Introduction
Obesity is the most common metabolic disease in
developed nations and has become a global epidemic in recent years
(1). Furthermore, obesity is
associated with a variety of chronic diseases, including glucose
intolerance, insulin resistance, dyslipidemia and hypertension. A
combination of these abnormalities is now referred to as the
metabolic syndrome (2). An
increase in fat mass is a result of an increase in adipocyte number
and size. Cellular and molecular studies focusing on the
development of obesity have shown that changes in the number of
adipocytes (hyperplasia) and adipocyte size (hypertrophy) can be
triggered by dietary factors (3,4).
The findings of a previous study indicated that an increased
adipocyte number during the aging process may contribute to the
increase in the incidence and severity of obesity observed in older
individuals (5). Thus,
hyperplasia of adipocytes may be an important factor in the
development of obesity.
Adipocytes are derived from mesenchymal stem cells,
which have the potential to differentiate into myoblasts,
chondroblasts, osteoblasts or adipocytes (6). Adipocyte differentiation involves an
elaborate network of transcription factors that regulate the
expression of numerous genes responsible for the phenotype of
mature adipocytes (7). Among the
various transcription factors that promote preadipocyte
differentiation and influence adipogenesis, peroxisome
proliferator-activated receptor γ (PPARγ) is considered the ‘master
regulator of adipogenesis’ (8–10).
Other adipogenic transcription factors include the CCAAT/enhancer
binding proteins (C/EBPα, C/EBPβ and C/EBPγ) (5,7,11).
These factors are necessary for the expression of
adipocyte-specific genes (adiponectin) (12). These transcription factors,
especially PPARγ and C/EBPα are regulated by the mitogen-activated
protein kinase (MAPK) pathway during adipogenesis (13–15).
Sphingosine-1-phosphate (S1P) is a member of an
important group of signaling sphingolipids now recognized to play a
role in a diverse array of cell processes, such as apoptosis, cell
motility, differentiation, and proliferation in a variety of cell
types including endothelial cells, smooth muscle cells and
macrophages (16,17). S1P is generated by the
phosphorylation of the sphingosine mediated by sphingosine
kinases-1 (Sphk-1) and Sphk-2 (18). S1P exerts most of its activity as
a ligand of G-protein-coupled receptors (GPCRs) (19). At present, five members of the S1P
receptor family have been identified in mammals, notably S1P1-5,
possessing distinct expression profiles and affinities towards S1P
(20,21).
S1P regulates the differentiation via MAPK pathways
in a variety of cell types including osteoclasts, monocytes,
placental trophoblasts, myoblasts and vascular smooth muscle cells
(16,19,22–24). In addition, a number of studies
have shown that S1P and sphingosine kinases have multifunctional
characteristics, including a correlation with weight gain in breast
cancer patients, a sensitivity to acute myeloid leukemia cells, a
chemotherapy sensor in prostate cancer and enhancing sensitivity to
hormone-resistant prostate cancer (25–28). ERK, p38 and JNK MAPKs are
intracellular signaling pathways that play a pivotal role in
numerous essential cell processes such as proliferation and
differentiation (3,13,24). Chemotherapy induces the
downregulation of S1P by inhibiting Sphk and this decrease of
circulating S1P by chemotherapy may switch S1P-mediated adipose
cell stasis to adipogenesis.
In the present study, we investigated whether S1P
inhibited adipocyte differentiation and regulated MAPK pathways
including ERK, p38 and JNK MAPKs. S1P was found to exert novel and
physiologically important biological effects on preadipocytes,
acting as an anti-differentiation agent.
Materials and methods
Reagents
S1P was purchased from Cayman Chemical (Ann Arbor,
MI, USA) and Sigma-Aldrich (St. Louis, MO, USA). S1P was prepared
as a 2 mM solution in 0.3 M NaOH or methanol or 125 μM solution in
fatty acid-free bovine serum albumin, subsequently diluted in cell
culture medium.
Cell culture and differentiation
3T3-L1 cells were maintained in Dulbecco’s modified
Eagle’s medium (DMEM) containing 10% calf serum and antibiotics
(100 μg/ml gentamycin and 100 μg/ml penicillin-streptomycin). To
induce differentiation, 2-day post confluent 3T3-L1 cells were
incubated in MDI induction media [DMEM containing 10% fetal bovine
serum, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 1 μm
dexamethasone and 1 μg/ml of insulin] for 2 days. In some
experiments, S1P (10 μM) was added at the time of the induction of
differentiation. The AdipoRed Assay and detection of glycerol
release contents were performed on day 7.
Quantification of lipid content
Lipid content was quantified using the commercially
available AdipoRed assay reagent (Lonza, Verviers, Belgium)
according to the manufacturer’s instructions. In brief,
preadipocytes grown in 24-well plates were incubated with MDI
medium or with the test compounds during the adipogenic phase and
on day 6, the culture supernatant was removed and carefully washed
with 500 μl phosphate-buffered saline (PBS). The wells were
subsequently filled with 300 μl PBS and 30 μl of AdipoRed reagent
were added followed by incubation for 10 min at 37°C. The AdipoRed
of the cells was photographed using a light microscope and
fluorescence was measured with an excitation at 485 nm and an
emission at 572 nm.
Adipolysis assay
Glycerol release was measured using a commercially
available Adipolysis assay kit (Cayman Chemical) according to the
manufacturer’s instructions. Briefly, the differentiated adipocytes
in a 96-well plate were stimulated with S1P or isoproterenol
solution used as a positive control for 24 h. After stimulation,
the cell culture supernatants were collected from each well and
stored until use at −20°C. A total of 100 μl of free glycerol assay
reagent was added to 25 μl of each supernatant. Following
incubation for 15 min at room temperature, the absorbance was
measured at 540 nm.
Quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from 3T3-L1 cells treated
with S1P using the Easy-spin™ total RNA extraction kit (Intron
Biotechnology, Seoul, Korea). cDNA synthesis was carried out
following the instructions of the Takara PrimeScript™ 1st Strand
cDNA synthesis kit (Takara Bio, Tokyo, Japan). For the RT-qPCR, 1
μl of gene primers with SYBR-Green (Bio-Rad Laboratories, Hercules,
CA, USA) in 20 μl of reaction volume was applied. The primer
sequences used for qPCR were: PPARγ (forward, 5′-CGGAAGCCCTTTGG
TGACTTTATG-3′ and reverse, 5′-GCAGCAGGTTGTCTT GGATGTC-3′), C/EBP-α
(forward, 5′-CGGGAACGCAAC AACATCGC-3′ and reverse,
5′-TGTCCAGTTCACGGCT CAGC-3′), adiponectin (forward,
5′-TGACGGCAGCACT GGCAAG-3′ and reverse, 5′-TGATACTGGTCGTAGGTGAA
GAGAAC-3′) β-actin (forward, 5′-TGAGAGGGAAATCG TGCGTGAC-3′ and
reverse, 5′-GCTCGTTGCCAATAGTGA TGACC-3′). All reactions with iTaq
SYBR-Green Supermix were performed on the CFX96 real-time PCR
detection system (both from Bio-Rad Laboratories).
Western blot analysis
The 3T3-L1 cells were lysed in a lysis buffer (25 mM
HEPES; pH 7.4, 100 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 0.1
mM dithiothreitol, and protease inhibitor mixture). Proteins were
electrophoretically resolved on an 8–15% sodium dodecyl sulfate
(SDS) gel, and immunoblotting was performed as previously described
(29). Images were captured using
the Fusion FX7 acquisition system (Vilber Lourmat, Eberhardzell,
Germany). Densitometry of the signal bands was analyzed using
Bio-1D (Vilber Lourmat) (30).
The antibodies used for immunoblotting were PPARγ (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), p-JNK and p-p38 (both
from Cell Signaling Technology Beverly, MA, USA) and β-actin
(Sigma-Aldrich).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean (SEM). Data were compared using the Student’s t-test,
analysis of variance (ANOVA) and Duncan test with the SAS
statistical package. The results were considered significant for
values of P<0.05 or P<0.01.
Results
S1P inhibits adipocyte differentiation of
3T3-L1 cells
Since S1P regulates the differentiation of various
cell types, the effect of S1P on adipocyte differentiation of the
3T3-L1 cells was investigated. Preadipocytes grown in 24-well
plates were incubated with MDI media with or without S1P during the
adipogenic differentiation phase. When 3T3-L1 cells differentiated
over 6 days in the presence of various concentrations of S1P in the
adipogenic medium, a reduction in lipid accumulation was observed
(Fig. 1A and B). The inhibition
effect of S1P was significantly detected at 0.5 μM and was maximal
at 50 μM. To confirm inhibition of triglyceride accumulation of
S1P, we measured the triglycerides directly in 3T3-L1 cells
differentiated over 6 days that were treated with S1P. S1P
treatment also inhibited triglyceride accumulation during the
differentiation of 3T3-L1 preadipocytes (Fig. 1C). To study the effect of S1P on
lipolysis, the differentiated adipocytes were incubated with
various concentrations of S1P for 24 h, and the glycerol level was
determined in the medium. However, S1P did not affect glycerol
release, marked to lipolysis of differentiated adipocytes (Fig. 1D), indicating that S1P inhibited
lipid accumulation by blocking adipogenic differentiation, not by
lipolysis of differentiated adipocytes.
We investigated whether the inhibition effects of
S1P are maintained in various dissolving solutions of S1P,
including fatty acid-free albumin and methyl alcohol. We tested the
adipogenic differentiation, lipid contents and glycerol release
assay using S1P dissolved in methyl alcohol (MeOH) and fatty
acid-free albumin stock solution in 3T3-L1 preadipocytes (Fig. 2). S1P dissolved in MeOH and in
fatty acid-free albumin inhibited lipid accumulation but did not
affect the glycerol release. The results demonstrated that S1P
inhibited lipid accumulation by inhibiting adipogenic
differentiation without regulating the lipolysis of adipocyte in
3T3-L1 cells.
S1P downregulates the transcriptional
factor, PPARγ, involved in adipocyte differentiation
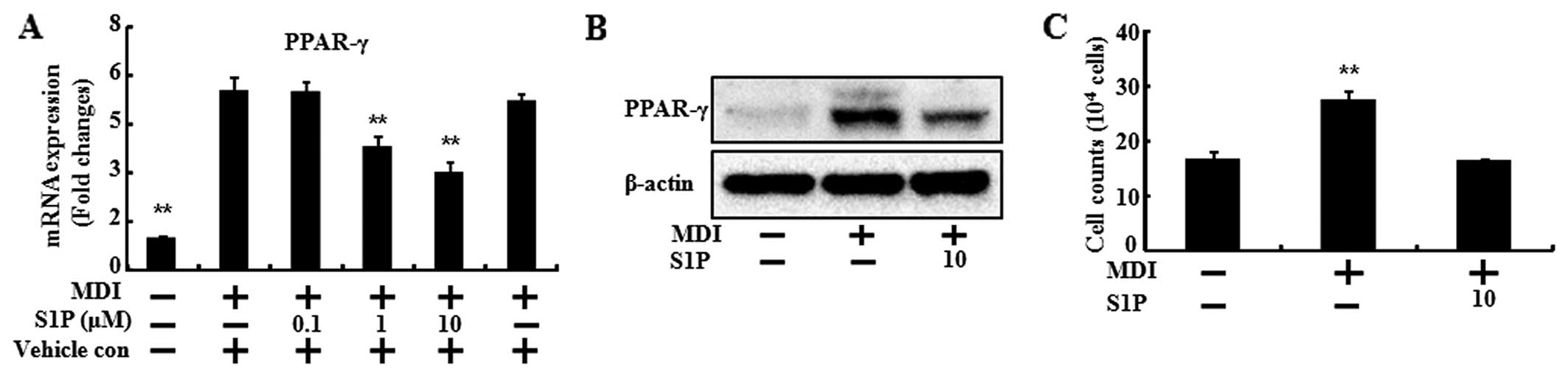
To confirm the inhibitory effects of SIP on
adipogenic differentiation, the mRNA levels of biochemical markers
of differentiation (PPARγ, C/EBPα and adiponectin) were determined
(Figs. 3 and 4). When the 3T3-L1 preadipocytes
differentiated with MDI treatment, the mRNA levels of the
biochemical markers of differentiation increased compared to the
control. However, S1P treatment led to a significant reduction by
increasing the dose of S1P in the mRNA level of PPARγ, C/EBPα and
adiponectin (Figs. 3A, 4A and 4B). In the case of PPARγ, the
protein expression was also decreased by S1P (Fig. 3B).
S1P is interconvertible with ceramide and it is a
critical mediator of apoptosis. Therefore, we investigated the cell
number of 3T3-L1 cells during the differentiation in the presence
of 10 μM of S1P. At 24 h after MDI induction, mitosis occurred,
thus the cell numbers were doubled (31). Consistent with results of that
study, in the present study, the cell numbers of the MDI induction
were doubled in the control and those of the S1P treatment were
similar to the control (Fig. 3C).
These results demonstrated that S1P exhibits anti-adipogenic
activity through downregulation of the transcription factors
involved in adipocyte differentiation.
S1P mediates its action on MAPK pathways
via the S1P2 receptor subtype
It is well known that preadipocyte differentiation
involves the activation of several key signaling pathways such as
JNK1/2 and p38 MAPK (13). To
gain insight into the molecular mechanisms responsible for the
observed biological effects of S1P, the ability of the sphingolipid
to inactivate these protein kinases was examined. The MDI
containing adipocyte differentiation cocktail induced the
phosphorylation of JNK1/2 and p38 MAPK, at 12, 6 and 3 h,
respectively, after MDI addition (Fig. 4C). However, 10 μM of S1P decreased
the phosphorylation of JNK1/2 and p38 MAPK at 12 h after the
addition of MDI. Taken together, these results showed that S1P
inhibited the adipocyte differentiation and lipid accumulation, and
the inhibition effects were mediated by the downregulation of
transcription factors and by inactivation of the MAPK signals.
Discussion
Excessive adipose tissue accumulation is a key
factor leading to insulin resistance, type 2 diabetes,
hyperlipidemia and an increased risk of cardiovascular disease.
Obesity is no longer considered to be only a cosmetic problem, but
is associated with an increased risk for the development of
numerous adverse health conditions (1,6).
The recruitment of new fat cells in adipose tissue requires the
differentiation of preadipocytes into adipocytes (adipogenesis), a
process closely controlled by the transcription factors PPARγ and
C/EBPα (32,33).
Studies on the effects of S1P on cell
differentiation are available. S1P acts as a regulator of
osteoclast differentiation (22)
as well as myogenic differentiation (24,34). S1P and the S1P1 receptor are
associated with angiogenic differentiation of vascular endothelial
cells (35). Although S1P has
been demonstrated to promote the differentiation of endothelial
cells and myocytes, the ability of S1P to affect cell
differentiation appears to be dependent on the cell type. In
placental trophoblasts and human monocytes, S1P shows
anti-differentiating effects. S1P inhibits the differentiation of
cytotrophoblasts into syncytiotrophoblasts through a G(i)-coupled
S1P receptor interaction (16).
In addition, S1P interferes with the differentiation of human
monocytes into competent dendritic cells (23). Results of previous studies are in
concordance with our results showing that S1P inhibits the
differentiation of preadipocytes into adipocytes in 3T3-L1 cells
(Figs. 1 and 2).
S1P levels inside cells are closely regulated by the
balance between its synthesis by sphingosine kinases and
degradation. S1P is interconvertible with ceramide, which is a
critical mediator of apoptosis. In the present study, a high dose
of S1P was utilized to determine the anti-adipogenic effect of S1P.
To verify whether the high dose of S1P can be converted to ceramide
and can equally activate the S1P receptors, further experiments are
required in future studies. The ceramide itself served as an
important second messenger in various stress responses and growth
mechanisms (36). While S1P
functions mainly via GPCR, ceramide and its metabolite appears to
bind directly to targets (36).
The p38 and JNK MAPKs are intracellular signaling
pathways that play a pivotal role in numerous essential cell
processes such as proliferation and differentiation (3,13,
24). MAPKs are activated by a
large variety of stimuli and one of their major functions is to
connect cell surface receptors to transcription factors in the
nucleus, which consequently triggers long-term cell responses
(13). Previously, it was
established that, the MAPK signaling pathway regulates the
expression of PPARγ and C/EBPα during adipogenesis in preadipocytes
(37). A well-known stimulus that
affects the MAPK signaling pathways is S1P. The results of this
study have shown that S1P inhibited MDI-induced phosphorylation of
p38 and JNK1/2 (Fig. 4C).
When induced to differentiate, growth-arrested
3T3-L1 preadipocytes synchronously re-enter the cell cycle and
undergo mitotic clonal expansion (MCE). MCE is a prerequisite for
the differentiation of 3T3-L1 preadipocytes into adipocytes
(31). Consistent with that
study, our results show that the number of cells at 24 h after MDI
induction was increased whereas the addition of S1P significantly
decreased cell populations (Fig.
3C). The results suggest that S1P inhibited the first round of
mitosis, thereby preventing the expression of adipogenic regulator
genes.
In conclusion, the results of this study have shown
that exposure of preadipocytes to S1P inhibited their
differentiation into adipocytes, as confirmed by a reduction in
triglyceride accumulation and a reduction in the expression of
adipocyte specific genes. Therefore, S1P functioned as an
anti-adipogenic compound. The results also suggest that the
adipogenic transcription factors and various MAPK pathways are a
potential therapeutic target for obesity.
Acknowledgements
This study was supported by a grant from the
National Research Foundation of Korea (NRF), funded by the Korean
government (2013R1A1A2063931).
References
|
1
|
Lei F, Zhang XN, Wang W, et al: Evidence
of anti-obesity effects of the pomegranate leaf extract in high-fat
diet induced obese mice. Int J Obes (Lond). 31:1023–1029. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyle KB, Hadaschik D, Virtue S, et al:
The transcription factors Egr1 and Egr2 have opposing influences on
adipocyte differentiation. Cell Death Differ. 16:782–789. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim KJ, Lee OH and Lee BY: Fucoidan, a
sulfated polysaccharide, inhibits adipogenesis through the
mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes.
Life Sci. 86:791–797. 2010. View Article : Google Scholar
|
|
4
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI
|
|
5
|
Yanagiya T, Tanabe A and Hotta K:
Gap-junctional communication is required for mitotic clonal
expansion during adipogenesis. Obesity (Silver Spring). 15:572–582.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rayalam S, Della-Fera MA and Baile CA:
Phytochemicals and regulation of the adipocyte life cycle. J Nutr
Biochem. 19:717–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee H, Lee YJ, Choi H, Ko EH and Kim JW:
Reactive oxygen species facilitate adipocyte differentiation by
accelerating mitotic clonal expansion. J Biol Chem.
284:10601–10609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wakabayashi K, Okamura M, Tsutsumi S, et
al: The peroxisome proliferator-activated receptor gamma/retinoid X
receptor alpha heterodimer targets the histone modification enzyme
PR-Set7/Setd8 gene and regulates adipogenesis through a positive
feedback loop. Mol Cell Biol. 29:3544–3555. 2009. View Article : Google Scholar
|
|
9
|
Tontonoz P and Spiegelman BM: Fat and
beyond: the diverse biology of PPARgamma. Annu Rev Biochem.
77:289–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosen ED and MacDougald OA: Adipocyte
differentiation from the inside out. Nat Rev Mol Cell Biol.
7:885–896. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nerurkar PV, Lee YK and Nerurkar VR:
Momordica charantia (bitter melon) inhibits primary human adipocyte
differentiation by modulating adipogenic genes. BMC Complement
Altern Med. 10:342010. View Article : Google Scholar
|
|
12
|
Xing Y, Yan F, Liu Y and Zhao Y: Matrine
inhibits 3T3-L1 preadipocyte differentiation associated with
suppression of ERK1/2 phosphorylation. Biochem Biophys Res Commun.
396:691–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bost F, Aouadi M, Caron L and Binétruy B:
The role of MAPKs in adipocyte differentiation and obesity.
Biochimie. 87:51–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alonso-Vale MI, Peres SB, Vernochet C,
Farmer SR and Lima FB: Adipocyte differentiation is inhibited by
melatonin through the regulation of C/EBPbeta transcriptional
activity. J Pineal Res. 47:221–227. 2009. View Article : Google Scholar
|
|
15
|
Chen TH, Chen WM, Hsu KH, Kuo CD and Hung
SC: Sodium butyrate activates ERK to regulate differentiation of
mesenchymal stem cells. Biochem Biophys Res Commun. 355:913–918.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnstone ED, Chan G, Sibley CP, Davidge
ST, Lowen B and Guilbert LJ: Sphingosine-1-phosphate inhibition of
placental trophoblast differentiation through a G(i)-coupled
receptor response. J Lipid Res. 46:1833–1839. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goetzl EJ, Wang W, McGiffert C, Liao JJ
and Huang MC: Sphingosine 1-phosphate as an intracellular messenger
and extracellular mediator in immunity. Acta Paediatr Suppl.
96:49–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He X, H’ng SC, Leong DT, Hutmacher DW and
Melendez AJ: Sphingosine-1-phosphate mediates proliferation
maintaining the multipotency of human adult bone marrow and adipose
tissue-derived stem cells. J Mol Cell Biol. 2:199–208. 2010.
View Article : Google Scholar
|
|
19
|
Schüppel M, Kürschner U, Kleuser U,
Schäfer-Korting M and Kleuser B: Sphingosine 1-phosphate restrains
insulin-mediated keratinocyte proliferation via inhibition of Akt
through the S1P2 receptor subtype. J Invest Dermatol.
128:1747–1756. 2008.PubMed/NCBI
|
|
20
|
Bieberich E: There is more to a lipid than
just being a fat: sphingolipid-guided differentiation of
oligodendroglial lineage from embryonic stem cells. Neurochem Res.
36:1601–1611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pyne S and Pyne NJ: Sphingosine
1-phosphate signalling in mammalian cells. Biochem J. 349:385–402.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y
and Kim HH: Sphingosine 1-phosphate as a regulator of osteoclast
differentiation and osteoclast-osteoblast coupling. EMBO J.
25:5840–5851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martino A, Volpe E, Auricchio G, et al:
Sphingosine 1-phosphate interferes on the differentiation of human
monocytes into competent dendritic cells. Scand J Immunol.
65:84–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Donati C, Meacci E, Nuti F, Becciolini L,
Farnararo M and Bruni P: Sphingosine 1-phosphate regulates myogenic
differentiation: a major role for S1P2 receptor. FASEB J.
19:449–451. 2005.PubMed/NCBI
|
|
25
|
Pchejetski D, Nunes J, Sauer L, et al:
Circulating sphingosine-1- phosphate inversely correlates with
chemotherapy-induced weight gain during early breast cancer. Breast
Cancer Res Treat. 124:543–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonhoure E, Pchejetski D, Aouali N, et al:
Overcoming MDR-associated chemoresistance in HL-60 acute myeloid
leukemia cells by targeting sphingosine kinase-1. Leukemia.
20:95–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pchejetski D, Golzio M, Bonhoure E, et al:
Sphingosine kinase-1 as a chemotherapy sensor in prostate
adenocarcinoma cell and mouse models. Cancer Res. 65:11667–11675.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sauer L, Nunes J, Salunkhe V, et al:
Sphingosine kinase 1 inhibition sensitizes hormone-resistant
prostate cancer to docetaxel. Int J Cancer. 125:2728–2736. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moon MH, Jeong JK, Seo JS, et al:
Bisphosphonate enhances TRAIL sensitivity to human osteosarcoma
cells via death receptor 5 upregulation. Exp Mol Med. 43:138–145.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seo JS, Moon MH, Jeong JK, et al: SIRT1, a
histone deacetylase, regulates prion protein-induced neuronal cell
death. Neurobiol Aging. 33:1110–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang QQ, Otto TC and Lane MD: Mitotic
clonal expansion: a synchronous process required for adipogenesis.
Proc Natl Acad Sci USA. 100:44–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simon MF, Daviaud D, Pradère JP, et al:
Lysophosphatidic acid inhibits adipocyte differentiation via
lysophosphatidic acid 1 receptor-dependent down-regulation of
peroxisome proliferator-activated receptor gamma2. J Biol Chem.
280:14656–14662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu L, Tang T, Miao Y, Zhang S, Qu Z and
Dai K: Stimulation of osteogenic differentiation and inhibition of
adipogenic differentiation in bone marrow stromal cells by
alendronate via ERK and JNK activation. Bone. 43:40–47. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meacci E, Cencetti F, Donati C, et al:
Down-regulation of EDG5/S1P2 during myogenic differentiation
results in the specific uncoupling of sphingosine 1-phosphate
signalling to phospholipase D. Biochim Biophys Acta. 1633:133–142.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Wada R, Yamashita T, et al: Edg-1,
the G protein-coupled receptor for sphingosine-1-phosphate, is
essential for vascular maturation. J Clin Invest. 106:951–961.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chalfant CE and Spiegel S: Sphingosine
1-phosphate and ceramide 1-phosphate: expanding roles in cell
signaling. J Cell Sci. 118:4605–4612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Prusty D, Park BH, Davis KE and Farmer SR:
Activation of MEK/ERK signaling promotes adipogenesis by enhancing
peroxisome proliferator-activated receptor gamma (PPARgamma ) and
C/EBPalpha gene expression during the differentiation of 3T3-L1
preadipocytes. J Biol Chem. 277:46226–46232. 2002. View Article : Google Scholar
|