Introduction
Chrysanthemum zawadskii var. latilobum (CZ),
is an annual and perennial herb with noteworthy flowers, which has
been used in traditional medicine for the treatment of inflammatory
diseases, gastroenteric disorders, bladder-related disorders and
hypertension (1). The effective
constituents comprised terpenoids and essential oils, as well as
flavonoids and polysaccharides. Terpenoids and flavonoids are
considered the active pharmaceutical ingredients (2), and flower extracts have been shown
to have numerous pharmacological properties, including
anti-allergic, anti-inflammatory and anticancer activities
(3–5).
The hair follicle (HF) is a mammalian skin organ
that produces hair that follows a specific growth cycle with three
distinct and concurrent phases: anagen, catagen and telogen
(6). Findings of previous studies
on HF morphogenesis demonstrated that bulge (a niche for adult stem
cells) stem cells migrate downwards and enter the matrix where they
rapidly proliferate and differentiate to form HFs (7–10).
During anagen, follicles are long and straight, and the
proliferating matrix cells have a cycle length of ~18 h. Dead cells
degenerate as they reach the upper follicle, releasing the hair
shaft which then continues through the skin surface. The duration
of anagen determines the hair shaft length and is dependent on the
continued proliferation and differentiation of matrix cells in the
matrix region of the HF (11).
Animal models comprising a variety of species,
including mice, rats, sheep, rabbits and monkeys, are an important
tool in the study of hair growth. However, murine models are widely
used for hair growth studies due to the availability of large
databases and specific mutants such as nude, hairless, rhino, and
severe combined immunodeficient mice (12,13). To achieve perfectly synchronized
anagen induction, black pigmentation of C57BL/6 or C3H mice in
dorsal skin is employed. This is the most commonly used site for
the observation of anagen initiation as the truncal epidermis in
this species lacks melanin-producing melanocytes and melanin
production is strictly connected with the anagen phase of hair
growth (14,15).
In recent years, traditional herb medicines, such as
eclipta alba, asiasari radix, panax ginseng
and schisandra nigra, have been widely used for hair growth
studies. Eclipta alba induced telogen to anagen transition
and was positive for FGF-7 and Shh and negative for BMP4 (12,16). Asiasari radix was
demonstrated to stimulate hair growth in C57BL/6 and C3H mice by
increasing the proliferation of HaCaT and human dermal papilla
cells (DPCs) and inducing the expression of VEGF in human DPCs
(17). The topical application of
the schisandra nigra extract enhanced hair growth by
increasing the expression of proliferating cell nuclear antigen
(PCNA), proliferation of immortalized vibrissa dermal papilla
cells, and downregulation of transforming growth factor-β2 (TGF-β2)
in the bulb matrix region (18).
The main molecular components in panax ginseng are
ginsenosides which are known to improve cell proliferation of human
DPCs through anti-apoptotic activation (19). In the present study, four
fractions of CZes were used for the induction of hair growth in
order to examine the mechanism of hair growth using
immunohistochemistry.
Materials and methods
Extract preparation
Dried whole plants of CZ were purchased from Jecheon
Medicinal Herb, Inc. (Jecheon, Korea). The dried plant of CZ was
crushed and extracted using 70% ethanol by ultrasonic extraction
for 2 h. The filtered liquid from the extraction was concentrated
in a rotary vacuum evaporator (Eyela N-N, Tokyo, Japan). The
residues were then diluted with deionized water. The extracted
sample was layered step by step using the dissolvents: petroleum
ether, diethyl ether, and n-butanol. Following collection,
concentration and quantification, petroleum ether, diethyl ether,
n-butanol and water fractions were dissolved in 70% ethanol with a
final concentration of 5%. A systematic investigation of cell
development on hair regeneration using water fraction was then
conducted.
Experimental procedures
Eight-week-old, female C57BL/6 mice were used for
all the experimental procedures. The animals were purchased from
Danhan Biolink, Inc. (Eumseong, Korea). Animal experiments were
conducted in accordance with the NIH guide for the care and use of
laboratory animals. The animals were housed under conventional
conditions with food/water available ad libitum and 12-h
light cycle. Mice were divided into five groups (n=7 per group).
All the mice were depilated topically with depilatory cream on
dorsal skin. Each mouse was administered a variety of CZe at a dose
of 1,600 mg/kg body weight by daily topical application for 30
days. The conversion of each phase was recorded on a daily
basis.
Bromodeoxyuridine (BrdU) labeling
BrdU is an analogue of thymidine, which is
incorporated into newly synthesized DNA of replicating cells
(S-phase of the cell cycle), substituting for thymidine during DNA
replication. It is commonly used in the detection of proliferating
and surviving cells in living tissues (20,21). As a proliferative marker of adult
epithelium, the timing of BrdU injection is a critical factor in
differentiating newly proliferated cells from epidermal
label-retaining cells (22). In
order to investigate cell development in different hair growth
stages and to ensure that the results reflected the true level of
cell proliferation and survival stimulated by CZes, a single and a
timed injection of BrdU were used for measuring cell proliferation
and survival independently in different time periods (Fig. 1). To detect cell proliferation,
BrdU was administered by intraperitoneal injection (160 μg/g of
body weight; Sigma, St. Louis, MO, USA) 24 h prior to mice being
sacrificed at day 8, 16 and 21. To detect cell survival, the mice
were injected with BrdU (160 μg/g of body weight) every 12 h for a
total of six injections prior to the 3 days of depilation, followed
by a chase of 19 days. At day 22, mice were then sacrificed and the
surviving BrdU-positive cells were detected as described in
subsequent sections.
Quantification of BrdU-positive
cells
The quantification and analysis of BrdU-positive
cells was performed as previously described with some modifications
(23,24). Briefly, a random selection of
seven complete HFs and equal length of epidermis tissues in each
skin section was counted. The BrdU-labeled cells in the different
parts of the HF were counted in each follicular section by an
observer who was blinded to the study. All counts were performed at
a magnification of ×400 and ×1,000 under a light microscope
(Olympus BX41; Olympus, Tokyo, Japan). A cell was counted as being
in the matrix region of the HF if it was in contact with or in the
matrix region. The mean number of labeled cells in each part of the
HF and epidermis were used for comparison.
Histology and immunohistochemistry
Paraffin-embedded skin tissues were sectioned and
attached to positively charged slides. Histology was carried out
using H&E (Mayer hematoxylin and eosin; Sigma) staining.
Immunohistochemical analysis of skin sections was performed as
previously described (25).
Briefly, the sections were deparaffinized and rehydrated in graded
ethanol and placed in 100 mM phosphate-buffered saline (PBS) for 10
min prior to initiating the staining procedure. The sections were
then sequentially incubated with peroxidase quenching solution (28%
H2O2: absolute pure methanol, 1:9, v:v) to
eliminate the endogenous peroxidase activity for 10 min. After
incubation with the blocking solution (Life Technologies, Carlsbad,
CA, USA) for 15 min, the blotted slides (without washing) were
incubated with anti-BrdU primary antibody (1:200; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), in a moist chamber, at
room temperature (RT) for 3 h. Subsequently, the sections were
incubated with a biotinylated secondary antibody (Life
Technologies) for 20 min at RT. After staining in
3,3′-diaminobenzidine (DAB), slides were dehydrated in a grade
series of alcohol and xylene. The sections were then mounted with
Histomount (Life Technologies).
Statistical analysis
Data were evaluated statistically using one-way
analysis of variance or the Student’s t-test. Data were expressed
as the mean number of proliferating and surviving cells ± the
standard deviation. The significant difference between the values
for the various experimental and control groups was set at
P<0.05.
Results
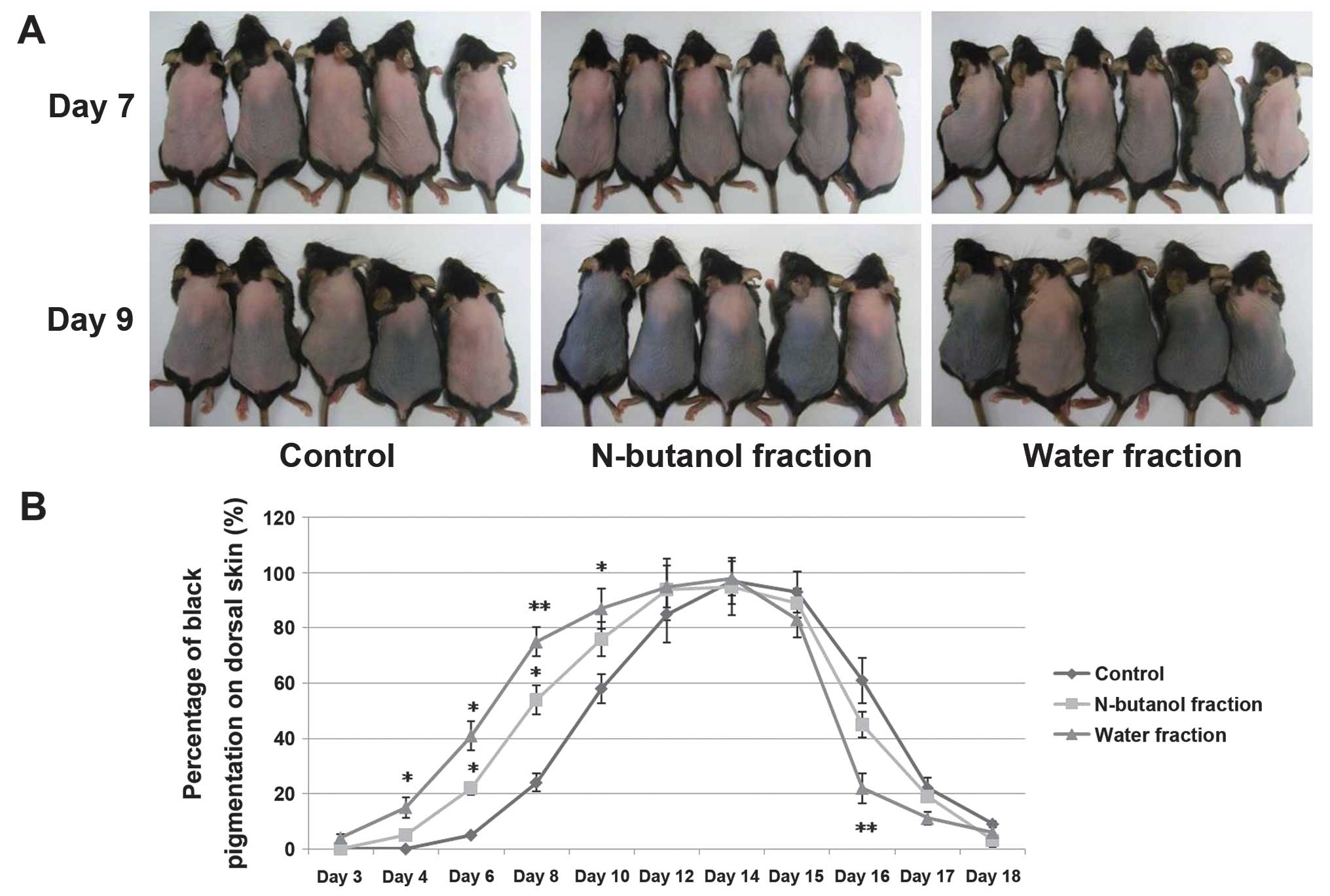
Water and n-butanol fractions of the CZe
induce early onset anagen transition from telogen
Hair growth initiation time was significantly
reduced following treatment with the water fraction of CZe. C57BL/6
murine dorsal hair is known to have a time-synchronized hair growth
cycle and the dorsal skin pigmentation was considered as evidence
for the transition of hair growth stages. Following topical
application onto the backs of mice for up to 7 days, the water
fraction of the CZe induced premature entry of telogen HFs into
anagen whereas the vehicle (70% ethanol, control) showed no anagen
induction until day 9 (Fig. 2A).
The percentage of black pigmentation was observed during
anagen-telogen development in the mice treated with CZes. These
results suggested that CZes, including the n-butanol and water
fractions, significantly induce anagen elongation (Fig. 2B). At day 22, the mean hair shaft
length was observed in the telogen stage (Table I), which showed that the water
fraction of the CZe stimulated hair fiber production. Statistical
analysis demonstrated that the water fraction of the CZe markedly
increased the HF size, and an increase was observed in the diameter
of the matrix region (P=0.053) and the HF length (Table I). In addition, no inflammation,
scaling or drying of skin was observed at the site of application
in any of the animals during the experimental procedure. The
petroleum ether and diethyl ether fractions did not show anagen
induction and hair shaft elongation (data not shown).
 | Table IIn vivo effect of
Chrysanthemum zawadskii extracts on hair growth. |
Table I
In vivo effect of
Chrysanthemum zawadskii extracts on hair growth.
| Anagen (Day 9) | Telogen (Day 22) |
|---|
|
|
|
|---|
| Measurements | N-butanol
fraction | Water fraction | Control | N-butanol
fraction | Water fraction | Control |
|---|
| Hair shaft length
(mm) | - | - | - | 10.57±0.50 | 11.37±0.55a | 10.40±0.76 |
| Hair follicle
counts (2.2 mm longitudinal section) | 17.00±3.58 | 15.33±2.58 | 17.33±2.25 | 11.37±1.77 | 12.00±1.41b | 9.75±1.49 |
| Diameter of
follicle matrix (μm) | 83.00±12.90 | 92.50±9.38c | 81.00±8.81 | 31.55±1.24 | 35.45±4.66 | 31.78±3.7 |
| Hair follicle
length (μm) | 779.00±86.99 |
909.83±82.64b | 756.67±98.24 | 234.93±13.21 | 240.13±19.49 | 224.15±9.63 |
| Dermis thickness
(μm) | 505.00±33.65 | 513.00±53.05 | 501.17±26.87 | 241.87±30.31 | 224.65±17.09 | 276.37±14.91 |
The water fraction of the CZe causes
hyperproliferation in the basal layer of the epidermis
During anagen initiation, the bulge (niche of
multipotent stem cells) stem cells migrate out of the follicle to
generate sebaceous glands and epidermis (9,26,27). In this study, the water fraction
of CZe induced a 2-fold increase in the growth of the epidermis
compared to the control and n-butanol fraction groups (Fig. 3A–D). To understand whether the
thickening of the epidermis was due to increased proliferation in
the basal layer of the epidermis, the proliferation index of BrdU
incorporated by using immunohistochemistry at day 9 was measured.
The results indicated that the water fraction of the CZe increased
proliferation in the basal layer of the epidermis (Fig. 3E–H).
The water fraction of the CZe increases
cell proliferation in anagen and telogen of matrix HFs
Cell proliferation and differentiation in the matrix
is required for HF formation. Pluripotent epidermal matrix cells,
which have dermal papilla at their base, in the hair bulb move
upward while cells at the center of the follicle become the medulla
of the hair shaft (26,28). To investigate whether the increase
of the hair shaft occurred to hyperproliferation in the matrix
region, we traced the cell development of hair matrix during the
anagen to telogen transition. The results suggested that the water
fraction of the CZe markedly increased cell proliferation during
the anagen phase of hair matrix (Fig.
4E–H). Additionally, the quantity of proliferating
BrdU+ cells in telogen demonstrated that the activation
of hyperproliferation in the matrix was maintained in the telogen
stage (Fig. 5I–L). Moreover, when
the quantification of cell proliferation and survival in the
epidermis was performed at day 22, the statistical index showed
that the water fraction of CZe stimulated proliferation in the
basal layer of the epidermis and not only in anagen (Fig. 5A–D), increasing the number of
surviving cells (Fig. 5E–H). In
addition, the n-butanol and water fractions of the CZe had no
effect on proliferation in the mid-section of the HFs (Fig. 4A–D) and no effect on cell survival
in the telogen stage of HF (data not shown).
Discussion
In the present study, we have shown that when the
shaved skin of C57BL/6 mice is treated with topical application of
water and n-butanol fractions of CZe for 25 days, new hair
morphogenesis is initiated in the shaved area, with the initiation
time for this process to occur also being significantly reduced
when compared to vehicle-treated mice. In order to elucidate the
biological mechanism by which CZes induce early onset anagen
transition; we investigated cell development in full-thickness skin
by using BrdU incorporation. The results indicate that the water
fraction of the CZe promoted cell proliferation in the basal layer
of the epidermis and induced the proliferation and differentiation
of epidermal matrix cells in anagen and telogen of HFs. During
anagen, the new HF morphogenesis was initiated by a number of
pluripotent stem cells that are normally present in the bulge
region, including epithelial stem cells. These stem cells migrate
downwards into the regenerating epithelium where they can
differentiate into at least eight different cell lines, forming the
ORS, companion layer, Henle’s layer, Huxley’s layer, cuticle of the
IRS, cuticle of the hair shaft, shaft cortex and shaft medulla
(29,30). It is also known that the lower
region of the whisker follicle contains cells that respond to skin
morphogenetic signals by upward and downward migration and form
epidermis, HFs and sebaceous glands (9,31).
Thus, we hypothesized that the hyperproliferation in the basal
layer of the epidermis may occur via stimulation of the migration
and differentiation of epithelial stem cells during anagen
initiation, and that the premature entry of telogen HFs into anagen
may occur through the induction of epithelial stem cell downward
migration and differentiation to form the new dermal papilla and
hair germ. In addition, stimulation of differentiation and
proliferation of pluripotent epidermal matrix cells in the matrix
region may be one of the mechanisms by which the water fraction of
CZes increase the hair shaft length and the HF size.
Previously, we had performed the screening test by
using crude extracts of herb medicines (17,18). The results indicated that ZC crude
extract showed a marked induction on anagen initiation. To
investigate the effective constituent on hair growth in CZ crude
extract, the petroleum ether, diethyl ether, n-butanol and water
fractions of CZe, were used in the present study. Petroleum ether
and diethyl ether fractions did not show induction on anagen
initiation and hair shaft production. Thus, liposoluble terpenoids,
sterols and essential oils, as the main components, did not
stimulate hair growth. By contrast, flavonoids, the main bioactive
constituents in n-butanol and water fractions, were considered as
effective constituents. Notably, quercetin, luteolin and linarin,
the main flavonoids in CZe, should be studied in future studies to
determine whether they serve as potential effective compounds.
HF is a mammalian skin organ that produces hair in a
process that occurs in phases, including the growth, regression and
resting phases. The migration and differentiation of epithelial
stem cells is principally responsible for hair production (27,32). Anagen is the active growth phase
responsible for forming the HF and hair fiber. During this phase
the roots of the hair divide rapidly and add to the hair shaft
(32). To understand the
mechanism of CZe stimulation of hair shaft production, we labeled
the cell proliferation in the different time-points and noted the
residence time of anagen. The results demonstrate that stimulation
of differentiation in hair matrix is probably initiated by the
water fraction of the CZe. The appearance of black pigmentation was
taken as the anagen initiation (33). Thus, the premature entry into
anagen was considered as an important symbol of migration and
differentiation of epithelial stem cells. Following treatment with
the water fraction of the CZe, the epithelial stem cells were
activated and migrated upward and downward, subsequently
differentiating rapidly to form the interfollicular epidermis and
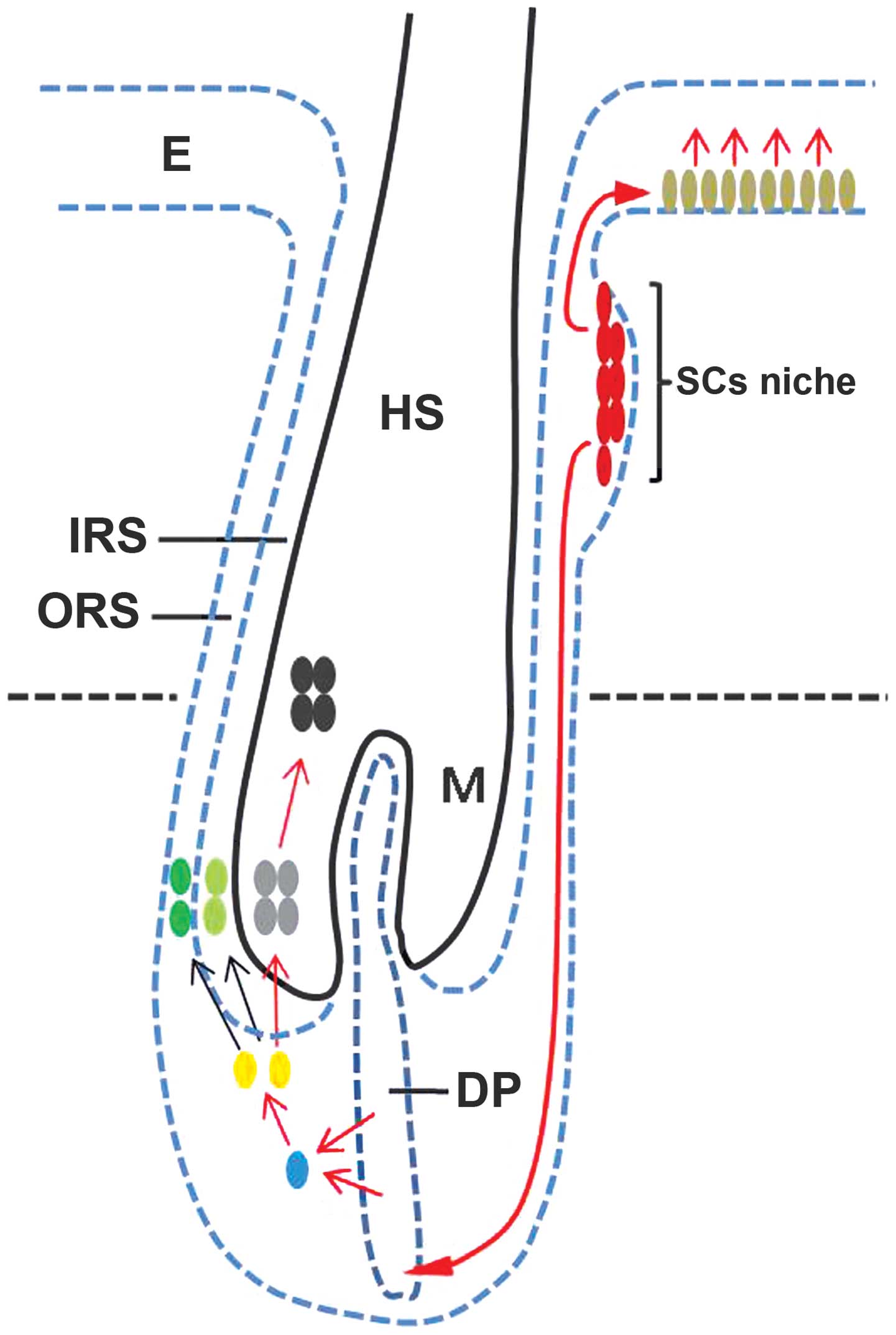
new HF during anagen phase. In consideration of these findings, we
suggest the hypothetical model shown in Fig. 6 (red arrows show the potential
pathway).
Hair cycle is regulated by the interplay of
stimulatory and inhibitory growth factors in mice and humans
(6). Previous studies on the
induction of hair growth as a result of CZ application, reported
that CZes promote proliferation of a human keratinocyte cell line
and human dermal papilla cells in vitro, and subsequently,
suggested that jaceosidin and eupatilin, which are both derived
from CZ, had the potential to promote hair growth through the
downregulation of TGF-β by the jaceosidin-dependent upregulation of
VEGF and Bcl-2 upregulation by eupatilin (34). In the present study, we did not
investigate the signaling pathways involved but performed a novel
evaluation in order to clarify the mechanism by which CZes promote
hair growth using cell labeling and tracing. It provided a more
precise measure of which fraction in CZes is responsible for hair
growth and may therefore provide a more reliable molecular basis
for potential clinical application.
In conclusion, as a traditional herb used as
medicine, Chrysanthemum zawadskii may have wide potential in
clinical application. It has few side effects; no local
inflammation, pruritus, edema, or scaling of skin were observed
during the application procedure. The present study has located the
effective region of hair growth, identified a more accurate
timeline by which growth occurs and provided a novel explanation of
the mechanism by which CZes promote hair growth through the
regulation of cell development at different time periods. Further
analysis of these results as well as a more detailed evaluation of
the molecular mechanism by which CZes induce proliferation of hair
growth are required before its potential can be completely realized
in the clinic.
Acknowledgements
The authors would like to thank Dr Kang-Ju Choi for
guidance in the process of herb extract and isolation. This study
was supported by the DBIO Science Food and Technology
Corporation.
References
|
1
|
Han S, Sung KH, Yim D, et al: The effect
of linarin on LPS-induced cytokine production and nitric oxide
inhibition in murine macrophages cell line RAW264.7. Arch Pharm
Res. 25:170–177. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shim SY, Kang HS, Sun HJ, et al: Isolation
and identification of flavonoids from Gujeolcho (Chrysanthemum
zawadskii var. latilobum) as inhibitor of histamine release.
Food Sci Biotechnol. 21:613–617. 2012. View Article : Google Scholar
|
|
3
|
Seo JY, Lim SS, Park J, et al: Protection
by Chrysanthemum zawadskii extract from liver damage of mice
caused by carbon tetrachloride is maybe mediated by modulation of
QR activity. Nutr Res Pract. 4:93–98. 2010.
|
|
4
|
Singh RP, Agrawal P, Yim D, Agarwal C and
Agarwal R: Acacetin inhibits cell growth and cell cycle
progression, and induces apoptosis in human prostate cancer cells:
structure-activity relationship with linarin and linarin acetate.
Carcinogenesis. 26:845–854. 2005. View Article : Google Scholar
|
|
5
|
Hsu YL, Kuo PL and Lin CC: Acacetin
inhibits the proliferation of Hep G2 by blocking cell cycle
progression and inducing apoptosis. Biochem Pharmacol. 67:823–829.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stenn KS and Paus R: Controls of hair
follicle cycling. Physiol Rev. 81:449–494. 2001.PubMed/NCBI
|
|
7
|
Li LH and Xie T: Stem cell niche:
Structure and function. Annu Rev Cell Dev Biol. 21:605–631. 2005.
View Article : Google Scholar
|
|
8
|
Blanpain C and Fuchs E: Epidermal stem
cells of the skin. Annu Rev Cell Dev Biol. 22:339–373. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gambardella L and Barrandon Y: The
multifaceted adult epidermal stem cell. Curr Opin Cell Biol.
15:771–777. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alonso L and Fuchs E: The hair cycle. J
Cell Sci. 119:391–393. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Datta K, Singh AT, Mukherjee A, Bhat B,
Ramesh B and Burman AC: Eclipta alba extract with potential
for hair growth promoting activity. J Ethnopharmacol. 124:450–456.
2009. View Article : Google Scholar
|
|
13
|
Sundberg JP and King LE Jr: Mouse models
for the study of human hair loss. Dermatol Clin. 14:619–632. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slominski A, Paus R and Costantino R:
Differential expression and activity of melanogenesis-related
proteins during induced hair growth in mice. J Invest Dermatol.
96:172–179. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Slominski A, Paus R, Plonka P, et al:
Melanogenesis during the anagen-catagen-telogen transformation of
the murine hair cycle. J Invest Dermatol. 102:862–869. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roy RK, Thakur M and Dixit VK: Hair growth
promoting activity of Eclipta alba in male albino rats. Arch
Dermatol Res. 300:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rho SS, Park SJ, Hwang SL, et al: The hair
growth promoting effect of Asiasari radix extract and its
molecular regulation. J Dermatol Sci. 38:89–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang JI, Kim SC, Hyun JH, et al: Promotion
effect of Schisandra nigra on the growth of hair. Eur J
Dermatol. 19:119–125. 2009.
|
|
19
|
Kim JH, Yi SM, Choi JE and Son SW: Study
of the efficacy of Korean red ginseng in the treatment of
androgenic alopecia. J Ginseng Res. 33:223–228. 2009. View Article : Google Scholar
|
|
20
|
Konishi T, Takeyasu A, Natsume T, Furusawa
Y and Hieda K: Visualization of heavy ion tracks by labeling 3′-OH
termini of induced DNA strand breaks. J Radiat Res. 52:433–440.
2011.PubMed/NCBI
|
|
21
|
Smith PJ, Howes EA and Treherne JE: Cell
proliferation in the repairing adult insect central nervous system:
incorporation of the thymidine analogue 5-bromo-2-deoxyuridine in
vivo. J Cell Sci. 95:599–604. 1990.
|
|
22
|
Nijhof JG, van Pelt C, Mulder AA, Mitchell
DL, Mullenders LH and de Gruijl FR: Epidermal stem and progenitor
cells in murine epidermis accumulate UV damage despite NER
proficiency. Carcinogenesis. 28:792–800. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blanpain C, Lowry WE, Geoghegan A, Polak L
and Fuchs E: Self-renewal, multipotency, and the existence of two
cell populations within an epithelial stem cell niche. Cell.
118:635–648. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sarin KY, Cheung P, Gilison D, et al:
Conditional telomerase induction causes proliferation of hair
follicle stem cells. Nature. 436:1048–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Hoang-Gia T, Wu H, et al:
Ginsenoside Rb1 improves spatial learning and memory by regulation
of cell genesis in the hippocampal subregions of rats. Brain Res.
1382:147–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alonso L and Fuchs E: Stem cells of the
skin epithelium. Proc Natl Acad Sci USA. 100:11830–11835. 2003.
View Article : Google Scholar
|
|
27
|
Cotsarelis G: Epithelial stem cells: a
folliculocentric view. J Invest Dermatol. 126:1459–1468. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spradling A, Drummond-Barbosa D and Kai T:
Stem cells find their niche. Nature. 414:98–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krause K and Foitzik K: Biology of the
hair follicle: the basics. Semin Cutan Med Surg. 25:2–10. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reynolds AJ and Jahoda CA: Hair follicle
stem cells? A distinct germinative epidermal cell population is
activated in vitro by the presence of hair dermal papilla cells. J
Cell Sci. 99:373–385. 1991.PubMed/NCBI
|
|
31
|
Oshima H, Rochat A, Kedzia C, Kobayashi K
and Barrandon Y: Morphogenesis and renewal of hair follicles from
adult multipotent stem cells. Cell. 104:233–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paus R and Cotsarelis G: The biology of
hair follicles. N Engl J Med. 341:491–497. 1999. View Article : Google Scholar
|
|
33
|
Peters EMJ, Botchkarev VA, Müller-Röver S,
Moll I, Rice FL and Paus R: Developmental timing of hair follicle
and dorsal skin innervation in mice. J Comp Neurol. 448:28–52.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pi LQ, Jin XH, Kim K, et al: Effects of
Chrysanthemum zawadskii extract on hair growth in vivo and
in vitro. Exp Dermatol. 19:5822010.
|