Introduction
Obesity is the most common metabolic disease
worldwide. It is characterized by an excessive storage of body fat,
and is associated with a number of metabolic complications,
including type 2 diabetes, hypertension and cardiovascular diseases
(CVD) (1). Such complications
could lead to higher mortality rates in obese as opposed to lean
patients.
Adipogenesis contributes to excess fat deposition in
adipocytes during the differentiation process from preadipocytes.
The molecular and cellular mechanisms of adipogenesis have been
extensively studied using the 3T3-L1 preadipocyte cell line as
these cells differentiate into adipocytes upon stimulation; this
process is similar to the development of obesity in humans
(2–5). Adipogenesis involves concerted
transcriptional and cellular events, including growth arrest,
re-entry into the cell cycle for mitotic clonal expansion (MCE) and
the start of transcription during differentiation (2). Numerous genes have been shown to be
involved in the development of obesity. For instance, peroxisome
proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding
protein (C/EBP)β, C/EBPα, C/EBPδ and sterol regulatory element
binding protein-1c (SREBP-1c) (2,6).
Furthermore, AMP-activated protein kinase (AMPK) has been suggested
to function as a sensor of cellular energy status and, when
activated, accelerates the ATP-producing catabolic pathways and
simultaneously reduces the anabolic pathways that consume ATP
(6,7). Several studies have identified AMPK
signaling as the target for the treatment of obesity and diabetes
(8–10). Moreover, mitogen-activated protein
kinases (MAPKs), namely, extracellular regulated kinase 1/2 (ERK
1/2), p38 and c-Jun-N-terminal kinase (JNK) are known to play a
crucial role in several cellular processes, including adipocyte
differentiation (11).
Dioscin (DS) is a steroidal saponin present in a
number of medicinal plants, such as Dioscorea nipponica
Makino and Dioscorea zingiberensis Wright. Traditionally,
saponins from Dioscorea plants are used to treat
cardiovascular diseases, rheumatoid arthritis, asthma and
hyperlipidemia (12). Several
studies have demonstrated that DS exerts anticancer (13–15), antifungal (16) and antiviral effects (17). A previous study reported that rats
fed a high-fat diet (HFD) containing 5% Dioscorea nipponica
Makino presented with reduced weight and adipose tissue gain when
compared to the control group (18). Similarly, mice fed an HFD
containing the aqueous extract of the Dioscorea plant,
Rhizoma Dioscoreae Tokoronis, showed lower body weight and adipose
tissue in comparison to the control mice (19), suggesting that Dioscorea
plants have anti-obesity effects. A recent study reported that a
steroidal saponin, pseudoprotodioscin, present in the
Dioscorea plant, inhibited adipogenesis in 3T3-L1 cells
(12). However, the potential
role of DS, an active compound in the Dioscorea plant, in
adipogenesis and its underlying mechanisms of action have not yet
been fully elucidated.
Materials and methods
Materials and reagents
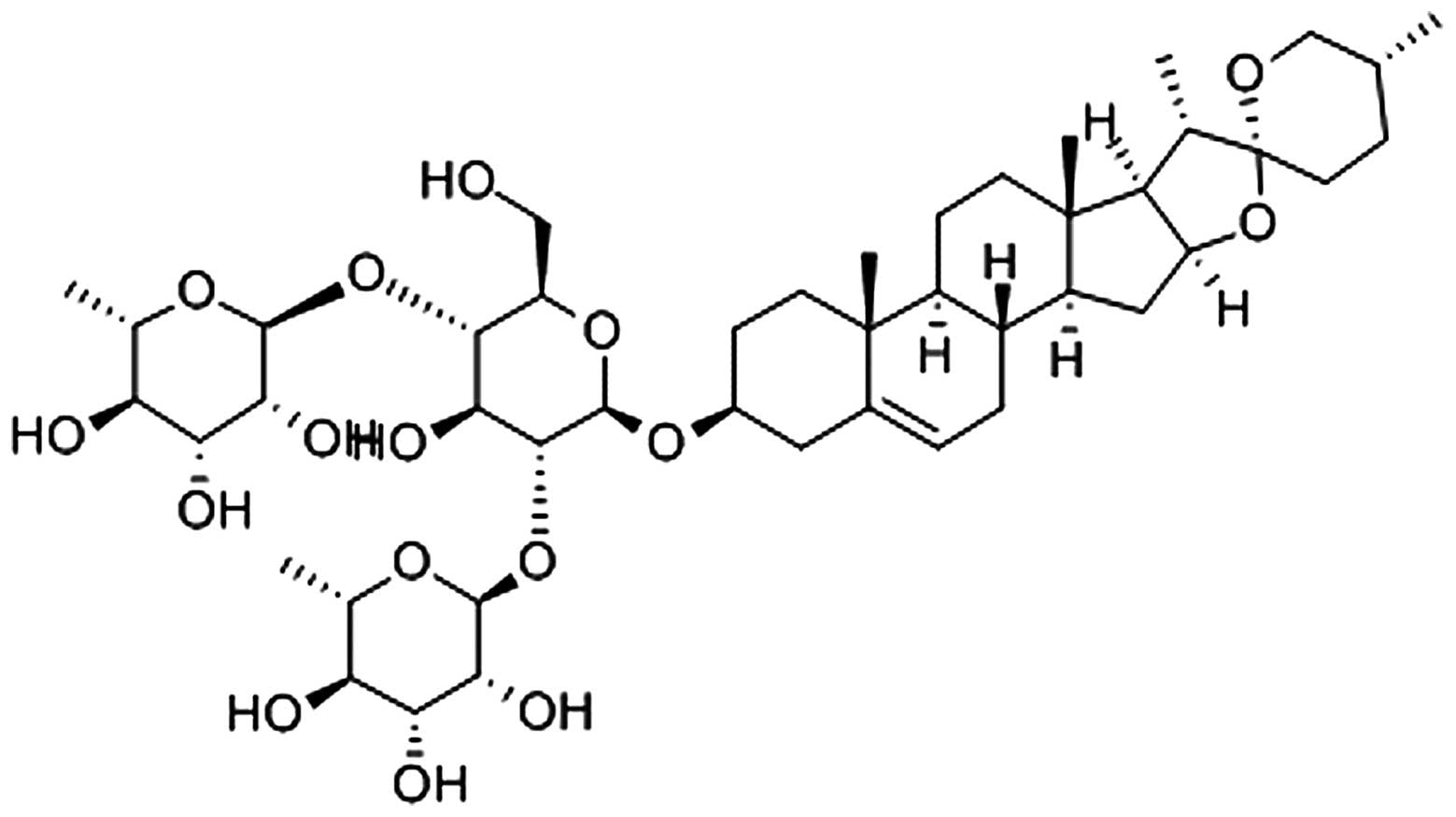
DS (PubChem CID: 119245) (Fig. 1, adopted from http://www.ncbi.nlm.nih.gov/pccompound)
was obtained from the Nanjing Zelang Medical Technology Co., Ltd.,
(Jiangsu, China) with 98% purity as determined by high performance
liquid chromatography, and was dissolved in demethyl sulfoxide
(DMSO) (Sigma, St. Louis, MO, USA) for the experiments.
Dexamethasone, insulin, propidium iodide,
3-isobutyl-1-methylxanthine (IBMX), RNase A, orlistat and Oil Red O
staining solution were purchased from Sigma. Dulbecco’s modified
Eagle’s medium (DMEM) and fetal calf serum (FCS) were from HyClone
(Logan, UT, USA). Zoletil 50 was obtained from Virbac Laboratories
(06516 Carros, France). TRIzol reagent and the SuperScript II kit
were obtained from Invitrogen (Carlsbad, CA, USA). The Cell
Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies (Rockville, MD, USA). Anti-AMPK, anti-p-AMPK,
anti-ACC, anti-phosphorylated (p)-ACC, anti-PPARγ, anti-C/EBPα,
anti-C/EBPβ, anti-C/EBPδ, anti-ERK, anti-p-ERK, anti-p38,
anti-p-p38, anti-JNK and anti-p-JNK antibodies were purchased from
Cell Signaling Biotechnology (Beverly, MA, USA). The protein assay
kit (RIPA buffer), rabbit and mouse secondary antibodies, and
anti-β-actin antibodies were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA).
Animals and diet
C57BL/6J male mice (5 weeks old) were purchased from
Samtako Inc. (Seoul, Korea). The animals were maintained in a room
under the following conditions: 12-h light/dark cycles, a
temperature pf 22±2°C and a relative humidity of 50±5% during the
whole experimenta period. Mice were fed a normal diet for 2 weeks
for adaptation. Subsequently, they were randomly divided into 5
groups (n=8). Two groups were fed either a normal diet (normal
control, NC) or an HFD (kcal%, protein 20, carbohydrate 35, fat 45;
D12451 Research Diets Inc., New Brunswick, NJ, USA). The other
groups were fed an HFD and were intragastrically adminstered with
DS or orlistat at the indicated concentrations dissolved in 0.5%
carboxymethyl cellulose (CMC) in distilled water. This solution was
freshly prepared each day prior to administration. The mice in the
control group were administered 0.5% CMC. The mice were treated as
described above for 7 weeks. Body weight was measured each week.
All animal experiments were approved by the Institutional Animal
Care and Use Committee at Chonbuk National University, Jeonju,
Korea.
Determination of body fat composition by
microtomoraphy or computed tomography (micro-CT) and abdominal fat
isolation
The mice were starved for 6 h and in vivo
micro-CT images of the anesthetized mice [Zoletil 50, 2 mg/kg,
intraperitoneally (i.p.)] were acquired using a Skyscan-1076
micro-CT scanner (Skyscan, Aartselaar, Belgium). CT was performed
using the following parameters: pixel size, 18 μm; source voltage,
48 kVp; and source current, 200 μA. The X-ray detector comprised a
12-bit water-cooled charge-coupled device high-resolution
(4,000×2,300-pixel) camera and an X-ray scintillator. The images
were acquired in increments of 0.6 degrees. The exposure time for
each view was 0.46 sec; a 0.5-mm aluminum energy filter was
used.
Following micro-CT scanning, the mice were
sacrificed by exposure to diethyl-ether, abdominal fat was isolated
and images were acquired using an Olympus SP-500UZ camera (Olympus,
Center Valley, PA, USA).
Cell culture and differentiation
3T3-L1 preadipocytes were obtained from the American
Type Culture Collection and maintained in DMEM containing 10% FCS
in a humidified atmosphere of 5% CO2 at 37°C. The
differentiation of the preadipocytes was induced 2 days
post-confluence (day 0) by the addition 0.5 mM IBMX, 1 μM
dexamethasone and 10 μg/ml insulin [multiple daily insulin (MDI)]
for 2 days. Subsequently, the culture medium was changed to
DMEM/10% FCS containing insulin. After 2 days, the medium was
replaced with DMEM/10% FCS and the cells were incubated for a
further 2 days. DS was added on day 0 during differentiation until
the cells were harvested for the experiments described below.
Cell viability
The cells were treated with MDI and various
concentrations of DS for the indicated periods of time, and cell
viability was measured using a CCK-8 kit according to the
manufacturer’s instructions. Absorbance was measured at 450 nm on a
microplate reader (Biochrom Anthos Zenyth 200; Biochrom Ltd.,
Cambridge, UK).
Oil Red O staining
The differentiation of the cells was induced as
described above. On day 6, the cells were stained with Oil Red O,
according to the manufacturer’s instructions to visualize lipid
accumulation in the cells. The intracellular lipid content was
measured by extracting Oil Red O with isopropanol, and the
absorbance at 520 nm was recorded using a spectrophotometer.
Triglyceride assay
Cellular triglyceride contents were measured using a
commercial triglyceride assay kit (Triglyzyme test; Wako Pure
Chemical Industries Ltd., Saitama, Japan), according to the
instructions provided by the manufacturer. Briefly, the cells were
washed twice with phosphate-buffered saline (PBS) and lysed in RIPA
lysis buffer. Following centrifugation at 3,000 × g for 5 min, the
supernatants were assayed for the triglyceride and protein content.
The triglyceride was content normalized to the protein
concentration determined using bovine serum albumin as the
standard.
Western blot analysis
The cells were lysed in ice-cold RIPA buffer for 20
min and centrifuged (15,000 × g) for 20 min at 4°C. The protein
concentration was measured using a bicinchoninic acid method.
Lysates (30 μg) were run on sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and transferred onto polyvinylidene difluoride
(PVDF) membranes (Amersham Pharmacia Biotech Inc., Piscataway, NJ,
USA). Subsequently, blocking was performed with 5% skimmed milk in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h at
room temperature. The membranes were probed with primary antibodies
as indicated at 4°C overnight, washed with TBST 4 times, and
subsequently incubated with horseradish peroxidase-conjugated
secondary antibody for 45 min. The membranes were washed again 3
times with TBST and the proteins were visualized using an enhanced
chemiluminescence detection kit (Amersham Pharmacia Biotech
Inc.).
Isolation of total RNA and quantitative
reverse transcription (RT-qPCR)
Total RNA was extracted using TRIzol reagent,
according to the manufacturer’s recommendations. Isolated RNA (1
μg/μl) was used for cDNA synthesis using the SuperScript II kit.
Aliquots of cDNA were amplified on the an ABI Real-Time PCR system
from Applied Biosystems Inc. (Forster City, CA, USA) using the
SYBR-Green Master Mix from Applied Biosystems. GAPDH was used as
the invariant control. Primers specific for the genes examined are
listed in Table I. The results
were presented as levels of expression relative to that of the
control.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Gene | Forward primer | Reverse primer |
|---|
| SREBP1c |
GGTTTTGAACGACATCGAAGA |
CGGGAAGTCACTGTCTTGGT |
| aP2 |
AGCCTTTCTCACCTGGAAGA |
TTGTGGCAAAGCCCACTC |
| FAS |
TGATGTGGAACACAGCAAGG |
GGCTGTGGTGACTCTTAGTGATAA |
| GLUT4 |
TGTGGCCTTCTTTGAGATTGG |
CTGAAGAGCTCGGCCCACCAA |
| Adiponectin |
GCAGAGATGGCACTCCTGGA |
CCCTTCAGCTCCTGTCATTCC |
| Leptin |
TCCAGAAAGTCCAGGATGACAC |
CACATTTTGGGAAGGCAGG |
| HSL |
GGAGCACTACAAACGCAACGA |
TCGGCCACCGGTAAAGAG |
| LPL |
AGTAGACTGGTTGTATCGGG |
AGCGTCATCAGGAGAAAGG |
| β-actin |
CCTAAGGCCAACCGTGAAAA |
GAGGCATACAGGGACAGCACA |
Cell cycle analysis
The cells were harvested at the indicated time
points following MDI stimulation with or without DS. Subsequently,
the cells were fixed overnight with 70% ethanol at −20°C, washed
twice with PBS, and stained with 50 μg/ml propidium iodide (IP)
solution containing 25 μg/ml RNase A for 30 min at 37°C. Cell cycle
analysis was performed using the FACSCalibur flow cytometry system
(BD Biosciences, San Diego, CA, USA), and data analysis was
performed using FlowJo v10 software (TreeStar, Inc., Ashland, OR,
USA).
Statistical αnalysis
All values are presented as the means ± SEM.
Statistical significance was determined using the Student’s t-test.
P-values <0.05 were considered to indicate statistically
significant differences.
Results
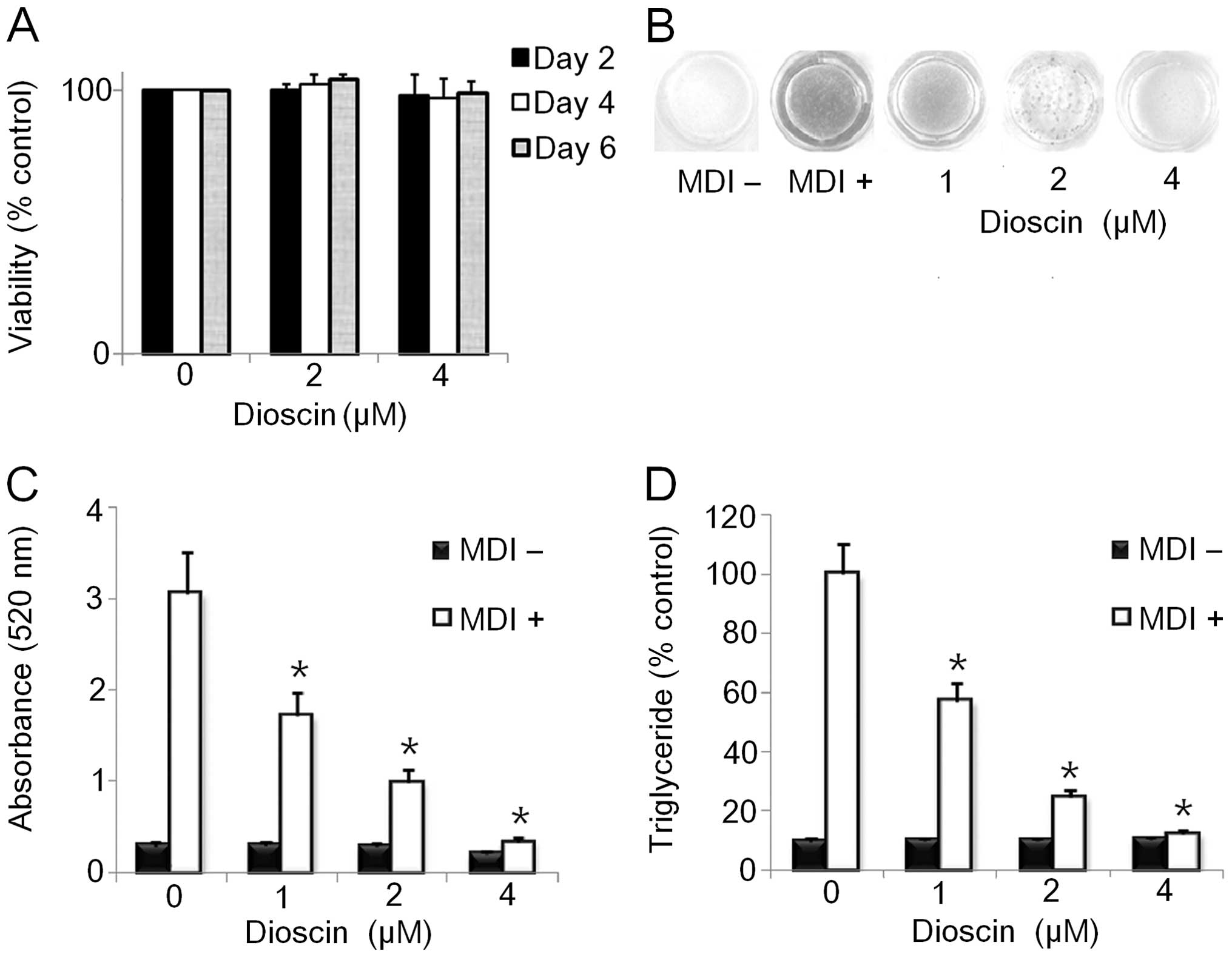
Effect of DS on adipocyte viability
Several studies have demonstrated the cytotoxic
effects of DS in a number of cell lines (14,15). This prompted us to examine the
possibility that the use of DS may result in cytotoxicity to 3T3-L1
cells. To examine this possibility, we examined the effects of DS
on the viability of 3T3-L1 cells by CCK-8 assay. DS (0–4 μM) showed
no significant cytotoxicity towards the differentiating
preadipocytes. However, 8 μM DS inhibited the viability of the
cells by approximately 50% at 24 h (Fig. 2A and data not shown). Therefore,
we selected the maximum dose of DS (4 μM) for further experiments
on the effects of DS on adipogenesis to rule out the possibility
that the inhibition of adipogenesis by DS may result from its
cytotoxic effects on 3T3-L1 cells.
DS inhibits lipid accumulation in 3T3-L1
adipocytes
We examined the effects of DS on the differentiation
of 3T3-L1 preadipocytes to adipocytes. The cells were stimulated
with MDI to initiate differentiation. The culture medium was
supplemented with various doses (0–4 μM) of DS for 6 days. DS
dose-dependently decreased intracellular fat accumulation compared
to the control as demonstrated by morphological and quantitative
analysis of intracellular lipids by Oil Red O staining (Fig. 2B and C). Consistent with these
results, the triglyceride content in the 3T3-L1 cells treated with
the indicated doses of DS was lower than that in the control cells
differentiated for 6 days (Fig.
2D).
DS suppresses MCE during the early phase
of adipogenesis
During adipogenesis, multiple rounds of cell cycle
progression contribute to the MCE process. Therefore, we sought to
examine the effects of DS on the cell cycle progression of 3T3-L1
cells during the MCE process. The results from flow cytometry
revealed that the DS-treated cells showed a delayed cell cycle
progression 48 h following stimulation with MDI (Fig. 3). The percentage of preadipocytes
in the G0/G1 phase was approximately 58%,
while 45% of the untreated adipocytes and 65% of the DS-treated
adipocytes were in the G0/G1 phase. These
observations suggested that DS inhibits clonal expansion of the
cells by inducing G0/G1 phase arrest.
DS inhibits the protein expression of
adipogenic transcription factors
The differentiation of preadipocytes into adipocytes
involves the sequential activation of several pro-adipogenic
transcription factors, such as, C/EBPα/β/δ and PPARγ. Thus, we
examined whether the reduced fat accumulation in the adipocytes was
due to the downregulation of the aforementioned adipogenic
transcription factors. As shown in Fig. 4, DS significantly inhibited the
protein expression of C/EBPα/β/δ and PPARγ, suggesting that DS
inhibits adipogenesis by suppressing the expression of adipogenic
transcription factors.
DS inhibits adipogenesis-related gene
expression in 3T3-L1 cells
As the adipogenic transcription factors were
downregulated by DS, we further examined the expression of other
adipogenesis-related genes involved in lipogenic and fatty acid
oxidation and glucose homeostasis pathways. As shown in Fig. 5A, DS (4 μM) significantly
inhibited the mRNA expression of SREBP-1c, activating protein 2
(aP2), fatty acid synthase (FAS), glucose transporter 4 (GLUT4) and
leptin. However, there was no significant difference in the mRNA
levels of hormone-sensitive lipase (HSL), lipoprotein lipase (LPL)
and adiponectin when compared to the controls (Fig. 5B).
DS regulates the AMPK pathway during
adipogenesis
AMPK and its target, acetyl Co-A carboxylase (ACC),
are the key regulators of preadipocyte differentiation and
adipogenesis. Our results revealed that DS enhanced the
phosphorylation of both AMPK and ACC (Fig. 6). These results suggest that DS
regulates the adipogenic process in adipocytes through the AMPK
pathway.
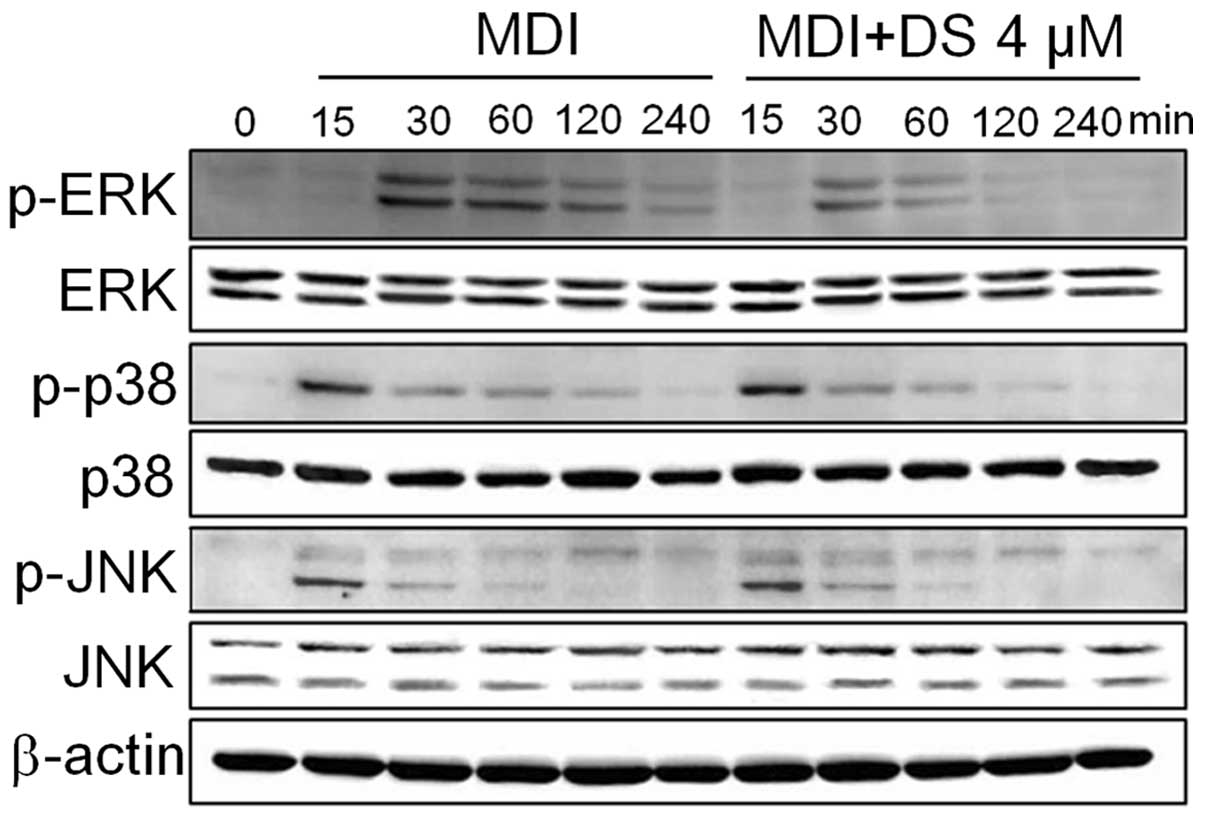
DS regulates the MAPK pathway during
adipogenesis
The suppression of ERK1/2 using pharmacological
inhibitors has been shown to correlate with the inhibition of cell
cycle progression, the partial blockage of MCE and the reduced
expression of adipogenic factors (37). On the other hand, the
pharmacological inhibition of the p38 pathway has been known to
suppress adipogenesis in 3T3-L1 cells by downregulating C/EBPβ
phosphorylation and its post-translational activation (20). Thus, we wished to determine
whether DS inhibits ERK and p38 activation in 3T3-L1 cells. The
results from western blot analysis revealed that DS inhibited the
phosphorylation of the ERK and p38 pathways. However, we did not
observe any inhibitory effect of DS on the phosphorylation of JNK
(Fig. 7).
DS suppresses fat accumulation in mice
with HFD-induced obesity
To assess the effects of DS on adipose tissue
accumulation, we compared body weight and abdominal fat content in
the different mouse groups. DS dose-dependently reduced body weight
compared with the group fed the HFD and not treated with DS. There
was a significant reduction in body weight after 4 weeks of DS (50
mg/kg) treatment in the mice HFD-induced obesity in comparison to
the controls. Moreover, treatment with DS (100 mg/kg) markedly
inhibited body weight gain in comparison to the controls from the
first weeks of treatment (Fig.
8A). This decrease in body weight was due to a significant
decrease in fat accumulation as established by micro-CT scanning
and macroscopy of abodominal fat (Fig. 8B).
Discussion
Obesity is a chronic, socially stigmatized and
costly disease that has become an epidemic worldwide. The drugs
currently available for the treatment of obesity have raised safety
concerns and have demonstrated poor efficacy. For instance,
orlistat is known to cause fetal fat loss and gastrointestinal
symptoms. Thus, it is necessary to identify new medicinal products
that are safe and effective in treating obesity (21,22).
The increased growth and proliferation of adipocyte
precursor cells lead to increased adipose mass, which represents
the size and number of adipocytes. Our results revealed that DS at
a dose of up to 4 μM did not affect the viability of
differentiating 3T3-L1 cells. This finding prompted us to eliminate
the possibility that DS-induced cytotoxicity accounts for the
inhibition of adipogenesis. Moreover, DS delayed the cell cycle
progression in differentiating 3T3-L1 cells during the early phase
of adipogenesis upon MDI stimulation. Further experiments
demonstrated that DS dose-dependently reduced adipogenesis in
3T3-L1 cells, accompanied by a decreased triglyceride content.
PPARs are ligand-activated transcription factors
that have 3 homologues: PPARα/β/γ, and have been reported to play a
role in glucose and protein metabolism. They also regulate the
proliferation and differentiation of preadipocytes (23–25). C/EBPs are crucial proteins
involved in preadipocyte differentiation. During the early steps of
adipocyte differentiation steps, the activation of C/EBPβ and δ is
known to result in the activation of C/EBPα and PPARγ, which in
turn control adipogenesis and insulin sensitivity in adipocytes
(26–28). Moreover, the insulin-dependent
glucose uptake requires the translocation of GLUT4, whose
expression is regulated by C/EBPs from intracellular storage sites
to the cell surface (7,11,29). Another transcription factor,
SREBP-1c, has been implicated in preadipocyte differentiation and
fatty acid metabolism (6).
C/EBPα, PPARγ and SREBP-1c activation is known to regulate the
adipocyte differentiation markers and genes associated with lipid
metabolism, such as aP2, fatty acid synthase (FAS), LPL and HSL
(30,31).
Our findings revealed that DS significantly
downregulated C/EBPα/β/δ, PPARγ, SREBP-1c and GLUT4 expression,
which is essential for adipogenesis. Consistent with our results,
previous studies have shown that ursolic acid and
resveratrol-amplified grape skin extracts inhibit adipogenesis in
3T3-L1 cells through the downregulation of PPARγ and C/EBP isoforms
(6,31). In accordance with our results, a
previous study reported that 4-hydroxyderricin and xanthoangelol,
plant-derived anti-obesity compounds, inhibited GLUT4 expression
during the differentiation of 3T3-L1 cells (11). We also observed reduced mRNA
levels of aP2 and FAS in adipocytes treated with DS, in accordance
with a previous study showing the anti-adipogenic effects of
resveratrol-amplified grape skin extracts on adipocytes (31). On the other hand, leptin is
secreted by adipocytes, suppresses food intake and promotes energy
expenditure. Thus, leptin levels are increased with adipogenesis
and obesity (32,33). In this study, decreased leptin
levels in 3T3-L1 cells treated with DS indicated that DS exerted
anti-adipogenic effects. Adiponectin, a vital hormone secreted by
adipose tissue has been suggested to exert anti-diabetic,
anti-inflammatory and anti-atherogenic effects (32). In our study, the increased
adiponectin expression in DS-treated 3T3-L1 cells was possibly the
result of the anti-inflammatory effects of DS on adipocytes. A
previous study suggested that high adiponectin production due to
the presence of niacin is the result of its direct
anti-inflammatory properties (34). However, further studies are
required to demonstrate the direct anti-inflammatory properties of
DS on adipocytes. LPL is an enzyme that hydrolyzes triglycerides in
order to facilitate the fatty acid uptake by cells. On the other
hand, HSL plays an important role in the mobilization of
triacylglycerol stored inside cells (35). In this study, although there was a
tendency towards an enhanced gene expression of LPL and HSL upon DS
treatment in differentiating adipocytes, the difference was not
statistically significant. However, this result may correlate with
the reduced adipogenesis in DS-treated 3T3-L1 cells, possibly due
to increased lipolysis in adipocytes.
Accumulating evidence suggests that AMPK is a major
protein that regulates cellular energy homeostasis and regulates a
number of biological pathways, such as carbohydrate and lipid
metabolism. Hence, it is one of the most important targets for
treating diabetes and obesity (8–10,36). ACC is important for the synthesis
and consumption of fatty acids and is a target of AMPK (11). In the present study, DS increased
the phosphorylation of AMPK and ACC during preadipocyte
differentiation. These results indicate that DS inhibits
adipogenesis through the AMPK pathway. In accordance with our
results, several compounds, such as chitin, ginsenosides,
epigallocatchenin gallate and aspigenin have been reported to
target AMPK for the inhibition of adipocyte differentiation
(6).
Previous studies have indicated that the MAPKs,
ERK1/2, JNK and p38, are involved in adipocyte differentiation
(11). The downregulation of
ERK1/2 or p38 by their inhibitors has been shown to result in
reduced adipocyte differentiation (20,37,38), suggesting that ERK1/2 and p38 are
essential for adipogenesis. Consistent with this result, our data
demonstrated that DS suppressed the phosphorylation of ERK1/2 and
p38. However, we did not observed any effect on JNK activation upon
DS treatment during adipocyte differentiation. There is limited
evidence on the role of JNK in adipocyte differentiation (20). However, contrary to our findings,
a previous study demonstrated that JNK phosphorylation is important
for adipocyte differentiation and that JNK inhibitor (SP600125)
reduced the lipid accumulation in adipocytes (11).
In the present study, we investigated the potential
role of DS in regulating the adipogenesis of 3T3-L1 preadipocytes.
We demonstrate that DS inhibits adipogenesis without exerting any
cytotoxic effects on differentiating preadipocytes. This
anti-adipogenic effect targets the MCE phase, where DS retards cell
cycle progression and decreases the expression of pro-adipogenic
transcription factors. The DS-associated blockage of the MCE phase
is accompanied by an inhibition of the phosphorylation of the
MAPKS, ERK1/2 and p38. Moreover, DS induced the phosphorylation of
AMPK. In our in vivo mouse model of obesity, DS
significantly suppressed body weight gain and abdominal fat
accumulation. This finding was consistent with previously reported
results showing that treatment with Dioscorea plant extract
reduced body weight and fat accumulation in mice with HFD-induced
obesity (18,19).
Taken together, our data demonstrate that DS is a
natural anti-adipogenic molecule that targets the AMPK/MAPK
pathway, inhibits the MCE phase and decreases the expression of
adipogenic transcription factors during the process of adipogenesis
in 3T3-L1 cells; it also modulates body weight and fat accumulation
in mice with HFD-induced obesity.
Acknowledgements
The present study was financially supported by the
Ministry of Knowledge Economy (MKE), Korea Institute for
Advancement of Technology (KIAT) through the Inter-ER Cooperation
Projects (R0002019).
References
|
1
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
2
|
Kwon JY, Seo SG, Heo YS, Yue S, Cheng JX,
Lee KW and Kim KH: Piceatannol, natural polyphenolic stilbene,
inhibits adipogenesis via modulation of mitotic clonal expansion
and insulin receptor-dependent insulin signaling in early phase of
differentiation. J Biol Chem. 287:11566–115678. 2012. View Article : Google Scholar
|
|
3
|
Green H and Kehinde O: An established
preadipose cell line and its differentiation in culture II. Factors
affecting the adipose conversion. Cell. 5:19–27. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI
|
|
5
|
Ntambi JM and Young-Cheul K: Adipocyte
differentiation and gene expression. J Nutr. 130:3122S–3126S.
2000.PubMed/NCBI
|
|
6
|
He Y, Li Y, Zhao T, Wang Y and Sun C:
Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through
LKB1/AMPK pathway. PLoS One. 8:e701352013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JO, Lee SK, Kim JH, et al: Metformin
regulates glucose transporter 4 (GLUT4) translocation through
AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in
3T3-L1 preadipocyte cells. J Biol Chem. 287:44121–44129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones RG, Plas DR, Kubek S, et al:
AMP-activated protein kinase induces a p53-dependent metabolic
checkpoint. Mol Cell. 18:283–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo Z, Saha AK, Xiang X and Ruderman NB:
AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci.
26:69–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song XM, Fiedler M, Galuska D, et al:
5-Aminoimidazole 4-carboxamide ribonucleoside treatment improves
glucose homeostasis in insulin-resistant diabetic (ob/ob) mice.
Diabetologia. 45:56–65. 2002. View Article : Google Scholar
|
|
11
|
Zhang T, Sawada K, Yamamoto N and Ashida
H: 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica
keiskei) suppresses differentiation of preadipocytes to
adipocytes via AMPK and MAPK pathways. Mol Nutr Food Res.
57:1729–1740. 2013.
|
|
12
|
Xiao J, Wang NL, Sun B and Cai GP:
Estrogen receptor mediates the effects of pseudoprotodiocsin on
adipogenesis in 3T3-L1 cells. Am J Physiol Cell Physiol.
299:C128–C138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura T, Komori C, Lee Y, Hashimoto F,
Yahara S, Nohara T and Ejima A: Cytotoxic activities of solanum
steroidal glycosides. Biol Pharm Bull. 19:564–566. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai J, Liu M, Wang Z and Ju Y: Apoptosis
induced by dioscin in Hela cells. Biol Pharm Bull. 25:193–196.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MJ, Wang Z, Ju Y, Zhou JB, Wang Y and
Wong RN: The mitotic-arresting and apoptosis-inducing effects of
diosgenyl saponins on human leukemia cell lines. Biol Pharm Bull.
27:1059–1065. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sata N, Matsunaga S, Fusetani N, Nishikawa
H, Takamura S and Saito T: New antifungal and cytotoxic steroidal
saponins from the bulbs of an elephant garlic mutant. Biosci
Biotechnol Biochem. 62:1904–1911. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda T, Ando J, Miyazono A, et al:
Anti-herpes virus activity of Solanum steroidal glycosides. Biol
Pharm Bull. 23:363–364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon CS, Sohn HY, Kim SH, et al:
Anti-obesity effect of Dioscorea nipponica Makino with
lipase-inhibitory activity in rodents. Biosci Biotechnol Biochem.
67:1451–1456. 2003.
|
|
19
|
Song MY, Lv N, Kim EK, et al: Antiobesity
activity of aqueous extracts of Rhizoma Dioscoreae Tokoronis on
high-fat diet-induced obesity in mice. J Med Food. 12:304–309.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bost F, Aouadi M, Caron L and Binétruy B:
The role of MAPKs in adipocyte differentiation and obesity.
Biochimie. 87:51–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bray GA and Tartaglia LA: Medicinal
strategies in the treatment of obesity. Nature. 404:672–677.
2000.PubMed/NCBI
|
|
22
|
Bray GA: A concise review on the
therapeutics of obesity. Nutrition. 16:953–960. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Christodoulides C and Vidal-Puig A: PPARs
and adipocyte function. Mol Cell Endocrinol. 318:61–68. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takahashi S, Tanaka T, Kodama T and Sakai
J: Peroxisome proliferator-activated receptor δ (PPARδ), a novel
target site for drug discovery in metabolic syndrome. Pharmacol
Res. 53:501–507. 2006.
|
|
25
|
Tontonoz P, Hu E and Spiegelman BM:
Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a
lipid-activated transcription factor. Cell. 79:1147–1156. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Darlington GJ, Ross SE and MacDougald OA:
The role of C/EBP genes in adipocyte differentiation. J Biol Chem.
273:30057–30060. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao Z, Umek RM and McKnight SL: Regulated
expression of three C/EBP isoforms during adipose conversion of
3T3-L1 cells. Genes Dev. 5:1538–1552. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Z, Rosen ED, Brun R, et al:
Cross-regulation of C/EBP alpha and PPAR gamma controls the
transcriptional pathway of adipogenesis and insulin sensitivity.
Mol Cell. 3:151–158. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu C, Zhu W, Shen CL and Gao W: Green tea
polyphenols reduce body weight in rats by modulating
obesity-related genes. PLoS One. 7:e383322012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Warnke I, Goralczyk R, Fuhrer E and
Schwager J: Dietary constituents reduce lipid accumulation in
murine C3H10 T1/2 adipocytes: a novel fluorescent method to
quantify fat droplets. Nutr Metab (Lond). 8:302011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang XH, Huang B, Choi SK and Seo JS:
Anti-obesity effect of resveratrol-amplified grape skin extracts on
3T3-L1 adipocytes differentiation. Nutr Res Pract. 6:286–293. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan G, Jia J, Di L, et al: Effects of
C-reactive protein on adipokines genes expression in 3T3-L1
adipocytes. Biochem Biophys Res Commun. 424:462–468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alvala R, Alvala M, Sama V, Dharmarajan S,
Ullas JV and BMR: Scientific evidence for traditional claim of
anti-obesity activity of Tecomella undulata bark. J
Ethnopharmacol. 148:441–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wanders D, Graff EC, White BD and Judd RL:
Niacin increases adiponectin and decreases adipose tissue
inflammation in high fat diet-fed mice. PLoS One. 8:e712852013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kanagasabapathy G, Malek SN, Mahmood AA,
Chua KH, Vikineswary S and Kuppusamy UR: Beta-glucan-rich extract
from Pleurotus sajor-caju (Fr.) Singer prevents obesity and
oxidative stress in C57BL/6J mice fed on a high-fat diet. Evid
Based Complement Alternat Med. 2013:1852592013.PubMed/NCBI
|
|
36
|
Zhang BB, Zhou G and Li C: AMPK: an
emerging drug target for diabetes and the metabolic syndrome. Cell
Metab. 9:407–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang QQ, Otto TC and Lane MD: Mitotic
clonal expansion: a synchronous process required for adipogenesis.
Proc Natl Acad Sci USA. 100:44–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Engelman JA, Lisanti MP and Scherer PE:
Specific inhibitors of p38 mitogen-activated protein kinase block
3T3-L1 adipogenesis. J Biol Chem. 273:32111–32120. 1998. View Article : Google Scholar : PubMed/NCBI
|