Introduction
The incidence of bladder cancer, a common urologic
cancer, continues to increase annually, ranking as the ninth most
common malignancy wordwide (1).
Although treatment with systemic chemotherapy is recommended, the
prognosis for patients with metastatic bladder cancer is poor.
Therefore, improvement of existing therapies and development of
alternative therapeutic approaches is critical. Recent advances in
the study on tumor-initiating cells, a small subpopulation of tumor
cells that contribute to tumor initiation, metastasis and
drug-resistance (2), suggest that
targeting these cells may lead to novel therapies that can be
utilized in the reduction of risk of tumor recurrence.
The peroxisome proliferator-activated receptor-γ
(PPAR-γ) is a member of the nuclear receptor superfamily that is
activated by its ligands. The activation of PPAR-γ may lead to cell
growth arrest, apoptosis, decrease of cell adhesion and migration,
and particularly, result in the differentiation of cancer cells
(3). The property of their
antigrowth and prodifferentiation renders natural and synthetic
ligands of PPAR-γ as attractive substances in cancer prevention and
treatment (3–6). However, given that PPAR-γ ligands
often trigger crosstalk with other signalling pathways (6–8),
use of PPAR-γ agonists alone on much more common advanced
epithelial malignancies has minimal clinical effect (9). Therefore, the combination of PPAR-γ
agonists with other drugs, such as EGFR inhibitor (10) or AKT inhibitor (11) has been examined for cancer
treatment.
One of the hallmarks of tumor cells is the ability
to evade apoptosis (12).
Overexpression of antiapoptotic genes is one of mechanisms to
escape cancer cell apoptosis. As an important member of the
inhibitor of apoptosis gene family, survivin can block the
activation of effector caspases in intrinsic and extrinsic pathways
of apoptosis. Survivin is absent in normal urothelium, whereas it
is present in 64–100% of bladder cancers (13). Moreover, the expression of
survivin is associated with high stage and grade as well as with an
increase risk of recurrence for patients with bladder cancer
(14–17). Thus, survivin has been suggested
as a suitable target for the development of specific treatment of
bladder cancer (15). In the
present study, we report that the combination of PPAR-γ activation
and survivin inhibition generates a more robust suppression in the
cell survival and stem cell properties of bladder cancer cells,
providing a basis for future studies testing the strategy for
experimental manipulation of bladder cancer.
Materials and methods
Cell culture
The human bladder cancer cell lines, T24 and 5637,
obtained from the American Type Culture Collection (Manassas, VA,
USA), were cultured in a maintenance medium containing DMEM with
high glucose supplemented with 10% fetal bovine serum and
penicillin/streptomycin [1% (v/v)] (all from Gibco, Grand Island,
NY, USA). The cells were then treated with 15d-PGJ2
(Sigma-Aldrich, St. Louis, MO, USA) for the indicated times (6
days). Cell cultures were maintained at 37°C in a humidified
atmosphere with 5% CO2.
siRNA of survivin
Survivin RNAi oligos and negative control high GC
oligo were purchased from Sigma-Aldrich. The siRNA sequences are
listed in Table I. One day prior
to the transfection, T24 and 5637 cells were seeded in 6-well
plates without antibiotics. Using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA), the siRNAs (60 nM) were transfected into the
cells according to the manufacturer’s instructions.
 | Table IList of siRNA sequences. |
Table I
List of siRNA sequences.
| Name | | siRNA sequence |
|---|
| siSurvivin1 | F |
GUCUGGACCUCAUGUUGUUdTdT |
| R |
AACAACAUGAGGUCCAGACdTdT |
| siSurvivin2 | F |
CCUCUACUGUUUAACAACAdTdT |
| R |
UGUUGUUAAACAGUAGAGGdTdT |
| siSurvivin3 | F |
GGUUUAUUCCCUGGUGCCAdTdT |
| R |
UGGCACCAGGGAAUAAACCdTdT |
Cell viability assay
To evaluate the effect of 15d-PGJ2 on T24
and 5637 cell growth, cell viability was determined by the
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) (Sigma-Aldrich) assay. In brief, a total of 1,000 5637 or
5,000 T24 cells/well were seeded in 96-well plates (BD Biosciences,
San Jose, CA, USA) in a volume of 200 μl. Subsequent to incubation
with 0.5 or 2 μg/ml 15d-PGJ2 for the indicated times, 20
μl MTT solution [5 mg/ml in phosphate-buffered saline (PBS)] was
added to each well and incubated for an additional 4 h at 37°C. MTT
solution was aspirated off, 150 μl dimethyl sulfoxide (DMSO) was
added to each well, and the absorbance was measured at 540 nm. Data
were recorded on a daily basis and the growth curve was drawn.
Cell cycle analysis
Cells were collected and centrifuged at 300 × g at
4°C for 5 min and resuspended by PBS in tubes. The abovementioned
steps were then repeated. The cells were fixed in ice-cold 70%
ethanol overnight. After washing with PBS twice, the cells were
labelled with propidium iodide (PI) (50 μg/ml; Sigma-Aldrich) and
treated with RNase A (100 μg/ml; Amresco, Solon, OH, USA) for 30
min in the dark. The cells were then analyzed using a FACSCalibur
flow cytometer (BD Biosciences).
Apoptosis assay
T24 and 5637 cells were collected, centrifuged at
300 × g at 4°C for 5 min, and washed twice with PBS containing 0.5%
BSA. The cells were dissociated in 1X binding buffer and the cell
concentration was adjusted to 1×106/ml. Cell suspension
(100 μl) was added with Annexin V-FITC (BD Biosciences) and 7-AAD
(Sigma-Aldrich) according to the manufacturer’s instructions, and
incubated for 20 min in the dark. Following the addition of 200 μl
1X binding buffer in the tube, FACS was performed.
Western blot analysis
Cells were lysed in a RIPA lysis buffer (Beyotime,
Nantong, China) with Protease Inhibitor Cocktail and PhosSTOP
(Roche, Monza, IT, USA). Proteins were detected using indicated
antibodies: anti-PPAR-γ, anti-survivin (all from Cell Signaling
Technology, Beverly, MA, USA); anti-GAPDH, anti-α-tubulin (both
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The
ChemiDoc™ XRS system (Bio-Rad Laboratories, Hercules, CA, USA) was
used for obtaining images.
Sphere formation assay
To assay sphere formation efficiency, single cells
were plated in Ultra Low Attachment plates (Costar, Corning, NY,
USA) and cultured in 1:1 DMEM:F12 (Gibco) supplemented with B27
(1:50; Invitrogen), 20 ng/ml epidermal growth factor and 20 ng/ml
basic fibroblast growth factor (R&D Systems, Minneapolis, MN,
USA). The cells were incubated in a CO2 incubator for
1–2 weeks, and spheres were counted under a stereomicroscope
(Olympus, Tokyo, Japan).
Measurement of reactive oxygen species
(ROS) accumulation
ROS was monitored by FACS using dihydroethidium
(DHE) (Invitrogen). Cells were incubated with 5 μM DHE at 37°C for
30 min, and fluorescence was measured by a FACSCalibur flow
cytometer.
Statistical analysis
Data are presented as the means ± SEM. Statistical
analyses were conducted using SPSS 13.0 for Windows. Data between
two groups were assessed using the Student’s t-test. P <0.05 was
considered to indicate statistical significance.
Results
15d-PGJ2 effectively inhibits
cell proliferation and stem cell-like properties of bladder cancer
cells
Since PPAR-γ agonists are known to inhibit cell
growth in various types of cancer cells (18–20), we first detected the effect of
15d-PGJ2, a natural PPAR-γ ligand, on the cell viability
of bladder cancer cells. T24 and 5637 cells were treated with
15d-PGJ2 at various concentrations for the indicated
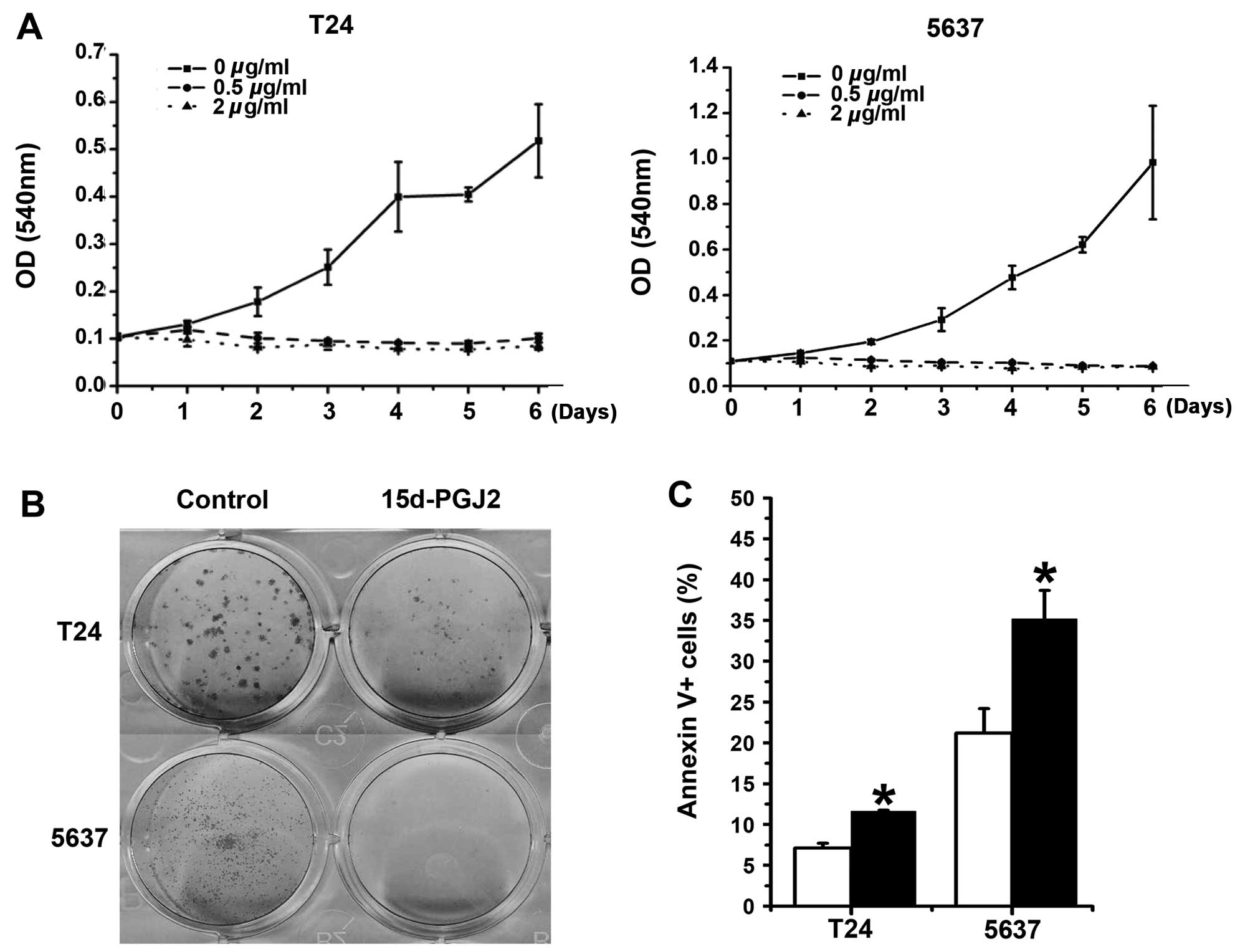
times (6 days). 15d-PGJ2 efficiently suppressed T24 and
5637 cell growth (Fig. 1A).
Similarly, we observed the inhibitory effect of 15d-PGJ2
on bladder cancer cells by measurement of foci formation and cell
apoptosis (Fig. 1B and C).
Since PPAR-γ agonists have been observed to regulate
differentiation of myxoid/round cell liposarcoma (21) and inhibit tumor-initiating cells
in brain and liver cancers (22),
we investigated the possibility that 15d-PGJ2 treatment
affects the stem cell-like properties of bladder cancer cells. The
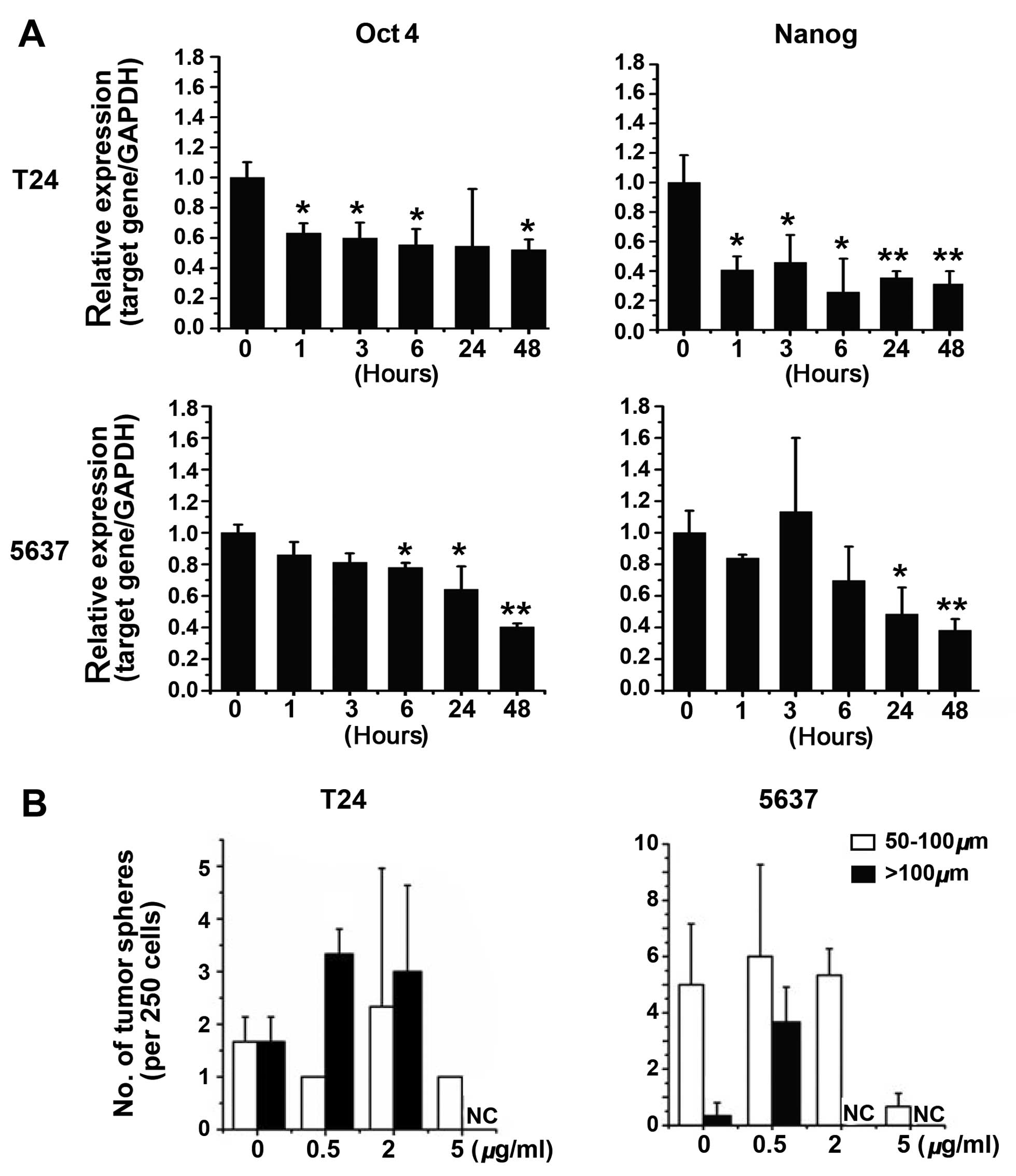
expression of the stemness-related genes, Oct4 and
Nanog, was significantly downregulated in T24 and 5637 cells
following the treatment of 15d-PGJ2 (Fig. 2A). Of note, the decreased
expression of Oct4 and Nanog genes was observed
starting ~1 h after treatment with 15d-PGJ2 in T24
cells, suggesting the key role of 15d-PGJ2 on the
repression of the stem-like phenotype of bladder cancer cells. We
also performed a speroid formation assay. The results showed that
15d-PGJ2 treatment at low concentrations had no
significant effect on the spheroid formation of T24 or 5637 cells
(Fig. 2B). Only the treatment
with a high dose of 15d-PGJ2 (up to 5 μg/ml) decreased
the spheroid number that bladder cancer cells formed (Fig. 2B), suggesting that the treatment
of 15d-PGJ2 alone is not sufficient to prevent bladder
cancer.
Survivin inhibition accelerates the
suppressive effect of 15d-PGJ2 on cell proliferation and
the stem cell-like properties of bladder cancer cells
The anti-apoptotic protein survivin has been
demonstrated as a promising biomarker for detection and prognosis
in bladder cancer. Thus, we hypothesized whether the combination of
15d-PGJ2 and survivin inhibition may more efficiently
inhibit cell growth and the stem-like phenotype of bladder cancer
cells as compared to the single treatment of 15d-PGJ2.
We first evaluated the expression of survivin in
15d-PGJ2-treated bladder cancer cells. The results
showed no significant difference in the expression of survivin
between the cells treated with or without 15d-PGJ2
(Fig. 3), suggesting that
downregulation of survivin may increase the efficiency of
15d-PGJ2 treatment. Survivin expression was depleted
with specific siRNAs in the T24 and 5637 cells and the
effectiveness of survivin siRNAs was validated by western blotting
(Fig. 4A). In the presence of
15d-PGJ2, we found that inhibition of survivin
expression by specific siRNAs increased cell apoptosis induced by
15d-PGJ2 (Fig. 4B).
Moreover, siRNAs against survivin strengthened the suppressive
effect of 15d-PGJ2 on the spheroid formation of T24
cells (Fig. 5A). Notably, the
downregulation of survivin by siRNA did not facilitate
15d-PGJ2-mediated inhibition of the stemness-related
genes in 5637 cells, even if survivin depletion alone affected the
expression of Oct4 and Nanog (Fig.
5B), suggesting that survivin inhibition by siRNAs exacerbated
the inhibitory effects of 15d-PGJ2 on bladder cancer
cells by directly inducing cell death.
15d-PGJ2 enhances survivin
inhibition-induced production of ROS in bladder cancer cells
Since oxidative stress is one of the most important
regulatory mechanisms for cell apoptosis and differentiation
(23–25), we evaluated the generation of ROS
in bladder cancer cells treated with 15d-PGJ2 or
transfected with survivin-specific siRNAs. Depleting the survivin
expression significantly induced the production of ROS in the T24
and 5637 cells, and 15d-PGJ2 further facilitated the
generation of ROS (Fig. 5C and
D). The upward trend in ROS was consistent with an increase of
cell apoptosis induced by 15d-PGJ2 and/or survivin
inhibition (Fig. 4B), suggesting
that generation of ROS may be responsible for the inhibition of
cell proliferation-mediated 15d-PGJ2 and/or survivin in
bladder cancer cells.
Discussion
PPAR-γ participates in multiple biological pathways,
such as lipid metabolism, energy homeostasis, cell proliferation,
death and differentiation (26,27), and various pathogenic processes
including inflammation, diabetes, atherosclerosis and cancer
(28–30). However, despite extensive studies
on the PPAR-γ agonists for tumor suppression, the effects of PPAR-γ
agonists in tumor-initiating cells (TICs) is still poorly defined.
In this study, we showed that 15d-PGJ2, the natural
ligand of PPAR-γ, impaired the maintenance and function of TICs in
bladder cancer cells. Moreover, the combination of survivin
inhibition and 15d-PGJ2 yielded greater inhibition of
cultured cell spheroid formation and cell growth of bladder cancer
cells.
It is becoming increasingly evident that TICs
overexpress multidrug resistance proteins (31,32), which provide a possible
explanation for the failure of standard chemotherapy (33–36). Our results have demonstrated that
15d-PGJ2 significantly repressed the spheroid formation
of bladder cancer cells, decreased the expression of
stemness-related genes, indicating that PPAR-γ agonists have a
marked inhibitory effect on tumor-initiating cells of human bladder
cancer. Survivin is a key biomarker for the detection of bladder
cancer metastasis (13,14). When we combined
15d-PGJ2 with survivin depletion, the cell proliferation
and spheroid formation were more efficiently suppressed than either
alone. These findings raise the possibility that the combination of
survivin suppressants and PPAR-γ agonists is likely a new therapy
for bladder cancer.
ROS play critical roles in the regulation of cell
proliferation, apoptosis, and transformation (24,37). It has recently been established
that 15d-PGJ2 negatively regulates cell proliferation by
eliciting the production of ROS (38–41). More importantly, previous studies
have demonstrated that the level of intracellular ROS is associated
with TICs (42–46). In the present results, we
demonstrated that 15d-PGJ2 upregulated the production of
ROS, and knockdown of survivin obviously enhanced the generation of
ROS stimulated by 15d-PGJ2, suggesting that
15d-PGJ2 and/or survivin inhibition restrained bladder
cancer stem-like phenotype and cell proliferation possibly by
upregulating ROS production. NADPH oxidases and mitochondria are
two major sources of ROS generation (47,48). A recent study has suggested that
PPAR-γ agonist may involve mitochondrial function (49). Additionally, it has been shown
that PPAR-γ agonists inhibit stem cell-like phenotype and cell
proliferation of liver cancer cells via NOX2-mediated oxidative
stress (50). Thus, the manner in which the PPAR-γ agonist is
involved in the production of ROS induced by 15d-PGJ2
and/or survivin suppression in bladder cancer cells remains to be
investigated.
In conclusion, we have shown that cotreatment of
15d-PGJ2 and survivin RNAi synergistically inhibit
bladder cancer stem-like phenotype and cell proliferation in
vitro. These observations suggest that the combined treatment
with survivin inhibitor and PPAR-γ agonists may be of therapeutic
importance in the clinical treatment of malignant tumors.
Acknowledgements
This study was funded by the Shanghai Minhang
Natural Science Foundation (NO. 2009MHZ109).
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basile KJ and Aplin AE: Resistance to
chemotherapy: short-term drug tolerance and stem cell-like
subpopulations. Adv Pharmacol. 65:315–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michalik L, Desvergne B and Wahli W:
Peroxisome-proliferator-activated receptors and cancers: complex
stories. Nat Rev Cancer. 4:61–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarraf P, Mueller E, Jones D, et al:
Differentiation and reversal of malignant changes in colon cancer
through PPARgamma. Nat Med. 4:1046–1052. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang TH and Szabo E: Induction of
differentiation and apoptosis by ligands of peroxisome
proliferator-activated receptor gamma in non-small cell lung
cancer. Cancer Res. 60:1129–1138. 2000.PubMed/NCBI
|
|
6
|
Keshamouni VG, Reddy RC, Arenberg DA, et
al: Peroxisome proliferator-activated receptor-γ activation
inhibits tumor progression in non-small-cell lung cancer. Oncogene.
23:100–108. 2004.
|
|
7
|
Chen YC, Shen SC and Tsai SH:
Prostaglandin D(2) and J(2) induce apoptosis in human leukemia
cells via activation of the caspase 3 cascade and production of
reactive oxygen species. Biochim Biophys Acta. 1743:291–304. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ray DM, Akbiyik F and Phipps RP: The
peroxisome proliferator-activated receptor gamma (PPARgamma)
ligands 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone induce
human B lymphocyte and B cell lymphoma apoptosis by
PPARgamma-independent mechanisms. J Immunol. 177:5068–5076. 2006.
View Article : Google Scholar
|
|
9
|
Girnun GD, Naseri E, Vafai SB, et al:
Synergy between PPARgamma ligands and platinum-based drugs in
cancer. Cancer Cell. 11:395–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mansure JJ, Nassim R, Chevalier S, et al:
A novel mechanism of PPARγ induction via EGFR signalling
constitutes rational for combination therapy in bladder cancer.
PLoS One. 8:e559972013.
|
|
11
|
Liu L, Yang Z, Xu Y, et al: Inhibition of
oxidative stress-elicited AKT activation facilitates PPARγ
agonist-mediated inhibition of stem cell character and tumor growth
of liver cancer cells. PLoS One. 8:e730382013.PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Margulis V, Lotan Y and Shariat SF:
Survivin: a promising biomarker for detection and prognosis of
bladder cancer. World J Urol. 26:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shariat SF, Ashfaq R, Karakiewicz PI, et
al: Survivin expression is associated with bladder cancer presence,
stage, progression, and mortality. Cancer. 109:1106–1113. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akhtar M, Gallagher L and Rohan S:
Survivin: role in diagnosis, prognosis, and treatment of bladder
cancer. Adv Anat Pathol. 13:122–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Atlasi Y, Mowla SJ and Ziaee SA:
Differential expression of survivin and its splice variants,
survivin-DeltaEx3 and survivin-2B, in bladder cancer. Cancer Detect
Prev. 32:308–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Wang T, Yang D, et al: Expression
of the IAP protein family acts cooperatively to predict prognosis
in human bladder cancer patients. Oncol Lett. 5:1278–1284.
2013.PubMed/NCBI
|
|
18
|
Lee N, Oh J, Ban J, et al:
4-O-methylhonokiol, a PPARγ agonist, inhibits prostate tumour
growth: p21-mediated suppression of NF-κB activity. Br J Pharmacol.
168:1133–1145. 2013.PubMed/NCBI
|
|
19
|
Smallridge RC, Copland JA, Brose MS, et
al: Efatutazone, an oral PPAR-γ agonist, in combination with
paclitaxel in anaplastic thyroid cancer: results of a multicenter
phase 1 trial. J Clin Endocrinol Metab. 98:2392–2400. 2013.
|
|
20
|
Ban JO, Oh JH, Son SM, et al:
Troglitazone, a PPAR agonist, inhibits human prostate cancer cell
growth through inactivation of NFκB via suppression of GSK-3β
expression. Cancer Biol Ther. 12:288–296. 2011.PubMed/NCBI
|
|
21
|
Charytonowicz E, Terry M, Coakley K, et
al: PPARγ agonists enhance ET-743-induced adipogenic
differentiation in a transgenic mouse model of myxoid round cell
liposarcoma. J Clin Invest. 122:886–898. 2012.
|
|
22
|
Pestereva E, Kanakasabai S and Bright JJ:
PPARγ agonists regulate the expression of stemness and
differentiation genes in brain tumour stem cells. Br J Cancer.
106:1702–1712. 2012.
|
|
23
|
Pereira L, Igea A, Canovas B, et al:
Inhibition of p38 MAPK sensitizes tumour cells to cisplatin-induced
apoptosis mediated by reactive oxygen species and JNK. EMBO Mol
Med. 5:1759–1774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lambeth JD: NOX enzymes and the biology of
reactive oxygen. Nat Rev Immunol. 4:181–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Owusu-Ansah E and Banerjee U: Reactive
oxygen species prime Drosophila haematopoietic progenitors
for differentiation. Nature. 461:537–541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Houseknecht KL, Cole BM and Steele PJ:
Peroxisome proliferator-activated receptor gamma (PPARgamma) and
its ligands: a review. Domest Anim Endocrinol. 22:1–23. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kersten S and Wahli W: Peroxisome
proliferator activated receptor agonists. EXS. 89:141–151.
2000.PubMed/NCBI
|
|
28
|
Koshiyama H, Shimono D, Kuwamura N, et al:
Rapid communication: inhibitory effect of pioglitazone on carotid
arterial wall thickness in type 2 diabetes. J Clin Endocrinol
Metab. 86:3452–3456. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lehmann JM, Moore LB, Smith-Oliver TA, et
al: An antidiabetic thiazolidinedione is a high affinity ligand for
peroxisome proliferator-activated receptor gamma (PPAR gamma). J
Biol Chem. 270:12953–12956. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su CG, Wen X, Bailey ST, et al: A novel
therapy for colitis utilizing PPAR-gamma ligands to inhibit the
epithelial inflammatory response. J Clin Invest. 104:383–389. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salmaggi A, Boiardi A, Gelati M, et al:
Glioblastoma-derived tumorospheres identify a population of tumor
stem-like cells with angiogenic potential and enhanced multidrug
resistance phenotype. Glia. 54:850–860. 2006. View Article : Google Scholar
|
|
33
|
Ding W, Mouzaki M, You H, et al:
CD133+ liver cancer stem cells from methionine adenosyl
transferase 1A-deficient mice demonstrate resistance to
transforming growth factor (TGF)-beta-induced apoptosis.
Hepatology. 49:1277–1286. 2009.
|
|
34
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kelly PN, Dakic A, Adams JM, et al: Tumor
growth need not be driven by rare cancer stem cells. Science.
317:3372007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adler V, Yin Z, Tew KD and Ronai Z: Role
of redox potential and reactive oxygen species in stress signaling.
Oncogene. 18:6104–6111. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kondo M, Oya-Ito T, Kumagai T, et al:
Cyclopentenone prostaglandins as potential inducers of
intracellular oxidative stress. J Biol Chem. 276:12076–12083. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim KY, Ahn JH and Cheon HG: Apoptotic
action of peroxisome proliferator-activated receptor-γ activation
in human non small-cell lung cancer is mediated via proline
oxidase-induced reactive oxygen species formation. Mol Pharmacol.
72:674–685. 2007.
|
|
40
|
Wang JJ and Mak OT: Induction of apoptosis
by 15d-PGJ2 via ROS formation: an alternative pathway
without PPARγ activation in non-small cell lung carcinoma A549
cells. Prostaglandins Other Lipid Mediat. 94:104–111.
2011.PubMed/NCBI
|
|
41
|
Shin SW, Seo CY, Han H, et al:
15d-PGJ2 induces apoptosis by reactive oxygen
species-mediated inactivation of Akt in leukemia and colorectal
cancer cells and shows in vivo antitumor activity. Clin Cancer Res.
15:5414–5425. 2009.
|
|
42
|
Ito K, Hirao A, Arai F, et al: Regulation
of oxidative stress by ATM is required for self-renewal of
haematopoietic stem cells. Nature. 431:997–1002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyamoto K, Araki KY, Naka K, et al:
Foxo3a is essential for maintenance of the hematopoietic stem cell
pool. Cell Stem Cell. 1:101–112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tothova Z, Kollipara R, Huntly BJ, et al:
FoxOs are critical mediators of hematopoietic stem cell resistance
to physiologic oxidative stress. Cell. 128:325–339. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yalcin S, Zhang X, Luciano JP, et al:
Foxo3 is essential for the regulation of ataxia telangiectasia
mutated and oxidative stress-mediated homeostasis of hematopoietic
stem cells. J Biol Chem. 283:25692–25705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ghaffari S: Oxidative stress in the
regulation of normal and neoplastic hematopoiesis. Antioxid Redox
Signal. 10:1923–1940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Alexandre J, Hu Y, Lu W, et al: Novel
action of paclitaxel against cancer cells: bystander effect
mediated by reactive oxygen species. Cancer Res. 67:3512–3517.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zolezzi JM, Silva-Alvarez C, Ordenes D, et
al: Peroxisome proliferator-activated receptor (PPAR) γ and PPARα
agonists modulate mitochondrial fusion-fission dynamics: relevance
to reactive oxygen species (ROS)-related neurodegenerative
disorders? PLoS One. 8:e640192013.
|