Introduction
The incidence of non-alcoholic steatohepatitis
(NASH) is rapidly increasing worldwide (1–4).
NASH can be caused by various pathogenic mechanisms, including
overeating, physical inactivity, diabetes mellitus, and medications
(5,6). The gut directly links to the liver
through the portal vein and is involved in the development of NASH
(7,8). The gut secretes various hormones in
the portal vein and regulates hepatic metabolism (9–11).
Glucagon-like peptide-1 (GLP-1) is a gut hormone and is known to
affect lipid metabolism in hepatocytes (9,11).
Exendin-4 (Ex-4) is a long-acting GLP-1 receptor
(GLP-1R) agonist. GLP-1R occurs in the pancreatic islets, kidney,
lung, heart, stomach, intestine, thyroid gland, and numerous
regions of the peripheral and central nervous system (12–14). GLP-1R also occurs in hepatocytes,
and treatment with Ex-4 substantially reduces triglyceride stores
in hepatoma cells (15).
Similarly, GLP-1R agonist reduces steatosis severity in certain
animal models of NASH (16–19). Findings of previous studies have
also shown that reduced hepatic accumulation of triglycerides is
mediated by GLP-1R agonist upregulation of hepatic
3-phosphoinositide-dependent kinase-1 activity, protein kinase C ζ
activity, peroxisome proliferator-activated receptor α activity,
and fatty acid β-oxidation (15–19).
Fatty acids are an important triglyceride component.
Fatty acids are a substrate of β-oxidation and yield large
quantities of adenosine 5′-triphosphate (20). In addition, some polyunsaturated
fatty acids (PUFAs) are a source of eicosanoids, which are
biologically active substances. n-3 PUFAs are precursors of
anti-inflammatory eicosanoids, including leukotriene B5,
prostaglandin E3, and thromboxane B3 (21). On the other hand, n-6 PUFA are
precursors of pro-inflammatory eicosanoids, including leukotriene
B4, prostaglandin E2, and thromboxane B2 (21). A reduced n-3/n-6 PUFA ratio is a
risk factor for chronic inflammatory diseases such as
cardiovascular disease, inflammatory bowel disease, rheumatoid
arthritis, and NASH (22–24). Thus, besides quantitative
abnormality in fatty acids, qualitative abnormality in fatty acids
is an important pathogenesis of NASH.
The production of pro- and anti-inflammatory
eicosanoids is regulated by desaturases, which are rate-limiting
enzymes of n-3 and n-6 PUFA cascades (25). Δ-5-desaturase, also known as fatty
acid desaturase 1, removes two hydrogen atoms from dihomo
γ-linolenic acid and synthesizes arachidonic acid. Upregulation of
Δ-5-desaturase activity promotes the production of pro-inflammatory
eicosanoids (26). Notably,
single-nucleotide polymorphisms in the Δ-5-desaturase gene are
associated with circulating high sensitivity C-reactive protein
levels in healthy young adults (27). Moreover, Δ-5-desaturase activity
is associated with aging (28),
development of type 2 diabetes mellitus (29), and NASH (30). However, the effects of Ex-4 on
hepatic fatty acid composition and Δ-5-desaturase activity remain
unclear.
The aim of this study was to investigate the effects
of Ex-4 on severity of steatohepatitis, hepatic fatty acid
composition, and Δ-5-desaturase index in a murine model of
NASH.
Materials and methods
Materials
Reagents were purchased from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan) unless otherwise indicated.
Animals
NASH was induced in db/db mice fed a
methionine-choline deficient (MCD) diet (31). Briefly, 5-week-old male db/db mice
(BKS.Cg- + Leprdb/+Leprdb/Jcl*) weighing 15–20 g were
purchased from CLEA Japan, Inc. (Tokyo, Japan). The mice were
housed individually in an air-conditioned room at 22±3°C and 55±10%
humidity with a 12-h light/dark cycle. The mice were fed a normal
diet during a 1-week quarantine and acclimatization period,
followed by the MCD diet (CLEA Japan, Inc.) and water ad
libitum throughout the experimental period. All the rat
experiments were conducted in accordance with the National
Institutes of Health Guidelines for the Care and Use of Laboratory
Animals and were approved by the University of Kurume Institutional
Animal Care and Use Committee.
Treatment
Ex-4 (20 μg/kg; no. 24463, AnaSpec, Inc., Fremont,
CA, USA) (Ex-4 group; n=4) or saline [control (CON) group; n=4] was
administered intraperitoneally under anesthesia every morning for 8
weeks. At week 14, the mice were sacrificed by using ether
anesthesia and the livers were obtained under anesthesia.
Measurement of body weight
Body weight was measured weekly, in the morning,
through week 14.
Liver histology
Random histological sampling was performed
throughout this study as previously described (32,33). Liver samples were fixed overnight
in 10% buffered formalin and embedded in paraffin. All sections
were cut at a thickness of 5 μm and stained with hematoxylin and
eosin (H&E) (34,35).
Hepatic triglyceride content
Liver samples were fixed overnight in 10% buffered
formalin. Sections were transferred to 70% ethanol and stained with
Sudan IV (0.1% Sudan IV dissolved in equal parts acetone and 70%
ethanol) to evaluate triglyceride content (36).
Non-alcoholic fatty liver disease (NAFLD)
activity
NAFLD activity was evaluated by the NAFLD activity
score, in which the following findings were evaluated
semi-quantitatively: steatosis (0–3 points), lobular inflammation
(0–2 points), hepatocellular ballooning (0–2 points), and fibrosis
(0–4 points) (37).
Fatty acid composition
Total liver fatty acids were extracted according to
Folch et al (38). Fatty
acid methyl esters were isolated and quantified by gas
chromatography furnished with a flame-ionization detector. The
fatty acids measured (and expressed as μg/g•liver) were: lauric,
myristic, myristoleic, palmitic, palmitoleic, stearic, oleic,
linoleic, γ-linolenic, linolenic, arachidic, eicosenoic,
eicosadienoic, 5,8,11-eicosatrienoic, dihomo γ-linolenic,
arachidonic, eicosapentaenoic, behenic, erucic, docosatetraenoic,
docosapentaenoic, lignoceric, docosahexaenoic, and nervonic
acid.
Classification of fatty acids
Fatty acids were classified as follows: saturated
fatty acids (SFAs), the sum of all identified SAFs; atherogenic
SFAs, the sum of lauric, myristic, and palmitic acids; thrombogenic
SFAs, the sum of myristic, palmitic, and stearic acids; medium
SFAs, the sum of SFAs containing 11–16 carbon atoms; long SFAs, the
sum of SFAs containing ≥16 carbon atoms; monounsaturated fatty
acids (MUFAs), the sum of all identified MUFAs; PUFAs, the sum of
all identified PUFAs; n-3 PUFAs, the sum of n-3 series PUFAs; n-6
PUFAs, the sum of n-6 series PUFA; Δ-5-desatulase index,
arachidonic acid/γ-linolenic acid.
Statistical analysis
Data were expressed as mean ± SD. Differences
between two groups were analyzed by the Wilcoxon test (JMP version
10.0.2, SAS Institute, Inc., Cary, NC). P≤0.05 was considered
statistically significant.
Results
Effects of Ex-4 on body weight,
appearance, and macroscopic appearance of the liver
In the CON group, body weight gradually increased to
~50 g at week 14 (Fig. 1A). In
the Ex-4 group, body weight gain stopped 1 week after the Ex-4
treatment and reached a plateau at ~40 g at week 7 (Fig. 1A). Ex-4 significantly suppressed
weight gain in MCD-fed db/db mice.
Representative mice from the CON and Ex-4 groups are
shown in Fig. 1B. The mouse from
the Ex-4 group was smaller and had a good coat of fur in comparison
to the mouse from the CON group (Fig.
1B).
A representative macroscopic image of the liver of
CON and Ex-4 mice is shown in Fig.
1C. CON livers exhibited xanthochromia with swelling, while the
Ex-4 livers were brown, with no swelling (Fig. 1C).
Effects of Ex-4 on hepatic histology,
hepatic triglyceride content, and the NAFLD activity score
Representative images of hepatic histology and Sudan
IV staining are shown in Fig. 2A.
Steatosis, lobular inflammation, and hepatocyte ballooning were
milder in the Ex-4 group compared to the CON group (Fig. 2A). Obvious hepatic fibrosis was
not evident in either group. Hepatic triglyceride content was
depleted in the Ex-4 group in comparison to the CON group (Fig. 2A).
The NAFLD activity score was significantly lower in
the Ex-4 group than in the CON group (Fig. 2B).
Effects of Ex-4 on hepatic SFA
There was no significant difference in the hepatic
SFA content of the CON and Ex-4 groups (Table I). No significant difference
between the groups was observed in the hepatic content of
atherogenic, thrombogenic, and medium-chain SFA. However,
long-chain SFA content was significantly higher in the Ex-4 group
compared to the CON group (Table
I).
 | Table IEffects of Ex-4 on hepatic SFA. |
Table I
Effects of Ex-4 on hepatic SFA.
| SFA type | Unit | CON | Ex-4 | P |
|---|
| SFA | μg/g•liver | 17838±3248 | 27541±9273 | N.S. |
| Atherogenic | μg/g•liver | 13414±2981 | 22457±8670 | N.S. |
| Thrombogenic | μg/g•liver | 17605±3244 | 27210±9260 | N.S. |
| Medium-chain | μg/g•liver | 15233±3554 | 25186±9799 | N.S. |
| Long-chain | μg/g•liver | 23240±955 | 31710±8436 | <0.05 |
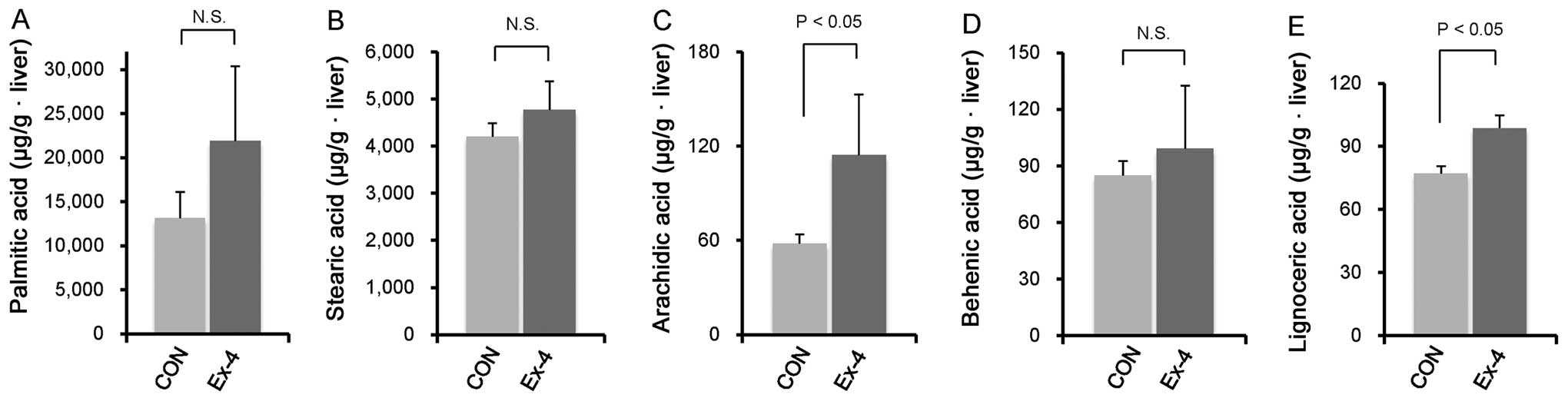
We also examined the hepatic content of each
long-chain SFA component and found no significant differences in
the palmitic, stearic, behenic, and lignoceric acid. However,
hepatic arachidic acid was significantly higher in the Ex-4 group
compared to the CON group (Fig.
3A–E).
Effects of Ex-4 on hepatic MUFAs and
PUFAs
Hepatic MUFA content did not significantly differ
between groups (Table II).
However, hepatic PUFA content was significantly higher in the Ex-4
group compared to the CON group. Similarly, hepatic n-6 PUFA
content and the n-3 PUFA/n-6 PUFA ratio were significantly higher
in the Ex-4 group compared to the CON group (Table II).
 | Table IIEffects of Ex-4 on hepatic MUFAs and
PUFAs. |
Table II
Effects of Ex-4 on hepatic MUFAs and
PUFAs.
| Acid type | Unit | CON | Ex-4 | P |
|---|
| MUFA | μg/g•liver | 20355±6701 | 34965±14485 | N.S. |
| PUFA | μg/g•liver | 18410±791 | 25986±8050 | <0.05 |
| n-3 PUFA | μg/g•liver | 2218.5±415.8 | 1992.4±288.7 | N.S. |
| n-6 PUFA | μg/g•liver | 16166±943 | 23937±7845 | <0.05 |
| n-3 PUFA/n-6
PUFA | Ratio | 13.83±3.15 | 8.73±1.95 | <0.05 |
We also assessed the hepatic content of each n-6
PUFA component and found no significant difference in arachidonic
acid. However, the hepatic content of linoleic acid, γ-linolenic
acid, and dihomo γ-linolenic acid was significantly higher in the
Ex-4 group compared to the CON group (Fig. 4A–D). By contrast, hepatic
Δ-5-desaturase index in the Ex-4 group was approximately one-third
of that in the CON group. Ex-4 treatment significantly reduced
hepatic Δ-5-desaturase index compared to the CON group (Fig. 4E).
Discussion
Results of this study have shown that Ex-4 inhibited
body weight gain and improved NASH in MCD diet-fed db/db mice. Ex-4
also altered hepatic fatty acid composition with a decrease in
Δ-5-desaturase index. Thus, Ex-4 may improve NASH by altering the
hepatic fatty acid composition in a murine model of NASH.
The effects of the GLP-1R agonist Ex-4 on NASH were
examined. The results showed that Ex-4 significantly suppressed
body weight gain and the NAFLD activity score in MCD diet-fed db/db
mice. GLP-1R expression is downregulated in a NASH rat model as
well as in patients with NASH (16). Moreover, GLP-1R agonist improves
NASH in various animal models, including high-fat diet-fed rats
(16), ob/ob mice (17,18), and diabetic male ApoE(−/−) mice
(19). The GLP-1R agonist also
reduced body weight and the NAFLD activity score in patients with
NASH (39). Thus, our results are
consistent with previous reports in this regard. Possible
mechanisms for GLP-1R agonist-induced NASH improvement include the
upregulation of insulin sensitivity, peroxisome
proliferator-activated receptor α activity, and fatty acid
β-oxidation (15,16,40,41). However, the effects of GLP-1R
agonist in hepatic fatty acid composition remain unclear.
In general, long-chain SFAs promote inflammation and
progression of NAFLD (42,43).
However, results of this study have shown that Ex-4 significantly
increased the hepatic content of long-chain SFAs, in particular the
arachidic and lignoceric acids. Although the reason for the
discrepancy between previous reports and our findings remains
unclear, certain SFAs, including arachidic and lignoceric acids are
not correlated with insulin resistance, a feature of NASH (44). Furthermore, arachidic acid
improves lipid metabolism by enhancing apoB secretion (45). Lignoceric acid is a precursor of
ceramide, thus an increase in hepatic lignoceric acid content
indicates a decrease in ceramide synthesis. Recently, Kurek et
al showed that inhibition of ceramide synthesis reduces hepatic
lipid accumulation in a rat model of NAFLD (46). This finding suggests that Ex-4
improves lipid metabolism through alterations in arachidic and
lignoceric acids in a murine model of NASH.
Although hepatic MUFA content was not altered by
Ex-4 treatment, hepatic PUFA content was increased. Ex-4 increased
the hepatic content of n-6 PUFAs such as linoleic acid, γ-linolenic
acid, and dihomo γ-linolenic acid. These n-6 PUFAs are precursors
of pro-inflammatory eicosanoids and are involved in the development
of NASH (22,47). Thus, our findings are different
from those of previous studies. However, a possible explanation for
the discrepancy is an Ex-4-induced alteration in n-6 PUFA
metabolism. Δ-5-desaturase is a rate-limiting enzyme of n-6 PUFA
metabolism that increases the production of pro-inflammatory
eicosanoids (48). An
oligonucleotide microarray analysis using human liver tissue showed
that Δ-5-desaturase is upregulated in patients with NASH (30). In this study, we have found that
the Δ-5-desaturase index was significantly reduced by Ex-4
treatment, indicating that Ex-4 inhibits Δ-5-desaturase activity
and subsequently suppresses the production of pro-inflammatory
eicosanoids (Fig. 5). In
addition, the inhibition of Δ-5-desaturase activity increases
hepatic contents of dihomo γ-linolenic acid, which is a precursor
of anti-inflammatory eicosanoids (Fig. 5). López-Vicario et al
recently showed that a Δ-5-desaturase inhibitor, CP-24879,
significantly reduces intracellular lipid accumulation and
inflammatory injury in hepatocytes in vitro (30), supporting our hypothesis. Thus,
our findings together with those of previous studies suggest that
suppression of Δ-5-desaturase activity could be a new therapeutic
strategy for NASH.
In conclusion, the results of the present study have
shown that Ex-4 suppressed body weight gain and improved
steatohepatitis in a murine model of NASH. Ex-4 also altered
hepatic fatty acid composition with a decrease in Δ-5-desaturase
index. These findings suggest that Ex-4 improves NASH by modulating
hepatic fatty acid metabolism.
Acknowledgements
This study was supported, in part, by Health and
Labour Sciences Research Grants for Research on Hepatitis from the
Ministry of Health, Labour and Welfare of Japan.
Abbreviations:
|
GLP-1
|
glucagon-like peptide-1
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
Ex-4
|
exendin-4
|
|
GLP-1R
|
GLP-1 receptor
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
PUFA
|
polyunsaturated fatty acid
|
|
MCD
|
methionine-choline deficient
|
|
SFA
|
saturated fatty acid
|
|
MUFA
|
monounsaturated fatty acid
|
References
|
1
|
Williams CD, Stengel J, Asike MI, et al:
Prevalence of nonalcoholic fatty liver disease and nonalcoholic
steatohepatitis among a largely middle-aged population utilizing
ultrasound and liver biopsy: a prospective study. Gastroenterology.
140:124–131. 2011. View Article : Google Scholar
|
|
2
|
Ono M and Saibara T: Clinical features of
nonalcoholic steatohepatitis in Japan: Evidence from the
literature. J Gastroenterol. 41:725–732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sumida Y, Yoneda M, Hyogo H, et al: A
simple clinical scoring system using ferritin, fasting insulin, and
type IV collagen 7S for predicting steatohepatitis in nonalcoholic
fatty liver disease. J Gastroenterol. 46:257–268. 2011. View Article : Google Scholar
|
|
4
|
Eguchi Y, Hyogo H, Ono M, et al:
Prevalence and associated metabolic factors of nonalcoholic fatty
liver disease in the general population from 2009 to 2010 in Japan:
a multicenter large retrospective study. J Gastroenterol.
47:586–595. 2012. View Article : Google Scholar
|
|
5
|
Nakahara T, Hyogo H, Yoneda M, et al: Type
2 diabetes mellitus is associated with the fibrosis severity in
patients with nonalcoholic fatty liver disease in a large
retrospective cohort of Japanese patients. J Gastroenterol. Nov
26–2013.(Epub ahead of print).
|
|
6
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imajo K, Fujita K, Yoneda M, et al:
Hyperresponsivity to low-dose endotoxin during progression to
nonalcoholic steatohepatitis is regulated by leptin-mediated
signaling. Cell Metab. 16:44–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith K: Microbiota: Gut microbiota
produce alcohol in patients with NASH. Nat Rev Gastroenterol
Hepatol. 9:6872012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mells JE and Anania FA: The role of
gastrointestinal hormones in hepatic lipid metabolism. Semin Liver
Dis. 33:343–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Itou M, Kawaguchi T, Taniguchi E, et al:
Altered expression of glucagon-like peptide-1 and dipeptidyl
peptidase IV in patients with HCV-related glucose intolerance. J
Gastroenterol Hepatol. 23:244–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itou M, Kawaguchi T, Taniguchi E and Sata
M: Dipeptidyl peptidase-4: a key player in chronic liver disease.
World J Gastroenterol. 19:2298–2306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drucker DJ: The biology of incretin
hormones. Cell Metab. 3:153–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gier B, Butler PC, Lai CK, Kirakossian D,
DeNicola MM and Yeh MW: Glucagon like peptide-1 receptor expression
in the human thyroid gland. J Clin Endocrinol Metab. 97:121–131.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Broide E, Bloch O, Ben-Yehudah G, Cantrell
D, Shirin H and Rapoport MJ: GLP-1 receptor is expressed in human
stomach mucosa: analysis of its cellular association and
distribution within gastric glands. J Histochem Cytochem.
61:649–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta NA, Mells J, Dunham RM, et al:
Glucagon-like peptide-1 receptor is present on human hepatocytes
and has a direct role in decreasing hepatic steatosis in vitro by
modulating elements of the insulin signaling pathway. Hepatology.
51:1584–1592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Svegliati-Baroni G, Saccomanno S,
Rychlicki C, et al: Glucagon-like peptide-1 receptor activation
stimulates hepatic lipid oxidation and restores hepatic signalling
alteration induced by a high-fat diet in nonalcoholic
steatohepatitis. Liver Int. 31:1285–1297. 2011. View Article : Google Scholar
|
|
17
|
Trevaskis JL, Griffin PS, Wittmer C, et
al: Glucagon-like peptide-1 receptor agonism improves metabolic,
biochemical, and histopathological indices of nonalcoholic
steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol.
302:G762–G772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhanesha N, Joharapurkar A, Shah G, et al:
Treatment with exendin-4 improves the antidiabetic efficacy and
reverses hepatic steatosis in glucokinase activator treated db/db
mice. Eur J Pharmacol. 714:188–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Panjwani N, Mulvihill EE, Longuet C, et
al: GLP-1 receptor activation indirectly reduces hepatic lipid
accumulation but does not attenuate development of atherosclerosis
in diabetic male ApoE(−/−) mice. Endocrinology. 154:127–139.
2013.PubMed/NCBI
|
|
20
|
Stumpf PK: Metabolism of fatty acids. Annu
Rev Biochem. 38:159–212. 1969. View Article : Google Scholar
|
|
21
|
Calder PC: N-3 polyunsaturated fatty acids
and inflammation: from molecular biology to the clinic. Lipids.
38:343–352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patterson E, Wall R, Fitzgerald GF, Ross
RP and Stanton C: Health implications of high dietary omega-6
polyunsaturated Fatty acids (Review). J Nutr Metab.
2012:e5394262012. View Article : Google Scholar
|
|
23
|
Puri P, Baillie RA, Wiest MM, et al: A
lipidomic analysis of nonalcoholic fatty liver disease. Hepatology.
46:1081–1090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Puri P, Wiest MM, Cheung O, et al: The
plasma lipidomic signature of nonalcoholic steatohepatitis.
Hepatology. 50:1827–1838. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poudel-Tandukar K, Sato M, Ejima Y, et al:
Relationship of serum fatty acid composition and desaturase
activity to C-reactive protein in Japanese men and women.
Atherosclerosis. 220:520–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chuang LT, Thurmond JM, Liu JW, Mukerji P,
Bray TM and Huang YS: Effect of conjugated linoleic acid on Delta-5
desaturase activity in yeast transformed with fungal Delta-5
desaturase gene. Mol Cell Biochem. 265:11–18. 2004. View Article : Google Scholar
|
|
27
|
Roke K, Ralston JC, Abdelmagid S, et al:
Variation in the FADS1/2 gene cluster alters plasma n-6 PUFA and is
weakly associated with hsCRP levels in healthy young adults.
Prostaglandins Leukot Essent Fatty Acids. 89:257–263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maniongui C, Blond JP, Ulmann L, Durand G,
Poisson JP and Bézard J: Age-related changes in delta 6 and delta 5
desaturase activities in rat liver microsomes. Lipids. 28:291–297.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kröger J and Schulze MB: Recent insights
into the relation of Δ5 desaturase and Δ6 desaturase activity to
the development of type 2 diabetes. Curr Opin Lipidol. 23:4–10.
2012.
|
|
30
|
López-Vicario C, González-Périz A, Rius B,
et al: Molecular interplay between Δ5/Δ6 desaturases and long-chain
fatty acids in the pathogenesis of non-alcoholic steatohepatitis.
Gut. 63:344–355. 2014.
|
|
31
|
Yamaguchi K, Yang L, McCall S, et al:
Inhibiting triglyceride synthesis improves hepatic steatosis but
exacerbates liver damage and fibrosis in obese mice with
nonalcoholic steatohepatitis. Hepatology. 45:1366–1374. 2007.
View Article : Google Scholar
|
|
32
|
Kawaguchi T, Sakisaka S, Sata M, Mori M
and Tanikawa K: Different lobular distributions of altered
hepatocyte tight junctions in rat models of intrahepatic and
extrahepatic cholestasis. Hepatology. 29:205–216. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawaguchi T, Sakisaka S, Mitsuyama K, et
al: Cholestasis with altered structure and function of hepatocyte
tight junction and decreased expression of canalicular
multispecific organic anion transporter in a rat model of colitis.
Hepatology. 31:1285–1295. 2000. View Article : Google Scholar
|
|
34
|
Kawaguchi T, Yoshida T, Harada M, et al:
Hepatitis C virus down-regulates insulin receptor substrates 1 and
2 through up-regulation of suppressor of cytokine signaling 3. Am J
Pathol. 165:1499–1508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawaguchi T, Ide T, Taniguchi E, et al:
Clearance of HCV improves insulin resistance, beta-cell function,
and hepatic expression of insulin receptor substrate 1 and 2. Am J
Gastroenterol. 102:570–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krishna SM, Seto SW, Moxon JV, et al:
Fenofibrate increases high-density lipoprotein and sphingosine 1
phosphate concentrations limiting abdominal aortic aneurysm
progression in a mouse model. Am J Pathol. 181:706–718. 2012.
View Article : Google Scholar
|
|
37
|
Kleiner DE, Brunt EM, Van Natta M, et al:
Design and validation of a histological scoring system for
nonalcoholic fatty liver disease. Hepatology. 41:1313–1321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Folch J, Lees M and Sloane Stanley GH: A
simple method for the isolation and purification of total lipides
from animal tissues. J Biol Chem. 226:497–509. 1957.PubMed/NCBI
|
|
39
|
Kenny PR, Brady DE, Torres DM, Ragozzino
L, Chalasani N and Harrison SA: Exenatide in the treatment of
diabetic patients with non-alcoholic steatohepatitis: a case
series. Am J Gastroenterol. 105:2707–2709. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ding X, Saxena NK, Lin S, Gupta NA and
Anania FA: Exendin-4, a glucagon-like protein-1 (GLP-1) receptor
agonist, reverses hepatic steatosis in ob/ob mice. Hepatology.
43:173–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gupta NA, Kolachala VL, Jiang R, et al:
The glucagon-like peptide-1 receptor agonist Exendin 4 has a
protective role in ischemic injury of lean and steatotic liver by
inhibiting cell death and stimulating lipolysis. Am J Pathol.
181:1693–1701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leamy AK, Egnatchik RA and Young JD:
Molecular mechanisms and the role of saturated fatty acids in the
progression of non-alcoholic fatty liver disease. Prog Lipid Res.
52:165–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsumori R, Miyazaki T, Shimada K, et al:
High levels of very long-chain saturated fatty acid in erythrocytes
correlates with atherogenic lipoprotein profiles in subjects with
metabolic syndrome. Diabetes Res Clin Pract. 99:12–18. 2013.
View Article : Google Scholar
|
|
44
|
Kusunoki M, Tsutsumi K, Nakayama M, et al:
Relationship between serum concentrations of saturated fatty acids
and unsaturated fatty acids and the homeostasis model insulin
resistance index in Japanese patients with type 2 diabetes
mellitus. J Med Invest. 54:243–247. 2007. View Article : Google Scholar
|
|
45
|
Arrol S, Mackness MI and Durrington PN:
The effects of fatty acids on apolipoprotein B secretion by human
hepatoma cells (HEP G2). Atherosclerosis. 150:255–264. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kurek K, Piotrowska DM, Wiesiolek-Kurek P,
et al: Inhibition of ceramide de novo synthesis reduces liver lipid
accumulation in rats with nonalcoholic fatty liver disease. Liver
Int. Sep 25–2013.(Epub ahead of print).
|
|
47
|
Araya J, Rodrigo R, Videla LA, et al:
Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in
relation to hepatic steatosis in patients with non-alcoholic fatty
liver disease. Clin Sci (Lond). 106:635–643. 2004. View Article : Google Scholar
|
|
48
|
de Gomez Dumm IN, de Alaniz MJ and Brenner
RR: Effect of dietary fatty acids on delta 5 desaturase activity
and biosynthesis of arachidonic acid in rat liver microsomes.
Lipids. 18:781–788. 1983.PubMed/NCBI
|