Introduction
Lung cancer is both the most common cancer and the
leading cause of cancer-related mortality in the majority of
countries, including China. Metastatic lung cancer is responsible
for >90% of lung cancer-related deaths (1). Adenocarcinoma is the most common
type of lung cancer, accounting for up to 30–40% of all cases.
Approximately 60 to 70% of patients with lung adenocarcinoma
already have a malignant pleural diffusion or distant metastasis at
the time of diagnosis, with poor prognosis (2). Therefore, the notable rise in the
incidence of lung adenocarcinoma and its lethal nature underscore
the importance of understanding the complex metastatic process.
Intensive efforts are underway to develop therapies to halt lung
cancer metastasis.
Hypoxia, or areas of low oxygen levels, is a
hallmark of solid tumors due to an imbalance between the oxygen
supply and consumption. Hypoxic stress induces a variety of
molecular responses through the activation of hypoxia-inducible
factors (HIFs), which regulate a large number of genes that are
exploited by tumor cells for several biological processes,
including cell proliferation, apoptosis, immortalization and
migration (3,4). Accumulating clinical and
experimental evidence has revealed a key role for intratumor
hypoxia in promoting metastatic progression (5,6).
Although tumor hypoxia is a major therapeutic concern, the precise
mechanisms of this process are poorly understood. It is hoped that
a better understanding of hypoxia-associated proteins that may be
involved in tumor progression and metastasis will lead to the
identification of more effective therapeutic approaches (7).
Transgelin (TAGLN) belongs to the calponin family of
actin-binding proteins, the members of which participate in diverse
cellular processes, including cell motility and migration (8,9).
We have previously demonstrated that TAGLN contributes to the
enhanced migration of hypoxic human pulmonary artery smooth muscle
cells (hPASMCs) (10). In
addition, TAGLN overexpression has been observed in gastric
malignancies (11,12) and pancreatic cancer (13). Such interesting results led us to
explore the largely unknown role of TAGLN in lung adenocarcinoma.
In the present study, we examined TAGLN expression in the lung
adenocarcinoma cancer cell lines, A549 and H358, following exposure
to hypoxia. RNA interference (RNAi) was employed to evaluate the
effects of TAGLN on cell migration under normoxic or hypoxic
conditions. We also investigated the expression patterns and
clinical significance of TAGLN protein levels in lung
adenocarcinoma samples.
Materials and methods
Cell lines and cell culture
The human lung adenocarcinoma cell lines used in
this study were originally obtained commercially from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China). The cells
were grown in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco/Invitrogen,
Carlsbad, CA, USA), 100 IU/ml penicillin and streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) and incubated in an air/5%
CO2 incubator set at 37°C in a humidified atmosphere.
The cells were routinely passaged every 2–3 days when they reached
approximately 80–90% confluence.
Cell treatment and protein
extraction
An appropriate number of cells (2×105)
was seeded into 6-cm dishes and exposed to hypoxia in a hypoxia
incubator (1% O2, 94% N2 and 5%
CO2) for 12, 24 or 48 h. The incubator was sealed, and
N2 was used to balance the reduced O2 level.
Parallel cultures were placed in a normoxia incubator. Following
incubation, whole-cell extracts were prepared by harvesting the
cells and lysing them in radioimmunoprecipitation assay (RIPA)
buffer [20 mM Tris-HCl, 137 mM NaCl, 1 mM MgCl2, 1 mM
CaCl2, 10% glycerol, 1% NP-40, 0.5% deoxycholate, 0.1%
sodium dodecyl sulfate (SDS) supplemented with 1 μg/ml aprotinin, 1
μg/ml leupeptin, 0.57 mM phenylmethanesulfonylfluoride (PMSF), 100
μM sodium vanadate and 20 mM β-glycerophosphate]. Following
incubation on ice for 30 min, the cells were scraped into a fresh
tube, and the cell lysate was centrifuged (12,000 rpm, 20 min, 4°C)
to remove cellular debris. The supernatant was harvested and stored
at −80°C.
Western blot analysis
Protein concentrations of cell lysates were
determined using the bicinchoninic acid (BCA) method using bovine
serum albumin as a standard. Equivalent cell extracts (20–40 μg of
protein) were resolved by 10% SDS-polyacrylamide gel
electrophoresis. The protein was transferred onto a polyvinylidene
difluoride (PVDF) membrane and blocked in Tris-buffered saline
(TBS) containing 5% non-fat dry milk and 0.2% Tween-20 for 1 h.
After blocking, the membrane was washed in TBS containing 0.2%
Tween-20 3 times, and then probed with anti-TAGLN polyclonal
antibody (Proteintech Group Inc., Chicago, IL, USA) at a dilution
of 1:1,000 or anti-HIF monoclonal antibody (Abcam, Cambridge, UK)
at a dilution of 1:1,000 at 4°C overnight, followed by incubation
with appropriate horseradish peroxidase (HRP)-conjugated secondary
antibodies. Proteins were detected using an enhanced
chemiluminescence detection kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). An antibody to glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as an internal loading control. Band
intensities were analyzed using Quality One software (Bio-Rad,
Hercules, CA, USA).
Inhibition of TAGLN expression by small
interfering RNA (siRNA)
TAGLN-specific siRNA and scrambled control siRNA
were designed and synthesized by GenePharma (Shanghai, China). The
sequence of TAGLN siRNA was as follows: forward,
5′-CCAAAAUCGAGAAGAAGUAdTdT-3′ and reverse,
5′-UACUUCUUCUCGAUUUUGGdTdT-3′. The corresponding control siRNA
sequence was: forward, 5′-UUC UCC GAA CGU GUC ACG U dtdt-3′ and
reverse, 5′-ACG UGA CAC GUU CGG AGA A dtdt-3′. Exponentially
growing cells were plated in 6-well plates at an appropriate
density and grown in antibiotic-free medium to 30–50% confluence
prior to siRNA transfection. The cells were then transiently
transfected with 100 pmol control siRNA or TAGLN siRNA (final
concentration of 33 nM RNA when added to the cells) using
Lipofectamine 2000 reagent (Invitrogen) in serum-free RPMI-1640
medium for 6 h as described by the manufacturer’s instructions.
Briefly, 100 pmol siRNA and 5 μl Lipofectamine 2000 per well were
diluted separately in serum-free Opti-MEM to a final volume of 250
μl, gently mixed, and incubated at room temperature for 5 min.
Subsequently, the diluted siRNA solution and the diluted
Lipofectamine 2000 were mixed gently and incubated at room
temperature for 20 min. The diluted siRNA/Lipofectamine 2000
complex was then added to the 6-well plates containing 1,500 μl
serum-free RPMI-1640 for 6 h.
Transwell in vitro migration assay
The cells were grown in 6-well plates, transfected
with 100 pmol TAGLN siRNA or control siRNA for 6 h and then
cultured for a further 24 h. The cells were trypsinized and
resuspended in RPMI-1640 medium supplemented with 1% FBS. The cell
suspension was adjusted to a concentration of 1×106/ml
for the A549 cells or 2.5×106/ml for the H358 cells.
Subsequently, 200 μl cell suspension was seeded in the upper
chambe220r of the Transwell (Corning Costar, Inc., Corning, NY,
USA). The lower chamber was filled with 600 μl RPMI-1640 medium
containing 10% FBS. After 24 h of incubation under normoxic or
hypoxic conditions at 37°C, the filter side of the upper chamber
was cleaned with a cotton swab. The cells that had migrated across
the filters were fixed for 5 min and stained for 20 min with
Crystal violet solution (Sigma-Aldrich). The filter was gently cut
from the chamber, and the number of migrated cells was counted in 4
high-power fields per insert (10×40). Three identical replicates
were performed for each migration condition.
Wound healing assay
The cells were treated with 100 pmol TAGLN siRNA or
control siRNA for 6 h and incubated for a further 24 h until
confluent. Subsequently, the cell monolayer was manually scraped
from one end of the well to the other with a sterile p200 pipet
tip. The medium and cell debris were aspirated away and replaced
with 2 ml fresh serum-free medium. The cells were incubated under
normoxic or hypoxic conditions, and their migration into the
scratch area was monitored for up to 24 h. Using a phase-contrast
microscope, images of the scratch wound in the same field were
captured 0 and 24 h after the wound was made. The relative width of
the wound was measured quantitatively using Adobe Photoshop 5.0
(Adobe Systems Inc., San Jose, CA, USA) with the width at 0 h as
the baseline. Wound closure (%) was determined as the distance
migrated after 24 h relative to the baseline width. Three identical
replicates were performed for each migration condition.
Study population and sample
preparation
A total of 75 patients who were diagnosed with lung
adenocarcinoma between August 2006 to December 2008 were included
in the current study. Matched tumor tissues and adjacent tumor-free
tissues were obtained during surgery. Tissue samples to be used for
immunohistochemistry (IHC) were fixed in formalin and embedded in
paraffin. Patient clinicopathological data regarding gender, age,
pathological TNM tumor stage, histology and grade were retrieved
from the medical records. The study was approved by the hospital
Ethical Committee, and written informed consent was obtained from
all participants prior to enrollment.
IHC and scoring methods
The IHC detection of TAGLN was carried out with a
lung adenocarcinoma tissue microarray containing matched duplicate
tumor and para-cancerous tissue cores from the 75 patients.
Briefly, 5-μm-thick microarray sections were deparaffinized in
xylene and then rehydrated in a series of alcohols. Antigen
retrieval was carried out by microwave treatment at 98°C for 10 min
in 1 mmol/l ethylenediaminetetraacetic acid (EDTA, pH 8.0). The
slide was then allowed to cool off at room temperature for 30 min.
Endogenous peroxidase activity was blocked in 3%
H2O2 in phosphate-buffered saline (PBS) for
30 min at room temperature. The slides were blocked with 10% bovine
serum albumin/1X PBS at room temperature for 30 min to reduce
non-specific background staining. Serum was removed from the
slides, which were then incubated with mouse anti-TAGLN monoclonal
antibody (1:100) at 4°C overnight in a humidity chamber. The slide
was then washed in PBS and incubated with biotinylated secondary
antibodies for 30 min in PBS buffer, followed by 3 washes in PBS
for 5 min each. Finally, the slides were incubated with
biotinylated alkaline phosphatase-streptavidin (StreptABComplex/AP)
for 20 min according to the manufacturer’s instructions (Dako
Denmark A/S, Glostrup, Denmark), developed with
3,3′diaminobenzidine (DAB) substrate, and counterstained with
hematoxylin before mounting and light microscopy examination.
Routine negative controls using non-immune serum instead of the
primary antibody were included to verify the specificity. All
immunoreactions were blindly evaluated by 2 independent experienced
pathologists to quantify TAGLN protein expression. TAGLN
immunoreactivity was scored on a 4-point scale as follows:
negative, 0; weak, 1; intermediate, 2; and strong, 3. Cells with a
staining intensity score of 1, 2 or 3 were regarded as positive,
while those with scores of 0 were regarded as negative. The
percentage of positive tumor cells (0%, negative; 1–50%, 1; 51–75%,
2; and ≥76%, 3) was assessed by counting >1,000 cancer cells in
10 randomly selected high-power fields (10×40). A combined staining
score for each compartment was obtained as the product of intensity
and extent of staining; a score of <4 was considered as a low
expression, and a score of ≥4 was considered as a high
expression.
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 for windows (SPSS Inc., Chicago, IL, USA). All results
are presented as the means ± standard error of the mean (SEM) from
at least 3 independent experiments with similar results.
Comparisons between 2 different groups were analyzed by using
Student’s t-tests. Correlations between TAGLN IHC scores and
clinicopathological characteristics were evaluated using Fisher’s
exact tests. For all tests, a value of p<0.05 was considered to
indicate a statistically significant difference.
Results
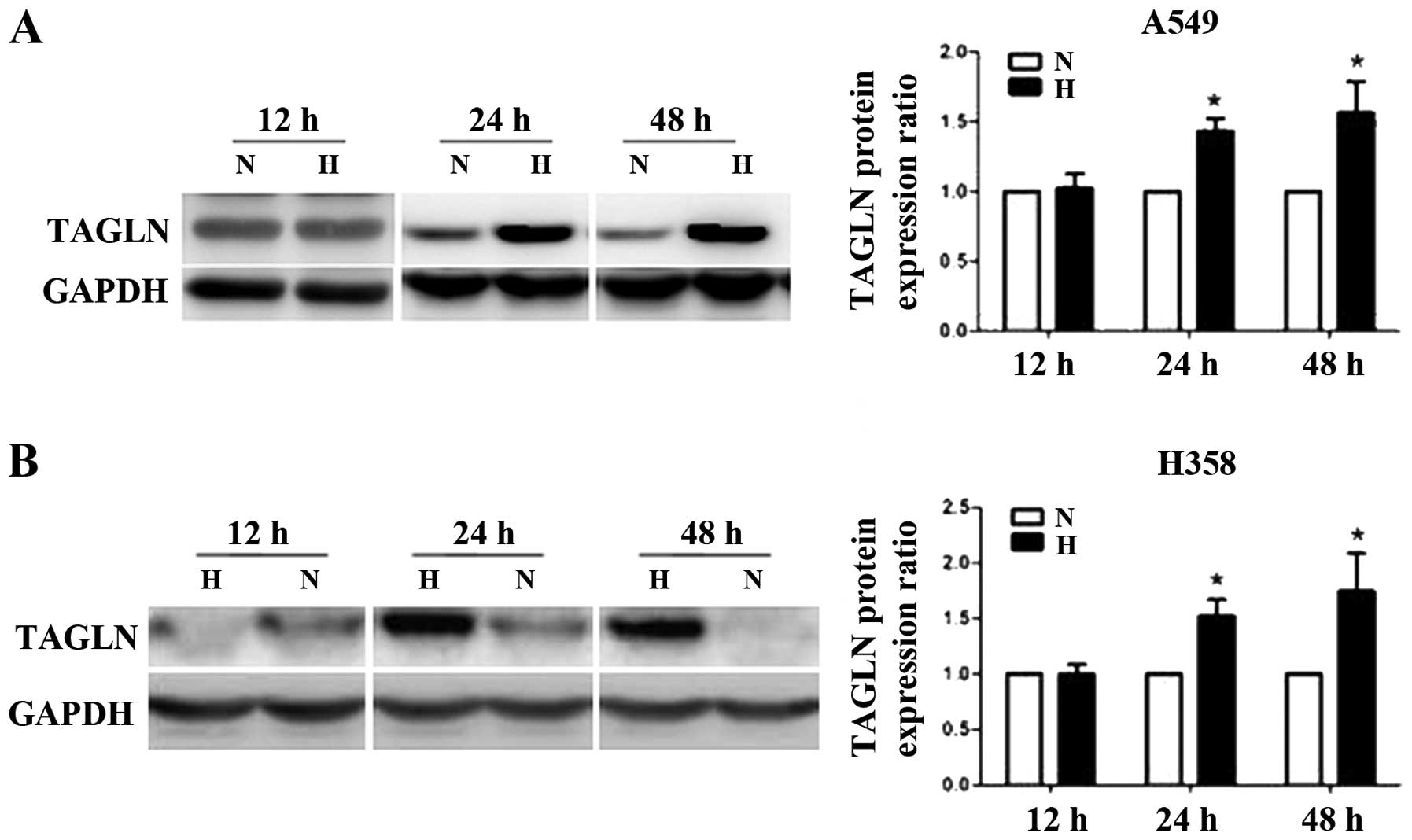
Hypoxia promotes TAGLN protein expression
in lung adenocarcinoma cells
The A549 and H358 cells were exposed to normoxia (5%
O2) or hypoxia (1% O2) for 12, 24 or 48.
TAGLN protein expression showed no changes at 12 h, but was
markedly elevated in the A549 and H358 cells at 24 h compared with
the corresponding normoxic controls. Its expression remained
elevated for up to 48 h (Fig.
1).
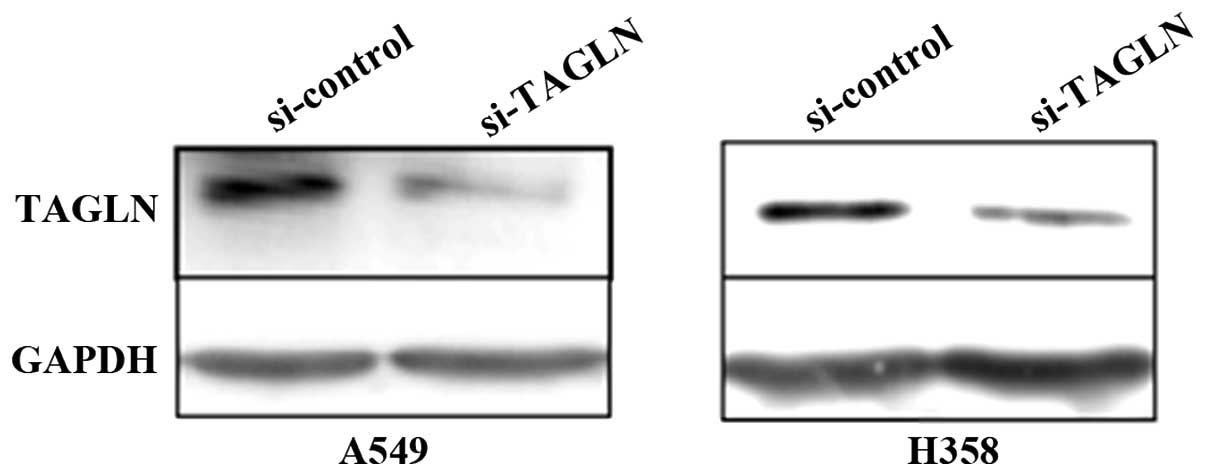
siRNA inhibition of TAGLN expression
In order to obtain the best silencing efficiency,
2×105 A549 cells/well or 5×105 H358
cells/well were transfected with 100 pmol TAGLN siRNA (si-TAGLN) or
control siRNA (si-control) using 5 μl Lipofectamine 2000 on the
basis of preliminary optimization experiments. At 48 h after
transfection, the silencing effect at the protein level was
determined by western blot analysis. si-TAGLN exhibited a potent
silencing effect up to 70% compared with the negative control
(Fig. 2).
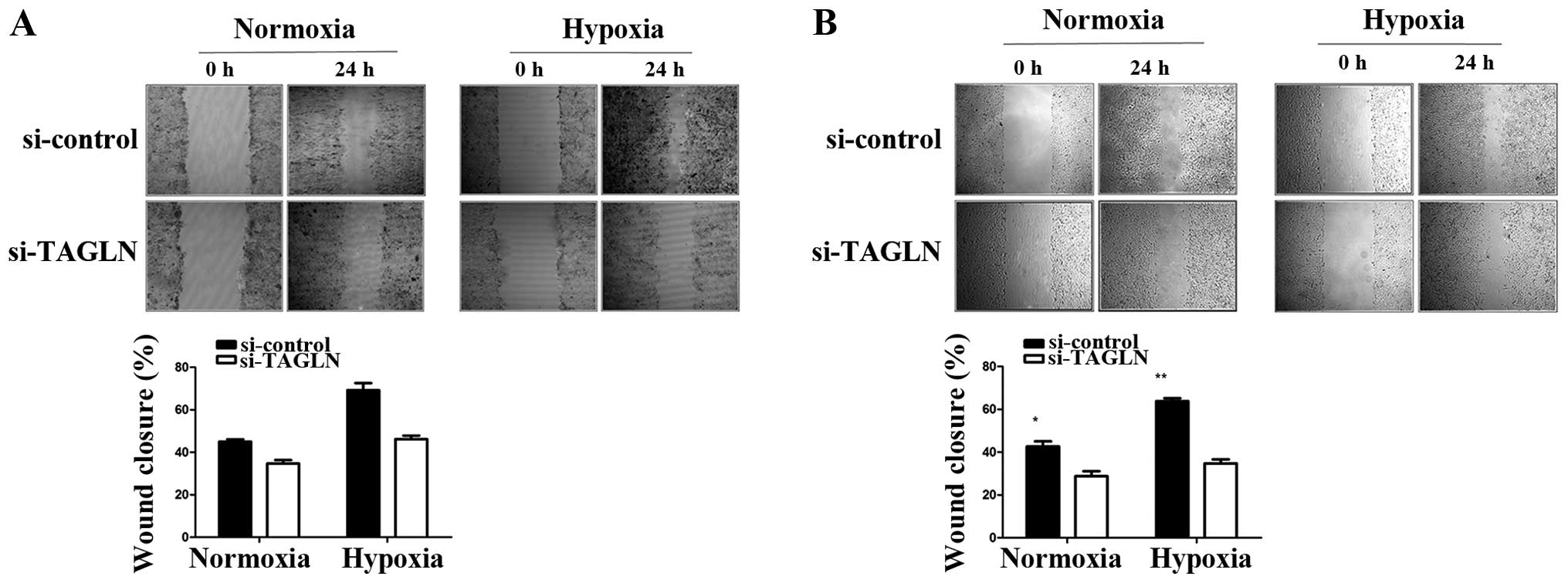
Inhibition TAGLN of expression reduces
lung adenocarcinoma cell migration
To explore the potential regulatory role of TAGLN in
cancer cell migration, we performed wound healing and Transwell
migration assays using the A549 and H358 cells under normoxic and
hypoxic conditions. As shown by wound healing assay (Fig. 3), compared with the negative
control, the cells transfected with si-TAGLN showed a slower wound
healing rate, particularly under hypoxic conditions. As shown by
Transwell assay, At 24 h, the cells transfected with si-control had
completely filled the gap with at least 80% wound closure under
both conditions, while the cells transfected with TAGLN-specific
siRNA showed 50% wound closure at most. These results are
consistent with those of the wound healing assay. As shown in
Fig. 4, there were fewer migrated
cells in the TAGLN-specific siRNA group than the negative control
group. TAGLN-specific siRNA reduced the migration ability of the
A549 cells by 28 and 39% under normoxic and hypoxic conditions,
respectively. For the H358 cells, the percentages were 50 and
67.3%, respectively. In addition, we found that the migration
ability of the A549 and H358 cells improved after 24 h of exposure
to hypoxia. Taken together, the results demonstrate that the
decreased TAGLN expression suppressed A549 and H358 cell migration
in vitro, particularly under hypoxic conditions. These
findings indicate that TAGLN protein expression may play a key role
in hypoxia-induced cell migration.
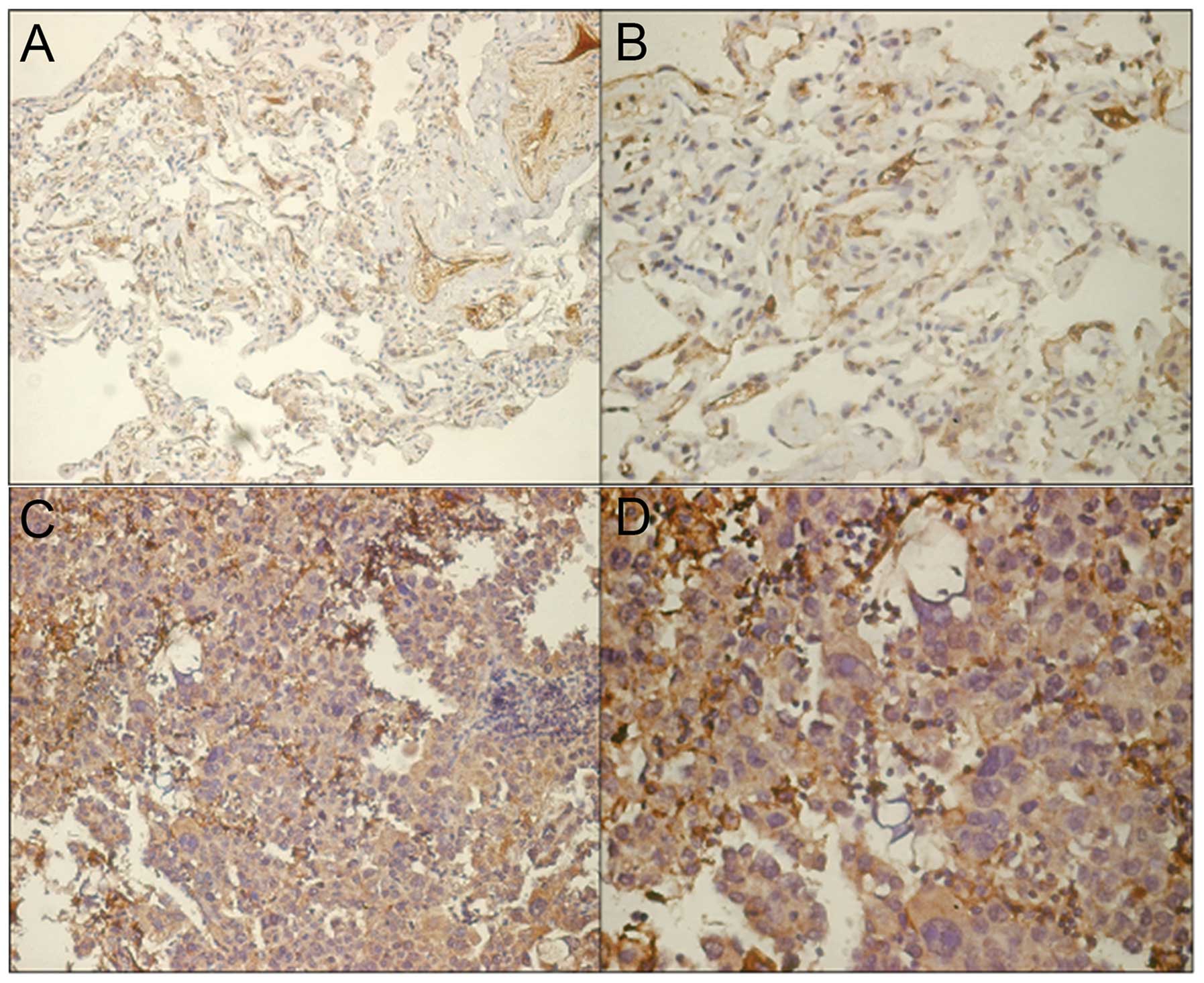
Correlation between TAGLN expression and
clinicopathological characteristics
Representative results for TAGLN protein expression
in tumor tissue and adjacent tumor-free tissue are shown in
Fig. 5. For the purpose of
analysis, TAGLN IHC scores were classified as low (<4) or high
(≥4). Using this classification, we detected significantly
increased expression rates of TAGLN protein in tumor tissue (51/75,
68%) compared to the adjacent tumor-free tissue (30/75, 40%)
(p=0.001, χ2=11.836). The association between TAGLN
protein expression and the clinicopathological characteristics of
the patients was investigated as shown in Table I. TAGLN expression was strongly
associated with tumor stage (p=0.023, χ2=6.117), lymph
node status (p=0.025, χ2=5.672) and differentiation
grade (p=0.014, χ2=6.63). A high TAGLN expression was
observed in 83.9% (26/31) of the cases with advanced tumor stage
and in 80% (32/40) of the lymph node-positive cases, while a low
expression of TAGLN was observed in 55% (11/20) of the poorly
differentiated cancers. No significant association was observed
between TAGLN expression and other clinicopathologic
characteristics, including gender, age and tumor size
(p>0.05).
 | Table ICorrelation between TAGLN expression
and clinicopathological characteristics in lung adenocarcinoma. |
Table I
Correlation between TAGLN expression
and clinicopathological characteristics in lung adenocarcinoma.
| TAGLN
immunoreactivity | | |
|---|
|
| | |
|---|
| Clinical
classification | Total no. | Low | High | p-value | χ2 |
|---|
| Gender |
| Male | 41 | 13 | 28 | 1 (Male vs.
female) | 0.004 |
| Female | 34 | 11 | 23 | | |
| Age at surgery
(years) |
| ≥60 | 46 | 17 | 29 | 0.313 (≥60
vs.<60) | 1.343 |
| <60 | 29 | 7 | 22 | | |
| T-primary tumor |
| T1 + T2 | 55 | 19 | 36 | 0.578 (T1 + T2 vs. T3
+ T4) | 0.614 |
| T3 + T4 | 20 | 5 | 15 | | |
| Lymph node
status |
| Negative | 35 | 16 | 19 | 0.025 (Negative vs.
positive) | 5.672 |
| Positive | 40 | 8 | 32 | | |
| Stage |
| I + II | 44 | 19 | 25 | 0.023 (I + II vs. III
+ IV) | 6.117 |
| III + IV | 31 | 5 | 26 | | |
| Grade |
| Well + moderate | 55 | 13 | 42 | 0.014 (Well +
moderate vs. poor) | 6.63 |
| Poor | 20 | 11 | 9 | | |
Discussion
Metastasis is a common phenomenon and the
predominant cause of mortaltiy in patients with lung
adenocarcinoma. There is growing evidence that hypoxia, which
occurs in a wide range of solid tumors, is associated with a
malignant tumor phenotype and augmented metastatic potential
(14,15). Hypoxia increases tumor
angiogenesis, metastasis and other biological responses by
activating the expression of relevant proteins through HIFs. It has
previously been shown that each step of the metastatic process can
potentially be regulated by hypoxia and the HIF system (16). Therefore, considerable research
into the mechanisms of tumor metastasis has focused on hypoxia and
hypoxia-associated proteins. We have previously reported that TAGLN
protein expression is significantly induced by hypoxia in hPASMCs,
which contributes to their increased motility under hypoxic
conditions (10). With this in
mind, we originally focused on the role of TAGLN in cancer. In the
present study, we demonstrated that TAGLN is increased in lung
adenocarcinoma cells under hypoxic conditions.
TAGLN, also known as SM22a, is a transformation- and
shape change-sensitive actin stress fiber-binding protein that
stabilizes actin gels (17). It
was originally described as predominantly expressed in smooth
muscle cells to bind to actin, suggesting its important roles in
cytoskeletal rearrangement and the phenotypic modulation of cells
(18,19). Yu et al showed that
increased TAGLN expression contributed to epithelial cell injury,
repair, and migration in lung fibrosis (20). However, little is known about the
potential functions of TAGLN in tumor progression. Therefore, in
this study, we examined whether its expression contributes to the
aberrant migration of lung adenocarcinoma cells, a prerequisite for
tumor cell invasion and metastasis. Our experiments revealed that
the inhibition of TAGLN expression using siRNA in A549 and H358
cells was accompanied by significantly impaired motility,
particularly under hypoxic conditions. We also demonstrated that
exposure to hypoxia stimulated A549 and H358 cell migration. Our
motility data are suggestive of the potential role for TAGLN in
lung adenocarcinoma cell migration and subsequent dissemination and
metastasis. However, the underlying mechanisms are not yet fully
understood. Gimona et al found that TAGLN is involved in
podosome formation through association with a specific
sub-population of actin filament bundles. They suggested that an
increase in TAGLN expression may favor podosome formation by
controlling the calponin/TAGLN ratio (21). Given the evidence that cell
migration is triggered by stimulating intracellular signaling
pathways that regulate actin cytoskeleton reorganization (22), we can infer that hypoxia or HIFs
play a role in cancer cell migration and that TAGLN is involved in
mediating the signaling pathways and can regulate the ability of
cells to migrate through the direct interaction with the actin
cytoskeleton. However, further studies are required to confirm this
hypothesis.
As an actin-binding protein that controls cell
motility, TAGLN may be valuable in assessing tumor progression or
prognosis. The expression patterns of TAGLN have been reported to
vary among different tumor types. It has been suggested that the
loss of TAGLN expression is closely associated with progression,
differentiation, metastasis and poor prognosis in colon cancer
patients (23). In addition, the
loss of TAGLN expression also occurs early in breast cancer
progression, and TAGLN can inhibit prostate cancer cell growth
(8,24). These results suggest that TAGLN
may act as a tumor suppressor. Indeed, the restoration of TAGLN
expression both in vitro and in vivo inhibits
carcinogenesis. This situation is reversed in other types of
cancer; TAGLN expression is significantly increased in
hepatocellular carcinoma (25),
as well as gastric (11) and
pancreatic cancer (26),
suggesting its potential as a tumor biomarker for certain cancer
types. These paradoxical roles of TAGLN in different forms of
cancer reveal the diverse functions of TAGLN, and also led us to
explore its expression patterns and clinical significance in lung
adenocarcinoma.
We found that TAGLN was overexpressed in >68% of
the tested lung adenocarcinoma tissues compared to the paired
adjacent tumor-free tissue. This overexpression did not correlate
with the gender, age or tumor size of the patients. However, it was
associated with TNM stage, lymph node status and differentiation
grade. A higher TAGLN overexpression was found in cases with
advanced TNM stages or lymph node-positive cases, which suggests
that TAGLN is involved in malignant progression. Conversely, TAGLN
expression was significantly lower in poorly differentiated tumors.
Collectively, these results indicate that TAGLN may be a useful
biomarker for tumor differentiation and for predicting lung
adenocarcinoma prognosis.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that TAGLN is upregulated
in human lung adenocarcinoma cell lines under hypoxic conditions,
and this can enhance cellular migration ability. Compared to
adjacent tumor-free tissues, lung adenocarcinoma tissues had
significantly increased TAGLN immunoreactivity. Moreover, a higher
TAGLN expression correlated with lymph node metastasis, TNM stage
and differentiation. These results indicate that TAGLN may be a
therapeutic target and a potential biomarker for predicting lung
adenocarcinoma prognosis. However, further studies are required to
fully elucidate the mechanisms involved.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81000019).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Silvestri GA, Alberg AJ and Ravenel J: The
changing epidemiology of lung cancer with a focus on screening.
BMJ. 339:b30532009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brahimi-Horn C and Pouysségur J: The role
of the hypoxia-inducible factor in tumor metabolism growth and
invasion. Bull Cancer. 93:E73–E80. 2006.PubMed/NCBI
|
|
4
|
Fraga A, Ribeiro R and Medeiros R: Tumor
hypoxia: the role of HIF. Actas Urol Esp. 33:941–951. 2009.(In
Spanish).
|
|
5
|
Cassavaugh J and Lounsbury KM:
Hypoxia-mediated biological control. J Cell Biochem. 112:735–744.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeClerck K and Elble RC: The role of
hypoxia and acidosis in promoting metastasis and resistance to
chemotherapy. Front Biosci. 15:213–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar
|
|
8
|
Assinder SJ, Stanton JA and Prasad PD:
Transgelin: an actin-binding protein and tumour suppressor. Int J
Biochem Cell Biol. 41:482–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lambrechts A, Van Troys M and Ampe C: The
actin cytoskeleton in normal and pathological cell motility. Int J
Biochem Cell Biol. 36:1890–1909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang R, Zhou L, Li Q, Liu J, Yao W and
Wan H: Up-regulation of two actin-associated proteins prompts
pulmonary artery smooth muscle cell migration under hypoxia. Am J
Respir Cell Mol Biol. 41:467–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Q, Chen W, Wang L, Lin W, Lin J and
Lin X: Identification of transgelin as a potential novel biomarker
for gastric adenocarcinoma based on proteomics technology. J Cancer
Res Clin Oncol. 134:1219–1227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryu JW, Kim HJ, Lee YS, et al: The
proteomics approach to find biomarkers in gastric cancer. J Korean
Med Sci. 18:505–509. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mikuriya K, Kuramitsu Y, Ryozawa S, et al:
Expression of glycolytic enzymes is increased in pancreatic
cancerous tissues as evidenced by proteomic profiling by
two-dimensional electrophoresis and liquid chromatography-mass
spectrometry/mass spectrometry. Int J Oncol. 30:849–855. 2007.
|
|
14
|
Arvelo F and Cotte C: Hypoxia in cancer
malignity. Review. Invest Clin. 50:529–546. 2009.(In Spanish).
|
|
15
|
Subarsky P and Hill RP: The hypoxic tumour
microenvironment and metastatic progression. Clin Exp Metastasis.
20:237–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giatromanolaki A and Harris AL: Tumour
hypoxia, hypoxia signaling pathways and hypoxia inducible factor
expression in human cancer. Anticancer Res. 21:4317–4324.
2001.PubMed/NCBI
|
|
17
|
Shapland C, Hsuan JJ, Totty NF and Lawson
D: Purification and properties of transgelin: a transformation and
shape change sensitive actin-gelling protein. J Cell Biol.
121:1065–1073. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu Y, Liu HW, Forsythe SM, et al:
Mutagenesis analysis of human SM22: characterization of actin
binding. J Appl Physiol. 89:1985–1990. 2000.PubMed/NCBI
|
|
19
|
Shanahan CM, Weissberg PL and Metcalfe JC:
Isolation of gene markers of differentiated and proliferating
vascular smooth muscle cells. Circ Res. 73:193–204. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu H, Königshoff M, Jayachandran A, et al:
Transgelin is a direct target of TGF-beta/Smad3-dependent
epithelial cell migration in lung fibrosis. FASEB J. 22:1778–1789.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gimona M, Kaverina I, Resch GP, Vignal E
and Burgstaller G: Calponin repeats regulate actin filament
stability and formation of podosomes in smooth muscle cells. Mol
Biol Cell. 14:2482–2491. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Wang H, Deng YJ, Wang S, Liu C,
Jin H and Ding YQ: Transgelin as a suppressor is associated with
poor prognosis in colorectal carcinoma patients. Mod Pathol.
22:786–796. 2009.PubMed/NCBI
|
|
24
|
Yang Z, Chang YJ, Miyamoto H, Ni J, Niu Y,
Chen Z, Chen YL, Yao JL, di Sant’Agnese PA and Chang C: Transgelin
functions as a suppressor via inhibition of ARA54-enhanced androgen
receptor transactivation and prostate cancer cell growth. Mol
Endocrinol. 21:343–358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi YY, Wang HC, Yin YH, et al:
Identification and analysis of tumour-associated antigens in
hepatocellular carcinoma. Br J Cancer. 92:929–934. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou L, Zhang R, Zhang L, Sun Y, Yao W,
Zhao A, Li J and Yuan Y: Upregulation of transgelin is an
independent factor predictive of poor prognosis in patients with
advanced pancreatic cancer. Cancer Sci. 104:4234302013. View Article : Google Scholar
|