Introduction
Pancreatic β cell dysfunction, mainly caused by
glucotoxicity, is an important factor leading to type 2 diabetes
(1,2). High glucose-induced free fatty acid
(FFA) synthesis leads to β cell apoptosis (3), impaired glucose-stimulated insulin
secretion (GSIS) (4) and lipid
accumulation (5). In recent
years, sterol regulatory element binding protein-1c (SREBP-1c), an
important lipogenic transcription factor (6), has been found to regulate genes
involving insulin secretion (7).
Insulin induced gene-1 (Insig-1) is a critical
upstream regulatory factor of SREBP-1c. Insig-1 prevents the SREBP
cleavage-activating protein (SCAP)/SREBP-1c complex to translocate
from the Golgi apparatus to the endoplasmic reticulum (ER),
subsequently decreases nuclear SREBP-1c (nSREBP-1c) expression, and
blocks the related gene transcription of SREBP-1c. It has been
demonstrated that the upregulation of Insig-1 decreases SREBP-1c
expression, thereby inhibiting lipid synthesis (8). In our previous study, we
demonstrated that the overexpression of Insig-1 leads to the
inhibition of SREBP-1c expression and the subsequent improvement of
β cell function (9).
Silibinin is a flavonoid extracted from milk thistle
(Silybum marianum), which has been used in the treatment of
liver disorders for over 2,000 years. Recently, silibinin has been
found to have antioxidant, anti-apoptotic and anti-inflammatory
properties, to have interactions with steroid hormone receptors,
and to be involved in the modulation of drug transporters. It has
also been shown that silibinin is able to improve β cell function
(10).
A number of in vivo and in vitro
studies have demonstrated the protective effects of silibinin on β
cell function. A clinical study demonstrated that silibinin reduced
the levels of fasting blood glucose, glycosylated hemoglobin,
cholesterol, triglycerides and low-density lipoprotein in patients
with type 2 diabetes (11). In
addition, silibinin decreases blood glucose levels in rats with
type 1 diabetes induced by streptozotocin (STZ); however, no
difference was observed in basal insulin secretion (12). Soto et al found that
silibinin improved the symptoms in alloxan-induced diabetic rats
through anti-oxidative stress and increased insulin secretion
through the upregulation of insulin and pancreatic and duodenal
homeobox 1 (PDX-1) mRNA expression (13). Silibinin has been shown to
suppress interleukin (IL)-1β and interferon (IFN)-γ-induced nitric
oxide (NO) production and ameliorate β cell dysfunction through the
suppression of c-Jun NH2-terminal kinase (JNK)/signal
transducer and activator of transcription (STAT) pathways (14). Silibinin has also been shown to
protect β cell damage induced by cyclosporine A and regulate the
physiological level of autophagy by promoting sirtuin (Sirt)-1
expression (15,16).
A previous study revealed that silibinin upregulates
the expression of Insig-1 in mouse 3T3-L1 pre-adipocytes (17). In this study, we further explored
the downstream effects of silibinin on the Insig-1/SREBP-1c
pathway, which may be a novel target in the protection of β cells
against glucotoxicity. The results clearly suggest that silibinin
protects β cells from glucotoxicity through the regulation of the
Insig-1/SREBP pathway.
Materials and methods
Materials
Silibinin was obtained from Sigma-Aldrich Shanghai
Trading Co., Ltd. (Shanghai, China); β-mercaptoethanol, HEPES,
L-glutamine and sodium pyruvate were purchased from Amresco, Inc.
(Cleveland, OH, USA). The
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay kit, Hoechst 33258, Annexin V-propidium iodide (PI) apoptosis
kit, total protein and nuclear protein extraction kits, enhanced
chemiluminescence detection (ECL) kit, and the bicinchoninic acid
(BCA) assay kit were all obtained from Beyotime Institute of
Biotechnology (Haimen, China). The rat insulin ELISA kit was
purchased from Linco Research, Inc. (St. Charles, MO, USA). TRIzol
reagent and reverse transcription kit were from Life Technologies
(Carlsbad, CA, USA). The SYBR®-Green PCR assay kit was
purchased from Toyobo Co., Ltd., (Osaka, Japan). The polyvinylidene
fluoride membrane was obtained from Millipore Corp. (Bedford, MA,
USA). The primary antibodies to Insig-1, SREBP-1 and GAPDH and the
secondary antibodies were all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Lipofectamine 2000 was
purchased from Invitrogen (Carlsbad, CA, USA). Oil Red O was
purchased from Beijing Solarbio Science and Technology Co., Ltd.,
(Beijing, China), and the FFA ELISA kit was from USCN Life Science
Inc. (Wuhan, China).
Cell culture
Rat insulinoma INS-1 cells were purchased from
Bioleaf Biotech Co., Ltd. (Shanghai, China). The INS-1 cells were
cultured in RPMI-1640 containing 11 mM glucose with 10 mM HEPES,
10% fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 50
μM β-mercaptoethanol, 100 IU/ml penicillin and 100 IU/ml
streptomycin and incubated at 37°C in a 5% CO2
atmosphere.
Measurement of cell viability
The INS-1 cells were incubated with or without 30 μM
silibinin in normal glucose (11.2 mM) or high glucose (25.0 mM)
RPMI-1640 for 0, 24 or 72 h. Cell viability was measured using an
MTT assay kit. Briefly, at different time points, 10 μl of MTT (5
mg/ml) were added to the culture medium in a 96-well plate and
incubated at 37°C for 4 h before 100 μl of formanzan was added to
dissolve the MTT. After 3 h, the absorbance was measured at 570
nm.
Apoptosis assay by flow cytometry
After the INS-1 cells were incubated with or without
30 μM silibinin in normal glucose (11.2 mM) or high glucose (25.0
mM) RPMI-1640 for 0, 24 or 72 h, the cells were collected, washed
with phosphate-buffered saline (PBS), resuspended in 200 μl of
binding buffer containing 5 μl of Annexin V, and incubated in the
dark for 10 min according to the manufacturer’s instructions. The
cells were then stained with 10 μl of PI, and the samples were
immediately analyzed using a flow cytometer (Epics XL; Beckman
Coulter, Brea, CA, USA).
Assessment of cell apoptosis by Hoechst
33258 staining
For the Hoechst 33258 staining assay, the cells were
washed twice with PBS, fixed with 4% paraformaldehyde for 30 min,
and then washed 3 times with PBS. Hoechst 33258 (10 μg/ml) was
added, and the cells were incubated in the dark at room temperature
for 30 min before being washed with PBS. The nuclear morphology was
observed under a fluorescence microscope (Olympus IX71; Olympus,
Tokyo, Japan). The cells with condensed chromatin and shrunken
nuclei were classified as apoptotic. One hundred nuclei from the
control (untreated cells) and each group were counted, and the
percentage of cleaved nuclei was calculated.
GSIS
The INS-1 cells (2×105) were seeded in
24-well cell culture plates containing RPMI-1640 medium with a
normal or high glucose concentration for different periods of time.
The cells were then washed twice with PBS and incubated in a 3 mM
glucose KRBB solution (114 mM NaCl, 4.4 mM KCl, 1.28 mM
CaCl2, 1 mM MgSO4, 29.5 mM NaHCO3,
10 mM HEPES,3 mM glucose, and 0.1% bovine serum albumin, pH 7.4) at
37°C for 1 h. After the supernatant was collected, 20 mM glucose
KRBB were added to each well, the cells were incubated at 37°C for
1 h, and the supernatant was collected. The insulin levels in the
supernatant were measured using a rat insulin ELISA kit.
Reverse transcription quantitative
PCR
Total RNA was extracted using TRIzol reagent.
Subsequently, 1 μg of total RNA was used for the synthesis of cDNA
using a reverse transcription kit according to the manufacturer’s
instructions. The SYBR-Green PCR assay (20 μl total volume)
contained 10 μl of QuantiTect SYBR-Green PCR Master mix, 2 μl of
cDNA, 1.2 μl of primer (10 μM) and 6.8 μl of RNase-free water. The
primer sequences including insulin receptor substrate-2 (IRS-2),
PDX-1 and uncoupling protein-2 (UCP-2) and PCR conditions were the
same as those used in our previous study (9). The PCR was performed on a
Mastercycler® ep realplex real-time PCR (Eppendorf,
Hamburg, Germany). Relative differences in gene expression between
groups were determined using the 2−ΔΔCT method.
Western blot analysis
The total protein and nuclear protein was extracted
from the cells according to the manufacturer’s instructions. The
protein concentration was determined using a BCA assay. Proteins
(50 μg) were separated in 8–10% criterion precast gels and 5%
polyacrylamide gels and transferred onto polyvinylidene fluoride
membranes. After blocking for 1 h, the membranes were incubated
with primary antibodies against Insig-1 (sc-51103, 1:200), SREBP-1
(sc-8984,1:300), or GAPDH (sc-47724,1:800) overnight at 4°C,
followed by incubation with horseradish peroxidase (HRP)-conjugated
secondary antibodies. GAPDH served as a loading control on the same
membrane. Peroxidase activity was visualized using the ECL kit. The
membranes were scanned and analyzed using Scion Image software
(Scion Corp., Frederick, MD, USA).
Oil Red O staining and measurement of FFA
content
The cells were washed twice in PBS and fixed with 4%
paraformaldehyde for 30 min, and subsequently stained with fresh
Oil Red O solution (60% Oil Red O stock solution consisting of 0.5%
Oil Red O in isopropanol and 40% H2O) for 15 min. After
staining, the lipid droplets were observed and photographed under a
microscope (TE2000-E; Nikon, Tokyo, Japan). The cell culture
supernatant was collected, and the FFA concentration was measured
using an ELISA kit according to the manufacturer’s
instructions.
Statistical analysis
The results are all presented as the means ±
standard error (SE). Statistically significant differences between
two groups was determined using the Student’s t-test. Groups of 3
or more were analyzed by one-way analysis of variance. Differences
were considered to be statistically significant at P<0.05.
Results
Silibinin protects against high
glucose-induced apoptosis in INS-1 cells
First, to define the suitable intervention
concentration, we examined the apoptotic rate by Annexin V and PI
double staining. The INS-1 cells were cultured in high glucose
(25.0 mM) medium for 72 h with various concentrations of silibinin
(0–80 μM). It was found that 10–20 μM of silibinin did not affect
the apoptotic rate (P>0.05); however, 30–80 μM silibinin
significantly decreased the apoptotic rate (P<0.05). A further
increase in the silibinin concentration did not exert a greater
inhibitory effect (P>0.05). In addition, Oil Red O staining
revealed that 0 and 10 μM silibinin did not inhibit intracellular
lipid accumulation, while 20–80 μM silibinin significantly
decreased lipid accumulation (data not shown). Therefore, we
selected 30 μM silibinin for the following experiments (Fig. 1A).
We then examined the apoptotic rate of the INS-1
cells using both Annexin V/PI double staining and Hoechst 33258
staining. Treatment of the cells with high glucose or high glucose
and silibinin for 72 h resulted in the apoptosis of the INS-1
cells, as determined by flow cytometry. However, the number of
apoptotic cells were significantly reduced in the high glucose and
silibinin group compared to the high glucose group (P<0.05)
(Fig. 1B). Hoechst 33258 staining
revealed that the apoptotic cells had condensed nuclei and DNA
fragmentation, and silibinin decreased the percentage of apoptotic
cells at both the 24 and 72 h intervals compared to the high
glucose group (P<0.05) (data from normal glucose without
silibinin for 24 and 72 h are not shown; Fig. 1C).
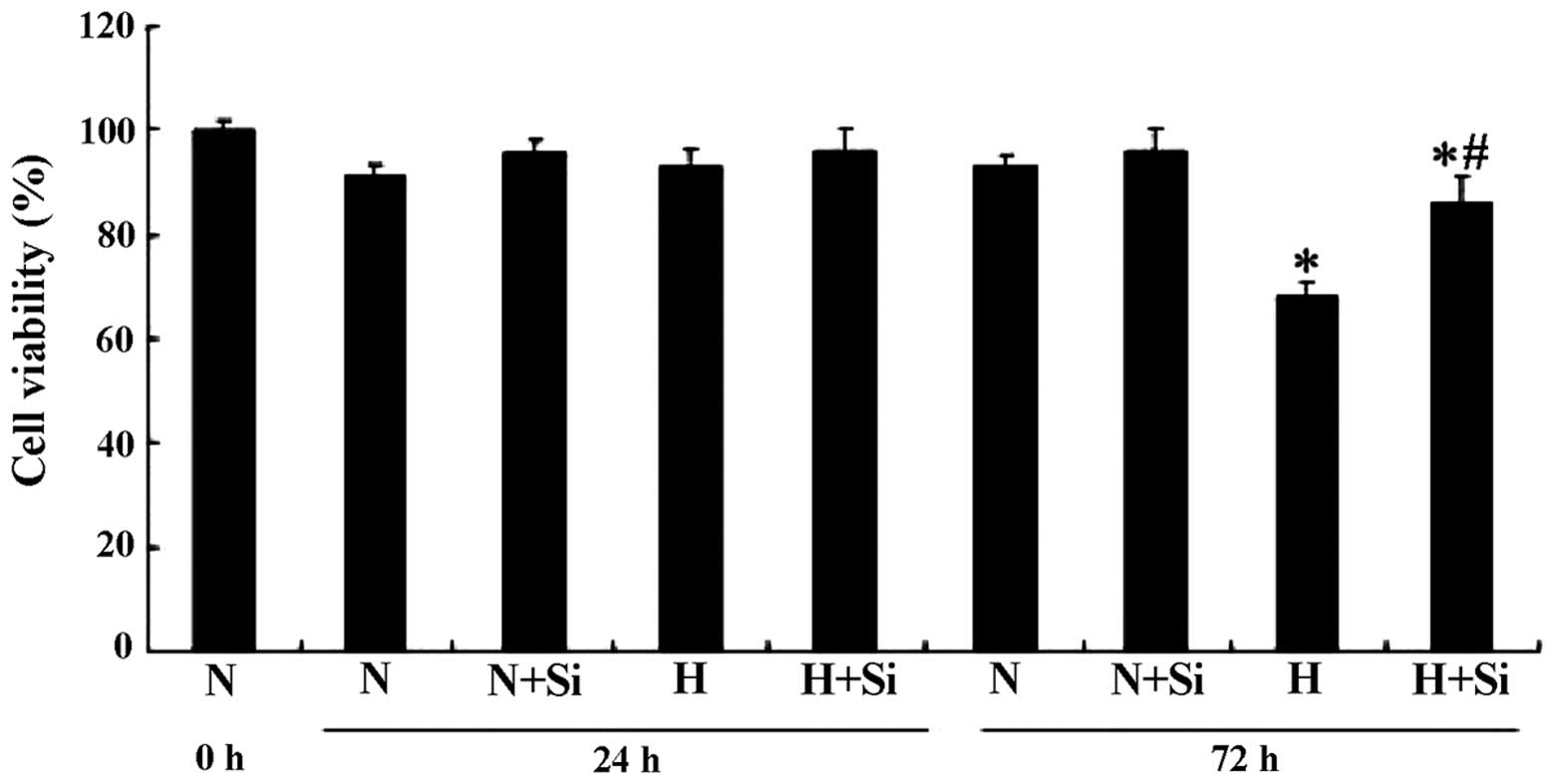
Silibinin improves INS-1 cell viability
under high glucose conditions
We used an MTT assay to determine whether silibinin
protects the cells from the high glucose-induced decrease in cell
viability. In the cells cultured in medium containing high glucose
(25.0 mM), significant cell death was observed after 72 h; however,
this was attenuated in the presence of 30 μM silibinin (P<0.05).
The cells incubated in culture medium containing a normal glucose
concentration (11.2 mM) did not show any obvious decrease in cell
viability (P>0.05) (Fig.
2).
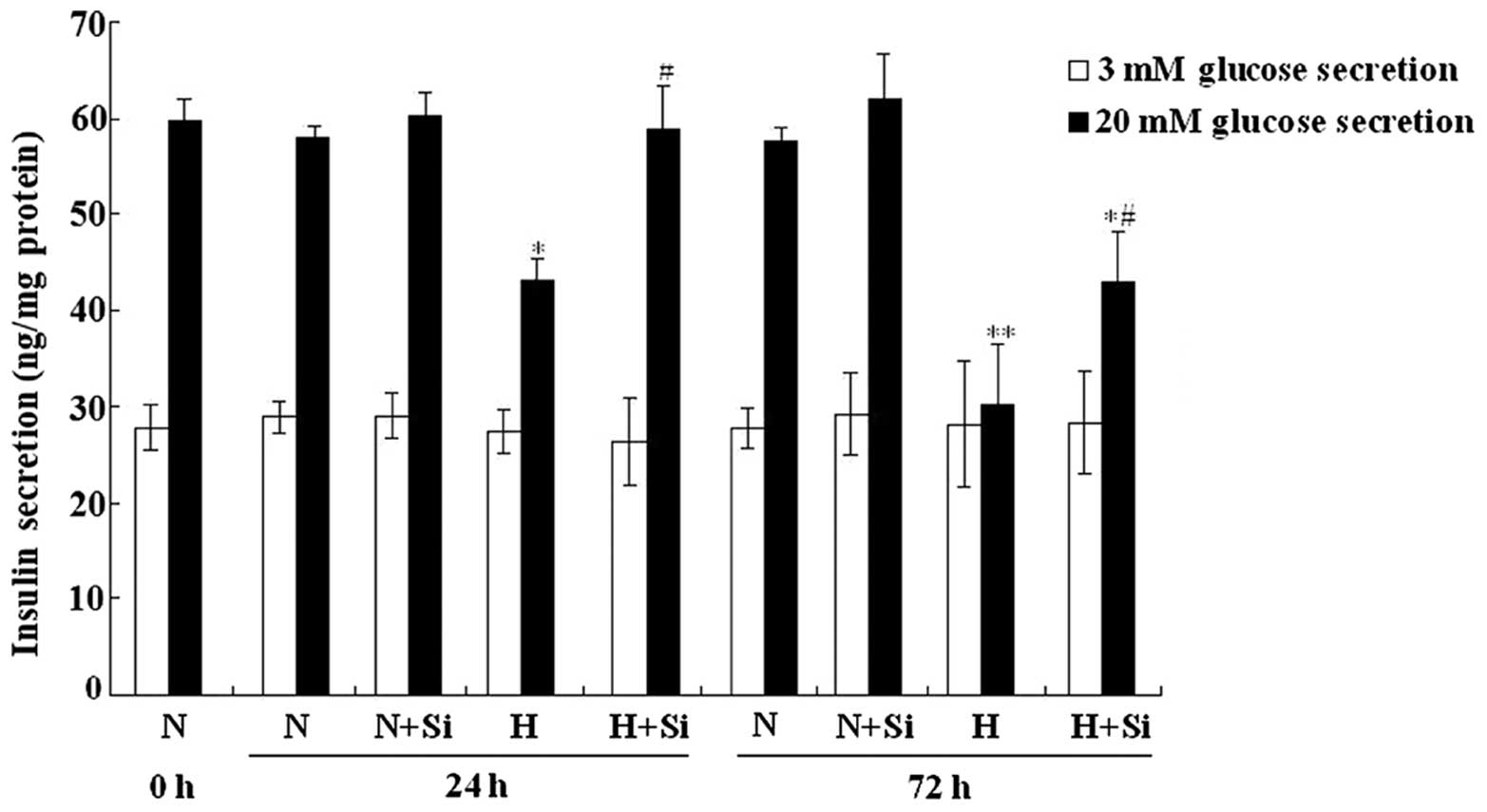
Silibinin improves insulin secretion
under high glucose conditions
The insulin secretion of the INS-1 cells was
measured following treatment with 3 or 20 mM glucose. No
significant difference in insulin secretion was observed following
treatment with 3 mM glucose (P>0.05). However, following
treatment with 20 mM glucose, the INS-1 cells cultured in high
glucose medium showed a significantly decreased insulin secretion
(24 h, P<0.05; 72 h, P<0.01). Furthermore, treatment with
silibinin partially restored the insulin secretion of the INS-1
cells cultured under high glucose conditions for both the 24- and
72-h periods (P<0.05) (Fig.
3).
Silibinin regulates the mRNA expression
of Insig-1/SREBP-1c and insulin secretion-related genes
Subsequently, we examined the expression of genes
related to the Insig-1/SREBP-1c signaling pathway and insulin
secretion. The INS-1 cells were incubated in medium containing
normal or high glucose concentrations for 72 h with or without
silibinin. The mRNA expression of Insig-1, SREBP-1c and fatty acid
synthetase (FAS) was significantly increased following exposure to
high glucose for 72 h (P<0.05, <0.05 and <0.01,
respectively) (Fig. 4A–C).
Silibinin further upregulated Insig-1 mRNA expression but
downregulated SREBP-1c (P<0.05) and FAS mRNA expression
(P<0.01). Similar results were observed for the expression of
insulin-related genes. High glucose induced the downregulation of
IRS-2, PDX-1 and insulin genes and the upregulation of UCP-2
(P<0.01 for all). Silibinin reversed the effects caused by high
glucose, markedly upregulating IRS-2, PDX-1 and insulin mRNA
expression and downregulating UCP-2 mRNA expression under high
glucose conditions (P<0.05 for all). By contrast, no difference
in the mRNA expression of these genes was observed when the INS-1
cells were incubated under normal glucose conditions for 72 h
(Fig. 4D–G).
 | Figure 4Silibinin upregulates insulin induced
gene-1 (Insig-1)/sterol regulatory element binding protein-1c
(SREBP-1c) expression and insulin secretion-related gene
expression. (A–G) Insig-1, SREBP-1c, fatty acid synthetase (FAS),
insulin receptor substrate 2 (IRS-2), pancreatic and duodenal
homeobox 1 (PDX-1), uncoupling protein-2 (UCP-2), and insulin mRNA
expression, respectively. *P<0.05,
**P<0.01 as compared with cells cultured under normal
glucose group; #P<0.05, ΔP<0.01 as
compared with cells cultured under high glucose group. N, normal
glucose group; N + Si, normal glucose + silibinin group; H, high
glucose group; H + Si, high glucose + silibinin group.
Representative data from 3 separate experiments are shown. |
Silibinin inhibits lipid droplet
accumulation and FFA synthesis
To further explore whether silibinin inhibits lipid
synthesis induced by high glucose, we used Oil Red O staining to
detect intracellular lipid accumulation and the FFA concentration
in the culture medium. The lipid droplets were stained bright red,
and silibinin did not affect lipid accumulation under normal
glucose conditions for 24 or 72 h (P>0.05). However, silibinin
significantly reduced lipid accumulation induced by culture under
high glucose conditions for both 24 and 72 h (P<0.05) (data of
normal glucose without silibinin for 24 and 72 h are not shown;
Fig. 5A). Similar results were
observed for the FFA concentration; silibinin decreased FFA
synthesis compared to cells cultured under high glucose conditions
for 24 and 72 h (P<0.01) (Fig.
5B).
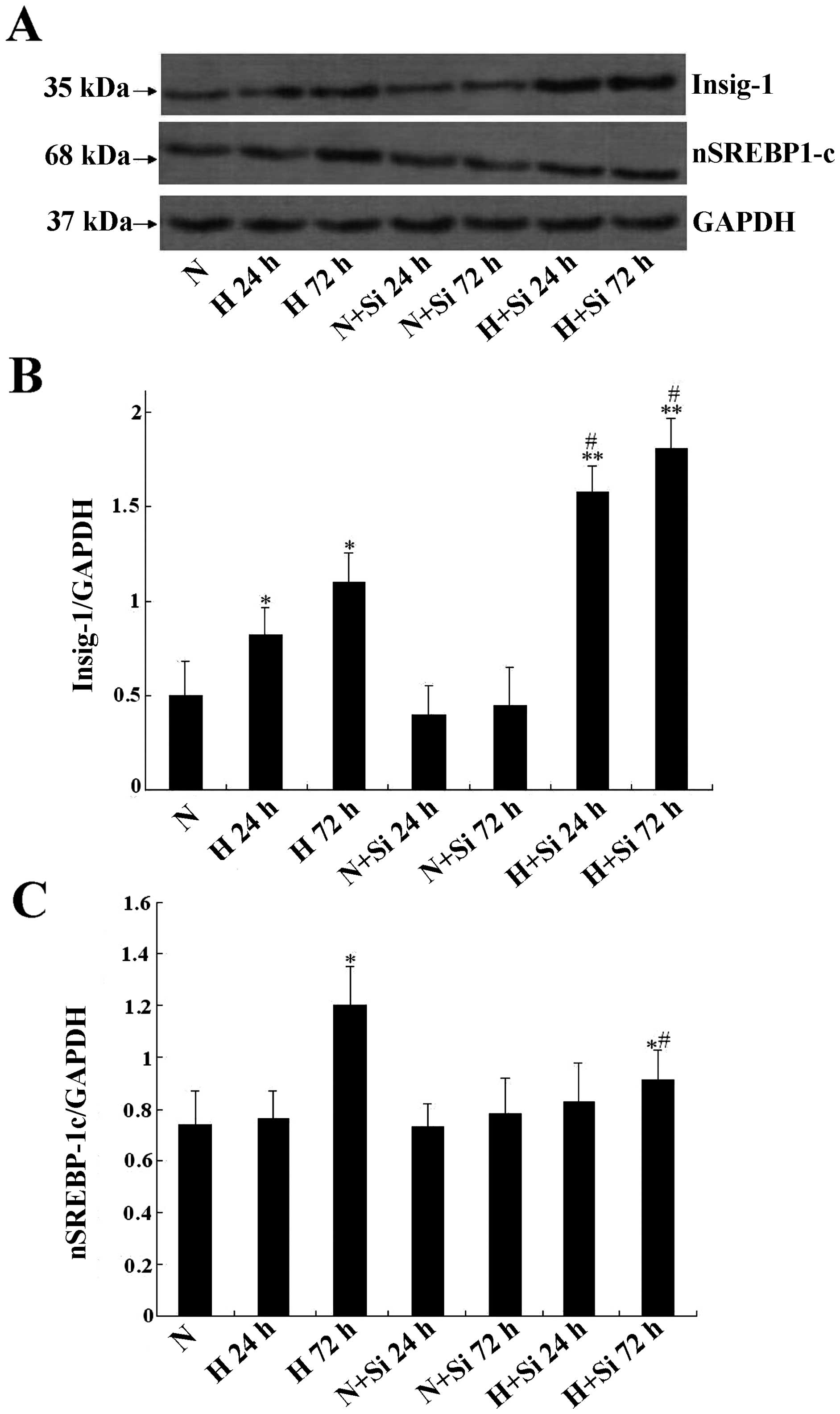
Silibinin regulation of the
Insig-1/SREBP-1c pathway in INS-1 cells
To explore the mechanisms through which silibinin
protects the INS-1 cells from glucotoxicity, we examined the
protein expression of Insig-1 and SREBP1-c in the INS-1 cells
cultured under different glucose concentrations. It was found that
high glucose induced Insig-1 upregulation at 24 and 72 h
(P<0.05), and silibinin further upregulated Insig-1 expression,
in line with the mRNA expression profile (P<0.05) (Fig. 6A and B). The expression of
SREBP-1c was significantly upregulated after the cells were treated
with high glucose or high glucose plus silibinin for 72 h compared
to the normal glucose group; however, in the high glucose plus
silibinin group, the expression was lower than that in the high
glucose group at 72 h. In addition, there was no significant
difference between the other groups and the normal glucose group
(Fig. 6C).
Discussion
In previous studies, some compounds have been
confirmed to upregulate Insig-1 expression, including the novel
hypocholesterolemic agent, LY295427, and the PPARγ agonist,
rosiglitazone, which both bind the Insig-1 promoter and upregulate
Insig-1 expression, thereby reducing lipogenesis in the liver and
white adipose tissue (18,19).
Sirolimus inhibits endogenous cholesterol synthesis through the
upregulation of Insig-1, Insig-2 and SREBP-1 expression in human
vascular smooth muscle cells (20). Recently, Ka et al (17) observed that silibinin upregulated
Insig-1 and Insig-2 expression at an early phase during
differentiation of 3T3-L1 preadipocytes to adipocytes. In their
study, 30 μM silibinin almost completely inhibited lipid synthesis
as well as certain important lipid metabolism factors, including
SREBP-1c, FAS, and the adipocyte-specific lipid binding protein
(aP2). These results indicated that silibinin may function through
the upregulation of Insig-1 and Insig-2 expression, thereby
inhibiting SREBP-1c transcription and finally leading to a decrease
in lipid synthesis (17). In
addition, Ka et al found that increasing the silibinin
concentration did not result in increased apoptosis in 3T3-L1
cells. We also found that 10–100 μM silibinin did not induce the
apoptosis of INS-1 cells (data not shown). Thus, it is likely that
silibinin has a low toxicity. Furthermore, Nassuato et al
reported that silibinin inhibited 3-hydroxymethyl-3-methylglutaryl
coenzyme A (HMG-CoA) reductase, a key enzyme of cholesterol
synthesis and an important downstream factor of the Insig-1/SREBP
pathway, in a dose-dependent manner (21).
In our previous study, we demonstrated that the
overexpression of Insig-1 protects β cell function against
glucotoxicity, including a decrease in lipid synthesis, an increase
in insulin secretion and a decrease in apoptosis (9). In this study, we further explored
whether silibinin upregulates the Insig-1/SREBP-1c pathway to
protect β cells against glucotoxicity. Several mechanisms of high
glucose-induced apoptosis in β cells have been implicated,
including ceramide formation (22,23), oxidative stress (24,25), inflammation (26) and ER stress (27–29). In this study, silibinin
significantly improved cell viability and decreased cell apoptosis
under high glucose conditions. Our previous study suggested that
the overexpression of Insig-1 protects β cells through the
regulation of the IRE1α pathway of ER-stress (9), possibly the same pathway through
which silibinin protects β cell function, as demonstrated in the
current study.
A clinical in vivo study revealed that
silibinin promotes insulin secretion and reduces blood glucose
levels (11). Similarly, our
results also indicated that silibinin partially improved insulin
secretion under high glucose conditions without affecting the basal
insulin secretion observed under normal glucose conditions.
Subsequently, we further explored the molecular mechanisms of
promoting insulin secretion in β cells. SREBP-1c is an important
nuclear transcription factor and plays a key role in the regulation
of insulin secretion; both in vivo and in vitro
studies have demonstrated that the overexpression of SREBP-1c
impairs insulin secretion (30).
SREBP-1c suppresses IRS-2 activity through direct binding to the
IRS-2 promoter, which may contribute to the GSIS of β cells
(31). PDX-1 is a crucial
transcription factor in the regulation of insulin secretion. In
addition, the overexpression of SREBP-1c suppresses PDX-1
expression both in vivo and in vitro. However, PDX-1
is upregulated when SREBP-1c is knocked down in islet cells
(32); UCP-2 may be a negative
regulator of cytoplasmic adenosine triphosphate (ATP)/adenosine
diphosphate (ADP), which is a key signaling molecule in GSIS.
SREBP-1c stimulates UCP-2 expression in β cells under a high
nutrition state (33). In our
study, Insig-1 expression was upregulated following culture under
high glucose conditions for 72 h. By contrast, SREBP-1c expression
was downregulated, and the insulin secretion-related genes, IRS-2,
PDX-1 and insulin, were upregulated but UCP-2 was downregulated.
Thus, silibinin may upregulate Insig-1, subsequently suppressing
SREBP-1c expression, further inhibiting the transcription of
insulin secretion-related genes.
Sandberg et al demonstrated that high
glucose-induced lipogenesis in β cells is regulated by SREBP-1
(34). Li et al found that
both Insig-1 and SREBP-1c were upregulated during the
differentiation of 3T3-L1 cells (35). In this study, we also proved that
Insig-1 and SREBP-1c were upregulated with lipid accumulation.
However, silibinin significantly decreased intracellular lipid
accumulation and FFA synthesis. We further explored the protein
expression following treatment with silibinin. Silibinin
upregulated the Insig-1 protein level after the cells were exposed
to high glucose conditions for 24 and 72 h. However, the SREBP-1c
protein level was only downregulated at 72 h. Further studies are
required to fully elucidate the mechanisms through which silibinin
upregulated Insig-1 expression.
In conclusion, we found that silibinin ameliorated β
cell dysfunction through the regulation of the Insig-1/SREBP-1c
pathway, including blocking β cell apoptosis and increasing cell
viability, improving insulin secretion and inhibiting lipid
synthesis. Investigation of the molecular mechanisms revealed that
silibinin may upregulate Insig-1 expression and downregulate
SREBP-1c transcription, both of which regulate the expression of
downstream insulin secretion-related genes and lipid synthesis,
further increasing insulin secretion and decreasing lipid
production. Thus, silibinin may be a novel therapeutic agent for β
cell dysfunction.
Acknowledgements
The present study was supported by grants from the
National Nature Science Foundation of China (no. 81200599) and
Hunan Province Nature Science Foundation of China (nos. 13JJ4027
and 06JJ5035). We thank Medjaden Bioscience Ltd. for assisting in
the preparation of this manuscript.
References
|
1
|
Imamura F, Mukamal KJ, Meigs JB, et al:
Risk factors for type 2 diabetes mellitus preceded by β-cell
dysfunction, insulin resistance, or both in older adults: the
Cardiovascular Health Study. Am J Epidemiol. 177:1418–1429.
2013.
|
|
2
|
Bensellam M, Laybutt DR and Jonas JC: The
molecular mechanisms of pancreatic β-cell glucotoxicity: Recent
findings and future research directions. Mol Cell Endocrinol.
364(1–2): 1–27. 2012.
|
|
3
|
Kwon MJ, Chung HS, Yoon CS, et al: Low
glibenclamide concentrations affect endoplasmic reticulum stress in
INS-1 cells under glucotoxic or glucolipotoxic conditions. Korean J
Intern Med. 28:339–346. 2013. View Article : Google Scholar
|
|
4
|
Somesh BP, Verma MK, Sadasivuni MK, et al:
Chronic glucolipotoxic conditions in pancreatic islets impair
insulin secretion due to dysregulated calciumdynamics, glucose
responsiveness and mitochondrial activity. BMC Cell Biol.
14:312013. View Article : Google Scholar
|
|
5
|
Filhoulaud G, Guilmeau S, Dentin R, et al:
Novel insights into ChREBP regulation and function. Trends
Endocrinol Metab. 24:257–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeon TI and Osborne TF: SREBPs: metabolic
integrators in physiology and metabolism. Trends Endocrinol Metab.
23:65–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sandberg MB, Fridriksson J, Madsen L, et
al: Glucose-induced lipogenesis in pancreatic beta cells is
dependent on SREBP-1. Mol Cell Endocrinol. 240:94–106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong XY and Tang SQ: Insulin-induced gene:
a new regulator in lipid metabolism. Peptides. 31:2145–2150. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen K, Jin P, He HH, et al:
Overexpression of Insig-1 protects β cell against glucolipotoxicity
via SREBP-1c. J Biomed Sci. 18:572011.
|
|
10
|
Gazák R, Walterová D and Kren V: Silybin
and silymarin-new and emerging applications in medicine. Curr Med
Chem. 14:315–338. 2007.PubMed/NCBI
|
|
11
|
Huseini HF, Larijani B, Heshmat R, et al:
The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in
the treatment of type II diabetes: a randomized, double-blind,
placebo-controlled, clinical trial. Phytother Res. 20:1036–1039.
2006.
|
|
12
|
Maghrani M, Zeggwagh NA, Lemhadri A, et
al: Study of the hypoglycaemic activity of Fraxinus
excelsior and Silybum marianum in an animal model of
type 1 diabetes mellitus. J Ethnopharmacol. 91:309–316. 2004.
|
|
13
|
Soto CP, Perez BL, Favari LP and Reyes JL:
Prevention of alloxan-induced diabetes mellitus in the rat by
silymarin. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol.
119:125–129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuda T, Ferreri K, Todorov I, et al:
Silymarin protects pancreatic beta-cells against cytokine-mediated
toxicity: implication of c-Jun NH2-terminal kinase and janus
kinase/signal transducer and activator of transcription pathways.
Endocrinology. 146:175–185. 2005. View Article : Google Scholar
|
|
15
|
Von Schönfeld J, Weisbrod B and Müller MK:
Silibinin, a plant extract with antioxidant and membrane
stabilizing properties, protects exocrine pancreas from cyclosporin
A toxicity. Cell Mol Life Sci. 53:917–920. 1997.PubMed/NCBI
|
|
16
|
Wang Q, Liu M, Liu WW, et al: In vivo
recovery effect of silibinin treatment on streptozotocin-induced
diabetic mice is associated with the modulations of Sirt-1
expression and autophagy in pancreatic β-cell. J Asian Nat Prod
Res. 14:413–423. 2012.PubMed/NCBI
|
|
17
|
Ka SO, Kim KA, Kwon KB, Park JW and Park
BH: Silibinin attenuates adipogenesis in 3T3-L1 preadipocytes
through a potential upregulation of the insig pathway. Int J Mol
Med. 23:633–637. 2009.PubMed/NCBI
|
|
18
|
Janowski BA: The hypocholesterolemic agent
LY295427 up-regulates INSIG-1, identifying the INSIG-1 protein as a
mediator of cholesterol homeostasis through SREBP. Proc Natl Acad
Sci USA. 99:12675–1280. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kast-Woelbern HR, Dana SL, Cesario RM, et
al: Rosiglitazone induction of Insig-1 in white adipose tissue
reveals a novel interplay of peroxisome proliferator-activated
receptor gamma and sterol regulatory element-binding protein in the
regulation of adipogenesis. J Biol Chem. 279:23908–23915. 2004.
View Article : Google Scholar
|
|
20
|
Ma KL, Varghese Z, Ku Y, et al: Sirolimus
inhibits endogenous cholesterol synthesis induced by inflammatory
stress in human vascular smooth muscle cells. Am J Physiol Heart
Circ Physiol. 298:H1646–H1651. 2010. View Article : Google Scholar
|
|
21
|
Nassuato G, Iemmolo RM, Strazzabosco M, et
al: Effect of Silibinin on biliary lipid composition. Experimental
and clinical study. J Hepatol. 12:290–295. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lupi R, Dotta F, Marselli L, et al:
Prolonged exposure to free fatty acids has cytostatic and
pro-apoptotic effects on human pancreatic islets: evidence that
beta-cell death is caspase mediated, partially dependent on
ceramide pathway, and Bcl-2 regulated. Diabetes. 51:1437–1442.
2002. View Article : Google Scholar
|
|
23
|
Maedler K, Oberholzer J, Bucher P, et al:
Monounsaturated fatty acids prevent the deleterious effects of
palmitate and high glucose on human pancreatic beta-cell turnover
and function. Diabetes. 52:726–733. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morgan D, Oliveira-Emilio HR, Keane D, et
al: Glucose, palmitate and pro-inflammatory cytokines modulate
production and activity of a phagocyte-like NADPH oxidase in rat
pancreatic islets and a clonal beta cell line. Diabetologia.
50:359–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Li H, De Leo D, et al: Gene and
protein kinase expression profiling of reactive oxygen
species-associated lipotoxicity in the pancreatic beta-cell line
MIN6. Diabetes. 53:129–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Busch AK, Cordery D, Denyer GS and Biden
J: Expression profiling of palmitate-and oleate-regulated genes
provides novel insights into the effects of chroniclipid exposure
on pancreatic beta-cell function. Diabetes. 51:977–987. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cunha DA, Hekerman P, Ladrière L, et al:
Initiation and execution of lipotoxic ER stress in pancreatic
beta-cells. J Cell Sci. 121:2308–2318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bachar E, Ariav Y, Ketzinel-Gilad M, et
al: Glucose amplifies fatty acid-induced endoplasmic reticulum
stress in pancreatic beta-cells via activation of mTORC1. Plos One.
4:e49542009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gwiazda KS, Yang TL, Lin Y and Johnson JD:
Effects of palmitate on ER and cytosolic Ca2+ homeostasis in
beta-cells. Am J Physiol Endocrinol Metab. 296:E690–E701. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimano H, Amemiya-Kudo M, Takahashi A, et
al: Sterol regulatory element-binding protein-1c and pancreatic
beta-cell dysfunction. Diabetes Obes Metab. 9(Suppl 2): 133–139.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eizirik DL, Cardozo AK and Cnop M: The
role for endoplasmic reticulum stress in diabetes mellitus. Endocr
Rev. 29:42–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amemiya-Kudo M, Oka J, Takeuchi Y, et al:
Suppression of the pancreatic duodenal homeodomain transcription
factor-1 (Pdx-1) promoter by sterol regulatory element-binding
protein-1c (SREBP-1c). J Biol Chem. 286:27902–27914. 2011.
View Article : Google Scholar
|
|
33
|
Medvedev AV, Robidoux J, Bai X, et al:
Regulation of the uncoupling protein-2 gene in INS-1 beta-cells by
oleic acid. J Biol Chem. 277:42639–42644. 2002.PubMed/NCBI
|
|
34
|
Sandberg MB, Bloksgaard M, Duran-Sandoval
D, et al: The gene encoding acyl-CoA-binding protein is subject to
metabolic regulation by both sterol regulatory element-binding
protein and peroxisome proliferator-activated receptor alpha in
hepatocytes. J Biol Chem. 280:5258–5266. 2005. View Article : Google Scholar
|
|
35
|
Li J, Takaishi K, Cook W, McCorkle SK and
Unger RH: Insig-1 ‘brakes’ lipogenesis in adipocytes and inhibits
differentiation of preadi-pocytes. Proc Natl Acad Sci USA.
100:9476–9481. 2003.
|