Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, and the majority of patients present with
metastatic stage IV disease at diagnosis (1,2).
The American Cancer Society estimated that 221,130 individuals in
the United States developed lung cancer in 2011, with the number of
deaths reaching 156,940 that year (3). Moreover, the relative 5-year
survival ratio of patients with lung or bronchus cancer remains
relatively low. Therefore, novel treatment strategies for lung
cancer are urgently required (4).
Chemotherapeutics are the mainstream strategy for
the treatment of localized and metastasized cancers. However, the
development of multidrug resistance (MDR) of cancer cells, and the
systemic toxic side-effects resulting from the unspecific
localization of anticancer drugs to non-tumor areas are major
obstacles to the success of chemotherapy in many types of cancer
(5–7). A new combination therapeutic
strategy for cancer, having the advantages of the co-delivery of
more than one therapeutic agent in one delivery system, has
recently been shown to be more effective than monotherapy by
providing the potential synergistic effects of different treatment
mechanisms. The co-delivery of nucleic acids and chemotherapeutics
has been suggested to achieve the combined effect of gene therapy
and chemotherapy (8–11). To date, attempts have been made to
simultaneously deliver genes and drugs into cancer cells using
liposomes (12), polymeric
nanoparticles (13,14), dendrimers (15), etc.
Our group has also worked on nanoparticulate
drug/gene delivery systems (16–19). Solid lipid nanoparticles (SLN)
have been widely developed in our laboratory as they have certain
advantages, such as being less toxic, having low immunogenicity,
and are easily modified (16,17). They also offer a number of
technological advantages, including better storage stability in
comparison to liposomes, the possibility of steam sterilization and
lyophilization, and large scale production with qualified
production lines (20,21).
Transferrin (Tf), an iron-binding glycoprotein, is a
well-studied ligand for delivering anticancer drugs/genes due to
the increased number of Tf receptors found in tumor cells as
compared to normal cells (22).
This receptor-mediated endocytosis may facilitate the delivery of
drugs/genes into cells (23,24). Polyethylene glycol
(PEG)-phosphatidylethanolamine (PEG-PE) conjugates with various PEG
lengths and terminal-targeted moieties can provide extremely
stable, long-circulating, and actively targeted nanocarriers, which
spontaneously accumulate at specific sites (25–27). In this study, Tf was linked to
PEG-PE to form Tf-PEG-PE as ligands for the surface modification of
nanocarriers.
In the present study, Tf-PEG-PE was synthesized and
modified onto the surface of the gene- and drug-loaded SLN. The
in vivo transfection efficiency and antitumor effects of the
novel modified vectors were evaluated using mice bearing A549
(human alveolar adenocarcinoma cell line) tumors. This system was
expected to achieve stable drug and gene loading capacity, prolong
the circulation time by PEG shielding, be recognized by the Tf
receptor on A549 cells and internalized through receptor-mediated
endocytosis, and finally, to achieve the co-delivery of both drug
and gene therapeutic effects.
Materials and methods
Materials
Human Tf (iron-free), stearic acid,
L-α-phosphatidylethanolamine (PE), 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT), 2-iminothiolane (Traut’s
reagent), doxorubicin hydrochloride (DOX·HCl), and
dimethyldioctadecylammonium bromide (DDAB), were purchased from
Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Injectable soya
lecithin was obtained from Shanghai Taiwei Pharmaceutical Co., Ltd.
(Shanghai, China). Maleimide-PEG2000-COOH was purchased
from Shanghai Yare Biotech, Inc. (Shanghai, China). The enhanced
green fluorescence protein plasmid (pEGFP)-N1 was provided by
Shandong University (Shandong, China). Quant-iT™
PicoGreen® dsDNA quantification reagent was obtained
from Invitrogen/Life Technologies (Carlsbad, CA, USA). A549 cells
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). All other chemicals were of analytical grade or
higher.
Animals
BALB/c mice (4–6 weeks old; weighing, 25–30 g) were
purchased from the Medical Animal Test Center of Shandong Province
and housed under standard laboratory conditions. All animal
experiments complied with the requirements of the National Act on
the Use of Experimental Animals (China).
Synthesis of Tf-PEG-PE
The Tf-PEG-PE ligands were synthesized as described
in a previous study of ours (17). Briefly,
maleimide-PEG2000-COOH was dissolved with dimethyl
sulfoxide (DMSO) and stirred with PE as a mixture.
1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride
(EDC·HCl) and triethylamine (TEA) were dissolved in DMSO and added
dropwise into the mixture in an ice bath, and stirred to produce
maleimide-PEG-CO-NH-PE. Tf was firstly modified with Traut’s
reagent to complete the thiolation of Tf. The thiolated Tf was then
added to the maleimide-PEG2000-COOH solution and the
mixture was incubated at room temperature with gentle stirring. The
product was dialyzed against Milli-Q water to form the Tf-PEG-PE
solution. The mixture was centrifuged and then resuspended in
phosphate-buffered saline (PBS, pH 7.4).
Preparation of SLN and SLN/DE
The SLN/pEGFP complexes were prepared as follows:
blank SLN was prepared following the nanoprecipitation method
(solvent displacement technique) as previouly described (16,17). Stearic acid (50 mg) and injectable
soya lecithin (30 mg) were accurately weighed and dissolved in 10
ml acetone. The organic phase was added dropwise into the 0.5% DDAB
solution being stirred at 600 rpm at room temperature. When
complete evaporation of the organic solvent occurred, the redundant
stabilizers were separated by ultracentrifugation at 1,000 × g, 4°C
for 20 min. The pellet was vortexed and resuspended in Milli-Q
water, washed 3 times, filtered through a 0.45-μm membrane, and
adjusted to pH 7.0 with sodium hydroxide. The SLN/pEGFP complexes
were prepared by incubating the SLN with pEGFP. Briefly, pEGFP was
mixed with SLN by vortexing the particles with a 5 mg/ml solution
of pEGFP for 30 sec. The mixture was incubated for 30 min at room
temperature to form SLN/pEGFP (Fig.
1).
The SLN/DOX complexes were prepared as follows:
DOX·HCl was stirred with TEA in DMSO overnight to obtain the DOX
base (28). DOX (5 mg) and
stearic acid (50 mg) and injectable soya lecithin (30 mg) were
dissolved in 10 ml warm ethanol. The resultant organic solution was
rapidly dispersed into distilled water under mechanical stirring at
600 rpm in a water bath at 70°C for 5 min. The obtained solution
was then cooled to room temperature to facilitate the formation of
SLN/DOX. The SLN/pEGFP solution was then added dropwise into the
SLN/DOX solution and stirred at 400 rpm to obtain SLN/DE (Fig. 1).
Preparation of T-SLN/DE
The Tf-PEG-PE ligands were dissolved in 20 ml of
PBS. The solution was then added dropwise into 40 ml of SLN/DE
complexes and stirred at 400 rpm at room temperature, leading to
the immediate modification. Subsequently, free Tf-PEG-PE was
removed from the modified SLN/DE by gel chromatography using a
Sephadex® G-50 column (GE Healthcare Life Sciences,
Piscataway, NJ, USA). The obtained complexes was resuspended in
Milli-Q water and filtered through a membrane with 0.45 μm pore
size to obtain T-SLN/DE. The T-SLN was prepared by the same
procedure using blank SLN without loading pEGFP and DOX (Fig. 1).
Characterization of SLN, SLN/DE and
T-SLN/DE
Physical-chemical characteristics
The mean particle size, polydispersity index (PDI)
and zeta potential of SLN, SLN/DE and T-SLN/DE were analyzed by
photon correlation spectroscopy (PCS) using a Zetasizer 3000
instrument (Malvern Instruments, Malvern, UK). The average particle
size was expressed as the volume mean diameter.
Gene loading capacity
The PicoGreen-fluorometry assay was used to quantify
the amount of pEGFP carried by SLN/DE and T-SLN/DE. The
concentration of pEGFP was determined by fluorescence, comparing
with the supernatant from blank SLN. The amount of pEGFP loaded in
the SLN was calculated according to the linear calibration curve of
pEGFP as follows: gene loading quantity (%) = (total amount of
pEGFP − the amount of free pEGFP)/total amount of pEGFP ×100.
Drug loading (DL) ability
encapsulation efficiency (EE) assay
The DL and EE of the SLN/DE and T-SLN/DE were
determined by a subtraction method. Briefly, 0.2 ml of T-SLN/DE
complexes solution was centrifuged through a filter (EMD Millipore,
Billerica, MA, USA) with a molecular weight cut-off of 3 kDa. Free
DOX could pass through the filter, but T-SLN/DE could not pass
through the filter. Unincorporated DOX in the solution was
quantified by determining the absorbance at 485 nm using a
spectrophotometer, as previously described (29). DL and EE were calculated using the
following equations: DL = [concentration of (total DOX − free DOX)]
× [concentration of (polymer + total DOX − free DOX)]−1
×100% (Equation 1); EE = concentration of (total DOX − free DOX) ×
concentration of total DOX−1 ×100% (Equation 2).
In vitro release assays
The in vitro gene release of SLN/DE and
T-SLN/DE was analyzed in PBS (pH 7.4). Typically, aliquots of
complexes were suspended in 1 ml of PBS (in Eppendorf®
tubes) and vortexed for 30 sec. The tubes were then placed in a
37°C shaking water bath (100 rpm). Separate tubes were used for
different data points. The suspensions were centrifuged at
pre-determined time intevals (1,000 × g for 30 min) and the amount
of pEGFP released into the supernatant was analyzed using the
PicoGreen assay mentioned above. Background readings were obtained
using the supernatants from the blank SLN.
The in vitro drug release of SLN/DE and
T-SLN/DE was analyzed using a modified dialysis method (30,31). Briefly, 0.5 ml complexes were
transferred into a dialysis membrane (MWCO 3000), and 0.5 ml of
free DOX solution in water (0.25 mg/ml) was used as the control.
The solutions were then dialyzed against 20 ml acetic acid sodium
buffer at pH 5.5 and PBS, both containing Tween-20 (0.5%), at 37°C
with gentle shaking. A total of 20 ml of the surrounding dialysis
medium was removed at pre-determined time points for analysis, and
20 ml of fresh buffer at the relevant pH were added to the dialysis
medium. The released DOX from the vectors was able to infiltrate
through the dialysis bag as the molecular weight of the DOX was
<3,000. The released DOX was quantified by determining the
absorbance at 485 nm using a spectrophotometer (F-2500; Hitachi,
Tokyo, Japan).
In vitro cytotoxicity evaluation
To examine the cytotoxicity, A549 cells were seeded
in 48-well plates at 1×105 cells/well and incubated for
24 h to allow cell attachment. The cells were incubated with SLN
and T-SLN complexes at various concentrations (10, 20, 50, 100 and
200 μg/ml) for 48 h at 37°C and a 5% CO2 atmosphere. The
cells without incubation were used as the negative controls. Cell
viability was assessed by MTT assay according to the manufacturer’s
instructions and the absorbance was measured at 570 nm using a
microplate reader (Model 680; Bio-Rad, Hercules, CA, USA). Cells
without the addition of MTT reagents were used as the blank to
calibrate the spectrophotometer to zero absorbance. The relative
cell viability (%) was calculated as (Abssample −
Absblank)/(Abscontrol − Absblank)
×100.
In vitro transfection analysis
For transfection efficiency analysis, the A549 cells
were seeded into 24-well plates at a density of 1×104
cells/well and transfected the following day at 80–90% confluency.
Prior to transfection, the media were replaced with 500 μl
transfection media containing T-SLN/DE. Naked pEGFP, blank SLN and
SLN/DE were used as controls. The original incubation medium was
replaced with 1 ml of complete medium following incubation at 37°C
for 4 h under a 5% CO2 atmosphere. The cells were
incubated and examined until 72 h post-transfection. To quantify
the transfection efficiency, the cells were washed with 1 ml of PBS
(100 g, 4°C for 5 min) and were detached with trypsin/EDTA. The
supernatant was discarded and resuspended with 300 μl of PBS, mixed
well and subjected to flow cytometry to determine the amount of
A549 cells which has been successfully transfected.
In vivo antitumor effects
Tumor-bearing mice were prepared by inoculating
(subcutaneously) a suspension of A549 cells (1×106
cells) into the right armpit of BALB/c mice (32). Briefly, the mice were acclimatized
at a temperature of 25±2°C and a relative humidity of 70±5% under
natural light/dark conditions for 1 week before dosing. The mice
were then subcutaneously injected in the right armpit with A549
cells suspended in PBS. Tumors were allowed to reach 4–5 mm in
diameter before initiation of the experiments.
The in vivo anticancer activity of T-SLN/DE
was evaluated against A549 solid tumors in mice. Five groups of
tumor-bearing mice (6 mice per group) were used. The mice were
injected with 10 mg/kg of T-SLN/DE, SLN/DE, SLN, free DOX solution,
and 0.9% sodium chloride solution (blank control). All drugs were
diluted with 0.9% sodium chloride (100 μl), and all were
administered through direct intratumoral injection. Following drug
administration, mortality was monitored daily and tumor growth was
determined by caliper measurement every 3 days. The tumor volume
was calculated as follows, according to a previously described
method (33): tumor volume
(mm3) = (length × width2)/2 (Equation 3).
Statistical analysis
All experiments were repeated 3 times and all
measurements were carried out in triplicate. The results are
reported as the means ± standard deviation (SD). Statistical
significance was analyzed using the Student’s t-test. The
differences between experimental groups was considered significant
when the P-value was <0.05 (P<0.05).
Results
Characterization of T-SLN/DE
The mean particle size, PDI, zeta potential, gene
loading ability, DL and EE of SLN, SLN/DE and T-SLN/DE were
characterized and are summarized in Table I.
 | Table IParticle size, zeta potential and
gene loading quantity of the different vectors. |
Table I
Particle size, zeta potential and
gene loading quantity of the different vectors.
| Sample
characteristics | SLN | SLN/DE | T-SLN/DE |
|---|
| Mean particle size
(nm) | 82.1±2.9 | 245.5±3.2 | 286.5±3.9 |
| Polydispersity
index (PDI) | 0.13±0.03 | 0.15±0.03 | 0.14±0.02 |
| Zeta potential
(mV) | +41.5±2.6 | +28.3±2.3 | +19.1±1.8 |
| Gene loading
quantity (%) | N/A | 82 | 81 |
| DL (%) | N/A | 8.9±0.8 | 8.7±0.9 |
| EE (%) | N/A | 82.6±1.5 | 81.9±1.3 |
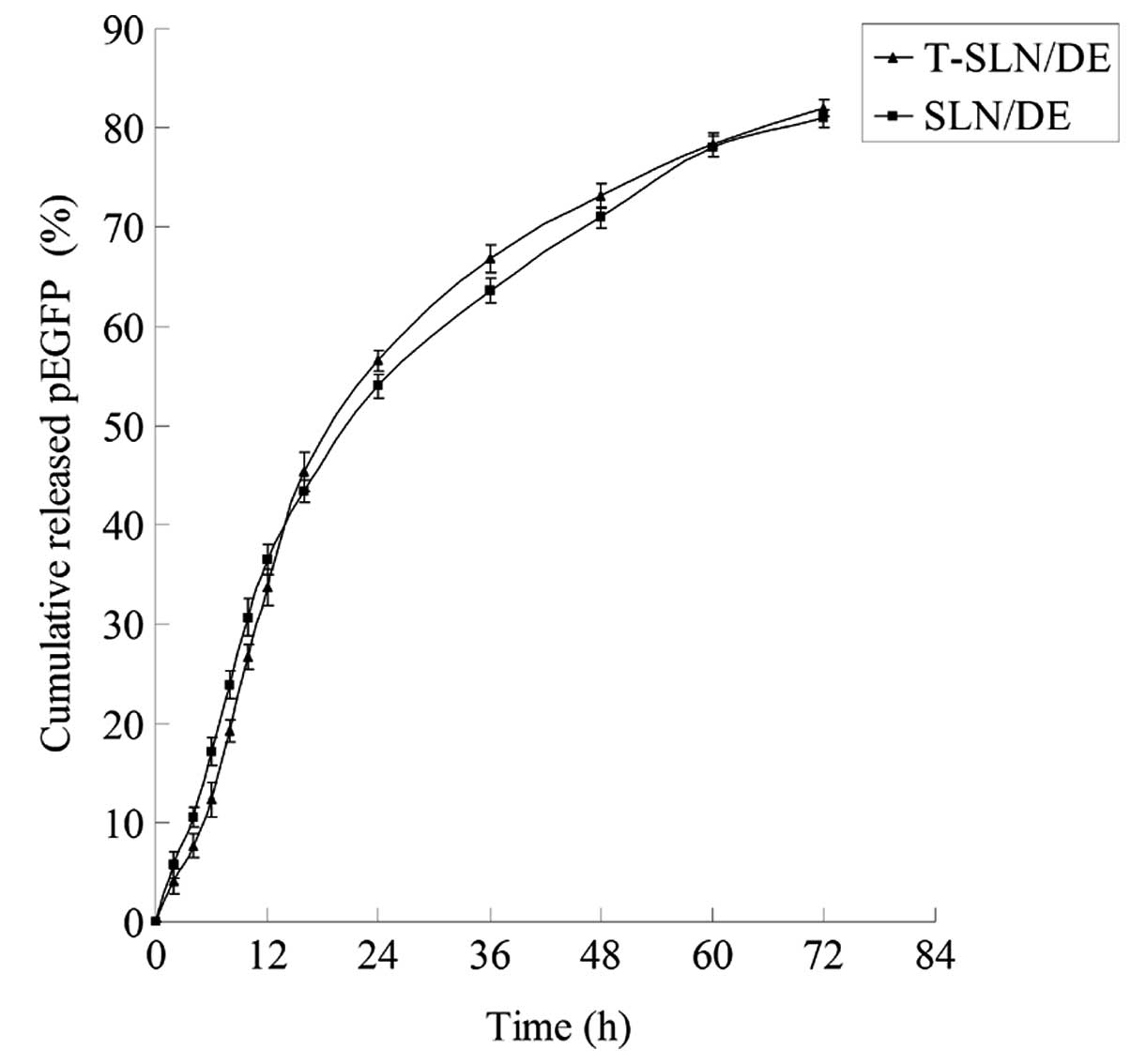
In vitro release assays
The in vitro gene and drug release profiles
of T-SLN/DE and SLN/DE are illustrated in Figs. 2 and 3. The gene and drug release of T-SLN/DE
and SLN/DE reached over 80% at the time point of 72 h.
In vitro cytotoxicity evaluation
The in vitro cytotoxicity of T-SLN and SLN at
various concentrations was evaluated by MTT assay in the A549
cells. As illustrated in Fig. 4,
the cell viability of the A549 cells transfected with the vectors
at the examined concentration range (10–200 μg/ml) was above 80%
compared with the controls (cells without incubation).
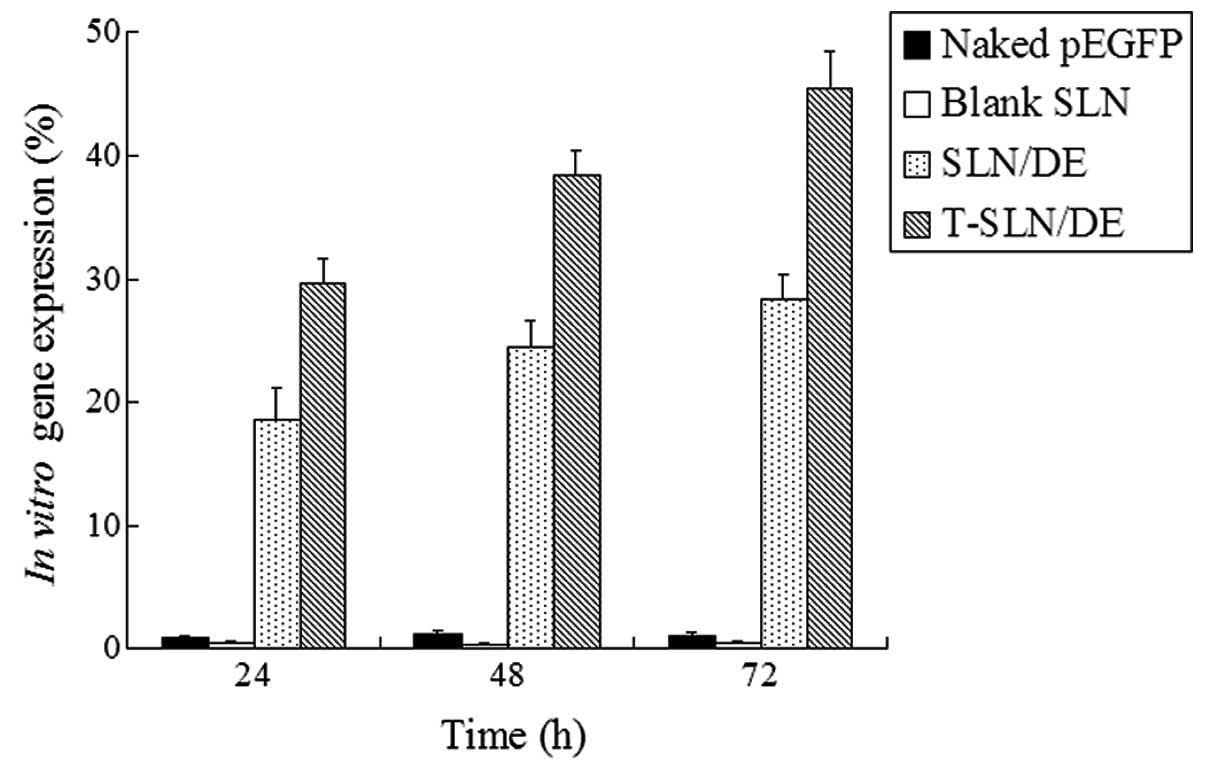
In vitro transfection analysis
The in vitro transfection efficiencies of
T-SLN/DE and SLN/DE were evaluated in the A549 cells until 72 h of
transfection (Fig. 5). T-SLN/DE
showed a higher transfection efficiency than SLN/DE and naked pEGFP
at 48 and 72 h post-transfection (P<0.05).
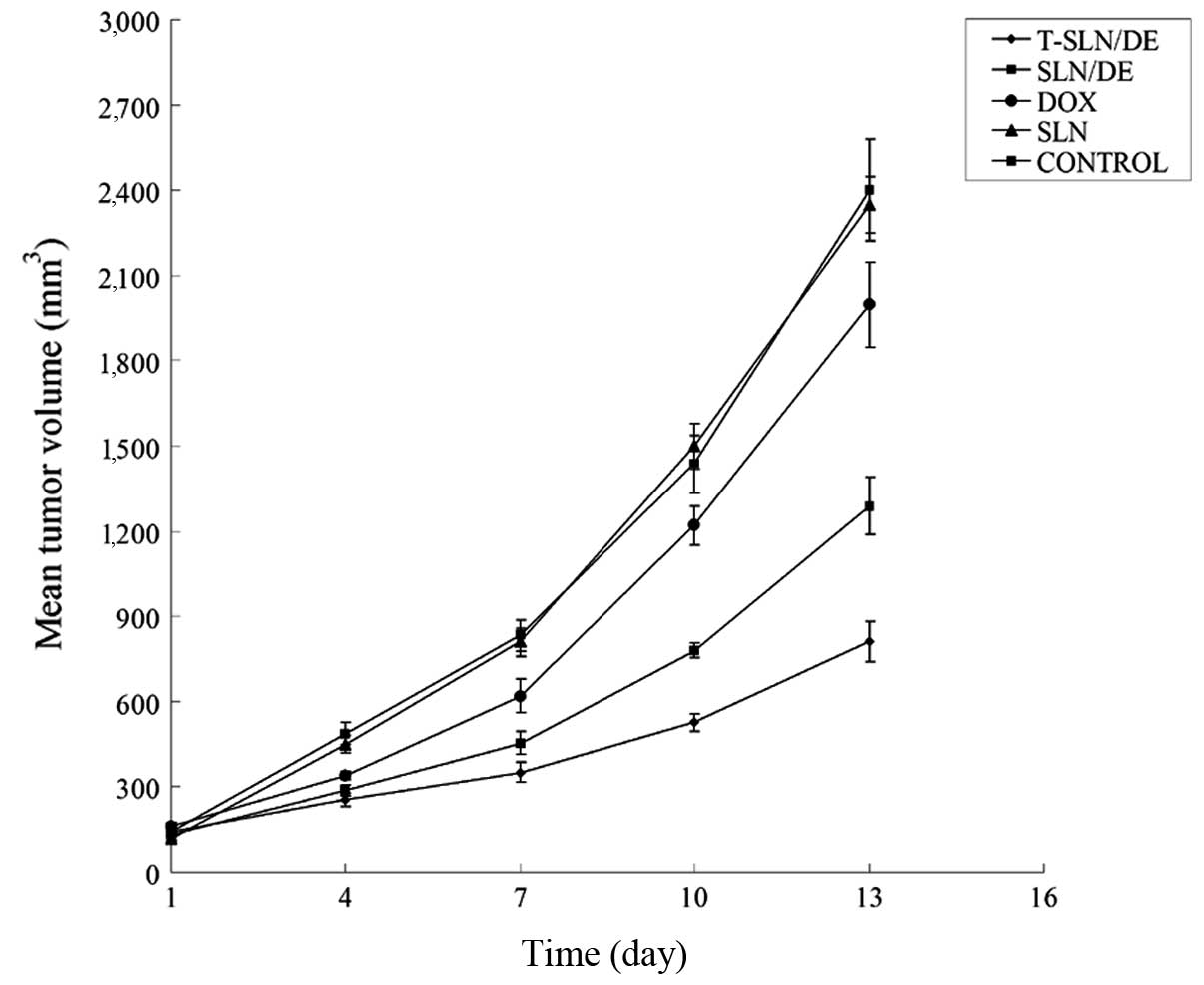
In vivo anticancer efficacy
The in vivo antitumor efficiency of T-SLN/DE
and SLN/DE was observed against A549 solid tumors in mice. The
tumor growth curves of each group are presented in Fig. 6. The results indicated that
treatment with SLN/DE resulted in smaller tumor volume compared
with free DOX; treatment with T-SLN/DE resulted in a smaller tumor
volume compared with the vectors. These results illustrate that the
encapsulation of DOX in SLN enhances the anticancer activity of DOX
in vivo; Tf-modified SLN/DE exerts more enhanced antitumor
effects compared with the vectors not modified with Tf.
Discussion
SLN have been previously developed by our group for
anticancer drug/gene delivery therapy (16–19). This has a number of technological
advantages, including rapid uptake by cells, protection of the
incorporated compound against chemical degradation and potential
for large-scale production (34,35). This study aimd to develop
surface-modified, co-encapsulated SLN containing pEGFP and DOX in
order to create a multifunctional delivery system that will target
lung cancer cells and increase the therapeutic efficacy.
To overcome the barriers of the cellular membrane
and achieve efficient gene therapy, Tf-PEG-PE was applied as a
modifier that was coated on the nanoparticle surface after the
preparation of the gene- and drug-loaded cationic SLN (SLN/DE). Tf
is an iron-binding glycoprotein, which is particularly useful in
targeting cancer cells, as many cancer cells overexpress Tf
receptor (TfR) on their surface (22,23,36). In this study, T-SLN/DE had a size
of 287 nm and a zeta potential of +19 mV (Table I).
PicoGreen-fluorometry assay was carried out to
determine the binding ability and in vitro gene release of
T-SLN/DE and SLN/DE. The gene loading efficiency of T-SLN/DE was
81%, which did not differ significantly from that of SLN/pEGFP
(82%) (Table I). The results
proved that the binding of the Tf-PEG-PE ligand did not detach the
pEGFP from the complexes. The in vitro release profile of
T-SLN/DE had almost the same behavior with SLN/DE (Fig. 2). During the first 12 h, T-SLN/DE
showed a slightly slower release activity than SLN/DE. This
phenomenon may be due to the surface coating of ligands initially
hindering the release of pEGFP. After 12 h and until the end of the
release analysis, the total amount of pEGFP delivered from the 2
types of vehicles was almost the same (over 80%). The DL and EE of
T-SLN/DE and SLN/DE were determined by a subtraction method. The DL
of SLN/DE and T-SLN/DE were approximately 9% and the EE of both
vectors was approximately 82% (Table
I). The results demonstrated that the binding of the Tf-PEG-PE
ligand did not detach the DOX from the complexes and that the
modified vectors were stable. The in vitro drug release
profile of T-SLN/DE a showed slightly slower release than that of
SLN/DE during the first 24 h (Fig.
3). At the end of the release analysis, the total amount of
drugs delivered from the 2 types of vehicles was almost the
same.
In vitro cytotoxicity and transfection
analyses were carried out using A549 cells. The viability of the
cells transfected with T-SLN and SLN at the examined concentration
range was over 80% compared with the controls (Fig. 4). T-SLN did not show a higher
cytotoxicity than SLN at all concentrations. In comparison to naked
pEGFP and SLN/DE, T-SLN/DE had a greater transfection efficiency at
48 and 72 h (P<0.05) (Fig. 5).
This may be explained by the receptor-mediated active targeting
mechanism: Tf on the SLN/DE surface was more likely to bind to the
A549 cells through the TfR on the cells and deliver the gene more
easily into the cells.
The antitumor efficacy of T-SLN/DE was further
examined in tumor-bearing mice. The mice were injected with 10
mg/kg of T-SLN/DE, SLN/DE, SLN, free DOX solution into the tumor
site; 0.9% sodium chloride solution was used as the blank control.
The tumor growth rate was not found to significantly decrease with
free DOX treatment and SLN (similar results were observed with 0.9%
sodium chloride solution). The tumor growth rate was significantly
decreased in the group treated with SLN/DE. Furthermore, T-SLN/DE
showed a greater antitumor effect than the unmodified SLN/DE in
vivo. These results indicate that Tf-modified drug- and
gene-loaded SLN have improved antitumor effects and an excellent
gene transfection efficiency. Therefore, T-SLN/DE can be used as a
promising vehicle for the delivery of antitumor drugs and genes and
may significantly contribute to cancer therapy.
In conclusion, in the current study, Tf-modified
co-encapsulated DOX- and pEGFP-loaded SLN were prepared and
characterized according to their size, loading efficiency and in
vitro drug release. The results revealed that T-SLN/DE may
significantly improve the gene transfection efficiency of the
vector and successfully control the tumor growth rate in
tumor-bearing mice. Conclusively, Tf may function as an excellent
active targeting ligand to improve the cell targeting ability of
carriers. Furthermore, the modified co-delivery system may function
comprehensively to improve the efficacy of anticancer therapy. Our
data indicate that this system may be an excellent carrier for the
delivery of both plasmid DNA and DOX, leading to the enhanced
efficacy of antitumor therapy.
References
|
1
|
Shepherd FA, Bunn PA and Paz-Ares L: Lung
cancer in 2013: state of the art therapy for metastatic disease. Am
Soc Clin Oncol Educ Book. 339–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sundaram S, Trivedi R, Durairaj C, Ramesh
R, Ambati BK and Kompella UB: Targeted drug and gene delivery
systems for lung cancer therapy. Clin Cancer Res. 15:7299–7308.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ward E, Brawley O and Jamel A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawabata A, Baoum A, Ohta N, Jacquez S,
Seo GM, Berkland C and Tamura M: Intratracheal administration of a
nanoparticle-based therapy with the angiotensin II type 2 receptor
gene attenuates lung cancer growth. Cancer Res. 72:2057–2067. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bajelan E, Haeri A, Vali AM, Ostad SN and
Dadashzadeh S: Co-delivery of doxorubicin and PSC 833 (Valspodar)
by stealth nanoliposomes for efficient overcoming of multidrug
resistance. J Pharm Pharm Sci. 15:568–582. 2012.PubMed/NCBI
|
|
6
|
Brannon-Peppas L and Blanchette JO:
Nanoparticle and targeted systems for cancer therapy. Adv Drug
Deliv Rev. 56:1649–1659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Liu F, Feng L, Li M, Zhang J and
Zhang N: The targeted co-delivery of DNA and doxorubicin to tumor
cells via multifunctional PEI-PEG based nanoparticles.
Biomaterials. 34:2547–2564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng C, Zheng M, Gong P, Deng J, Yi H,
Zhang P, Zhang Y, Liu P, Ma Y and Cai L: Polypeptide cationic
micelles mediated co-delivery of docetaxel and siRNA for
synergistic tumor therapy. Biomaterials. 34:3431–3438. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loh XJ, Ong SJ, Tung YT and Choo HT:
Co-delivery of drug and DNA from cationic dual-responsive micelles
derived from poly(DMAEMA-co-PPGMA). Mater Sci Eng C Mater Biol
Appl. 33:4545–4550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao J, Mi Y and Feng SS: Targeted
co-delivery of docetaxel and siPlk1 by herceptin-conjugated vitamin
E TPGS based immunomicelles. Biomaterials. 34:3411–3421. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shim G, Han SE, Yu YH, et al: Trilysinoyl
oleylamide-based cationic liposomes for systemic co-delivery of
siRNA and an anticancer drug. J Control Release. 155:60–66. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Z, Zhang Z, Chen Y, Chen L, Lin L and
Li Y: The characteristics and performance of a multifunctional
nanoassembly system for the co-delivery of docetaxel and iSur-pDNA
in a mouse hepatocellular carcinoma model. Biomaterials.
31:916–922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu B, Ji M, Song X, et al: Co-delivery of
docetaxel and endostatin by a biodegradable nanoparticle for the
synergistic treatment of cervical cancer. Nanoscale Res Lett.
7:6662012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaneshiro TL and Lu ZR: Targeted
intracellular codelivery of chemotherapeutics and nucleic acid with
a well-defined dendrimer-based nanoglobular carrier. Biomaterials.
30:5660–5666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Z, Sun C, Yin Z, Zhou F, Ge L, Liu X
and Kong F: Comparison of two kinds of nanomedicine for targeted
gene therapy: premodified or postmodified gene delivery systems.
Int J Nanomedicine. 7:2019–2031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Zhou F, Ge L, Liu X and Kong F:
Transferrin-PEG-PE modified dexamethasone conjugated cationic lipid
carrier mediated gene delivery system for tumor-targeted
transfection. Int J Nanomedicine. 7:2513–2522. 2012.
|
|
18
|
Kong F, Ge L, Liu X, Huang N and Zhou F:
Mannan-modified PLGA nanoparticles for targeted gene delivery. Int
J Photoenergy. 2012:9267542012. View Article : Google Scholar
|
|
19
|
Kong F, Zhou F, Ge L, Liu X and Wang Y:
Mannosylated liposomes for targeted gene delivery. Int J
Nanomedicine. 7:1079–1089. 2012. View Article : Google Scholar
|
|
20
|
Olbrich C, Bakowsky U, Lehr CM, Müller RH
and Kneuer C: Cationic solid-lipid nanoparticles can efficiently
bind and transfect plasmid DNA. J Control Release. 77:345–355.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vighi E, Ruozi B, Montanari M, Battini R
and Leo E: Re-dispersible cationic solid lipid nanoparticles (SLNs)
freeze-dried without cryoprotectors: characterization and ability
to bind the pEGFP-plasmid. Eur J Pharm Biopharm. 67:320–328. 2007.
View Article : Google Scholar
|
|
22
|
Bellocq NC, Pun SH, Jensen GS and Davis
ME: Transferrin-containing, cyclodextrin polymer-based particles
for tumor-targeted gene delivery. Bioconjug Chem. 14:1122–1132.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H and Qian ZM: Transferrin/transferrin
receptor-mediated drug delivery. Med Res Rev. 22:225–250. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maruyama K: Intracellular targeting
delivery of liposomal drugs to solid tumors based on EPR effects.
Adv Drug Deliv Rev. 63:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torchilin VP: Multifunctional
nanocarriers. Adv Drug Deliv Rev. 58:1532–1555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Torchilin VP: Recent advances with
liposomes as pharmaceutical carriers. Nat Rev Drug Discov.
4:145–160. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lukyanov AN, Gao Z, Mazzola L and
Torchilin VP: Polyethylene glycol-diacyllipid micelles demonstrate
increased acculumation in subcutaneous tumors in mice. Pharm Res.
19:1424–1429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miao J, Du YZ, Yuan H, Zhang XG and Hu FQ:
Drug resistance reversal activity of anticancer drug loaded solid
lipid nanoparticles in multi-drug resistant cancer cells. Colloids
Surf B Biointerfaces. 110:74–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao X, Wang B, Wei X, et al: Preparation,
characterization and application of star-shaped PCL/PEG micelles
for the delivery of doxorubicin in the treatment of colon cancer.
Int J Nanomedicine. 8:971–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Men K, Liu W, Li L, et al: Delivering
instilled hydrophobic drug to the bladder by a cationic
nanoparticle and thermo-sensitive hydrogel composite system.
Nanoscale. 4:6425–6433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang BL, Gao X, Men K, et al: Treating
acute cystitis with biodegradable micelle-encapsulated quercetin.
Int J Nanomedicine. 7:2239–2247. 2012.PubMed/NCBI
|
|
32
|
Li P, Liu D, Miao L, Liu C, Sun X, Liu Y
and Zhang N: A pH-sensitive multifunctional gene carrier assembled
via layer-by-layer technique for efficient gene delivery. Int J
Nanomedicine. 7:925–939. 2012.PubMed/NCBI
|
|
33
|
Jia Y, Yuan M, Yuan H, et al:
Co-encapsulation of magnetic Fe3O4
nanoparticles and doxorubicin into biodegradable PLGA nanocarriers
for intratumoral drug delivery. Int J Nanomedicine. 7:1697–1708.
2012.PubMed/NCBI
|
|
34
|
He SN, Li YL, Yan JJ, Zhang W, Du YZ, Yu
HY, Hu FQ and Yuan H: Ternary nanoparticles composed of cationic
solid lipid nanoparticles, protamine, and DNA for gene delivery.
Int J Nanomedicine. 8:2859–2869. 2013.PubMed/NCBI
|
|
35
|
Mehnert W and Mäder K: Solid lipid
nanoparticles: production, characterization and applications. Adv
Drug Deliv Rev. 47:165–196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh M: Transferrin as a targeting ligand
for liposomes and anticancer drugs. Curr Pharm Des. 5:443–451.
1999.PubMed/NCBI
|