Introduction
Organic nitrates are a class of drugs that have been
clinically used in the treatment of myocardial ischemia, as well as
congestive heart failure for more than a hundred years. Nitrates
release nitric oxide (NO), an endothelium-derived relaxing factor,
executing a broad range of functions, including antioxidant,
anti-inflammatory, antithrombotic and anti-atherogenic effects
(1). Furthermore, NO has been
shown to ameliorate the aging of cells (2), and small interfering RNA (siRNA)
targeting endothelial NO synthase (eNOS) have been shown to reduce
the anti-senescence effects of insulin (3). However, whether organic nitrates, a
source of NO production, play a protective role against the
senescence of bone marrow-derived mesenchymal stem cells (MSCs),
which have potential for the repair of damaged tissue (4), remains to be clarified.
We have previously reported that MSCs are a superior
stem cell source for cellular treatment and biomedical engineering
due to their unique paracrine and immunosuppressive properties
(5). However, cell aging, which
can be induced by various pathophysiological states, including high
glucose (HG), oxidative stress and inflammatory attack, can
severely impair the therapeutic potential of MSCs in tissue
regerenation (6). Therefore, the
investigation of possible interventions to attenuate MSC senescence
and the further investigation of the potential mechanisms involved
is of critical clinical significance for patients with diabetes
accompanied by organ injury, currently awaiting the regeneration of
‘viable’ MSCs (7,8).
The results of previous studies have led us to
hypothesize that organic nitrates may prevent the onset of MSC
senescence. Lauer et al (9) demonstrated that the lack of nitrite
in the plasma was mainly responsible for the inability of exercise
to reverse age-dependent endothelial dysfunction. Ward et al
(10) also noted that the
overexpression of eNOS, which results in endogenous NO production,
boosted the capability of bone marrow cells, leading to a
substantial improvement in left ventricular ejection fraction at 6
weeks. Accordingly, in the present study, we examined the effects
of isosorbide dinitrate (ISDN), a commonly used organic nitrate, on
the senescence of MSCs triggered by HG. The characteristics of
cellular aging, such as increased expression of
senescence-associated galactosidase (SA-β-gal), cell enlargement
and the upregulation of p21 expression, were investigated. In
addition, the mRNA expression of the typical senescence-associated
cellular biomarkers (11),
including DNA methyltransferase 1 (DNMT1) and differentiated embryo
chondrocyte expressed gene 1 (DEC1) was detected to characterize
growth arrest. We also investigated the underlying mechanisms
responsible for the effects of ISDN based on the knowledge of the
key roles of ERK phosphorylation (12) and forkhead box M1 (FOXM1)
regulation (13) in cell cycle
modulation, and further clarified the association between them.
Considering that cellular senescence has recently been implicated
in regulation of microRNAs (miRNAs or miRs) (14), crucial factors of diverse
pathophysiological processes, we wished to explore the potential
role of senescence-associated miRNAs in the effects of ISDN on MSC
senescence.
Materials and methods
All animal treatments were performed in strict
accordance with the Guidelines for the Care and Use of Laboratory
Animals published by the National Academy Press (NIH Publication
no. 85–23, revised 1996). The study was approved by the Animal Care
and Use Committee of Zhongshan Hospital, Fudan University.
Isolation and culture of MSCs
Bone marrow-derived MSCs were purified from
4-week-old male Sprague-Dawley (SD) rats as previously described
(15). Briefly, the bone marrow
in the tibias and femurs was flushed using Dulbecco’s modified
Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA). Following
incubation in DMEM supplemented with 10% fetal bovine serum (FBS;
Gibco) at 37°C in an atmosphere of 5% CO2 for 24 h, the
bone marrow solution was discarded, and the adherent cells were
subsequently cultured. Subsequently, the spindle-shaped, adherent
MSCs were isolated and expanded, and the culture medium was
regularly changed every 3–4 days. Passage 4 MSCs, which were
previously demonstrated to express typical MSC-related cell surface
antigens (15), were used in the
subsequent experiments.
Experimental manipulation of MSCs
Native MSCs were starved for serum and glucose
overnight and then exposed to medium with HG or ISDN (both from
Sigma-Aldrich, St. Louis, MO, USA) at the indicated concentrations
or mannitol (33 mM) for 24 h. The untreated MSCs served as the
control group and those exposed to mannitol served as the negative
control group. To examine the effects of ISDN on cell aging, the
starved MSCs were treated with ISDN (50 μM) for 6 h prior to HG
treatment. To explore the underlying mechanisms responsible for the
effects of ISDN, the MSCs were incubated with PD98059 (ERK
inhibitor, 20 μM; Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA) or DMSO (dimethyl sulfoxide; Sigma-Aldrich) for 30 min prior
to ISDN treatment or were subjected to FOXM1 knockdown using siRNA,
followed by subsequent treatment with ISDN and HG.
Western blot analysis
After the indicated treatments, the MSCs were lysed
in RIPA lysis buffer containing 50 mM Tris-HCl (pH 7.4), 1% NP-40,
1 mM phenylmethylsulphonyl fluoride, 150 mM NaCl, 1 mM EDTA and 1%
sodium deoxycholate. Protein lysates were separated by 10% SDS-PAGE
and transferred onto polyvinylidene fluoride membranes. The
membranes were washed 3 times with TBS (pH 7.6) buffer, soaked in
5% non-fat dry milk for 2 h and incubated overnight at 4°C with
anti-p21 (#2947), anti-phosphorylated (p-)ERK (#4370) (both from
Cell Signaling Technology, Danvers, MA, USA; both diluted 1:1,000)
and anti-FOXM1 (sc-271746; Santa Cruz Biotechnology Inc.)
polyclonal antibodies (diluted 1:300), followed by incubation with
a horseradish peroxidase-conjugated secondary antibody (diluted
1:5,000) for 2 h at room temperature. The immune complexes were
visualized using enhanced chemiluminescence detection reagents, and
the band intensity was measured, quantified and analyzed using an
Image System (Bio-Rad, Hercules, CA, USA). The β-actin (#8457; Cell
Signaling Technology) band intensity served as the control for p21
and FOXM1 expression; similarly, total-ERK (#4695; Cell Signaling
Technology) served as the control for p-ERK expression.
Reverse transcription-quantitative
(real-time) polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cultured MSCs using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Spectrophotometric
OD260 measurements were used to determine the 2 μg RNA
input for cDNA synthesis and cDNA was generated using a High
Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster
City, CA, USA) as recommended by the manufacturer. Real-time PCR
was performed using the SYBR® ExScript™ PCR kit (Takara
Biochemicals, Kyoto, Japan) in a total volume of 10 μl in a Bio-Rad
iQ5 optical module. The primer sequences used for the genes
(DNMT1, DEC1 and β-actin) were synthesized and
are listed as follows: DNMT1 forward, 5′-GTG AAG GAG
AAA TTG AAT CTC TT-3′ and reverse, 5′-GAG GAA GCT GCT
AAG GAC-3′; DEC1 forward, 5′-CCA GGA AAC CAT
TGG ACT CAG -3′ and reverse, 5′-AGA GGT CGG ATA CCA
GCA TTT-3′; β-actin forward, 5′-CCA TTG AAC
ACG GCA TTG-3′ and reverse, 5′-TAC GAC CAG AGG CAT
ACA-3′. The PCR amplification consisted of 40 cycles (95°C
for 5 sec, 59.5°C for 30 sec) following an initial denaturation at
95°C for 30 sec. Melting curves were obtained at the end of the
reaction by stepwise increases in temperature of 1°C/min from 59.5
to 95°C over a period of 35 min. The threshold cycle (Ct) value was
defined as the fractional cycle number at which the fluorescence
passed a fixed threshold. The fold -change in target mRNA
expression was calculated using the 2−ΔΔCt method
following normalization to β-actin expression.
For microRNA detection, total RNA was extracted from
the MSCs using the mirVana™ miRNA Isolation kit (Ambion, Austin,
TX, USA) according to the manufacturer’s instructions and reverse
transcribed into cDNA using the PrimeScript RT reagent kit (Takara
Biochemicals). miR-130a/b was quantified using TaqMan®
MicroRNA Assay kits (Applied Biosystems) with U6 small nuclear RNA
as an endogenous control. All PCR reactions were performed in
triplicate.
RNA interference
FOXM1 and control siRNA (scramble) were purchased
from Santa Cruz Biotechnology, Inc. After the MSCs were transfected
with FOXM1 or control siRNA using Lipofectamine™ 2000 (Invitrogen)
as previously described by Wang et al (16), the cells were collected and
processed for subsequent analysis by western blot analysis and
quantitative PCR. The untreated MSCs served as the control
group.
SA-β-gal assay
MSC senescence was determined based on SA-β-gal
activity, which was measured using a β-galactosidase staining kit
(BioVision, Palo Alto, CA, USA) following the manufacturer’s
instructions. Briefly, the treated MSCs were washed in PBS, fixed
in 0.5 μl of fixative solution for 10–15 min at room temperature,
and incubated with the staining solution mix overnight at 37°C.
Green-stained cells and total cells were then counted under a
microscope, and the percentage of β-galactosidase-positive and
enlarged cells was calculated.
miR-130b silencing by antagomiR-based
treatment
Chemically modified antisense oligonucleotides
(antagomiR-130b; RiboBio, Guangzhou, China) have been previously
used to inhibit miR-130b expression in MSCs in vitro
(17). Antagomir oligonucleotides
were transfected into the MSCs at doses of 100 nmol/l using
Lipofectamine 2000 (Invitrogen) according to the manufacturer’s
instructions. As the controls, an unrelated negative control (NC;
RiboBio) was transfected into the MSCs. Transfection efficiencies
were determined by quantitative PCR, and the cells were processed
for subsequent intervention or analysis on day 1 following
transfection.
Statistical analysis
The results are expressed as the means ± standard
error of the mean (SEM). Statistical analysis, which included an
unpaired Student’s t-test for comparisons between 2 groups and
analysis of variance (ANOVA) with Bonferroni’s correction for
multiple comparisons, was performed using SPSS software (version
14; SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
HG induces senescence in MSCs
To determine the effects of HG on MSC senescence, we
performed a dose-effect experiment. Upregulated p21 expression,
decreased DNMT1 mRNA expression and increased DEC1 mRNA expression
were used as markers to evaluate cellular senescence. Incubation
with HG for 24 h significantly enhanced p21 expression in the MSCs
in a concentration-dependent manner by 155.3% (P<0.05) and
268.1% (P<0.01) in the 15 and 33 mM HG groups, respectively,
compared with the control group; however, culture with mannitol at
an osmotic pressure equal to that provided by 33 mM HG had no
significant pro-senescence effects (P>0.05) on MSCs (Fig. 1A and B).
Quantitative PCR confirmed the effects of 33 mM HG
on MSC senescence, as indicated by a 43.9% decrease (P<0.05) in
DNMT1 mRNA expression and a 346.2% increase in DEC1 mRNA expression
(P<0.01) compared with the control group (Fig. 1C and D). Analogously, the control,
mannitol, did not have a notable effect on the mRNA expression of
either DNMT1 or DEC1. Based on these results and those of a
previous study (18), we selected
the HG dose of 33 mM to establish an experimental model of MSC
senescence in the subsequent experiments.
ISDN treatment reduces HG-induced MSC
senescence
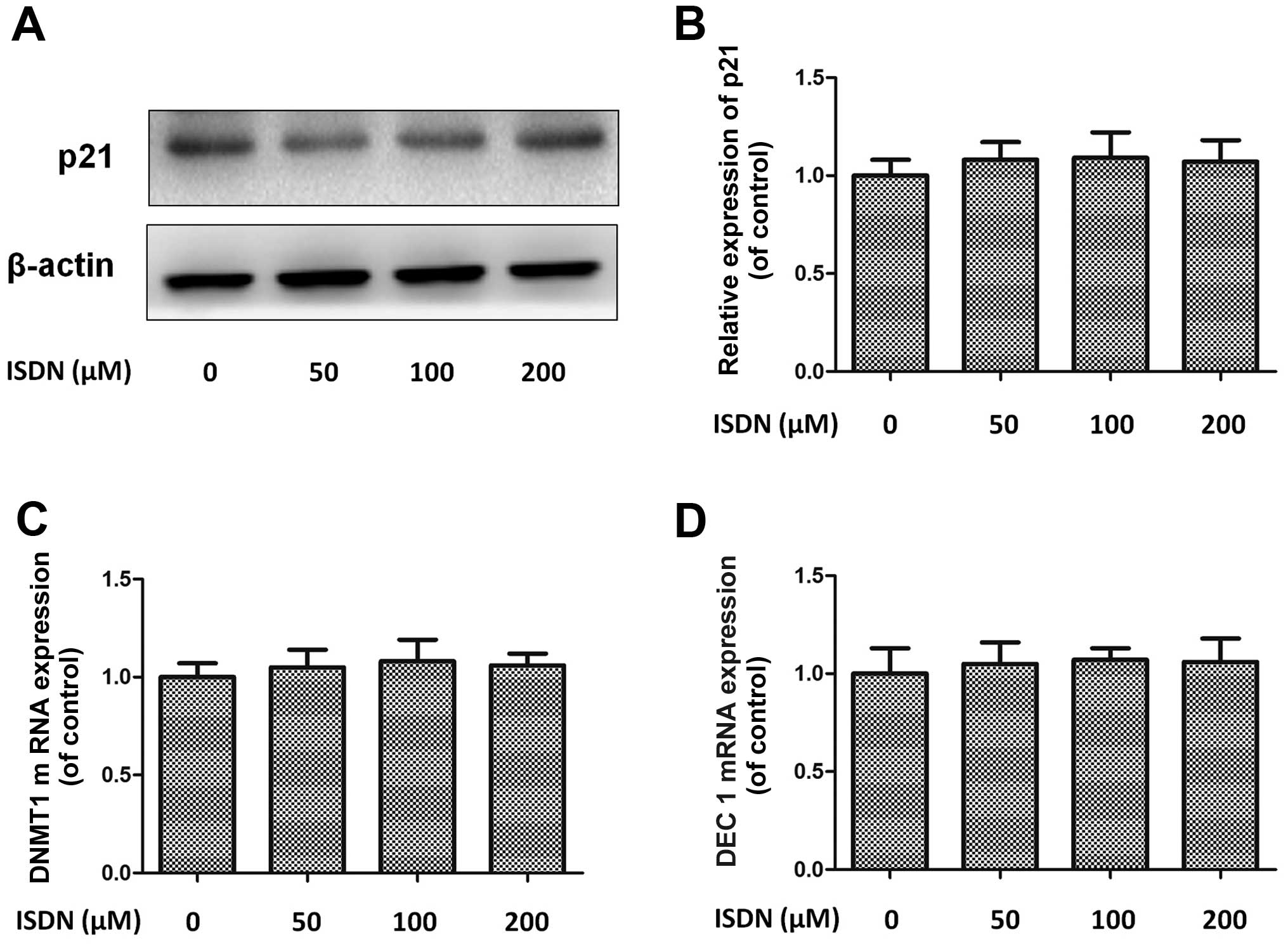
To observe the effect of ISDN pre-treatment on
HG-induced MSC senescence, the MSCs were first cultured with ISDN
at a concentration of 50, 100 or 200 μM for 24 h to determine the
appropriate intervention dose of ISDN. At these concentrations,
ISDN did not exert a significant effect on MSC senescence compared
with the control group, as evidenced by p21 expression and DNMT1
and DEC1 mRNA expression (all P>0.05) (Fig. 2).
Subsequently, the MSCs were incubated with ISDN (50
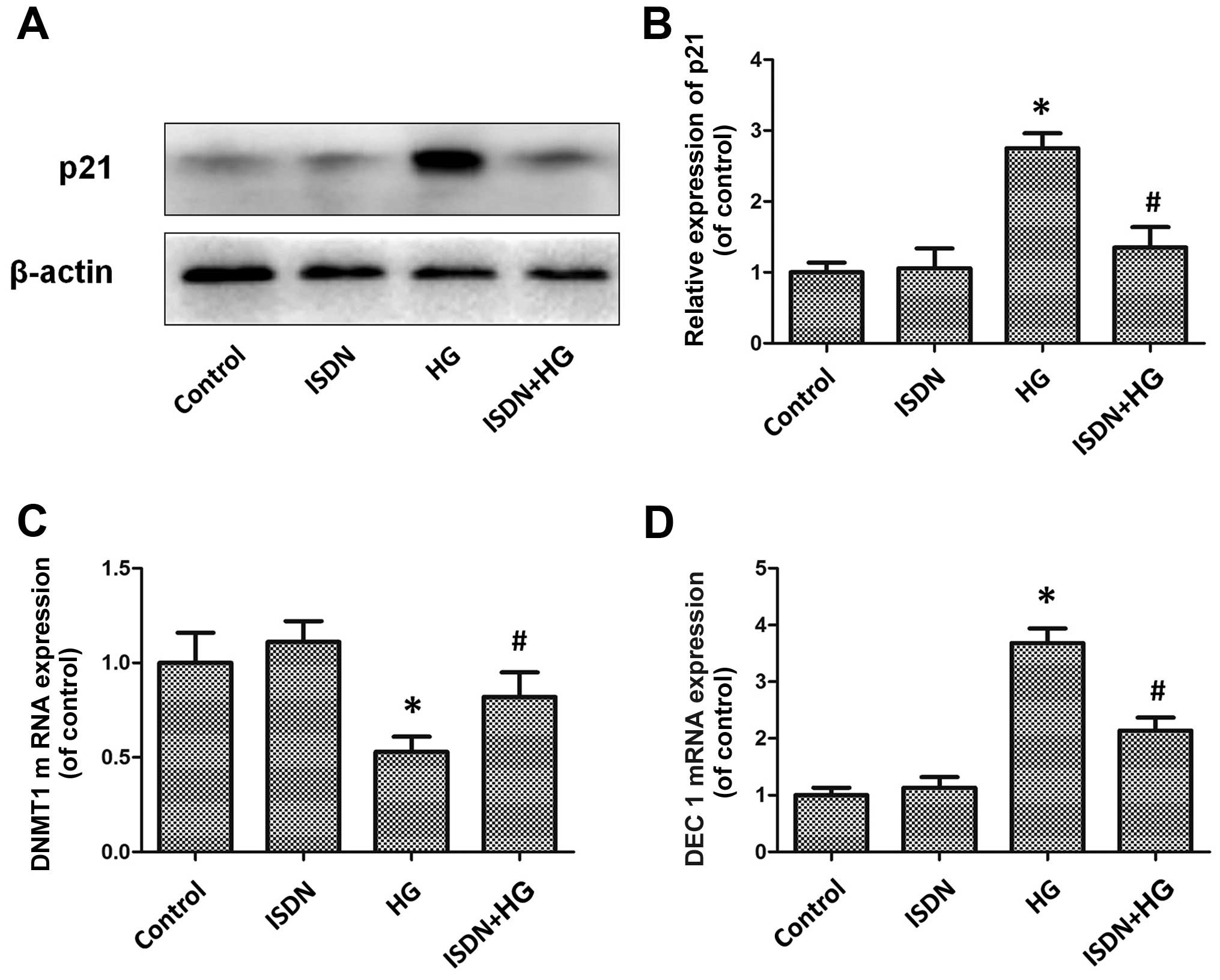
μM) for 6 h and then cultured with 33 mM HG for an additional 24 h.
Western blot analysis demonstrated that although incubation with 50
μM ISDN did not affect p21 expression [ISDN group vs. control
(untreated) group, 1.06±0.28 vs. 1.00±0.14; P>0.05], treatment
with 33 mM HG significantly upregulated p21 expression in the MSCs
[HG group vs. control (untreated) group, 2.75±0.21 vs. 1.00±0.14;
P<0.05], which was markedly attenuated after pre-conditioning
with ISDN (ISDN + HG group vs. HG group, 1.35±0.29 vs. 2.75±0.21;
P<0.05) (Fig. 3A and B). DNMT1
mRNA expression was significantly increased in the ISDN + HG group
compared to the HG group (0.82±0.13 vs. 0.53±0.08, P<0.05)
(Fig. 3C); DEC1 mRNA expression
was decreased in the ISDN + HG group compared to the HG group
(2.14±0.23 vs. 3.68±0.26, P<0.05) (Fig. 3D).
The ERK/FOXM1 pathway is involved in the
protective effects of ISDN against MSC senescence
The ERK/FOXM1 pathway plays a key role in cellular
senescence (19), and thus we
investigated changes occurring in this pathway in MSCs following
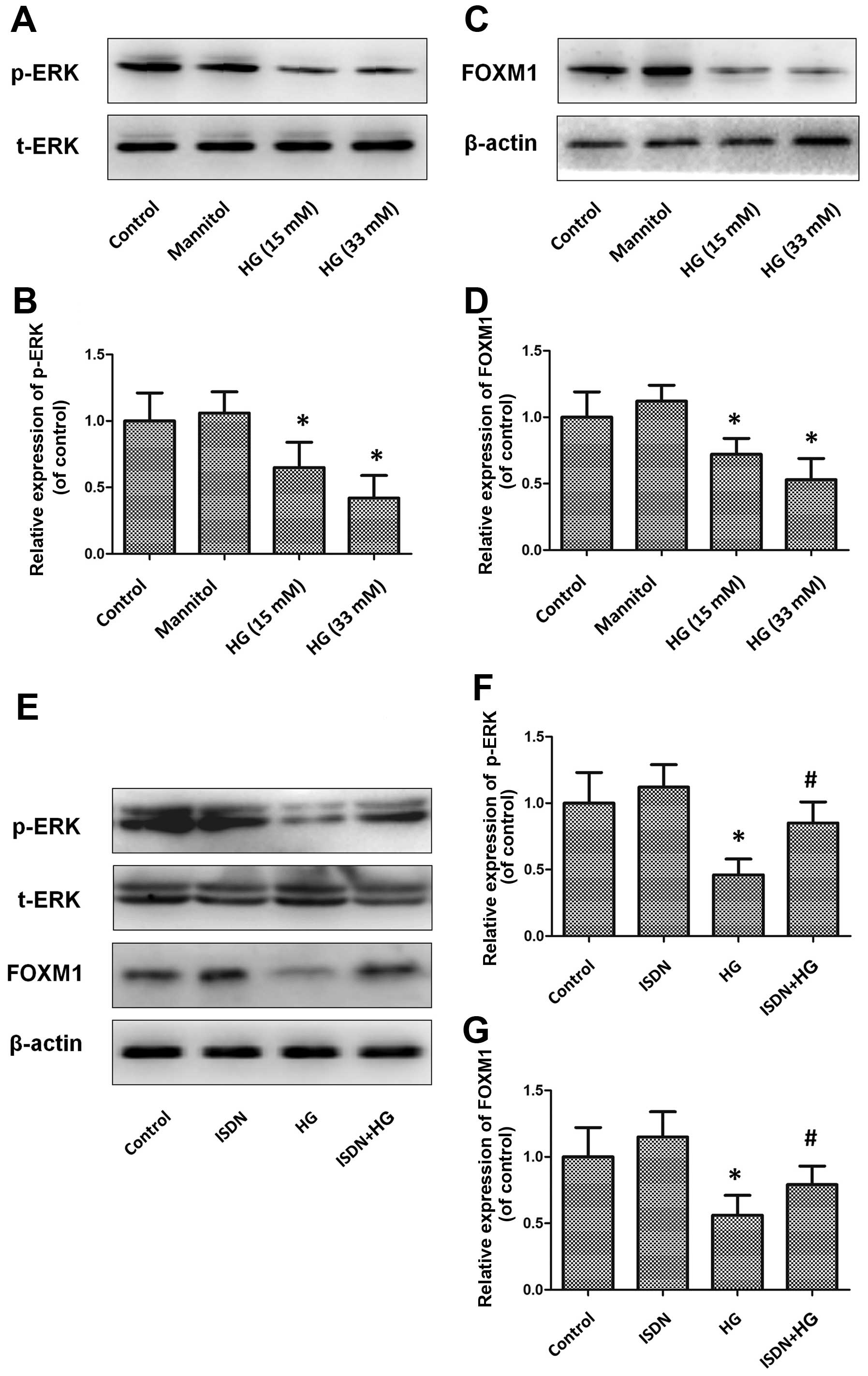
treatment with HG with or without pre-incubation with ISDN. HG
induced the downregulation of ERK phosphorylation and FOXM1
expression in a dose-dependent manner (all P<0.05) (Fig. 4A–D). At a concentration of 33 mM,
HG decreased ERK phosphorylation and FOXM1 expression by 53.8 and
43.9%, respectively, compared with the control (untreated) group
(both P<0.05); however, the suppressive effects on the ERK/FOXM1
pathway were markedly reversed by pre-incubation with ISDN (ERK
phosphorylation: ISDN + HG group vs. HG group, 0.85±0.16 vs.
0.46±0.12, P<0.05; FOXM1 expression: ISDN + HG group vs. HG
group, 0.79±0.14 vs. 0.56±0.15, P<0.05) (Fig. 4E–G).
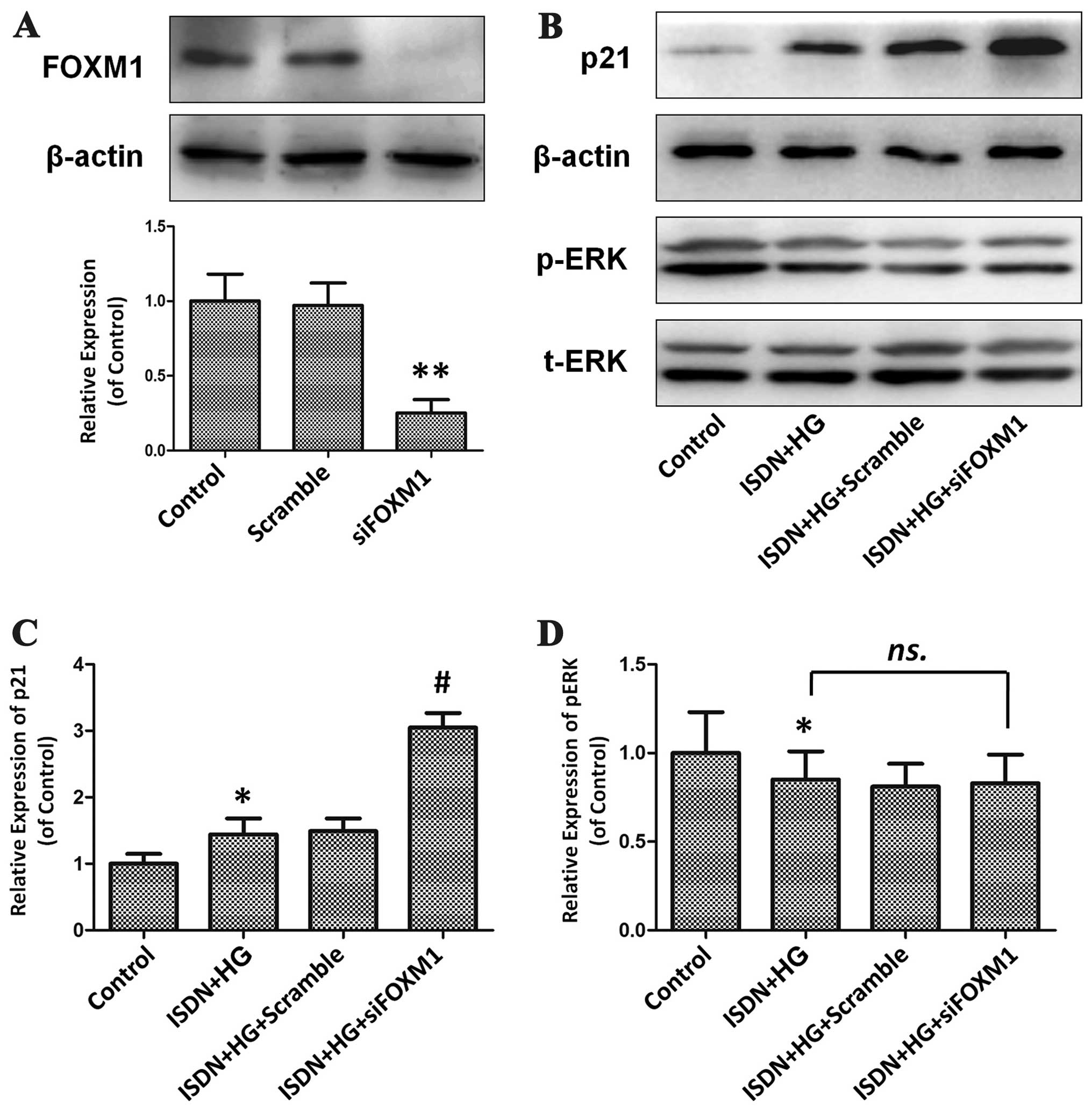
Activation of the ERK/FOXM1 pathway
mediates the effects of ISDN on MSC senescence
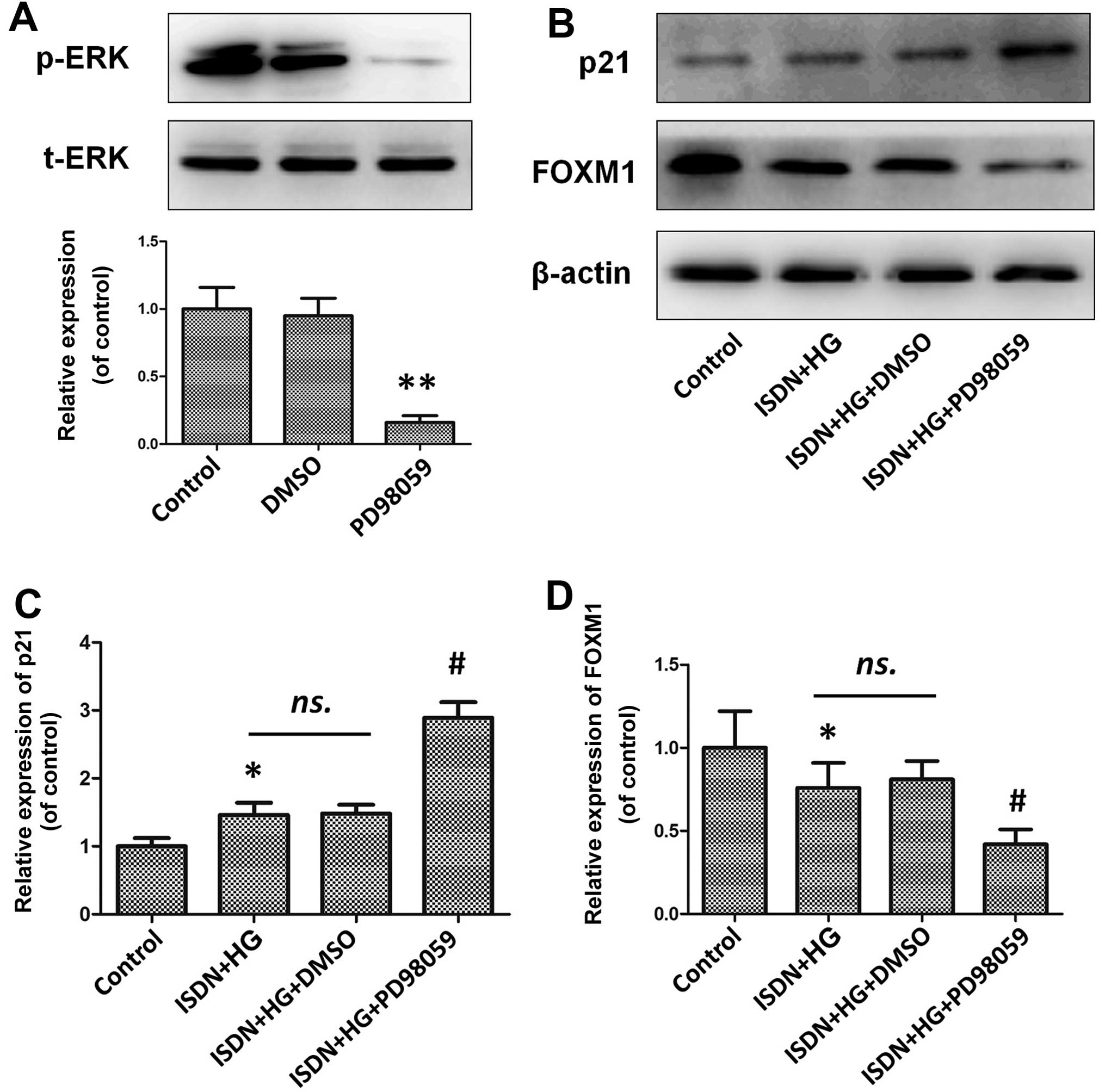
To determine the role of the ERK/FOXM1 pathway in
the effects of ISDN on HG-induced MSC senescence, PD98059, a
specific inhibitor of the ERK pathway, and siRNA were used to
inhibit the activity of the ERK/FOXM1 pathway (Figs. 5A and 6A). PD98059 effectively inhibited ERK
phosphorylation by 83.6% compared with the control (untreated)
group (P<0.01), which significantly inhibited the beneficial
effects of ISDN on MSC senescence, as indicated by the upregulation
of p21 expression in the ISDN + HG + PD98059 group compared with
the ISDN + HG group (2.89±0.23 vs. 1.46±0.18, P<0.05) (Fig. 5B and C). Similarly, following the
knockdown of FOXM1 in the MSCs by 74.8% using RNA interference, the
protective effects of ISDN on HG-induced MSC senescence were
significantly attenuated, as indicated by a comparison of the ISDN
+ HG + siFOXM1 and ISDN + HG groups; there was an increase in p21
expression in the ISDN + HG + siFOXM1 group compared with the ISDN
+ HG group (3.05±0.22 vs. 1.44±0.24, P<0.05) (Fig. 6B and C).
A senescence-associated SA-β-gal assay was performed
to verify the beneficial effects of ISDN on MSC senescence as
revealed above. A higher percentage of senescent MSCs with a
typically flattened and enlarged cell shape accompanied by SA-β-gal
positivity was observed in the HG group compared to the control
group (P<0.05) (Fig. 7A and
B). Of note, pre-incubation with ISDN markedly decreased the
HG-induced MSC senescence (ISDN + HG group vs. HG group, 10.9±3.72
vs. 32.8±6.83%; P<0.05). However, treatment with PD98059
significantly attenuated the effects of ISDN on MSC aging,
indicated by the increased number of SA-β-gal-positive MSCs
(29.4±5.81 vs. 10.9±3.72%, P<0.05) (Fig. 7A and B), as well as the decrease
in DNMT1 mRNA expression (0.45±0.05 vs. 0.75±0.09, P<0.05)
(Fig. 7C), and the increase in
DEC1 mRNA expression (3.78±0.18 vs. 2.26±0.21, P<0.05) (Fig. 7D) in the ISDN + HG + PD98059 group
compared with the ISDN + HG group. Accordingly, FOXM1 knockdown
markedly abolished the effects of ISDN in MSC senescence, which was
indicated by the increase in the number of MSCs with SA-β-gal
positivity (28.3±5.25 vs. 10.9±3.72%, P<0.05) (Fig. 7A and B), as well as the decrease
in DNMT1 mRNA expression (0.42±0.08 vs. 0.75±0.09, P<0.05)
(Fig. 7C), and an increase in
DEC1 mRNA expression (3.64±0.24 vs. 2.26±0.21, P<0.05) (Fig. 7D) in the ISDN + HG + siFOXM1 group
compared with the ISDN + HG group.
Upregulation of miR-130b is associated
with the activation of the ERK/FOXM1 pathway
The key regulatory role of the miR-130 family,
including miR-130a and miR-130b, in cellular growth arrest has
previously been demonstrated (14). In this study, we therefore
examined the potential involvement of miR-130a and miR-130b in the
response to HG treatment and the effects of ISDN. HG gradually
suppressed miR-130b expression (all P<0.05) in a
concentration-dependent manner, but had no detectable effect on
miR-130a expression (all P>0.05) (Fig. 8A and B). Pre-treatment with ISDN
reversed the decrease in miR-130b expression induced by HG
stimulation (ISDN + HG group vs. HG group, 0.75±0.11 vs. 0.39±0.07;
P<0.05), but had no apparent effect on miR-130a expression (ISDN
+ HG group vs. HG group, 0.92±0.13 vs. 0.93±0.11; P>0.05)
(Fig. 8C and D). Furthermore,
inhibition of the ERK/FOXM1 pathway by either PD98059 or FOXM1
siRNA substantially abolished the recovery of miR-130b expression
following pre-treatment with ISDN (ISDN + HG + PD98059 group vs.
ISDN + HG group: 0.32±0.09 vs. 0.75±0.11, P<0.05; ISDN + HG +
siFOXM1 group vs. ISDN + HG group: 0.35±0.08 vs. 0.75±0.11,
P<0.05) (Fig. 8E and F).
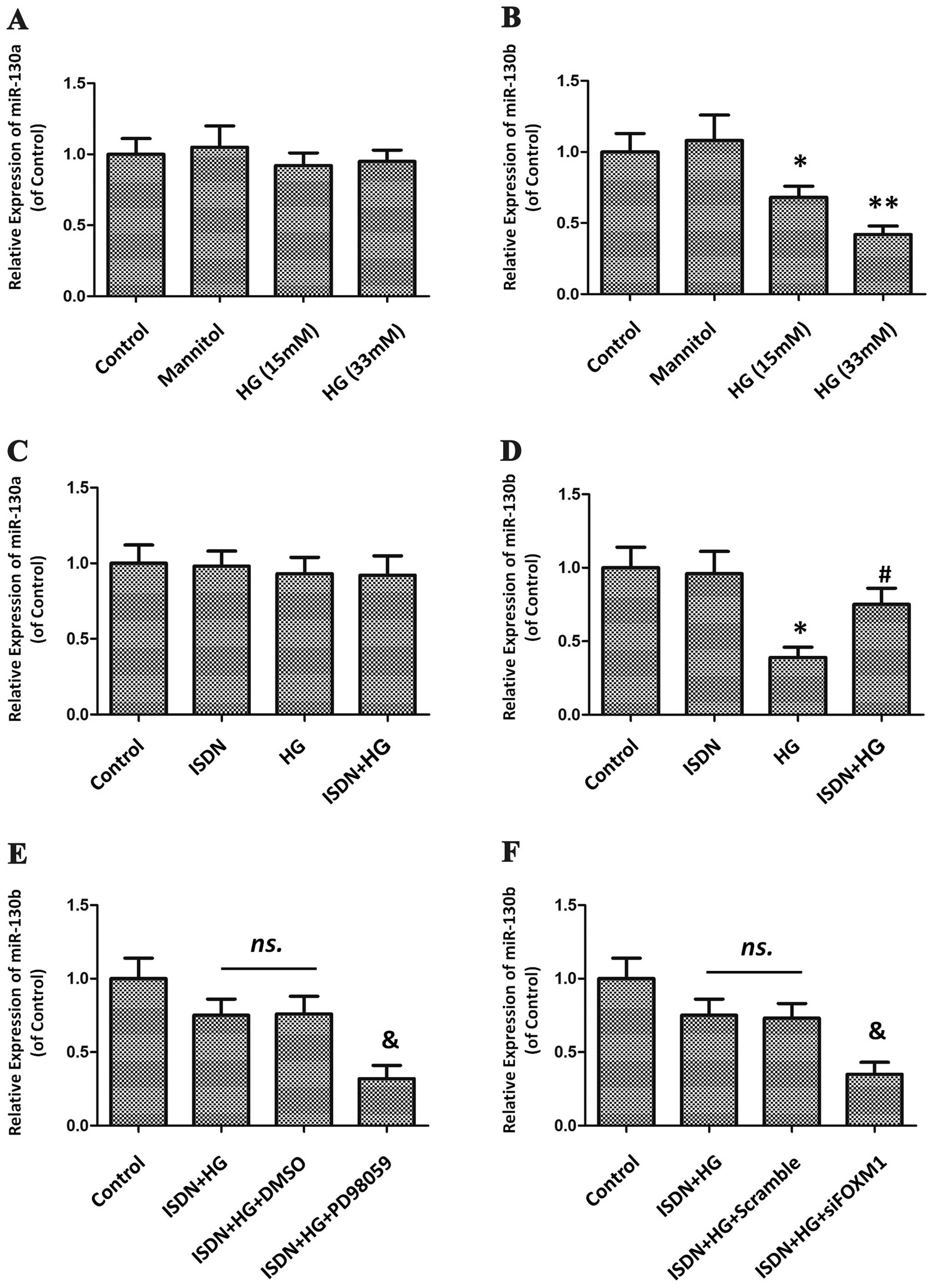 | Figure 8miR-130b, not miR-130a, is
upregulated by the activation of the ERK/FOXM1 cascade during the
protection of high glucose (HG)-induced mesenchymal stem cell (MSC)
senescence by isosorbide dinitrate (ISDN). (A and B) MSCs were
exposed to HG at progressively increasing concentrations for 24 h
or to mannitol (33 mM), which served as a negative control with an
osmotic pressure equal to that of 33 mM HG. The expression of the
miR-130 family, including miR-130a and -130b, was determined by
quantitative PCR and is shown in the histogram. The data are
presented as the means ± standard error of the mean (SEM); n=3 in
each experiment. *P<0.05, **P<0.01 vs.
the control group. (C and D) MSCs were incubated with or without
ISDN (50 μM) for 6 h, followed by HG treatment (33 mM) for an
additional 24 h. The expression of miR-130a/b was analyzed by
quantitative PCR, and the results are presented in the histogram.
The data are presented as the means ± standard error of the mean
(SEM); n=3 in each experiment. *P<0.05 vs. the
control group; #P<0.05 vs. the HG group. (E and F)
MSCs were cultured with PD98059 (20 μM) for 30 min prior to ISDN
treatment (50 μM, 6 h), followed by HG treatment (33 mM, 24 h).
Alternatively, MSCs were treated with PD98059 or subjected to FOXM1
knockdown by siRNA and then treated with ISDN and HG. miR-130b
expression was evaluated by quantitative PCR, and the results are
shown in the bar diagram. The data are presented as the means ±
SEM; n=3 in each experiment. ns., not significant;
&P<0.05 vs. the ISDN + HG group. |
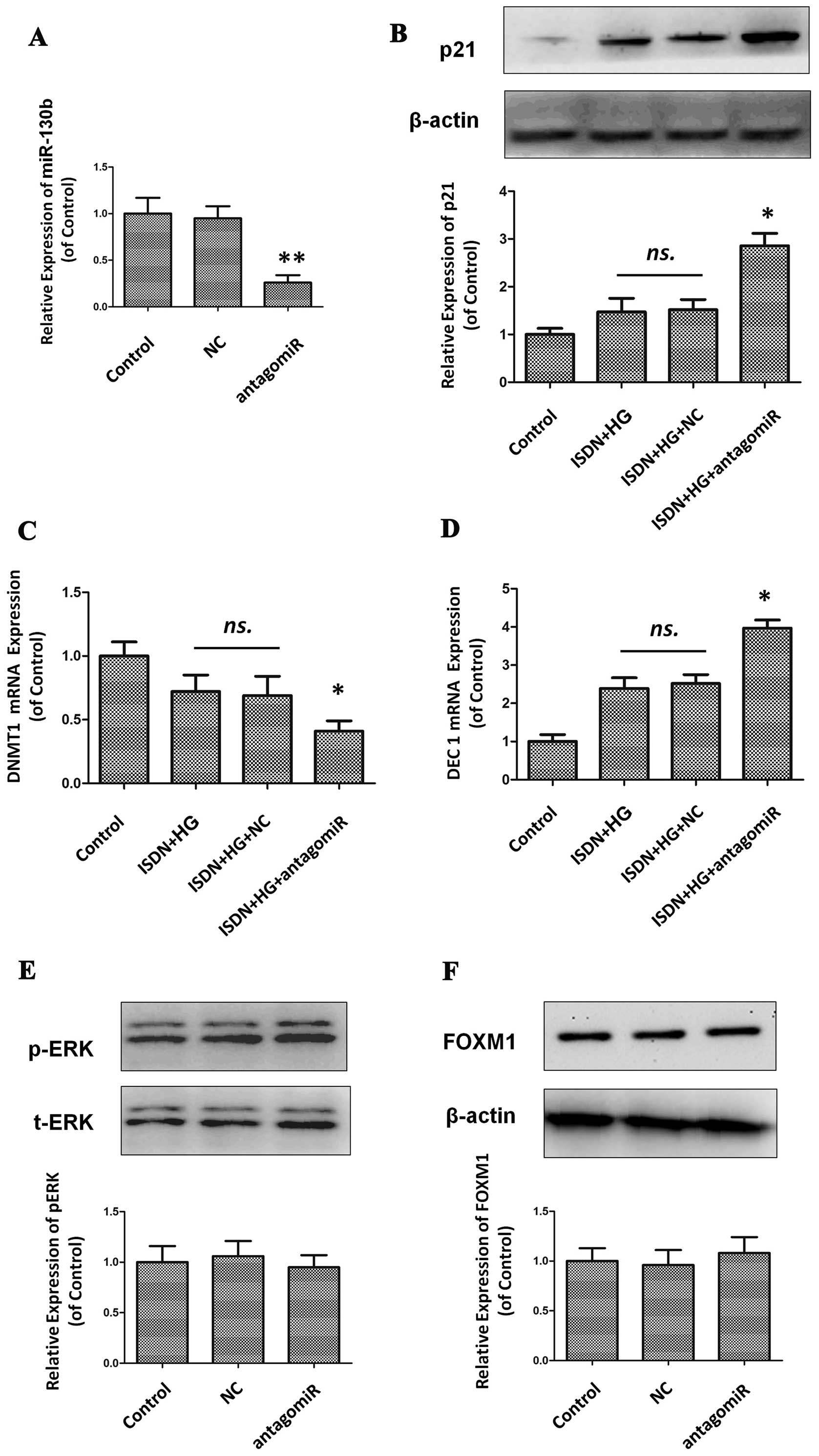
miR-130b is the key mediator of the
effects of ISDN on MSC senescence
To determine whether miR-130b plays a key role in
the protective effects of ISDN against MSC senescence, miR-130b was
knocked down using antagomiR transfection and senescence-associated
assays were then performed. It was found that after miR-130b
expression was effectively downregulated by 74.3% compared to the
control (Fig. 9A), the beneficial
effects of ISDN on MSC senescence were markedly abolished, which
was confirmed by the analysis of p21 expression (1.47±0.29 vs.
2.86±0.26, P<0.05), DNMT1 mRNA expression (0.72±0.13 vs.
0.41±0.08, P<0.05) and DEC1 mRNA expression (2.39±0.28 vs.
3.97±0.21, P<0.05) (Fig. 9B–D)
when comparing the ISDN + HG group with the ISDN + HG + antagomiR
group.
ERK phosphorylation and FOXM1 activity were examined
following the downregulation of miR-130b, indicating that there
were no signficant changes between the control group and the
antagomiR group, both in phosphorylated ERK (1.00±0.16 vs.
0.95±0.12, P>0.05) and in FOXM1 expression (1.00±0.13 vs.
1.08±0.16, P>0.05) (Fig. 9E and
F).
Discussion
This study aimed to investigate the effects of ISDN
on HG-induced MSC senescence, demonstrating that ISDN
preconditioning decreases SA-β-Gal activity in MSCs, decreases the
expression of p21 protein and DEC1 mRNA, and increases the
expression of DNMT1 mRNA, consistent with our original assumption
that ISDN inhibits MSC aging triggered by HG. The activation of the
ERK/FOXM1 pathway plays a pivotal role in this pathophysiological
process, as evidenced by the attenuation of the favourable effects
of ISDN by either the inhibition of ERK phosphorylation or the
knockdown of FOXM1 in the MSCs. As a senescence-associated
modulator, miRNA-130b was identified to function downstream of the
ERK/FOXM1 pathway, collectively contributing to the protective
effects of ISDN against MSC aging.
Diabetic patients frequently suffer from micro- or
macrovascular abnormalities, including neuropathy, nephropathy,
retinopathy and accelerated ischemic heart diseases, which mobilize
MSCs to repair and regenerate damaged tissue. Although there is a
complex condition which induces MSC growth arrest, it is
undoubtedly considered that HG is the primary trigger. Accordingly,
HG incubation, not oxidative stress or ultraviolet B radiation
(UVB) treatment, was developed to establish a cellular senescence
model in MSCs, which may better simulate the true
pathophysiological state in diabetes mellitus (DM) (20), and may have great practical
implications for the treatment of patients with DM accompanied by
its various complications.
Organic nitrates are one of the most common types of
drugs used for the long-term treatment of acute coronary syndrome
and acute or chronic congestive heart failure, as well as coronary
heart diseases (21). The release
of NO is the main function of ISDN (22) and leads to the activation of
soluble guanylate cyclase and the reduction of the intracellular
calcium content, subsequently resulting in the relaxation of target
cells, primarily the vascular smooth muscle (23). However, a comprehensive
understanding of the mechanisms of action of organic nitrates,
particularly other non-hemodynamic effects on other various cells,
has not yet been established (24). Given the critical role of MSCs in
cardiovascular diseases, we wished to determine whether treatment
with ISDN affects the biological characteristics of MSCs.
Senescent MSCs exhibit a significantly reduced
ability to self-renew, growth is terminated at an early stage at
approximately 40–50 population doublings, and a decline in
differentiation potential and proliferation rate is observed
(25). Furthermore, senescent
MSCs provide a weak protective effect due to the decreased activity
of repair and antioxidant enzymes in aged MSCs, resulting in the
unsatisfactory effects of MSC-based treatments for tissue repair
(26). Several pathophysiological
states, such as HG and oxidative stress, are considered critical
contributors to cell senescence, as well as predominant risks or
accompanying factors in cardiac ischemia, which is regularly
treated with organic nitrates (27). Therefore, our identification of
ISDN as a modulator of MSC senescence may provide insight into the
protective mechanisms of organic nitrates in cardiovascular
diseases. However, an investigation using DM models in vivo,
comparing MSC senescence before and after receiving organic nitrate
treatment, is required in order to further confirm the in
vitro above findings.
The ERK/FOXM1 pathway is involved in cell cycle
regulation and senescence modulation (12,13). However, whether it accelerates or
delays cell aging is dependent on the cell type and
pathophysiological conditions. Ling et al (28) observed that the activated ERK
phosphorylation and upregulated FOXM1 expression were essential for
the protective effects of low-power laser irradiation against
NIH3T3 cell senescence induced by UVB. By contrast, Wang et
al (19) demonstrated that
the N terminus of ASPP2 binds to Ras, enhancing Ras/Raf/MEK/ERK
activation to facilitate oncogene-induced senescence; Zeng et
al (29) discovered that
FOXM1 is upregulated in gastric cancer and that its inhibition led
to termination of the cell cycle at the G0/G1 phase, partially
dependent on p27 kip1. In light of these results, we examined
changes in the activities of ERK and FOXM1 in HG-treated MSCs and
in response to the protective effects of ISDN. HG significantly
suppressed ERK phosphorylation and FOXM1 expression, which were
both attenuated by ISDN preconditioning. Furthermore, the
inhibition of ERK phosphorylation or the knockdown of FOXM1
attenuated the favourable effects of ISDN on MSC aging, indicating
that ERK and FOXM1 are key mediators of the effects of ISDN. While
the inhibition of ERK phosphorylation markedly decreased the
upregulation of FOXM1, the downregulation of FOXM1 did not exert a
significant effect on ERK activity, revealing the regulatory
association between these two proteins.
Considering the crucial function of miRNAs in cell
biology, we also investigated the senescence-associated miRNAs
based on the review by Abdelmohsen et al (14), including those that elicit their
actions through the pRB/p16 pathway (miR-106, miR-130 and miR-24)
or the p53 pathway (miR-34), affect senescence-associated secretory
phenotypes (SASP) (miR-146) and modulate other senescence
regulatory proteins (miR-29, miR-30 and miR-519). As demonstrated
by quantitative PCR (data not shown), we determined that only
miR-130b and not miR-130a is involved in HG-induced MSC aging, as
well as the subsequent ISDN protective effects. Subsequently, we
examined miR-130b expression following the suppression of the
ERK/FOXM1 cascade and hence deduced that miR-130b functions
downstream of this pathway. Based on previous studies, it has been
demonstrated that miR-130b inhibits the expression of p21, which is
regarded to be antagonistic to p53/p21 pathway activity, leading to
a recovery of growth arrest in proliferative cells (30,31). Borgdorff et al (32) established an important role for
the cyclin-dependent kinase (CDK) inhibitor, p21 (Waf1/Cip1), in
the growth control of human mammary epithelial cells (HMECs) and
analyzed the repertoire of miRNAs that modulate the activity of
this tumor suppressor, demonstrating that miR-130b reduced p21
expression and in turn blocked Ras (G12V)-induced senescence. They
further revealed that the overexpression of miR-130b rescued HMECs
from Ras-induced senescence through the prevention of Ras-induced
upregulation of p21, verifying the key role of miR-130b during
proliferative cell senescence, which powerfully supported the
underlying mechanisms found in our study. Considering that the
regulation of miRNAs differs depending on cell type, we wished to
determine whether the inhibition of miR-130b reduces the protective
effects of ISDN on MSC senescence. We therefore silenced miR-130b
expression in MSCs, revealing that miR-130b downregulation markedly
attenuated the advantageous effects of ISDN on MSC senescence,
which was indicated by the detection of p21 expression and the mRNA
expression of DNMT1 and DEC1. Furthermore, we revealed that both
ERK phosphorylation and FOXM1 expression were not significantly
affected by miR-130b suppression, together with our prior finding
that the suppression of either ERK phosphorylation or FOXM1
expression markedly reduced miR-130b expression, collectively
indicating that miR-130b resides downstream of the ERK/FOXM1
pathway. However, the exploration of the role of miR-130b in MSC
senescence in the current study is preliminary, and further
research focusing on the presumed target of miR-130b is required,
including the prediction of informatics algorithm, luciferase
report assay and functional complementary experiments. We aim to
resolve these issues in our future research, which may broaden our
understanding of the association between MSC senescence and miRNA
modulation.
Support for our hypothesis can also be found in
previous studies of the pharmacological function of organic
nitrates on cell senescence or growth arrest. Huang et al
(33) demonstrated that
nitroglycerine (NTG) within a concentration range of 0.1–10 μmol/l
notably increased BrdU incorporation into human MSCs in a
dose-dependent manner, suggesting that NTG enhances the cell growth
rate and activity. The authors further determined that the
promotion of NO release in MSCs by NTG was a key mechanism
underlying this effect, suggesting that organic nitrate stimulates
NO production in MSCs, leading to the increased cell viability.
Moreover, the use of organic nitrates in phytophysiology (34) has indicated that nitrate
limitation or starvation markedly induces leaf senescence based on
a combined 15N tracing/proteomics analysis (35) and SAG12-transgenic plant
construction (36). In addition,
it has been reported that the long-term inhibition of NO synthase
by L-arginine analogues, such as N(ω)-nitro-l-arginine methyl ester
(L-NAME), accelerates vascular senescence in vitro and
systemic hypertension and arteriosclerosis in vivo (37). Furthermore, it has been
demonstrated that pre-treatment with various Chinese traditional
medicines promotes the renovation of
H2O2-stimulated senescence in human umbilical
endothelial cells via nitrate production (38); and that elevated NO production,
which can also be promoted by organic nitrates, in MSCs
overexpressing eNOS enhances cellular activity and migration
through the upregulation of stromal cell-derived factor-1α
(39), which conformably
evidenced the significance and reasonability of our novel
finding.
In conclusion, to the best of our knowledge, the
present study provides evidence that ISDN exerts protective effects
agaisnt HG-induced MSC senescence, and that the underlying
mechanisms involve the activation of the ERK/FOXM1 pathway and the
upregulation of miR-130b. The data presented in this study may
prove to be of clinical value for patients with DM accompanied by
diverse clinical complications.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (project nos. 81100145,
81370003, 81300082, 81470467 and 81370322), the National Science
Foundation for Post-doctoral Scientists of China (project nos.
2013M531124 and 2014T70391), the Medical Guiding Foundation of
Science and Technology Commission of Shanghai (project no.
124119a7701), the Chinese Medical Doctor Association Foundation for
Cardiovascular Research (project nos. DFCMDA201259 ands
DFCMDA201255), the Key Specialty Construction of Medical Program in
Shanghai (ZK2012A24) and the National Basic Research Program of
China (973 Program, project no. 2012CB518605).
References
|
1
|
Sung YH, Shin MS, Ko IG, et al:
Ulinastatin suppresses lipopolysaccharide-induced prostaglandin E-2
synthesis and nitric oxide production through the downregulation of
nuclear factor-κB in BV2 mouse microglial cells. Int J Mol Med.
31:1030–1036. 2013.PubMed/NCBI
|
|
2
|
Navascues J, Perez-Rontome C, Gay M, et
al: Leghemoglobin green derivatives with nitrated hemes evidence
production of highly reactive nitrogen species during aging of
legume nodules. Proc Natl Acad Sci USA. 109:2660–2665. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsui-Hirai H, Hayashi T, Yamamoto S, et
al: Dose-dependent modulatory effects of insulin on glucose-induced
endothelial senescence in vitro and in vivo: a relationship between
telomeres and nitric oxide. J Pharmacol Exp Ther. 337:591–599.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ransohoff JD and Wu JC: Imaging stem cell
therapy for the treatment of peripheral arterial disease. Curr Vasc
Pharmacol. 10:361–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu J, Qian J, Xie X, et al: High density
lipoprotein cholesterol promotes the proliferation of bone-derived
mesenchymal stem cells via binding scavenger receptor-B type I and
activation of PI3K/Akt, MAPK/ERK1/2 pathways. Mol Cell Biochem.
371:55–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hou M, Liu J, Liu F, Liu K and Yu B: C1q
tumor necrosis factor-related protein-3 protects mesenchymal stem
cells against hypoxia- and serum deprivation-induced apoptosis
through the phosphoinositide 3-kinase/Akt pathway. Int J Mol Med.
33:97–104. 2014.
|
|
7
|
Severino V, Alessio N, Farina A, et al:
Insulin-like growth factor binding proteins 4 and 7 released by
senescent cells promote premature senescence in mesenchymal stem
cells. Cell Death Dis. 4:e9112013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Li Z, Liu T, et al: Impaired
cardioprotective function of transplantation of mesenchymal stem
cells from patients with diabetes mellitus to rats with
experimentally induced myocardial infarction. Cardiovasc Diabetol.
12:402013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lauer T, Heiss C, Balzer J, et al:
Age-dependent endothelial dysfunction is associated with failure to
increase plasma nitrite in response to exercise. Basic Res Cardiol.
103:291–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ward MR, Connelly KA, Vijayaraghavan R, et
al: eNOS overexpressing bone marrow cells are safe and effective in
a porcine model of myocardial regeneration following acute
myocardial infarction. Cardiovasc Ther. 31:e72–e78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang MY, Lin PM, Liu YC, et al: Induction
of cellular senescence by doxorubicin is associated with
upregulated miR-375 and induction of autophagy in K562 cells. PLoS
One. 7:e372052012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonet C, Giuliano S, Ohanna M, et al:
Aurora B is regulated by the mitogen-activated protein
kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling
pathway and is a valuable potential target in melanoma cells. J
Biol Chem. 287:29887–29898. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu K, Xu X, Liu C, et al: Negative
regulation of transcription factor FoxM1 by p53 enhances
oxaliplatin-induced senescence in hepatocellular carcinoma. Cancer
Lett. 331:105–114. 2013. View Article : Google Scholar
|
|
14
|
Abdelmohsen K, Srikantan S, Kang MJ and
Gorospe M: Regulation of senescence by microRNA biogenesis factors.
Ageing Res Rev. 11:491–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu J, Qian J, Xie X, et al: High density
lipoprotein protects mesenchymal stem cells from oxidative
stress-induced apoptosis via activation of the PI3K/Akt pathway and
suppression of reactive oxygen species. Int J Mol Sci.
13:17104–17120. 2012. View Article : Google Scholar
|
|
16
|
Wang Z, Ahmad A, Banerjee S, et al: FoxM1
is a novel target of a natural agent in pancreatic cancer. Pharm
Res. 27:1159–1168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Popovic R, Riesbeck LE, Velu CS, et al:
Regulation of mir-196b by MLL and its overexpression by MLL fusions
contributes to immortalization. Blood. 113:3314–3322. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong W, Zou G, Gu J and Zhang J:
L-arginine attenuates high glucose-accelerated senescence in human
umbilical vein endothelial cells. Diabetes Res Clin Pract.
89:38–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Liu Y, Takahashi M, et al: N
terminus of ASPP2 binds to Ras and enhances Ras/Raf/MEK/ERK
activation to promote oncogene-induced senescence. Proc Natl Acad
Sci USA. 110:312–317. 2013. View Article : Google Scholar :
|
|
20
|
Yokoi T, Fukuo K, Yasuda O, et al:
Apoptosis signal-regulating kinase 1 mediates cellular senescence
induced by high glucose in endothelial cells. Diabetes.
55:1660–1665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Munzel T: Recent studies on nitrates:
their action, bioactivation and development of tolerance. Dtsch Med
Wochenschr. 133:2277–2282. 2008.(In German).
|
|
22
|
Wang ZQ, Xu JF, Wang JP, Zhao WJ and Zeng
M: Involvement of guanylate cyclase and K+ channels in
relaxation evoked by ferulate nitrate in rat aorta artery. J
Pharmacol Sci. 118:521–530. 2012. View Article : Google Scholar
|
|
23
|
Thum T, Wiebking V, Ertl G and Bauersachs
J: Organic nitrates differentially modulate circulating endothelial
progenitor cells and endothelial function in patients with
symptomatic coronary artery disease. Antioxid Redox Signal.
15:925–931. 2011. View Article : Google Scholar
|
|
24
|
Gori T and Daiber A: Non-hemodynamic
effects of organic nitrates and the distinctive characteristics of
pentaerithrityl tetranitrate. Am J Cardiovasc Drugs. 9:7–15. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnston PV, Sasano T, Mills K, et al:
Engraftment, differentiation, and functional benefits of autologous
cardiosphere-derived cells in porcine ischemic cardiomyopathy.
Circulation. 120:1075–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams AR and Hare JM: Mesenchymal stem
cells: biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oelze M, Schuhmacher S and Daiber A:
Organic nitrates and nitrate resistance in diabetes: the role of
vascular dysfunction and oxidative stress with emphasis on
antioxidant properties of pentaerithrityl tetranitrate. Exp
Diabetes Res. 2010:2131762010. View Article : Google Scholar
|
|
28
|
Ling Q, Meng C, Chen Q and Xing D:
Activated ERK/FOXM1 pathway by low-power laser irradiation inhibits
UVB-induced senescence through down-regulating p21 expression. J
Cell Physiol. 229:108–116. 2014.
|
|
29
|
Zeng J, Wang L, Li Q, et al: FoxM1 is
up-regulated in gastric cancer and its inhibition leads to cellular
senescence, partially dependent on p27 kip1. J Pathol. 218:419–427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gorospe M and Abdelmohsen K:
MicroRegulators come of age in senescence. Trends Genet.
27:233–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su X, Chakravarti D, Cho MS, et al: TAp63
suppresses metastasis through coordinate regulation of Dicer and
miRNAs. Nature. 467:986–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borgdorff V, Lleonart ME, Bishop CL, et
al: Multiple microRNAs rescue from Ras-induced senescence by
inhibiting p21(Waf1/Cip1). Oncogene. 29:2262–2271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang L, Qiu N, Zhang C, et al:
Nitroglycerin enhances proliferation and osteoblastic
differentiation in human mesenchymal stem cells via nitric oxide
pathway. Acta Pharmacol Sin. 29:580–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guiboileau A, Yoshimoto K, Soulay F,
Bataille MP, Avice JC and Masclaux-Daubresse C: Autophagy machinery
controls nitrogen remobilization at the whole-plant level under
both limiting and ample nitrate conditions in Arabidopsis. New
Phytol. 194:732–740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Desclos M, Etienne P, Coquet L, et al: A
combined N15 tracing/proteomics study in Brassica napus reveals the
chronology of proteomics events associated with N remobilisation
during leaf senescence induced by nitrate limitation or starvation.
Proteomics. 9:3580–3608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sykorova B, Kuresova G, Daskalova S, et
al: Senescence-induced ectopic expression of the A. tumefaciens ipt
gene in wheat delays leaf senescence, increases cytokinin content,
nitrate influx, and nitrate reductase activity, but does not affect
grain yield. J Exp Bot. 59:377–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boe AE, Eren M, Murphy SB, et al:
Plasminogen activator inhibitor-1 antagonist TM5441 attenuates
Nω-nitro-L-arginine methyl ester-induced hypertension and vascular
senescence. Circulation. 128:2318–2324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu DH, Chen YM, Liu Y, et al: Ginsenoside
Rb1 reverses H2O2-induced senescence in human
umbilical endothelial cells: involvement of eNOS pathway. J
Cardiovasc Pharmacol. 59:222–230. 2012. View Article : Google Scholar
|
|
39
|
Li N, Lu X, Zhao X, et al: Endothelial
nitric oxide synthase promotes bone marrow stromal cell migration
to the ischemic myocardium via upregulation of stromal cell-derived
factor-1alpha. Stem Cells. 27:961–970. 2009. View Article : Google Scholar : PubMed/NCBI
|