neural
progenitor cells in the subventricular zone after stroke. J Biol
Chem. 284:22680–22689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SW, Moskowitz MA and Sims JR: Sonic
hedgehog inversely regulates the expression of angiopoietin-1 and
angiopoietin-2 in fibroblasts. Int J Mol Med. 19:445–451.
2007.PubMed/NCBI
|
|
27
|
Lok J, Gupta P, Guo S, et al: Cell-cell
signaling in the neurovascular unit. Neurochem Res. 32:2032–2045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arai K and Lo EH: Oligovascular signaling
in white matter stroke. Biol Pharm Bull. 32:1639–1644. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mukouyama YS, Shin D, Britsch S, Taniguchi
M and Anderson DJ: Sensory nerves determine the pattern of arterial
differentiation and blood vessel branching in the skin. Cell.
109:693–705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carmeliet P and Tessier-Lavigne M: Common
mechanisms of nerve and blood vessel wiring. Nature. 436:193–200.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arai F, Hirao A, Ohmura M, et al:
Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell
quiescence in the bone marrow niche. Cell. 118:149–161. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SW, Won JY, Kim WJ, et al: Snail as a
potential target molecule in cardiac fibrosis: paracrine action of
endothelial cells on fibroblasts through snail and CTGF axis. Mol
Ther. 21:1767–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dohle E, Fuchs S, Kolbe M, Hofmann A,
Schmidt H and Kirkpatrick CJ: Sonic hedgehog promotes angiogenesis
and osteogenesis in a coculture system consisting of primary
osteoblasts and outgrowth endothelial cells. Tissue Eng Part A.
16:1235–1237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Straface G, Aprahamian T, Flex A, et al:
Sonic hedgehog regulates angiogenesis and myogenesis during
post-natal skeletal muscle regeneration. J Cell Mol Med.
13:2424–2435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abou-Khalil R, Le Grand F, Pallafacchina
G, et al: Autocrine and paracrine angiopoietin 1/Tie-2 signaling
promotes muscle satellite cell self-renewal. Cell Stem Cell.
5:298–309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamahara K, Sone M, Itoh H, et al:
Augmentation of neovascularization [corrected] in hindlimb ischemia
by combined transplantation of human embryonic stem cells-derived
endothelial and mural cells. PLoS One. 3:e16662008.
|
|
37
|
Palladino M, Gatto I, Neri V, et al:
Combined therapy with sonic hedgehog gene transfer and bone
marrow-derived endothelial progenitor cells enhances angiogenesis
and myogenesis in the ischemic skeletal muscle. J Vasc Res.
49:425–431. 2012. View Article : Google Scholar
|