Introduction
Selenium (Sel) is an ubiquitous trace element in
nature that has been shown to be essential to various aspects of
human health (1). This trace
element has also been shown to be required for normal growth and
reproduction during spermatogenesis (2). Furthermore, Sel deficiency induces
multiple diseases associated with oxidative damage, such as fatal
cardiomyopathy, which is endemic in Keshan (China) (3), and muscular dystrophy in patients
subjected to long-term unsupplemented parenteral nutrition
(4). It is also well known that
vitamin E can partially replace Sel deficiency (5,6).
Sel exists naturally in organic (such as selenomethionine and
selenocysteine) and inorganic forms (such as selenite, selenate and
selenide) (7). However, these
compounds require catabolizing into inorganic precursors prior to
insertion into proteins, and the rare amino acid selenocysteine
(Sec) is essential for the catalytic function of selenoenzymes
(8). Sec, which is the 21st
proteinogenic amino acid, was not initially recognized in the
classical genetic code as it is encoded by the UGA ‘STOP’ codon.
For Sec insertion at UGA codons in the translation process, a
specific RNA stem-loop structure is required. In eukaryotes, this
loop resides in the 3′-untranslated region of the mRNA, known as
the Sec-insertion-sequence (9).
Sel has also been shown to be an essential part of mammalian
enzymes, such as glutathione peroxidase (GPx), thyroid hormone
deiodinase and thioredoxin reductase. Thus far, 25 genes encoding
selenoproteins in the sequenced human genome have been identified
(10). Selenoprotein M (SelM) was
first reported as a 0.7-kb cDNA gene that encoded a new
selenoprotein identified from the mammalian EST database. This gene
has a 145-amino acid open reading frame beginning with an ATG codon
in a favorable Kozak context and contains an in-frame TGA as the
Sec codon. Furthermore, homologous proteins have been identified in
the rat, zebra fish and other vertebrates, and Sec was conserved in
these homologs (11). There have
also been several functional studies of SelM. For example, the
study by Müller et al (12) showed that this protein plays a
major role in spicule formation in the demosponge Suberites
domuncula. In addition, Hwang et al (13) indicated that SelM plays a
suppressive or protective role in the pathology of patients with
Alzheimer’s disease (AD). However, there have been no studies for
whether SelM overexpression could affect the changes in global gene
expression in CMV/hSelM transgenic (Tg) rats following Sel
treatment.
Sel is maintained at high levels in the brain, even
upon prolonged dietary Sel deficiency (14). Changes in Sel concentration in the
brain and blood have been detected in AD, Parkinson’s disease (PD),
multiple sclerosis and brain tumors. Several studies have shown
that Sel treatment leads to reduced seizures, improved
electroencephalogram recordings (15), protection against the depletion of
striatal dopamine (16) and a
reduction in the progression of neurodegeneration (17). Furthermore, several selenoproteins
have been expressed in the brain. Among these proteins, GPx has
been localized in glial cells, and its expression level was
significantly upregulated in damaged areas in PD (18). High expression of selenoprotein P
(SelP) was also observed in the olfactory bulb, hippocampus and
frontal cortex (19).
Genetically-engineered models, including Tg and knock-out models,
can provide a platform to define the in vivo function of
genes and to also study the molecular events responding to
environmental changes (20).
Previous studies using a knock-out model of SelP showed that it had
the important function of Sel delivery into the brain tissue
(21,22). Other selenoproteins, including
selenoprotein W, thioredoxin reductase, 15-kDa selenoprotein and
SelP, have also been detected in the brain. However, numerous
questions regarding the roles of these proteins in neuronal
function remain.
In the present study, the global change of gene
expression affected by SelM overexpression and Sel treatment in the
brain cortex was investigated. Two-dimensional electrophoresis
(2-DE) analysis showed that eight proteins were significantly
changed in CMV/hSelM Tg rats. These results indicated that the
information isolated from SelM overexpression and Sel treatment may
be useful for studying the association between antioxidant
conditions and brain disease, which shows a higher level of
oxidative stress condition in specific tissues.
Materials and methods
Maintenance and identification of
CMV/hSelM Tg rats
The CMV/hSelM Tg rats used in the study, showing
high antioxidant status in various tissues, were developed by
microinjection of the CMV/hSelM recombinant gene into fertilized
rat eggs (13). The animal
protocol was reviewed and approved based on the ethical and
scientific care procedures of the Korea Food and Drug
Administration (KFDA)-Institutional Animal Care and Use Committee.
All rats were maintained in an accredited KFDA animal facility in
accordance with AAALAC International Animal Care policies
(Accredited Unit-KFDA; unit No. 000996). The rats were provided a
standard irradiated chow diet (Purina Mills Inc., St. Louis, MO,
USA) ad libitum and maintained in a specified pathogen-free
state under a strict light cycle (lights on at 06:00 h and off at
18:00 h). All the pedigrees were hemizygous for their
transgenes.
Experimental design and Sel
treatment
Sodium selenite (NaSeO3) purchased from
Sigma-Aldrich Co., (St. Louis, MO, USA) was dissolved in distilled
water to a final concentration of 0.2 μmol/μl (23,24). Ten-week-old rats were randomly
divided into two subgroups (n=6 per group). The first subgroup of
the CMV/hSelM Tg and non-Tg rat groups each received a comparable
volume of distilled water daily via intraperitoneal injection
(vehicle-treated CMV/hSelM Tg and non-Tg groups), whereas rats in
the second subgroup each received 5 μmol/kg body weight/day of
sodium selenite via intraperitoneal injection for three weeks
(Sel-treated CMV/hSelM Tg and non-Tg groups). At three weeks after
Sel-solution injection, the animals were immediately euthanized
using CO2 gas, following which the cortex samples from
their brains were prepared and stored in Eppendorf tubes at −70°C
until assayed.
Analysis of GPx and superoxide dismutase
(SOD) activities and total oxidized products concentration
The levels of GPx and SOD in the brain cortex of
CMV/hSelM Tg and non-Tg rats were detected by following the
colorimetric assay procedure using Bioxytech SOD-525 and Bioxytech
GPx-340 kits (OxisResearch™, Portland, OR, USA). The level of total
oxidized products in the sera of CMV/hSelM Tg and non-Tg rats was
detected using a Total Antioxidant Status kit (Randox Laboratories
Ltd., Antrim, UK) as previously described (13).
γ-secretase activity analysis
The γ-secretase activity was detected with a
γ-Secretase Activity kit (R&D System Inc., Minneapolis, MN,
USA) using the manufacturer’s instructions. Initially, cortex
tissue was homogenized with a glass homogenizer in cold 1X
extraction buffer to yield a final protein concentration of roughly
0.5–2.0 mg. These mixtures were incubated on ice for 10 min, after
which they were centrifuged at 10,000 × g for 1 min to remove the
unbroken fragments. The final supernatants collected from the
centrifuged mixture were subsequently used for detection of
secretase activity, and this process was carried out in the
microplate provided by the manufacturer. To perform the enzyme
reaction, 50 μl tissue lysate was added to each well, followed by
50 μl 2X reaction buffer and 5 μl substrate. The microplates were
gently mixed and incubated in the dark at 37°C for 1–2 h to induce
an enzyme-substrate reaction. Final fluorescence produced by the
reaction was read on a fluorescent microplate reader using a filter
that allowed EDANS excitation at between 335–355 nm.
Sample preparation for 2-DE
Analyses of global protein expression by 2-DE were
performed according to the methods established by our laboratory in
previous studies (25–27). The cortex samples isolated from
the brain were homogenized in liquid nitrogen, following which the
homogenized tissues were lysed in buffer [7 M urea, 2 M thiourea,
4% w/v CHAPS, 40 mM Tris and 100 mM dithioerythritol (DTE)]. The
sample mixtures were subsequently centrifuged at 45,000 × g at 4°C
for 1 h, after which protein concentrations were determined by the
Bradford protein assay (Bio-Rad, Hercules, CA, USA). In this
process, a cortex sample was generated from a pool of the six
animals in each group. The pooled samples were analyzed three
times.
2-DE analysis
One-dimensional isoelectric focusing (IEF) was
performed using 24 cm immobilized pH gradient (IPG) strips (GE
Healthcare, Uppsala, Sweden) in a pH range of 3.0–10.0
(non-linear). Protein (1-mg) was loaded in a total volume of 450
μl, following which the samples were diluted with rehydration
solution [7 M urea, 2 M thiourea, 4 % w/v CHAPS, 40 mM Tris, 100 mM
DTE and 2% IPG buffer (pH 3.0–10.0)]. After rehydration for 13 h,
the strips were focused at 30 V for 2 h, 100 V for 2 h, 200 V for 1
h, 500 V for 1 h, 1,000 V for 1 h and finally at 8,000 V for 22 h
to obtain ~100,000 VHr (IPGphor; GE Healthcare). Once IEF was
completed, the strips were equilibrated in 6 M urea containing 20%
glycerol, 2% SDS and 0.01% bromophenol blue with 10 mM tributyl
phosphine. Two-dimensional SDS-PAGE was performed using 8–18%
linear gradient acrylamide gels on an EttanDalt system (GE
Healthcare). Proteins were visualized by staining with Coomassie
blue G-250 (Bio-Rad).
To analyze changes in protein expression between the
types of rats according to SelM level, an average gel representing
non-Tg rats was compared to an average gel representing the
CMV/hSelM Tg rats. Only the filtered spots exceeding an intensity
threshold of a 1.5 or 2-fold increase or decrease between non-Tg
and CMV/hSleM Tg rats were studied further, whereas the threshold
regulation factor for the significance level was set at P≤0.05.
Furthermore, any spot showing a significant difference in
expression between non-Tg and CMV/hSleM Tg rats was analyzed in all
the rats to map changes in expression according to Sel-related
factors. Finally, the spots showing significant changes in
expression were subsequently identified by mass spectrometry.
Identification of protein spots
The stained gels were scanned with a GS800
densitometer (Bio-Rad) and analyzed using Image master™ (Swiss
Institute of Bioinfomatics, Geneva, Switzerland). The spots were
digested using trypsin, following which supernatant peptide
mixtures were loaded onto a Poros R2 column (Applied Biosystems,
Foster City, CA, USA) that had been washed with the following
solutions: i) 70% acetonitrile in 5% formic acid; ii) 100%
acetonitrile; and iii) 5% formic acid. Peptides were eluted using 5
μl of α-cyano-4-hydroxycinnamic acid and analyzed with a
matrix-assisted laser desorption/ionization time-of-flight
(MALDI-TOF) mass spectrometer (Voyager DE-PRO; Applied Biosystems).
For protein identification, masses of peptides determined by
MALDI-TOF were matched with theoretical peptides in the NCBI
(http://www.ncbi.nih.gov/) database using the
MASCOT (http://www.matrixscience.com) and
ProFound programs (http://prowl.rockefeller.edu).
Western blot analysis
Total proteins prepared from a cortex sample of
CMV/hSelM Tg and non-Tg rats were separated by electrophoresis on a
4–20% SDS-PAGE gel for 3 h and subsequently transferred to
nitrocellulose membranes for 2 h at 40 V. Each membrane was
incubated separately with anti-creatine kinase antibody (Abcam,
Cambridge, UK), anti-synaptotagmin antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), and anti-actin
(Sigma-Aldrich) antibodies overnight at 4°C. The membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (Zymed Laboratories, Inc., South San Francisco,
CA, USA) at a 1:1,000 dilution at room temperature for 2 h. The
membrane blots were developed using a Chemiluminescence Reagent
Plus kit (ECL; Amersham Pharmacia Biotech, Inc., Piscataway, NJ,
USA).
Statistical analysis
Tests for significance between vehicle- and
Sel-treated rats were performed using a one-way analysis of
variance test of variance (SPSS for Windows, Release 10.10,
Standard Version; SPSS, Inc., Chicago, IL, USA). Tests for
significance between CMV/hSelM Tg and non-Tg rats were performed
using a post-hoc test (SPSS for Windows, Release 10.10, Standard
Version) of variance, and significance levels are provided
throughout. All the values are reported as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Enhancement of SOD and GPX activity in
the brain cortex of CMV/hSelM Tg rats
To confirm whether or not alteration of
antioxidative conditions is induced by Sel treatment and SelM
overexpression in the cortex region of rat brains, the activity of
SOD and GPX was measured in the brain tissue of CMV/hSelM Tg and
non-Tg rats using a detection kit containing a specific substrate.
In the vehicle-treated group, SOD/GPX activity in the CMV/hSelM Tg
rats showed a higher level of enzyme activity than that of the
non-Tg rats, although they showed differing rates of increase
(Fig. 1A and C). Following Sel
treatment, the activity of these enzymes increased significantly in
the CMV/hSelM Tg and non-Tg rats. However, the rate of increase in
the CMV/hSelM Tg rats was greater than that of the non-Tg rats
(Fig. 1A and C). These results
indicate that SelM overexpression and Sel treatment induced an
increase of antioxidant protection in the brain cortex from
CMV/hSelM Tg rats.
Change in total oxidized products
concentration in the brain of CMV/hSelM Tg rats
To determine whether or not enhancement of SOD and
GPX activity was accompanied by a decreased level of oxidized
products, the concentration of total oxidized products was
determined in the serum of CMV/hSelM Tg and non-Tg rats following
Sel treatment using an ELISA kit. For the vehicle-treated group,
the concentration of total oxidized products at the basal level was
significantly lower in the CMV/hSelM Tg rats compared to the non-Tg
rats. Following Sel treatment, these levels decreased
simultaneously in the CMV/hSelM Tg and non-Tg rats, although the
concentration of total oxidized products in the CMV/hSelM Tg rats
was consistently maintained at a low level compared to the non-Tg
rats. In particular, the decreasing rate of total oxidized products
concentration in the Sel-treated group was significantly greater
compared to the vehicle-treated group (Fig. 1B). These results indicate that the
overactivation of SOD and GPX induced by SelM overexpression and
Sel treatment contributes to the increase in the functional
activity for oxidized products protection in the brain cortex of
CMV/hSelM rats.
Change of γ-secretase activity in the
brain cortex of CMV/hSelM Tg rats
Furthermore, γ-secretase plays an important role in
the production of Aβ-42 peptide in the pathogenesis of AD (28). To investigate the effects of Sel
treatment and SelM overexpression on the physiological changes in
neurodegenerative pathology, the activity of γ-secretase, a
critical enzyme of Aβ-42 peptides production, was measured in the
brains of CMV/hSelM Tg and non-Tg rats using a detection kit
containing a specific substrate. For the vehicle-treated group, the
activity of γ-secretase in the CMV/hSelM Tg rats was slightly
higher compared to non-Tg rats, although this difference was not
significant. However, following Sel treatment, these activities
were significantly lower in the two groups of rats. Furthermore,
the rate of decrease in γ-secretase activity in the CMV/hSelM Tg
rats was higher compared to the non-Tg rats (Fig. 1D). These findings indicate that
SelM overexpression and Sel treatment may lead to a decreased
incidence of neurodegenerative disease in the brain cortex through
the regulation of γ-secretase activity.
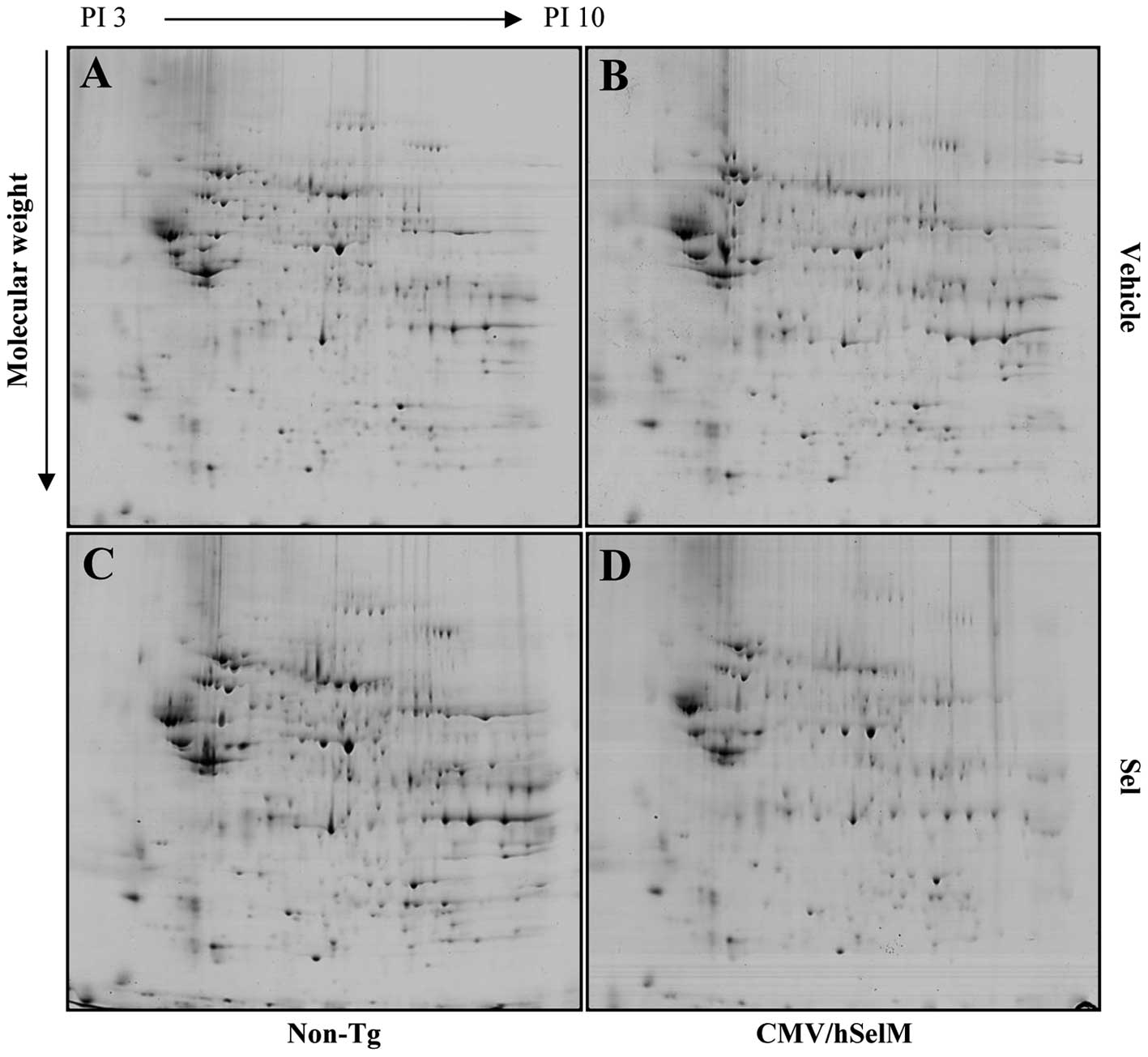
Proteome analysis of total proteins in
brain cortex from of CMV/hSelM Tg rats
To characterize the changes in global protein
expression in the brain cortex of CMV/hSelM Tg rats in response to
the induced bioavailability of Sel treatment and SelM
overexpression, whole proteins extracted from the brain cortex of
10-week old CMV/hSelM Tg and non-Tg rats were analyzed on analytic
2-DE gels. Computer analysis of gel images showed good matching in
four analytical replicates, including vehicle-treated non-Tg,
Sel-treated non-Tg, vehicle-treated CMV/hSelM Tg and Sel-treated
CMV/hSelM Tg rats. The 2-DE protein maps of the samples from the
four groups are shown in Fig. 2.
Approximately 270 spots were detected in one gel from brain cortex.
The spots that showed significantly different expression were
selected for further analysis. Eight spots were identified as key
proteins differentially expressed in four experimental groups.
Furthermore, in the vehicle-treated group, eight spots were
classified into two groups, including upregulated spots (five
spots) and downregulated spots (three spots), according to their
expression level in CMV/hSelM Tg rats. As shown in Table I, the five upregulated proteins
included creatine kinase B-type (B-CK), synaptotagmin-15 (SytXV),
E3 ubiquitin-protein ligase RING1 (RING1 finger protein 1), lactate
dehydrogenase B (LDH-B) and eukaryotic translation initiation
factor 4H (eIF-4H), whereas the three downregulated included
centromere protein N (CENP-N), proteasome subunit K and
dihydropyrimidinase-related protein 2 (DRP-2).
 | Table IList of the differentially expressed
proteins in the four experimental groups. |
Table I
List of the differentially expressed
proteins in the four experimental groups.
| Spot no. | Protein name | Gene name | Accession no. | Sequence coverage,
% | Mw, Da/pI | Mascot score | Vehicle | Selenium |
|---|
|
|
|---|
| Non-Tg | Tga | Non-Tga | Tga |
|---|
| 1 | Creatine kinase
B-type (EC 2.7.3.2) (Creatine kinase B chain) (B-CK) | Ckb | P07335 | 53 | 42698/5.39 | 124 | 1 | 3.07±0.31 | 2.19±0.18 | 3.87±0.25 |
| 2 | E3
ubiquitin-protein ligase RING1 (EC 6.3.2.-) (Polycomb complex
protein RING1) (RING finger protein 1) | Ring1 | Q6MGB6 | 23 | 42634/5.54 | 30 | 1 | 2.72±0.18 | 2.98±0.21 | 3.62±0.28 |
| 3 | Synaptotagmin-15
(Synaptotagmin XV) (SytXV) | Syt15 | P59926 | 21 | 47562/8.45 | 32 | 1 | 1.66±0.12 | 2.37±0.18 | 1.25±0.11 |
| 4 | L-lactate
dehydrogenase B chain (EC 1.1.1.27) (LDH-B) (LDH heart subunit)
(LDH-H) | Ldhb | P42123 | 14 | 36589/5.70 | 76 | 1 | 2.24±0.15 | 3.19±0.25 | 1.57±0.19 |
| 5 | Eukaryotic
translation initiation factor 4H (eIF-4H) (Williams-Beuren syndrome
chromosomal region 1 protein homolog) | Eif4h | Q5XI72 | 51 | 27307/6.67 | 26 | 1 | 1.78±0.22 | 1.10±0.29 | 1.09±0.13 |
| 6 | Proteasome subunit
alpha type-3 (EC 3.4.25.1) (Proteasome component C8) (Proteasome
subunit K) (Macropain subunit C8) (Multicatalytic endopeptidase
complex subunit C8) | Psma3 | P18422 | 58 | 28401/5.29 | 25 | 1 | 0.71±0.05 | 0.86±0.07 | 0.70±0.06 |
| 7 | Centromere protein
N (CENP-N) | Cenpn | Q5U2W4 | 31 | 39434/8.09 | 37 | 1 | 0.53±0.04 | 1.14±0.25 | 0.49±0.05 |
| 8 |
Dihydropyrimidinase-related protein 2
(DRP-2) (Turned on after division, 64 kDa protein) (TOAD-64)
(Collapsin response mediator protein 2) (CRMP-2) | Dpysl2 | P47942 | 32 | 62239/5.95 | 126 | 1 | 0.34±0.03 | 1.17±0.23 | 0.36±0.03 |
Following Sel treatment, the eight spots showed
different patterns. The five-upregulated spots were classified into
three groups according to their expression pattern. Two neighboring
spots in the first group showed a marked increase in response to
Sel treatment and were identified as B-CK and RING1 finger protein
1 (Fig. 3). The volume ratio of
these two spots was significantly higher in the CMV/hSelM Tg rats
compared to non-Tg rats. Following Sel treatment, these volumes
were markedly increased in the two groups relative to the
vehicle-treated group. Even though the volume of these two spots
increased in the Sel-treated group, they remained at a higher level
in the CMV/hSelM Tg rats compared to the non-Tg rats (Fig. 3). The next group consisted of two
spots that showed moderate changes in SytXV and LDH-B (Table I). The spot identified as SytXV
was expressed at higher levels in the CMV/hSelM Tg rats than non-Tg
rats that had received vehicle treatment. Sel treatment induced an
increase in spot volume in non-Tg rats, whereas it was
significantly decreased in CMV/hSelM Tg rats (Fig. 4). The spot isolated as LDH-B
showed a change similar to the spot of SytXV. However, the basic
level of these spots was higher in the CMV/hSelM Tg rats compared
to the non-Tg rats (Fig. 4). The
third group was not affected by Sel treatment and was only induced
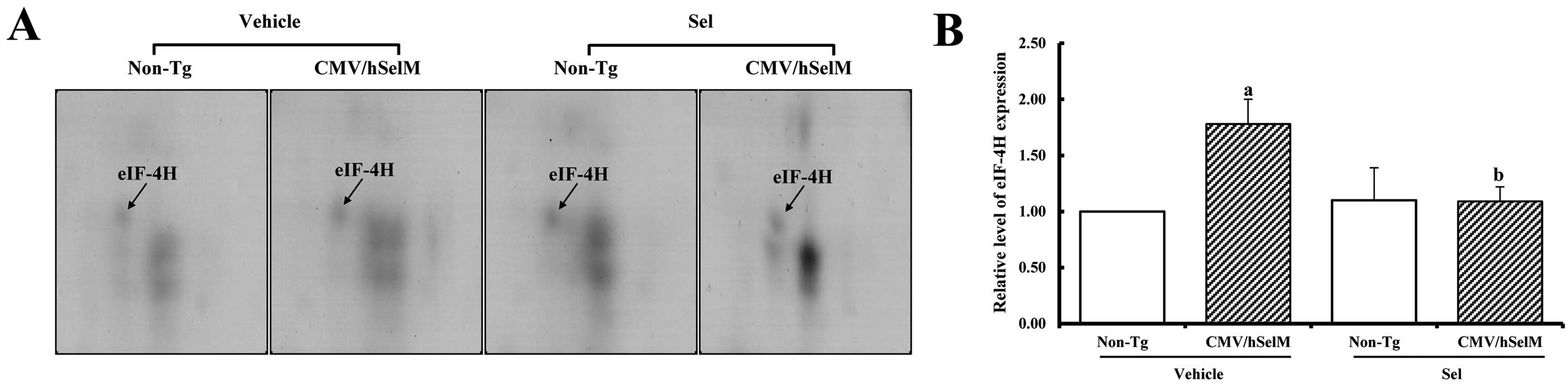
by SelM overexpression. Spot eIF-4H showed a significantly higher
level in CMV/hSelM Tg rats, but was significantly downregulated
following Sel treatment, whereas it remained unchanged in the
non-Tg rats (Fig. 5).
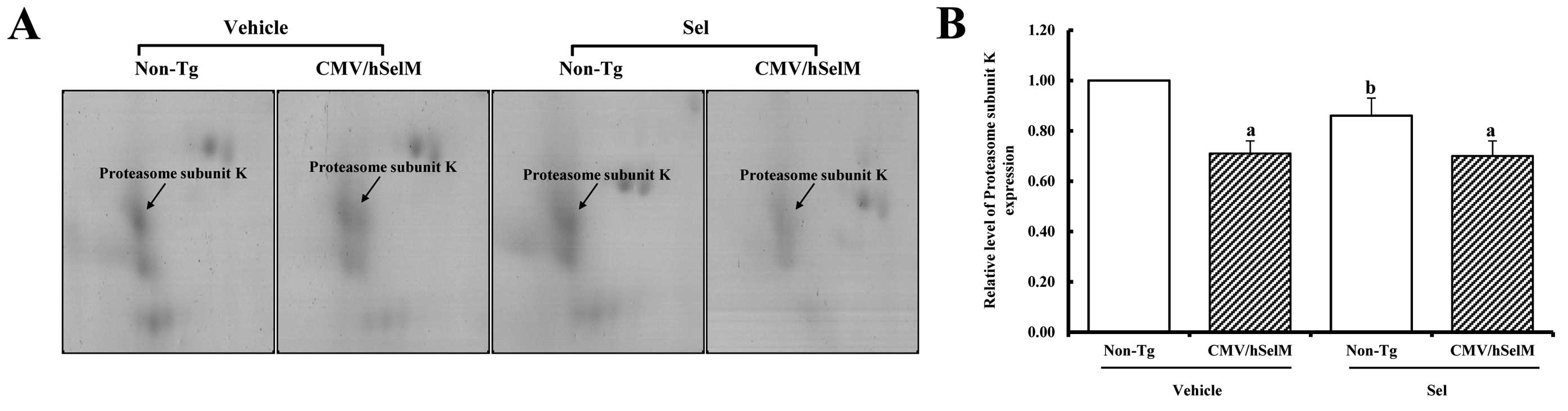
The group that showed downregulated SelM was further
classified into two subgroups. One large spot known as proteasome
subunit K belonged to the first subgroup. The volume of this spot
was lower in CMV/hSelM Tg rats than non-Tg rats under
vehicle-treated conditions. However, Sel treatment induced a
decrease in the proteasome subunit K level in non-Tg rats, whereas
it remained at a constant level in the CMV/hSelM Tg rats (Fig. 6). In the second subgroup, two
spots identified as CENP-N and DRP-2 showed similar patterns under
the two conditions (Table I),
with a lower level being observed for CMV/hSelM Tg rats than non-Tg
rats. These levels were not changed following Sel treatment in
either group (Fig. 7).
Confirmation of B-CK and SytXV
expression
Western blot analysis was conducted to validate the
changes in the protein expression levels of the two selected spots
(B-CK and SytXV) identified by 2-DE. As shown in Fig. 8, the expression of B-CK was
significantly higher in the CMV/hSelM Tg rats than non-Tg rats.
Following Sel treatment, its level increased further in the two
groups, although its total expression pattern was maintained. In
the case of SytXV, its expression level was higher in CMV/hSelM Tg
rats compared to non-Tg rats under vehicle treatment conditions.
However, expression of this protein increased significantly in
response to Sel treatment in non-Tg rats, whereas CMV/hSelM rats
showed no significant difference in expression. The expression
pattern of the above proteins observed upon western blot analysis
was extremely similar to that in the 2-DE gel image, indicating
that the alteration of protein spots detected by 2-DE reflects
changes in protein expression in the brains of the CMV/hSelM Tg and
non-Tg rats.
Discussion
Sel deficiency is associated with a variety of
serious diseases, including infectious disease, cardiovascular
disease, cancer and neurodegenerative disorders (7). Savaskan et al (29) provided direct evidence that Sel
plays a pivotal role in neuronal susceptibility to excitotoxic
lesions. The results of this study indicated that the
neuroprotective effects of Sel are not directly mediated via the
antioxidative effects of selenite, but require de novo
protein synthesis. Therefore, Sel deficiency in the brain tissue
leads to increased oxidative stress with subsequent NK-κB
activation and neuronal cell death. Several studies reported that
intracellular ETKs were activated by either sodium selenite, an
inorganic salt known to activate ERK (30), or transfected with a
constitutively active mutant of MEK1, an immediate upstream
activator of ERKR (31,32). The present study investigated
whether the high level of antioxidants induced by Sel treatment and
SelM overexpression affected the change in the cortex proteins of
CMV/hSelM Tg and non-Tg rats. The CMV/hSelM Tg rats used in the
study showed a higher level of antioxidant enzymes, such as SOD and
GPX, compared to non-Tg rats. Additionally, the concentration of
total oxidized products regulated by these enzymes was
significantly decreased in the brain cortex of the CMV/hSelM Tg
rats compared to non-Tg rats. These results indicate that the
antioxidant condition was induced by Sel treatment and SelM
overexpression in CMV/hSelM Tg rats. Furthermore, these findings
are concordant with those of our previous study (13).
Aβ-42 peptide in the cortex of the brain is closely
correlated with deposition at neuritic plaques in the pathogenesis
of AD (28,33). The production of these peptides is
tightly regulated by γ-secretase, which is composed of presenilin
and four additional cofactors, nicastrin, Aph-1, Pen-2 and TMP-21,
to create a multimeric protease complex (34,35). Thus far, limited studies
investigating the correlation of Sel and γ-secretase have been
conducted. The present study investigated whether the level of
γ-secretase activity could be changed by Sel treatment and SelM
overexpression in the brain of CMV/hSelM Tg rats. The results
showed that the γ-secretase activity significantly decreased in
response to Sel treatment. Therefore, the protein spot detected in
the study may be correlated with the production of Aβ-42 peptides
in CMV/hSelM Tg rats.
The study identified eight spots that were
differentially expressed among four groups, which were
vehicle-treated non-Tg, vehicle-treated CMV/hSelM Tg, Sel-treated
non-Tg and Sel-treated CMV/hSelM Tg rats. Among these spots,
creatine kinase was significantly upregulated in response to Sel
treatment and SelM overexpression. B-CK, one of the creatine
kinases, catalyzed the conversion of creatine to phosphocreatine,
which was expressed in various tissue types (36). Generally, this enzyme is known as
a buffering system of cellular ATP concentration and used as a
marker of several diseases, including myocardial infarction,
rhabdomyolysis, muscular dystrophy and renal failure (37). Decreased creatine kinase activity
was recently reported to be tightly associated with
neurodegenerative pathways in neurodegenerative diseases (38), ischemia (39) and other diseases (40,41). Creatine kinase and pyruvate kinase
are crucial for energy homeostasis and antioxidant defense through
inhibition of two enzyme activities (42). In the present study, the
expression of B-CK was higher in CMV/hSelM Tg rats overexpressing
SelM protein than non-Tg rats under vehicle-treated conditions. In
addition, Sel treatment induced an increase of this enzyme
expression in the two groups. Furthermore, this change pattern was
observed in the spot identified as RING1. This enzyme, which is
known as a Parkin, appeared to be part of the cell’s defense
against damage caused by environmental insults (43). This enzyme ubiquitinates itself
and promotes its own degradation, and the impairment of ubiquitin
ligase function may cause familial autosomal recessive PD.
Furthermore, oxidative and nitrosative stress were closely
associated with the ubiquitin ligase function of PD and the
associated α-synucleinopathy (44).
SytXV and LDH-B showed similar protein change
patterns on the 2-DE gels. Syt constitutes a family of
membrane-trafficking proteins that are characterized by an
N-terminal transmembrane region, a variable linker and two
C-terminal domains (45,46). This protein is known as a
Ca2+ sensor that regulates Ca2+-dependent
membrane trafficking, including endocrine exocytosis (47,48), synaptic vesicle exocytosis
(49,50), plasma membrane repair (51,52) and acrosomal reaction (53). Several studies have also
documented the correlation between neurotransmitter release and Syt
function (54,55). However, limited studies of whether
selenoprotein and Sel treatment were affected by SytXV expression
have been conducted thus far. In the present study, the expression
of this protein was significantly increased in response to SelM
overexpression compared to non-Tg rats. However, in the CMV/hSelM
Tg rats, Sel treatment did not induce a significant change in SytXV
expression in the brain cortex. These results indicate that SelM
overexpression could be affected by Ca2+-dependent
membrane trafficking through the regulation of Syt expression.
Another spot was identified as LDH-B, which catalyzes the
interconversion of pyruvate and lactate with concomitant
interconversion of NADH and NAD+ (56). Nazam Ansari et al (57) showed that serum lactate
dehydrogenase and GPX were significantly lower in rats with
ischemia. However, in the present study, LDH-B was increased by
SelM overexpression in CMV/hSelM Tg rats, but increased by Sel
treatment in non-Tg rats, indicating that selenoprotein and Sel
treatment are extremely important for normal brain function.
eIF-4H was the last member in the groups showing
upregulated expression in response to SelM overexpression. This
protein regulates protein synthesis through facilitating the
binding of initiator tRNA to ribosomes (58). Several stress-responding genes
induced by ultraviolet light, ER stress and reactive oxygen species
were tightly regulated by eIF2. Furthermore, an abnormally high
concentration of NO in the nervous system increases the eIF2α level
of phosphorylated forms, thus suppressing protein synthesis in
neurons (59). The eIF2α level of
the phosphorylated form was also increased in hippocampal CA1
neurons following ischemia (60).
In the present study, SelM overexpression induced increased eIF2α
expression, but Sel treatment did not induce any change in either
group.
The expression of CENP-N was extremely similar to
that of DRP-2 upon 2-DE analysis. CENP-N was first reported as a
member of three new human centromere proteins (CENP-M, CENP-N and
CENP-T), which comprised a CENP-A nucleosome-associated complex
(CENP-A NAC) (61). Additionally,
disruption of CENP-A NAC by reduction of these three proteins
induced a mitotic defect or significant mitotic delay in cells.
However, there have been no studies of the correlation between
CENP-N and antioxidant status. Therefore, to the best of our
knowledge, the present study is the first to indicate that SelM
overexpression led to a significant decrease in the expression of
CENP-N.
DRP-2 is turned on following division of the 64-kDa
protein or collapsing response mediator protein 2 (CRMP-2). This
protein is highly expressed in the adult brain, is an important
molecule in neurite outgrowth and axonal guidance, and plays a role
in several neurological diseases, including AD, epilepsy and
ischemia (62,63). Furthermore, a dramatic decrease of
intact CRMP-2 was observed following maitotoxin treatment,
N-methyl-D-asparatate treatment and ischemia induction,
accompanied by the appearance of distinct breakdown products of
CRMP-2 (63). The present study
showed that SelM overexpression led to significant induction of the
decrease of DRP-2 in CMV/hSelM Tg rats compared to non-Tg rats.
These results indicate that SelM could be referred to as a
regulator of DRP-2, although further study of the degradation
process is necessary.
All the aforementioned results showed that SelM
overexpression and Sel treatment play a crucial role in the
regulation of brain function via modulation of eight associated
proteins. In addition, our proteomic analysis allowed the
verification of the change in protein patterns of brain antioxidant
regulation. However, intensive study is required to define the role
and detailed mechanism of SelM and Sel in the cortex region of the
brain.
Acknowledgements
The authors wish to acknowledge the animal
technicians, S.M. Choi (BS) and Ms. J.L. Song, for directing the
Animal Facility and Care at the Division of Laboratory Animal
Resources. The present study was supported by the grants from the
KFDA. This study was supported by the Bio and Medical Technology
Development Program of the National Research Foundation (NRF)
funded by the Ministry of Science, ICT and Future Planning
(2012M3A9B6055344), and also supported by Bio-industry Technology
Development Program, Ministry of Agriculture, Food and Rural
Affairs (311054-03-3-HD110) to Professor Je Kyung Seong.
References
|
1
|
Wilber CG: Toxicology of selenium: a
review. Clin Toxicol. 17:171–230. 1980. View Article : Google Scholar
|
|
2
|
Smith AM and Picciano MF: Evidence for
increased selenium requirement for the rat during pregnancy and
lactation. J Nutr. 116:1068–1079. 1986.PubMed/NCBI
|
|
3
|
Yang GQ, Chen JS, Wen ZM, Ge KY, Zhu LZ,
Chen XC and Chen XS: The role of selenium in Keshan disease. Adv
Nutr Res. 6:203–231. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Rij AM, Thomson CD, McKenzie JM and
Robinson MF: Selenium deficiency in total parenteral nutrition. Am
J Clin Nutr. 32:2076–2085. 1979.PubMed/NCBI
|
|
5
|
Schwarz K and Foltz CM: Selenium as an
integral part of factor 3 against dietary necrotic liver
degeneration. J Am Chem Soc. 79:3292–3293. 1957. View Article : Google Scholar
|
|
6
|
Reddy PG, Morill JL, Minocha HC and
Stevenson JS: Vitamin E is immunostimulatory in calves. J Dairy
Sci. 70:993–999. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Birringer M, Pilawa S and Flohé L: Trends
in selenium biochemistry. Nat Prod Rep. 19:693–718. 2002.
View Article : Google Scholar
|
|
8
|
Carlson BA, Novoselov SV, Kumaraswamy E,
Lee BJ, Anver MR, Gladyshev VN and Hatfield DL: Specific excision
of the selenocysteine tRNA[Ser]Sec (Trsp) gene in mouse liver
demonstrates an essential role of selenoproteins in liver function.
J Biol Chem. 279:8011–8017. 2004.
|
|
9
|
Berry MJ, Banu L, Chen YY, Mandel SJ,
Kieffer JD, Harney JW and Larsen PR: Recognition of UGA as a
selenocysteine codon in type I deiodinase requires sequences in the
3′ untransalated region. Nature. 353:273–276. 1991.PubMed/NCBI
|
|
10
|
Kryukov GV, Castellano S, Novoselov SV,
Lobanov AV, Zehtab O, Guigó R and Gladyshev VN: Characterization of
mammalian selenoproteomes. Science. 300:1439–1443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Korotkov KV, Novoselov SV, Hatfield DL and
Gladyshev VN: Mammalian selenoprotein in which selenocysteine (Sec)
incorporation is supported by a new form of Sec insertion sequence
element. Mol Cell Biol. 22:1402–1411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Müller WE, Borejko A, Brandt D, et al:
Selenium affects biosilica formation in the demosponge Suberites
domuncula. Effect on gene expression and spicule formation. FEBS J.
272:3838–3852. 2005.PubMed/NCBI
|
|
13
|
Hwang DY, Sin JS, Kim MS, et al:
Overexpression of human selenoprotein M differentially regulates
the concentrations of antioxidants and H2O2,
the activity of antioxidant enzymes, and the composition of white
blood cells in a transgenic rat. Int J Mol Med. 21:169–179.
2008.PubMed/NCBI
|
|
14
|
Chen J and Berry MJ: Selenium and
selenoproteins in the brain and brain diseases (Review). J
Neurochem. 86:1–12. 2003. View Article : Google Scholar
|
|
15
|
Ramaekers VT, Calomme M, Vanden Berghe D
and Makropoulos W: Selenium deficiency triggering intractable
seizures. Neuropediatrics. 25:217–223. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imam SZ, el-Yazal J, Newport GD, Itzhak Y,
Cadet JL, Slikker W Jr and Ali SF: Methamphetamine-induced
dopaminergic neurotoxicity: role of peroxynitrite and
neuroprotective role of antioxidants and peroxynitrite
decomposition catalysts. Ann NY Acad Sci. 939:366–380. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zafar KS, Siddiqui A, Sayeed I, Ahmad M,
Salim S and Islam F: Dose-dependent protective effect of selenium
in rat model of Parkinson’s disease: neurobehavioral and
neurochemical evidences. J Neurochem. 84:438–446. 2003.PubMed/NCBI
|
|
18
|
Takizawa S, Matsushima K, Shinohara Y,
Ogawa S, Komatsu N, Utsunomiya H and Watanabe K:
Immunohistochemical localization of glutathione peroxidase in
infarcted human brain. J Neurol Sci. 122:66–73. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saijoh K, Saito N, Lee MJ, Fujii M,
Kobayashi T and Sumino K: Molecular cloning of cDNA encoding a
bovine selenoprotein P-like protein containing 12 selenocysteines
and a (His-Pro) rich domain insertion, and its regional expression.
Brain Res Mol Brain Res. 30:301–311. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim IY, Shin JH and Seong JK: Mouse
phenogenomics, toolbox for functional annotation of human genome.
BMB Rep. 43:79–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schomburg L, Schweizer U, Holtmann B,
Flohé L, Sendtner M and Köhrle J: Gene disruption discloses role of
selenoprotein P in selenium delivery to target tissues. Biochem J.
370:397–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill KE, Zhou J, McMahan WJ, Motley AK,
Atkins JF, Gesteland RF and Burk RF: Deletion of selenoprotein P
alters distribution of selenium in the mouse. J Biol Chem.
278:13640–13646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang DY, Seo SJ, Kim YK, et al: Selenium
acts as an insulin-like molecule for the downregulation of diabetic
symptoms via endoplasmic reticulum stress and insulin signalling
proteins in diabetes-induced non-obese diabetic mice. J Biosci.
32:723–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yim SY, Chae KR, Shim SB, et al: ERK
activation induced by selenium treatment significantly
downregulates beta/gamma-secretase activity and Tau phosphorylation
in the transgenic rat overexpressing human selenoprotein M. Int J
Mol Med. 24:91–96. 2009.
|
|
25
|
Goo JS, Kim YN, Choi KM, et al: Proteomic
analysis of kidneys from selenoprotein M transgenic rats in
response to increased bioability of selenium. Clin Proteomics.
10:102013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park JY, Seong JK and Paik YK: Proteomic
analysis of diet-induced hypercholesterolemic mice. Proteomics.
4:514–523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim BH, Park EY, Yoo KH, Choi KM, Kim Y,
Seong Jk and Park JH: N-myc downstream-regulated gene 1 is involved
in the regulation of cystogenesis in transgenic mice overexpressing
human PKD2 gene. Proteomics. 13:134–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Selkoe DJ: Alzheimer’s disease: genes,
proteins, and therapy. Physiol Rev. 81:741–766. 2001.
|
|
29
|
Savaskan NE, Bräuer AU, Kühbacher M, et
al: Selenium deficiency increases susceptibility to
glutamate-induced excitotoxicity. FASEB J. 17:112–114. 2003.
|
|
30
|
Hu H, Jiang C, Li G and Lü J: PKB/AKT and
ERK regulation of caspase-mediated apoptosis by methylseleninic
acid in LNCaP prostate cancer cells. Carcinogenesis. 26:1374–1381.
2005. View Article : Google Scholar
|
|
31
|
Jaaro H, Rubinfeld H, Hanoch T and Seger
R: Nuclear translocation of mitogen-activated protein kinase kinase
(MEK1) in response to mitogenic stimulation. Proc Natl Acad Sci
USA. 94:3742–3747. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seger R, Seger D, Reszka AA, et al:
Overexpression of mitogen-activated protein kinase kinase (MAPKK)
and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement
in cellular proliferation is regulated by phosphorylation of serine
residues in its kinase subdomains VII and VIII. J Biol Chem.
269:25699–25709. 1994.
|
|
33
|
Kim SK, Park HJ, Hong HS, Baik EJ, Jung MW
and Mook-Jung I: ERK1/2 is an endogenous negative regulator of the
gamma-secretase activity. FASEB J. 20:157–159. 2006.PubMed/NCBI
|
|
34
|
Iwatsubo T: The gamma-secretase complex:
machinery for intramembrane proteolysis. Curr Opin Neurobiol.
14:379–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen F, Hasegawa H, Schmitt-Ulms G, et al:
TMP21 is a presenilin complex component that modulates
gamma-secretase but not epsilon-secretase activity. Nature.
440:1208–1212. 2006. View Article : Google Scholar
|
|
36
|
Bessman SP and Carpenter CL: The
creatine-creatine phosphate energy shuttle. Annu Rev Biochem.
54:831–862. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schnyder T, Winkler H, Gross H,
Eppenberger HM and Wallimann T: Crystallization of mitochondrial
creatine kinase. Growing of large protein crystals and electron
microscopic investigation of microcrystals consisting of octamers.
J Biol Chem. 266:5318–5322. 1991.
|
|
38
|
David S, Shoemaker M and Haley BE:
Abnormal properties of creatine kinase in Alzheimer’s disease
brain: correlation of reduced enzyme activity and active site
photolabeling with aberrant cytosol-membrane partitioning. Brain
Res Mol Brain Res. 54:276–287. 1998.PubMed/NCBI
|
|
39
|
Tomimoto H, Yamamoto K, Homburger HA and
Yanagihara T: Immunoelectron microscopic investigation of creatine
kinase BB-isoenzyme after cerebral ischemia in gerbils. Acta
Neuropathol. 86:447–455. 1993.PubMed/NCBI
|
|
40
|
Gross WL, Bak MI, Ingwall JS, Arstall MA,
Smith TW, Balligand JL and Kelly RA: Nitric oxide inhibits creatine
kinase and regulates rat heart contractile reserve. Proc Natl Acad
Sci USA. 93:5604–5609. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hamman BL, Bittl JA, Jacobus WE, Allen PD,
Spencer RS, Tian R and Ingwall JS: Inhibition of the creatine
kinase reaction decreases the contractile reserve of isolated rat
hearts. Am J Physiol. 269:H1030–H1036. 1995.PubMed/NCBI
|
|
42
|
Rech VC, Feksa LR, Fleck RM, Athaydes GA,
Dornelles PK, Rodrigues-Junior V and Wannmacher CM: Cysteamine
prevents inhibition of thiol-containing enzymes caused by cystine
or cystine dimethylester loading in rat brain cortex. Metab Brain
Dis. 23:133–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Steece-Collier K, Maries E and Kordower
JH: Etiology of Parkinson’s disease: genetics and environment
revisited. Proc Natl Acad Sci USA. 99:13972–13974. 2002.
|
|
44
|
Dawson TM: Parkin and defective
ubiquitination in Parkinson’s disease. J Neural Transm Suppl.
70:209–213. 2006.
|
|
45
|
Fukuda M and Mikoshiba K:
Synaptotagmin-like protein 1–3: a novel family of C-terminal-type
tandem C2 proteins. Biochem Biophys Res Commun. 281:1226–1233.
2001.
|
|
46
|
Fukuda M, Saegusa C and Mikoshiba K: Novel
splicing isoforms of synaptotagmin-like proteins 2 and 3:
identification of the Slp homology domain. Biochem Biophys Res
Commun. 283:513–519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sugita S, Shin OH, Han W, Lao Y and Südhof
TC: Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors
with distinct Ca(2+) affinities. EMBO J. 21:270–280. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fukuda M, Kanno E, Ogata Y, Saegusa C, Kim
T, Loh YP and Yamamoto A: Nerve growth factor-dependent sorting of
synaptotagmin IV protein to mature dense-core vesicles that undergo
calcium-dependent exocytosis in PC12 cells. J Biol Chem.
278:3220–3226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chapman ER: Synaptotagmin: a Ca(2+) sensor
that triggers exocytosis? Nat Rev Mol Cell Biol. 3:498–508. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mikoshiba K, Fukuda M, Moreira JE, Lewis
FMT, Sugimori M, Niinobe M and Llinás R: Role of the C2A domain of
synaptotagmin in transmitter release as determined by specific
antibody injection into the squid giant synapse preterminal. Proc
Natl Acad Sci USA. 92:10703–10707. 1995. View Article : Google Scholar
|
|
51
|
Detrait ER, Yoo S, Eddleman CS, Fukuda M,
Bittner GD and Fishman HM: Plasmalemmal repair of severed neurites
of PC12 cells requires Ca(2+) and synaptotagmin. J Neurosci Res.
62:566–573. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Reddy A, Caler EV and Andrews NW: Plasma
membrane repair is mediated by Ca(2+)-regulated exocytosis of
lysosomes. Cell. 106:157–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Michaut M, De Blas G, Tomes CN, Yunes R,
Fukuda M and Mayorga LS: Synaptotagmin VI participates in the
acrosome reaction of human spermatozoa. Dev Biol. 235:521–529.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schiavo G, Osborne SL and Sgouros JG:
Synaptotagmins: more isoforms than functions? Biochem Biophys Res
Commun. 248:1–8. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fukuda M and Mikoshiba K: The function of
inositol high polyphosphate binding proteins. Bioessays.
19:593–603. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Philibert RA, Nelson JJ, Sandhu HK, Crowe
RR and Coryell WH: Association of an exonic LDHA polymorphism with
altered respiratory response in probands at high risk for panic
disorder. Am J Med Genet B Neuropsychiatr Genet. 117:11–17. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nazam Ansari M, Bhandari U, Islam F and
Tripathi CD: Evaluation of antioxidant and neuroprotective effect
of ethanolic extract of Embelia ribes Burm in focal cerebral
ischemia/reperfusion-induced oxidative stress in rats. Fundam Clin
Pharmacol. 22:305–314. 2008.PubMed/NCBI
|
|
58
|
Kim HS, Choi Y, Shin KY, et al: Swedish
amyloid precursor protein mutation increases phosphorylation of
eIF2alpha in vitro and in vivo. J Neurosci Res.
85:1528–1537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Petrov T, Underwood BD, Braun B, Alousi SS
and Rafols JA: Upregulation of iNOS expression and phosphorylation
of eIF-2alpha are paralleled by suppression of protein synthesis in
rat hypothalamus in a closed head trauma model. J Neurotrauma.
18:799–812. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hayashi T, Saito A, Okuno S, et al:
Oxidative damage to the endoplasmic reticulum is implicated in
ischemic neuronal cell death. J Cereb Blood Flow Metab.
23:1117–1128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Foltz DR, Jansen LE, Black BE, Bailey AO,
Yates JR 3rd and Cleveland DW: The human CENP-A centromeric
nucleosome-associated complex. Nat Cell Biol. 8:458–469. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang Z, Ottens AK, Sadasivan S, Kobeissy
FH, Fang T, Hayes RL and Wang KK: Calpain-mediated collapsin
response mediator protein-1, -2, and -4 proteolysis after
neurotoxic and traumatic brain injury. J Neurotrauma. 24:460–472.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chung MA, Lee JE, Lee JY, Ko MJ, Lee ST
and Kim HJ: Alteration of collapsin response mediator protein-2
expression in focal ischemic rat brain. Neuroreport. 16:1647–1653.
2005. View Article : Google Scholar : PubMed/NCBI
|