Introduction
Prion diseases are a family of progressive
neurodegenerative disorders, which are fatal in the majority of
cases and affect both humans and domestic animals. Prion diseaes
are also termed as transmissible spongiform encephalopathy (TSE)
and are characterized by the spongiform degeneration of the central
nervous system (1). Prion
diseases involve the conversion of normal cellular prion protein
(PrPc) into the scrapie isoform of prion protein (PrPsc) (2). PrPsc is widely known as an
infectious agent of prion diseases (3). In addition, it is more prone to be
accumulated in insoluble fibrils and thereby disrupts normal
neuronal functions (4).
The neuronal aggregation of PrPSc or neuronal cells
exposed to prion protein (PrP) fragment [PrP (106–126)] induces
mitochondrial malfunction, which has been reported to be major
hallmark of neurodegenerative diseases, including Huntington’s
disease and Alzheimer’s disease (5–9). A
previous study demonstrated that treatment with gingerol prevents
PrP (106–126)-mediated mitochondrial neurotoxicity through the
regulation of hypoxia-inducible factor-1α (HIF-1α) activation
(10). Xie et al (11) also demonstrated that the exposure
of neurons to low oxygen activated HIF-1α and inhibited the
activation of the mitochondrial apoptotic pathway induced by nerve
growth factor (NGF) deprivation. These observations suggest that
regulators of mitochondrial homeostasis, including HIF-1α, may be
key factors for the protection against prion-related diseases.
A peptide corresponding to the residues 106–126 of
the PrP sequence [PrP (106–126)] has been widely used to examine
the mechanisms of prion-mediated neurotoxicity (12). Moreover, PrP (106–126) has been
found to induce neuronal apoptosis in primary cultures of
hippocampal, cortical and cerebellar neurons (13,14). Therefore, PrP (106–126) is a
useful experimental model for in vitro studies of
prion-induced neuronal apoptosis (15).
Typically, mammalian cells have the ability to
recognize changes in the local availability of oxygen (16), which is a key element for cell
survival. Under hypoxic conditions, whereby low oxygen levels are
low, the hypoxic response pathway is activated. This leads to the
increased levels of HIF-1 and its stabilization (17).
HIF-1 is a heterodimer that consists of α- and
β-subunits (18). The α-subunit
consists of 3 subunits, HIF-1α, HIF-2α and HIF-3α, all of which are
structurally similar. Of these, HIF-1 is involved in the regulation
of iron metabolism, angiogenesis and cell survival (19). Furthermore, in a previous study,
we demonstrated that HIF-1α is involved in the regulation of the
expression of prion protein to protect neurons (20). Under normoxic conditions, however,
HIF-1α is degraded through the ubiquitin-proteasome pathway when
the von Hippel-Lindau tumor suppressor protein (pVHL) binds to the
oxygen degradation domain (ODD) mediated by the HIF prolyl
hydroxylase domain-containing proteins (PHDs) (21). In mammalian cells, PHDs are
composed of 3 isoforms, PHD1, PHD2 and PHD3, all of which have been
shown to cause the hydroxylation of the key proline residues
(Pro402 and Pro564) of HIF-1α in an in vitro setting
(21,22). Furthermore, the HIF-1α protein can
be stabilized by PHD enzyme inhibitors, such as deferoxamine (DFO)
and dimethyloxalylglycine (DMOG) (23). Recent major advances have shown
that HIF PHD2 is involved in the regulation of the
ubiquitin-proteasome pathway associated with HIF-1α (3). Moreover, it is also a key oxygen
sensor that creates low steady-state levels of HIF-1α under
normoxic conditions (3).
Gingerol is the active constituent of fresh ginger,
and it has been widely used as a Chinese herbal medicine in the
treatment of a variety of diseases, including inflammation
(24). Moreover, it is one of the
pivotal bioactive products of ginger and has various
pharmacological properties, such as anti-inflammatory and
antioxidant activities. Furthermore, it is also involved in cell
survival and neuroprotection (10,24,25). It has been reported that gingerol
increases the protein levels of HIF-1α (10). However, little is known about the
molecular mechanisms through which gingerol mediates the expression
of HIF-1α in the pathogenesis of prion diseases.
Given the above background, we hypothesized that
gingerol may inhibit the catalytic activity of PHD2 and thereby
prevent the occurrence of PrP (106–126)-induced neuronal apoptosis
through the upregulation of the protein expression of HIF-1α. To
examine this hypothesis, in the present study, we investigated the
effects of gingerol on PHD2 catalytic activity and assessed its
role in the effects of HIF-1α on the occurrence of PrP
(106–126)-induced neuronal apoptosis.
Materials and methods
Cell culture
The SH-SY5Y human neuroblastoma cell line was
obtained from the American Type Culture Collection (ATCC,
Rockville, MD, USA). The murine neuronal cell lines, ZW 13-2 and
Zpl 3–4, established from the hippocampus of ICR
(Prnp+/+) and Zürich I
(Prnp−/−) mice, respectively, were kindly donated
by Professor Yong-Sun Kim (Hallym University, Chuncheon, Korea).
The SH-SY5Y cells were cultured in minimum essential medium (MEM;
Invitrogen-Gibco, Grand Island, NY, USA), whereas the ZW 13-2 and
Zpl 3–4 cells were cultured in Dulbecco’s modified Eagle’s medium
(DMEM; HyClone, Logan, UT, USA) that contained 10% fetal bovine
serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) and
penicillin-streptomycin (both 100 units/ml) in a humidified
incubator maintained at 37°C and 5% CO2.
Construction of HIF-1α short hairpin
(sh)RNA plasmid
The shRNA against HIF-1α was kindly donated by Dr
Yong-Nyun Kim (National Cancer Center, Goyang, Korea). The shRNA
plasmid constructs for HIF-1α (shHIF-1α) were generated in the
lentiviral vector, pL-UGIP. The shRNA for HIF-1α was obtained with
the following oligonucleotide sequences: forward,
5′-CTGATGACCAGCAACTTGA-3′ and reverse, 5′-TCAA GTTGCTGGTCATCAG-3′.
The SH-SY5Y cells were transfected with shHIF-1α, and stable
transfectants were selected with puromycin after a 24-h recovery in
standard growth medium. SH-SY5Y cells transfected with the mock
vector (shMOCK) were used as the controls.
Reagents
Gingerol (0.625, 1.25 and 2.5 nM) was purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA); DMOG (500 μM) and
DFO (100 μM) and doxorubicin (DOX, 40 nM) were purchased from
Sigma-Aldrich. In addition, cycloheximide (CHX, 50 μM) was
purchased from Santa Cruz Biotechnology.
PrP (106–126) treatment
The synthetic PrP (106–126) (sequence,
Lys-Thr-Asn-Met-Lys-His-Met-Ala-Gly-Ala-Ala-Ala-Ala-Gly-Ala-Val-Val-Gly-Gly-Leu-Gly)
was synthesized by Peptron Inc. (Daejon, Korea). The peptide was
dissolved in sterile dimethyl sulfoxide (DMSO) at a concentration
of 10 mM and then stored at −80°C.
Western blot analysis
The SH-SY5Y cells were lysed in buffer containing 25
mM HEPES; pH 7.4, 100 mM NaCl, 1 mM EDTA, 5 mM MgCl2,
0.1 mM dithiothreitol (DTT) and protease inhibitor mixture.
Proteins were electrophoretically resolved by 10–15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
immunoblotting was performed as previously described. Equal amounts
of lysate protein were similarly electrophoretically resolved and
electrophoretically transferred onto a nitrocellulose membrane.
Immunoreactivity was detected through sequential incubation with
horseradish peroxidase-conjugated secondary antibody and enhanced
chemiluminescence reagents. Antibodies used for immunoblotting were
HIF-1α (Pierce Biotechnology, Rockford, IL, USA), HO-HIF-1α (Cell
Signaling Technology, Boston, MA, USA), PHD2 (Abcam, Cambridge, MA,
USA), PrPc (Millipore, Billerica, MA, USA) and β-actin (Santa Cruz
Biotechnology).
Annexin V assay
Apoptosis was assessed using a commercial Annexin V
assay (Santa Cruz Biotechnology) according to the manufacturer’s
instructions using a flow cytometry method. The Annexin V content
was determined by measuring fluorescence at an excitation
wavelength of 488 nm and emission wavelengths of 525 and 530 nm
using a Guava easyCyte™ flow cytometer (Millipore, Bedford, MA,
USA).
Immunofluorescence staining
The SH-SY5Y cells cultured on glass coverslips were
treated with PrP (106–126). The cells were washed with
phosphate-buffered saline (PBS) and fixed with cold acetone for 90
sec. The cells were then washed with PBS, blocked with 5% fetal
bovine serum in Tris-buffered saline containing Tween-20, and
incubated with anti-HO-HIF-1α (2 μg/ml) and PHD2 (2 μg/ml) and
anti-HIF-1α (2 μg/ml) monoclonal antibodies for 48 h at 20°C.
Unbound antibody was removed by an additional PBS wash, and the
cells were incubated with labeled anti-rabbit Alexa
Fluor® 546 (for anti-HO-HIF-1α and PHD2) IgG antibody (4
μg/ml) and Alexa Fluor 488 (for HIF-1α) IgG antibody (4 μg/ml) for
2 h at 20°C. Finally, the cells were counterstained with DAPI
(blue) and mounted with DakoCytomation fluorescent medium (Dako,
Glostrup, Denmark) and visualized under a fluorescence
microscope.
RNA interference (RNAi)
The SH-SY5Y cells were transfected with HIF-1α small
interfering RNA (siRNA; oligo ID HSS104775; Invitrogen, Carlsbad,
CA, USA) using Lipofectamine™ 2000 according to the instructions of
the manufacturer. Following culture for 48 h, the knockdown
efficiency was typically measured at the protein level by an
immunoblot assay.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the SH-SY5Y cells using
easy-spin™ Total RNA Extraction kits (Intron Biotechnology, Seoul,
Korea). cDNA synthesis was carried out following the instructions
provided with the Takara PrimeScript® First Strand cDNA
Synthesis kit (Takara Bio, Inc., Shiga, Japan). For RT-qPCR, 1 μl
of gene primer with SYBR®-Green (Bio-Rad Laboratories,
Hercules, CA, USA) in 20 μl of reaction volume was applied. The
sequences of the primers used for the qPCR were as follows: HIF-1α
forward, 5′-AGAAACCAC CTATGACCTGC-3′ and reverse,
5′-GTCGTGCTGAATAAT ACCACTC-3′; PHD2 forward, 5′-CAAGGACATCCGAGG
CGATAAG-3′ and reverse, 5′-CCGTTACAGTGGCGTATC AGG-3′; and β-actin
(as an internal control) forward, 5′-GCAA GCAGGAGTATGACGAG3′ and
reverse, 5′-CAAATAAA GCCG CCAATC-3′. All reactions with iTaq™
SYBR-Green Supermix (Bio-Rad Laboratories) were performed using the
CFX96™ real-time PCR detection system (from Bio-Rad
Laboratories).
Statistical analysis
All data are expressed as the means ± standard
deviation (SD), and were compared using the Student’s t-test and
ANOVA with the Duncan test using the SAS statistical package (SAS
Institute, Inc., Cary, NC, USA). Statistical significance was set
at P<0.05 or P<0.01.
Results
The gingerol-induced increase in the
expression of HIF-1α prevents PrP (106–126)-induced
neurotoxicity
It has recently been demonstrated that the
gingerol-induced expression of HIF-1α inhibits the occurrence of
human prion peptide-mediated neurotoxicity (20). Based on this report, we
reconfirmed the protective effects of gingerol on PrP
(106–126)-induced neuronal apoptosis (Fig. 1). The SH-SY5Y cells were
pre-treated for 12 h with various concentrations of gingerol and
then exposed to 100 μM PrP (106–126) for 12 h (Fig. 1A). The Annexin V-negative cell
population was decreased following treatment with PrP (106–126).
Following pre-treatment with gingerol, however, there was an
increase in the PrP (106–126)-induced Annexin V-negative SH-SY5Y
neuronal cell population (Fig. 1A and
B). These results indicate that gingerol prevents PrP
(106–126)-induced neurotoxicity.
In our previous studies, we demonstrated that HIF-1α
is involved in the regulation of the expression of prion protein to
protect neurons (20,26). Indeed, gingerol increases the
protein levels of HIF-1α (15).
To determine whether HIF-1α is involved in the protective effects
of gingerol, we assessed the protein and mRNA levels of HIF-1α in
the SH-SY5Y cells (Fig. 1C–E).
The SH-SY5Y cells were pre-treated with various concentrations of
gingerol for 12 h (Fig. 1C) and
then exposed to PrP (106–126) for 8 h. In the cells treated with
gingerol, there was an increase in the protein levels of HIF-1α
(Fig. 1C). These results were
confirmed by immunofluorescence staining (Fig. 1D). To determine whether gingerol
is involved in the regulation of the expression of the HIF-1α gene,
the SH-SY5Y cells were incubated for 24 h in the presence of
gingerol. The expression of HIF-1α gene was then measured by
RT-qPCR. Following treatment with gingerol, there was a
dose-dependent increase in the mRNA expression of HIF-1α (Fig. 1E).
We used DOX as an HIF-1α inhibitor in order to
examine the effects of gingerol in preventing the occurrence of PrP
(106–126)-induced neurotoxicity through HIF-1α. The SH-SY5Y cells
were incubated with PrP (106–126) for 8 h following exposure to 2.5
nM of gingerol (12 h) and/or 40 nM of DOX for 1 h prior to
treatment with gingerol. As shown in Fig. 2A, the protein levels of HIF-1α
were increased following treatment with gingerol. However, DOX
inhibited the effects of gingerol on the protein levels of HIF-1α.
Moreover, DOX inhibited the effects of gingerol on PrP
(106–126)-induced neurotoxicity in the SH-SY5Y cells (Fig. 2B). These results were confirmed by
RNAi experiments using Annexin V assay. As shown in Fig. 2C, gingerol prevented the
occurrence of PrP (106–126)-induced neurotoxicity in the cells
transfected with non-specific siRNA (NS siRNA), whereas treatment
with gingerol had no effect on PrP (106–126)-induced neurotoxicity
in the cells transfected with HIF-1α siRNA. These results were
confirmed by shRNA experiments using the Annexin V assay for cell
viability (Fig. 2D).
Gingerol promotes the stabilization of
HIF-1α through the inhibition of HIF PHD2 catalytic activity
Under normoxic conditions, HIF-1α is degraded by
catalytically activated PHD2 (2).
Furthermore, the molecular mechanisms responsible for the
gingerol-mediated induction of HIF-1α expression are not yet fully
understood. In the present study, in order to examine the effects
of gingerol on the hydroxylation of HIF-1α, the SH-SY5Y cells were
treated with various concentrations of gingerol, and DMOG 500 μM or
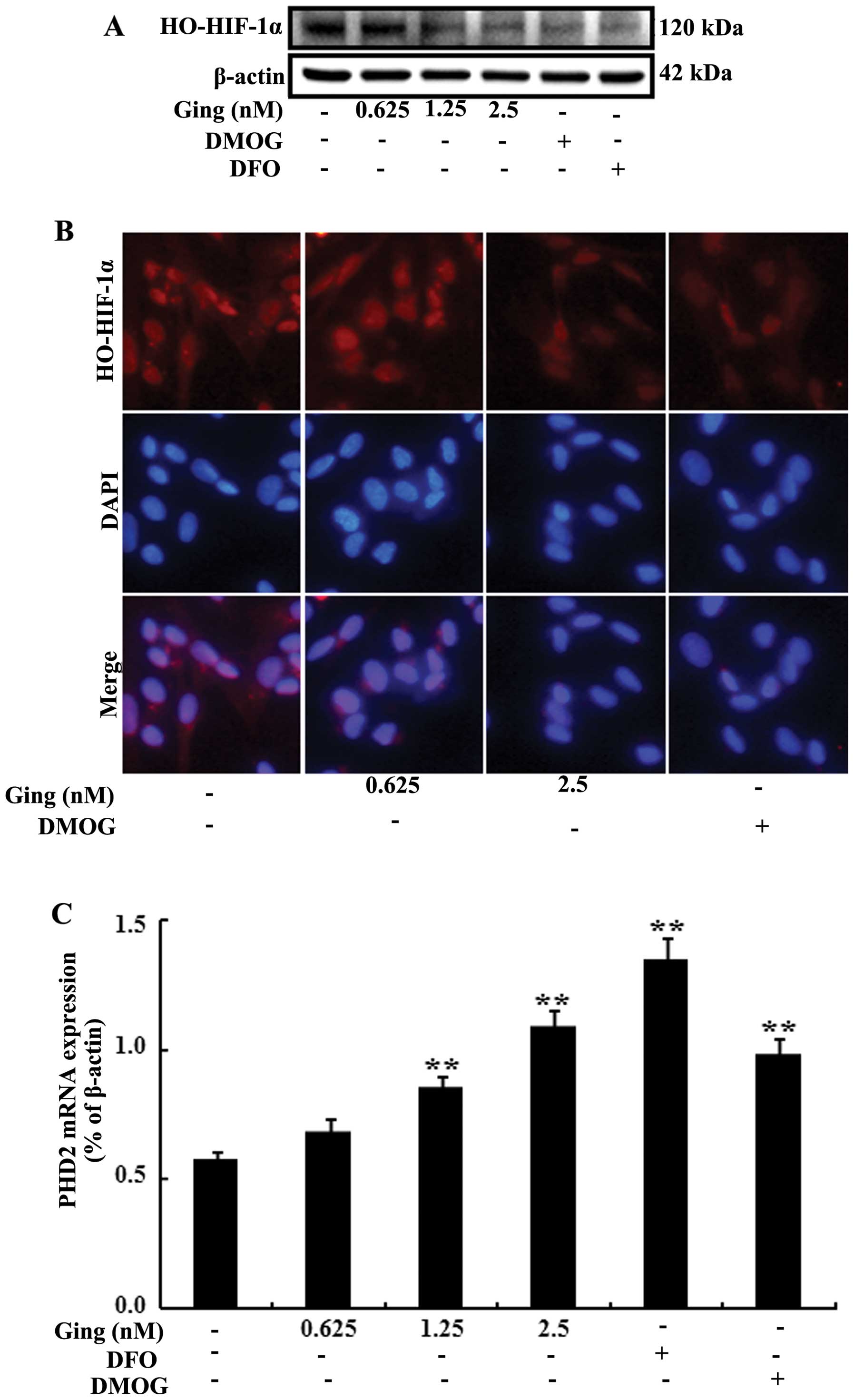
DFO (PHD enzyme inhibitors). As shown in Fig. 3A, following treatment with
gingerol, there was a dose-dependent decrease in HIF-1α
hydroxylation. Consistent with these results, immunofluorescence
staining also revealed that HIF-1α hydroxylation was inhibited
following treatment with gingerol (Fig. 3B). Furthermore, to determine
whether gingerol is involved in the regulation of the mRNA
expression of PHD2, the SH-SY5Y cells were incubated for 24 h in
the presence of gingerol, DMOG or DFO. Subsequently, the mRNA
expression of PHD2 was measured by RT-qPCR. The mRNA expression of
PHD2 was increased in the SH-SY5Y cells following treatment with
gingerol (Fig. 3C). In addition,
following treatment with gingerol, there was an increase in the
protein levels of PHD2 (Fig. 3D).
We then assessed whether the gingerol-induced upregulation of PHD2
is related to HIF-1α stabilization by using CHX. For this purpose,
the SH-SY5Y cells were incubated with PrP (106–126) for 8 h
following exposure to 2.5 nM gingerol (12 h) and/or 50 μM CHX for 1
h prior to treatment with gingerol. In the CHX-treated group, there
was a decrease in the protein levels of HO-HIF-1α following
treatment with gingerol (Fig.
3E). These results suggest that the catalytic activity of PHD2
is inhibited by treatment with gingerol.
Gingerol-induced HIF-1α expression
upregulates the expression of PrPc in neurons
HIF-1α is involved in the regulation of prion
protein expression to protect neurons (15). Moreover, it has also been
suggested that the gingerol-induced expression of HIF-1α is a key
factor in attenuating hypoxia-induced embryo toxicity (27). In our study, to determine whether
HIF-1α is stabilized by gingerol-mediated PrPc expression, ZW 13-2
and Zpl 3–4 murine neuronal cells were incubated with 2.5 nM
gingerol for 20 h. As shown in Fig.
4A, following treatment with gingerol, the expression of HIF-1α
and PrPc was increased in the ZW 13-2 cells. However, PrPc protein
expression was not detected in the Zpl 3–4 cells (Fig. 4A). To confirm the increase in the
expression of PrPc following treatment with gingerol and that this
caused a beneficial effect on the ZW 13-2 and Zpl 3–4 cells, the
cells were incubated with PrP (106–126) 100 μM for 12 h following a
12-h exposure to 2.5 nM gingerol. Cell viability was measured by
Annexin V assay and flow cytometry. In the ZW 13-2 murine neuronal
cells, gingerol had a neuroprotective effect on the PrP
(106–126)-induced neuronal apoptosis. In the Zpl 3–4 cells,
however, it had no neuroprotective effects (Fig. 4B and C).
These results demonstrate that treatment with
gingerol promotes the stabilization of HIF-1α and the expression of
the HIF-1α gene. Furthermore, the gingerol-induced expression of
HIF-1α increased the expression of PrPc in neurons. Taken together,
these results indicate that gingerol exerts a protective effect on
PrP (106–126)-induced neurotoxicity in neurons through the
upregulation of HIF-1α-mediated by the expression of PrPc.
Discussion
We conducted the present study in order to examine
the effects of gingerol on PrP (106–126)-induced neurotoxicity and
the interactions between gingerol and HIF-1α. Our results indicated
that gingerol has therapeutic potential for use in the treatment or
prevention of neurodegenerationve diseases, such as prion-related
diseases.
Prion diseases involve the conversion of normal
cellular prion protein into the scrapie isoform of prion protein
(PrPsc) (2,14). PrPsc is widely known as an
infectious agent of prion diseases (3). In addition, it is more prone to be
accumulated in insoluble fibrils and thereby disturbs normal
neuronal functions (2). PrP
(106–126) retains the neurotoxic properties of the entire
pathological PrPsc and it is generally used as a model to study the
mechanisms responsible for prion diseases (12,28). However, little is known about the
molecular mechanisms through which PrP (106–126)-mediated neuronal
apoptosis occurs. In our previous study, we demonstrated that
HIF-1α is involved in the regulation of the expression of prion
protein to protect neurons (20).
Furthermore, with the gingerol-induced expression of HIF-1α, human
prion peptide-mediated neurotoxicity has been shown to be inhibited
in neurons (15). However, little
is known about the molecular mechanisms through which gingerol
mediates the expression of HIF-1α. We therefore hypothesized that
gingerol induces the expression of HIF-1α by inhibiting the
catalytic activity of PHD2. Under normoxic conditions, HIF-1α is
degraded by the hydroxylation of 2 prolines (Pro 402 and Pro 564)
located within the ODD (21). In
addition, HIF PHDs induce the hydroxylation of HIF-1α under
normoxic conditions. Moreover, recent major advances have shown
that HIF PHD2 is involved in the regulation of the
ubiquitin-proteasome pathway associated with HIF-1α (3). Moreover, it is a key oxygen sensor
that creates low steady-state levels of HIF-1α under normoxic
conditions (3). Our results
demonstrated that gingerol promoted the stabilization of HIF-1α
through the inhibition of the catalytic activity of HIF PHD2
(Fig. 3). Furthermore, gingerol
inhibited the hydroxylation of HIF-1α (Fig. 3A, B and E). In our study,
following treatment with gingerol, there was an increase in the
expression of the PHD2 gene and its products (Fig. 3C and D). These results indicated
that the catalytic activity of PHD2 was inhibited as there was an
increase in the gingerol-mediated expression of HIF-1α. To examine
the inhibitory effects of gingerol on the catalytic activity of
PHD2, we used CHX. As shown in Fig.
4E, there was a decrease in the hydroxylation of HIF-1α
following treatment with gingerol in the CHX-treated group.
As shown in Figs.
1 and 2, gingerol induced the
expression of HIF-1α and thereby protected the cells against PrP
(106–126)-induced neurotoxicity. Moreover, it was also involved in
the regulation of the catalytic activity of PHD2 by inducing the
expression of HIF-1α. These results indicate that gingerol inhibits
the catalytic activity of PHD2 and that this leads to the decreased
hydroxylation of HIF-1α. This eventually leads to an increase in
the stabilization of HIF-1α under normoxic conditions.
In a previous study, it was demonstrated that PrP
(106–126)-induced neuronal apoptosis is prevented under hypoxic
conditions by the activation of HIF-1α and that HIF-1α is involved
in the regulation of the expression of prion protein to protect
neurons (17). Furthermore,
HIF-1α is involved in the regulation of the expression of PrPc in
neurons (29). As shown in
Fig. 4, gingerol induced the
expression of HIF-1α in the ZW 13-2 murine neuronal cells, but not
in the Zpl 3–4 cells. Thus, gingerol is involved in the
upregulation of the expression of PrPc in ZW 13-2 neuronal cells.
In the ZW 13-2 murine neuronal cells, gingerol had a
neuroprotective effect on PrP (106–126)-induced neuronal apoptosis.
In the Zpl 3–4 cells, however, it had no neuroprotective effects
(Fig. 4B and C). These results
indicate that gingerol mediates the expression of PrPc and thereby
exerts a protective effect, thus, suggesting the existence of a
correlation between the expression of HIF-1α and that of PrPc, both
of which are induced by treatment with gingerol.
Briefly, our results demonstrated that gingerol
prevented the occurrence of PrP (106–126)-induced neuronal
apoptosis by upregulating the expression of PrPc. Moreover,
gingerol induced the stabilization of HIF-1α by inhibiting the
catalytic activity of PHD2. In conclusion, our results indicate
that gingerol has therapeutic potential for use in the treatment or
prevention of prion diseases by exerting inhibitory effects on the
catalytic activity of PHD2, thus stabilizing HIF-1α.
Acknowledgements
The present study was supported by a grant from the
National Research Foundation of Korea (NRF), funded by the Korean
government (2013R1A2A2A01009614).
References
|
1
|
Aguzzi A and Calella AM: Prions: protein
aggregation and infectious diseases. Physiol Rev. 89:1105–1152.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Solomon IH, Schepker JA and Harris DA:
Prion neurotoxicity: insights from prion protein mutants. Curr
Issues Mol Biol. 12:51–61. 2010.PubMed/NCBI
|
|
3
|
Sajnani G, Silva CJ, Ramos A, et al:
PK-sensitive PrP is infectious and shares basic structural features
with PK-resistant PrP. PLoS Pathog. 8:e10025472012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakudo A and Ikuta K: Prion protein
functions and dysfunction in prion diseases. Curr Med Chem.
16:380–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeong JK, Moon MH, Bae BC, et al:
Autophagy induced by resveratrol prevents human prion
protein-mediated neurotoxicity. Neurosci Res. 73:99–105. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong JK, Moon MH, Lee YJ, Seol JW and
Park SY: Autophagy induced by the class III histone deacetylase
Sirt1 prevents prion peptide neurotoxicity. Neurobiol Aging.
34:146–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aiken JM, Williamson JL and Marsh RF:
Evidence of mitochondrial involvement in scrapie infection. J
Virol. 63:1686–1694. 1989.PubMed/NCBI
|
|
8
|
Quintanilla RA, Dolan PJ, Jin YN and
Johnson GV: Truncated tau and Aβ cooperatively impair mitochondria
in primary neurons. Neurobiol Aging. 33:619.e625–619.e635.
2012.
|
|
9
|
Freixes M, Rodriguez A, Dalfo E and Ferrer
I: Oxidation, glycoxidation, lipoxidation, nitration, and responses
to oxidative stress in the cerebral cortex in Creutzfeldt-Jakob
disease. Neurobiol Aging. 27:1807–1815. 2006. View Article : Google Scholar
|
|
10
|
Jeong JK, Moon MH, Park YG, et al:
Gingerol-induced hypoxia-inducible factor 1 alpha inhibits human
prion peptide-mediated neurotoxicity. Phytother Res. 27:1185–1192.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie L, Johnson RS and Freeman RS:
Inhibition of NGF deprivation-induced death by low oxygen involves
suppression of BIMEL and activation of HIF-1. J Cell Biol.
168:911–920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O’Donovan CN, Tobin D and Cotter TG: Prion
protein fragment PrP-(106–126) induces apoptosis via mitochondrial
disruption in human neuronal SH-SY5Y cells. J Biol Chem.
276:43516–43523. 2001.
|
|
13
|
Forloni G, Angeretti N, Chiesa R, et al:
Neurotoxicity of a prion protein fragment. Nature. 362:543–546.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melo JB, Agostinho P and Oliveira CR:
Prion protein aggregation and neurotoxicity in cortical neurons.
Ann NY Acad Sci. 1096:220–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sponne I, Fifre A, Koziel V, Kriem B,
Oster T and Pillot T: Humanin rescues cortical neurons from
prion-peptide-induced apoptosis. Mol Cell Neurosci. 25:95–102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lando D, Peet DJ, Gorman JJ, Whelan DA,
Whitelaw ML and Bruick RK: FIH-1 is an asparaginyl hydroxylase
enzyme that regulates the transcriptional activity of
hypoxia-inducible factor. Genes Dev. 16:1466–1471. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dery MA, Michaud MD and Richard DE:
Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic
activators. Int J Biochem Cell Biol. 37:535–540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madan A, Varma S and Cohen HJ:
Developmental stage-specific expression of the alpha and beta
subunits of the HIF-1 protein in the mouse and human fetus. Mol
Genet Metab. 75:244–249. 2002. View Article : Google Scholar
|
|
19
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong JK, Seo JS, Moon MH, Lee YJ, Seol JW
and Park SY: Hypoxia-inducible factor-1α regulates prion protein
expression to protect against neuron cell damage. Neurobiol Aging.
33:1006 e1001–1010. 2012.
|
|
21
|
Bruick RK: Oxygen sensing in the hypoxic
response pathway: regulation of the hypoxia-inducible transcription
factor. Genes Dev. 17:2614–2623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Page EL, Chan DA, Giaccia AJ, Levine M and
Richard DE: Hypoxia-inducible factor-1alpha stabilization in
nonhypoxic conditions: role of oxidation and intracellular
ascorbate depletion. Mol Biol Cell. 19:86–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Groenman FA, Rutter M, Wang J, Caniggia I,
Tibboel D and Post M: Effect of chemical stabilizers of
hypoxia-inducible factors on early lung development. Am J Physiol
Lung Cell Mol Physiol. 293:L557–L567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dugasani S, Pichika MR, Nadarajah VD,
Balijepalli MK, Tandra S and Korlakunta JN: Comparative antioxidant
and anti-inflammatory effects of [6]-gingerol, [8]-gingerol,
[10]-gingerol and [6]-shogaol. J Ethnopharmacol. 127:515–520.
2010.
|
|
25
|
Masuda Y, Kikuzaki H, Hisamoto M and
Nakatani N: Antioxidant properties of gingerol related compounds
from ginger. Biofactors. 21:293–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo JS, Seol JW, Moon MH, Jeong JK, Lee YJ
and Park SY: Hypoxia protects neuronal cells from human prion
protein fragment-induced apoptosis. J Neurochem. 112:715–722. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yon JM, Baek IJ, Lee BJ, Yun YW and Nam
SY: Emodin and [6]-gingerol lessen hypoxia-induced embryotoxicities
in cultured mouse whole embryos via upregulation of
hypoxia-inducible factor 1alpha and intracellular superoxide
dismutases. Reprod Toxicol. 31:513–518. 2011.
|
|
28
|
Selvaggini C, De Gioia L, Cantu L, et al:
Molecular characteristics of a protease-resistant, amyloidogenic
and neurotoxic peptide homologous to residues 106–126 of the prion
protein. Biochem Biophys Res Commun. 194:1380–1386. 1993.PubMed/NCBI
|
|
29
|
Jeong JK and Park SY: Transcriptional
regulation of specific protein 1 (SP1) by hypoxia-inducible factor
1 alpha (HIF-1α) leads to PRNP expression and neuroprotection from
toxic prion peptide. Biochem Biophys Res Commun. 429:93–98.
2012.
|