Introduction
Three defining clinical symptoms of autism are
aberrant reciprocal social interactions, deficits in social
communication and repetitive/stereotypic behavior (1,2).
The etiology of autism remains largely unclear thus far. With the
continuous accumulation of research on the molecular pathogenesis
of autism, more attention is being paid to the canonical Wnt
signaling pathway (3,4). The canonical Wnt pathway has been
implicated in a variety of cellular functions, such as cell
proliferation, differentiation, apoptosis and neuronal migration
during embryonic development (5).
Given this broad spectrum of roles, it is no surprise that the
dysregulation of the canonical Wnt signaling pathway may cause a
similarly broad spectrum of deleterious effects on neuronal
development, and largely on this basis may thereby contribute to
the pathogenesis of neurodevelopmental disorders, such as
autism.
A number of previous studies have suggested that the
canonical Wnt pathway is involved in autism and the dysfunction of
the Wnt signaling pathway induces morphological and functional
abnormalities similar to those observed in autistic individuals.
For example, transgenic mice expressing active stabilized forms of
β-catenin in neuronal precursor cells have been shown to develop
grossly enlarged brains with an increased cerebral cortical volume
(6), whereas the inactivation of
the β-catenin gene causes marked brain malformation and the failure
of craniofacial development (7).
The upregulation of β-catenin expression, as well as that of its
downstream target genes (3), has
been observed in rat models of autism (3,4),
and the expression levels of β-catenin are abnormal with changes
over time and space. The mutation of genes, such as Wnt1, that
modestly increase the activation of this downstream signaling
pathway would therefore be expected to result in neurodevelopmental
and behavioral phenotypes. A recent study suggested that a Wnt1
missense variant leading to the Wnt signaling pathway activation
contributes to autism in the context of other genetic and
non-genetic factors that increase susceptibility to autism
(8).
Recent studies have focused, in particular, on the
role of oxidative stress in autism. In fact, oxidative stress has
also been implicated in the pathogenesis of other neuropsychiatric
diseases, including schizophrenia (9,10),
a disease that may be the genetic opposite of autism (11). Accordingly, accumulating evidence
suggests a role of oxidative stress in the development and clinical
manifestation of autism (1,2).
Sulindac is an FDA-approved non-steroidal
anti-inflammatory drug (NSAID) with documented anticancer
activities. Sulindac, as a NSAID with a number of functions, has
been used as a specific Wnt pathway inhibitor and is known as a
pharmacological inhibitor of β-catenin for the prevention of
metastasis (12,13). Since the upregulation of the
canonical Wnt pathway has been associated with the pathogenisis of
autism and valproic acid (VPA) is known to generate autism
phenotypes, in this study, we therefore used VPA to generate
conditions similar to those observed in autism. We aimed to
determine whether sulindac ameliorates autism-like behavioral
abnormalities, such as repetitive/stereotypic-like movement
deficits that develop in rats prenatally exposed to valproic acid
(VPA) as an animal model of autism. In addition, we aimed to
identify the possible mechanisms responsible for the development of
autism using primary cortical neurons.
Materials and methods
Animals and exposure to VPA
All animal experiments were carried out in
accordance with the National Institutes of Health guidelines for
the Care and Use of Laboratory Animals and the regulations of Fudan
University (Shanghai, China) for animal experimentation. The
experimental protocol was approved by the Shanghai Medical
Experimental Animal Care and Use Committee. As described in our
previous study (4), with some
modifications, Female Wistar rats (Shanghai Institute of Materia
Medica, Chinese Academy of Sciences, Shanghai, China) were mated
overnight, and the morning on which spermatozoa were found was
designated as the first day of gestation. The female fats received
a single intraperitoneal injection of 600 mg/kg VPA (CAS no:
1069665, Sigma, St. Louis, MO, USA) or 5 mg/kg sulindac
(CAS:38194502, Sigma) following the administration of 600 mg/kg VPA
for 30 min on day 12.5 after conception, and the control female
rats were injected with physiological saline or 5 mg/kg sulindac at
the same time point. The offspring were weaned on postnatal day
(PND)23, and rats of either gender were housed separately.
Repetitive/stereotypic-like behavioral tests were conducted for all
animals during the adolescent period. The behavioral experiments
were performed in the light phase between 09:00 and 15:00.
Primary neuronal culture and experimental
treatments
Wistar rats were used in the present study. The
experiments were carried out in accordance with the regulations of
Fudan University for animal experimentation. Cortices were
dissected from timed-pregnant (embryonic day 18) Wistar rat pups,
dissociated in a solution of 2.5 mg/ml trypsin (Gibco, Carlsbad,
CA, USA) for 15 min, and resuspended following centrifugation for 5
min in DMEM/F12 (Gibco) supplemented with 10% fetal bovine serum,
1% penicillin/streptomycin and 0.5 mmol/l glutamine. The cells were
plated on 6-well plates pre-treated with poly-L-lysine (100 μg/ml,
CAS no: 25988630, Sigma). Twenty-four hours later, the culture
medium was completely replaced with NB/B27 (neurobasal medium
supplemented with 2% B27, 1% penicillin/streptomycin and 0.5 mmol/l
glutamine). Subsequently, approximately one-third of the volume was
removed and replaced with an equal volume of fresh NB (0050128DJ,
Gibco)/B27 (10889-038, Gibco) twice per week. On the 7th day of
plating, the primary cultured neurons were exposed to VPA. After 24
h, VPA was replaced with the antioxidant, N-acetylcysteine (NAC;
CAS no: 616911, Sigma), or sulindac for 1 h. The neurons were then
harvested for further assay.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
RT-qPCR. RT-qPCR was carried out as described in our
previous study with minor modifications (4). Total RNA was isolated from the
cultured cortical neurons using TRIzol reagent (15596-018, Gibco).
Subsequently, cDNA was prepared using the PrimeScript™ RT reagent
kit (DRR019A, Takara Biotechnology, Dalian, China). The primer
sequences for RT-qPCR are listed in Table I. PCR parameters were as follows:
29 cycles of denaturation at 95°C for 30 sec, annealing at 57°C for
30 sec and extension at 72°C for 30 sec. The real-time detection of
the emission intensity of SYBR-Green I bound to double-stranded DNA
was performed using the Rotor-Gene 3000 real-time DNA analysis
system (RG-3000, Corbett Research, Sydney, Australia). At the end
of the runs, melting curves were obtained to make sure there were
no primer-dimer artifacts.
 | Table IPrimer sets used in RT-qPCR. |
Table I
Primer sets used in RT-qPCR.
| Primer name | Accession no. | Sequence 5′→3′ |
|---|
| GSK-3β | NM_032080.1 | Forward:
GTTGGTGGAAATAATAAAGG
Reverse: AAGTTGAAGAGGGCAGGT |
| β-catenin | NM_053357.2 | Forward:
CTGAGAAACTTGTCCGATGC
Reverse: CGGTAATGTCCTCCCTGT |
| GAPDH | NM_017008.3 | Forward:
TATCGGACGCCTGGTTAC
Reverse: TGCTGACAATCTTGAGGGA |
The PCR assay was performed simultaneously with
standard samples to construct each standard curve. Referring to the
standard curve, the relative mRNA expression level was normalized
to the expression level of the endogenous reference, GAPDH, to
calibrate the possible variability in the initial amount of total
RNA in each sample.
Western blot analysis
Western blot analysis was performed as previously
described (4), with some
modifications. Briefly, equal amounts of protein (25–30 μg) from
cell lysates were separated by sodium dodecylsufate
(SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred
onto PVDF membranes (Millipore Corp., Bedford, MA, USA). The blots
were incubated with the primary antibodies in 5% BSA or fat-free
milk in TBST buffer overnight at 4°C. Bound primary antibodies were
visualized using horseradish peroxidase-labeled secondary
antibodies and were detected by enhanced chemiluminescence (ECL).
The densities of the protein bands were quantified using ImageQuant
software (LAS-4000, GE Healthcare, Fairfield, CT, USA). GAPDH
(1:5000, Kangen Biotechnology Co., Shanghai, China) was used to
confirm equal loading. The primary antibodies used were as follows:
anti-glycogen synthase kinase (GSK)-3β (1:1000, Cat no. 9315; Cell
Signaling Technology, Danvers, MA, USA), phospho-GSK-3β (Ser9)
(1:500, Cat no. 9336; Cell Signaling Technology), anti-β-catenin
(1:1000, sc-7199; Santa Cruz Biotechnology, San Diego, CA, USA),
phospho-β-catenin (Ser33/37/Thr41) (1:1000, Cat no. 9561; Cell
Signaling Technology) and anti-4-hydroxynonenal (4-HNE) (1:500,
MAB3249; R&D Systems, Minneapolis, MN, USA).
Cell viability assay
Cell survival was evaluated by MTT assay as
previously described (14).
Briefly, the cells were plated into 96-well microtiter plates and
exposed to VPA or sulindac for 48 h. Following exposure to VPA or
sulindac, 10 μl of MTT labeling reagents were added to each well
and the plates were incubated at 37°C for 4 h. The cultures were
then solubilized, and the spectrophotometric absorbance of the
samples was detected using a microtiter plate reader. The
absorbance was read at 570 nm.
Measurement of intracellular ROS
levels
The average level of intracellular ROS was evaluated
in the cells loaded with the redox-sensitive dye,
2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Molecular
Probes, Eugene, OR, USA). An increase in the fluorescence emission
of dichlorofluorescein (DCF), a derivative of DCFH-DA, reflects the
enhanced cellular oxidative stress. All the experimental cells were
washed 3 times with PBS for 5 min, stained in the dark for 20 min
with 10 μmol/l DCFH-DA and harvested. Fluorescence was observed
under fluorescence light microscope, and measured at an excitation
and emission wavelength of 485 and 530 nm, respectively using a
fluorescence activated cell sorting (FACS) on fluorescence
spectrometer (HTS 7000, Perkin Elmer, Boston, MA, USA).
Behavioral testing: open field test
Exploratory activity was assessed in an automated
open field apparatus (RS Medical Associates, PA, USA) for rats. All
rats were tested one by one in an open field apparatus with an area
of 100×100 cm2 and a height of 40 cm. Testing was
conducted under dim light (approximately 80 lux) during the morning
hours in an undisturbed room and no stressor was applied to the
animals for at least 12 h before the test. Two rats were tested at
one time, one animal per chamber. A rat was placed in the center of
the apparatus and then allowed to behave and move freely for 30
min. At the same time, movements were recorded using a digital
video camera and recorded for further analysis. Data were assessed
by comparing the changes in the mean activity across a 30-min
testing session divided into 6 time bins (5 min each). Grooming and
rearing behaviors were also scored in the same paradigm by an
observer blinded to the treatments. The time spent self-grooming
and the number of rearing episodes were scored, as previously
described (15). A rearing
episode is defined as the number of times the rat stands upright on
its hind limbs. Repetitive/stereotypic-like behaviors were scored
and quantified by an experimenter blinded to the treatments.
Statistical analysis
All values are presented as the means ± SEM.
Multiple group comparisons were performed by one-way analysis of
variance (ANOVA) with post-hoc correction. Repeated measures
between 2 factors were examined using a repeated two-way analysis
of variance (ANOVA) and Fisher’s least-significant difference post
hoc comparison using SPSS 16.0 software (SPSS, Inc., Chicago, IL,
USA). Values of p<0.05 were considered to indicate statistically
significant differences.
Results
Decreased protein levels of GSK-3β and
increased β-catenin expression
Whether VPA activates the canonical Wnt pathway
remains unknown. To determine the effects of VPA on the activity of
the Wnt/β-catenin signaling pathway, dissociated primary cortical
neurons were cultured. Primary cultured cortical neurons were
exposed to VPA (1, 5 and 10 mmol/l) for 24 h. The cortical neurons
were continuously exposed to growth medium as the control.
Subsequently, all the abovementioned cultured cortical neurons were
processed for protein extraction. Quantitative analysis of the
protein content by western blotting demonstrated that VPA induced a
concentration-dependent decrease in the expression of GSK-3β, but
an increase in the expression of β-catenin when compared to the
controls (Fig. 1A and B). As
shown in Fig. 1A, 5 mmol/l and 10
mmol/l VPA increased the protein levels of β-catenin compared with
the controls. We then investigated GSK-3β, a negative regulatory
factor of β-catenin. The results revealed that GSK-3β expression
was lower in the 1, 5 and 10 mmol/l VPA-treated groups in
comparison to the controls; however, 5 mmol/l and 10 mmol/l VPA
significantly decreased the protein levels of GSK-3β (P<0.001;
Fig. 1B). Therefore, we selected
the dose of 5 mmol/l VPA as an ideal concentration for further
experiments.
Effects of NAC on VPA-treated primary
cultured neurons
Primary cultured neurons were exposed to VPA (5
mmol/l). After 24 h, VPA was replaced with the antioxidant, NAC (1
mM), for 1 h. The neurons were then harvested for further assay.
Western blot analysis, RT-qPCR and flow cytometry (FACS analysis)
were used to further investigate the levels of 4-HNE, GSK-3β,
β-catenin and ROS. The results from western blot analysis and FACS
revealed that treatment with VPA increased the levels of 4-HNE and
ROS as compared with the controls (Fig. 2A and B). However, both VPA and NAC
decreased the expression levels of 4-HNE and ROS (Fig. 2A and B). The above results
demonstrated that NAC attenuated oxidative stress in the
VPA-treated primary cultured neurons. Whether the decrease in
oxidative stress downregulates the canonical Wnt signaling pathway
in VPA-exposed primary cortical neurons remains unknown. Therefore,
in further experiments, we investigated the changes occurring in
the mRNA expression levels of β-catenin and GSK-3β. The results
from RT-qPCR revealed that GSK-3β expression was lower and
β-catenin expression was higher in the VPA-treated group when
compared with the controls; however treatment with both NAC and VPA
did not alter the mRNA levels of GSK-3β and β-catenin in comparison
to the VPA-treated group (Fig. 2C and
D).
Effects of sulindac on VPA-exposed
primary cultured neurons
Sulindac is a known pharmacological inhibitor of
β-catenin, known for inhibiting β-catenin expression through
proteasome-dependent degradation and suppressing tumorigenesis
in vivo by downregulating β-catenin signaling (16,17). GSK-3β destabilizes β-catenin by
phosphorylating its inhibitory sites, such as Ser33, Ser37 and
Thr41 (18). In this study, we
thus detected the changes occurring in the Wnt/β-catenin pathway,
oxidative stress markers and cell viability in primary cortical
neurons exposed to VPA following treatment with sulindac. Primary
cultured neurons were exposed to VPA (5 mmol/l). After 24 h, VPA
was replaced with sulindac (1, 3 and 5 mmol/l) for 1 h. The neurons
were then harvested for RNA and protein extraction. The results
from RT-qPCR revealed that treatment with sulindac decreased the
mRNA levels of β-catenin (Fig.
3A). As shown in Fig. 3A,
treatment with 3 and 5 mmol/l sulindac decreased the mRNA levels of
β-catenin compared with the controls. Thus, we selected the dose of
3 mmol/l sulindac as the optimal concentration for further
experiments. Whether treatment with 3 mmol/l sulindac inactivates
the canonical Wnt pathway requires further verification. Therefore,
we examined the phosphorylation levels of GSK-3β and β-catenin.
Western blot analysis demonstrated that VPA activated the canonical
Wnt pathway based on the increased phosphorylation levels of
GSK-3β, and the decreased phosphorylation levels of β-catenin in
comparison to the controls (Fig. 3D
and E). However, treatment with both sulindac and VPA
inactivated the canonical Wnt pathway, as indicated by the
decreased phosphorylation levels of GSK-3β and the increased
phosphorylation levels of β-catenin compared with the VPA-treated
group (Fig. 3D and E). We then
detected the effects of sulindac on the viability of primary
cultured cortical neurons. The results revealed that exposure to
VPA decreased cell viability (Fig.
3B). However, co-incubation of the neurons with VPA and
sulindac increased cell viability (Fig. 3B).
In order to investigate the effects of sulindac on
oxidative stress, we determined the expression levels of the
oxidative stress marker, 4-HNE. Western blot analysis revealed that
the protein expression of 4-HNE was higher in the VPA-treated group
than in the controls, whereas it was lower in the VPA plus
sulindac-treated group than in the VPA-treated group. However, the
expression of 4-HNE was not altered in the VPA plus
sulindac-treated group compared with the control group (Fig. 3C).
Effects of sulindac on
repetitive/stereotypic-like movements in Wistar rats prenatally
exposed to VPA
The VPA-treated rats were hyperactive at the 0–5,
5–10 and 20–25 min intervals, demonstrating that they spent more
time engaged in repetitive/stereotypic-like behaviors and had more
repetitive/stereotypic-like movements, with the results approaching
statistical significance at the 0–5, 5–10 and 10–15 min intervals
in comparison to the controls (Fig.
4B and C). An increase in the distance traveled during the 0–5,
5–10 and 20–25 min intervals was also observed (Fig. 4A). Compared with the VPA-treated
rats, the VPA plus sulindac-treated rats were hypoactive showing a
decrease in distance traveled during the 0–5, 5–10 and 20–25 min
intervals (Fig. 4A), a decrease
in the number of repetitive/stereotypic-like behaviors during the
0–5, 5–10 and 10–15 min intervals, as well as in the time engaged
in repetitive/stereotypic-like movements during the 0–5, 10–15 and
20–25 min intervals (Fig. 4B and
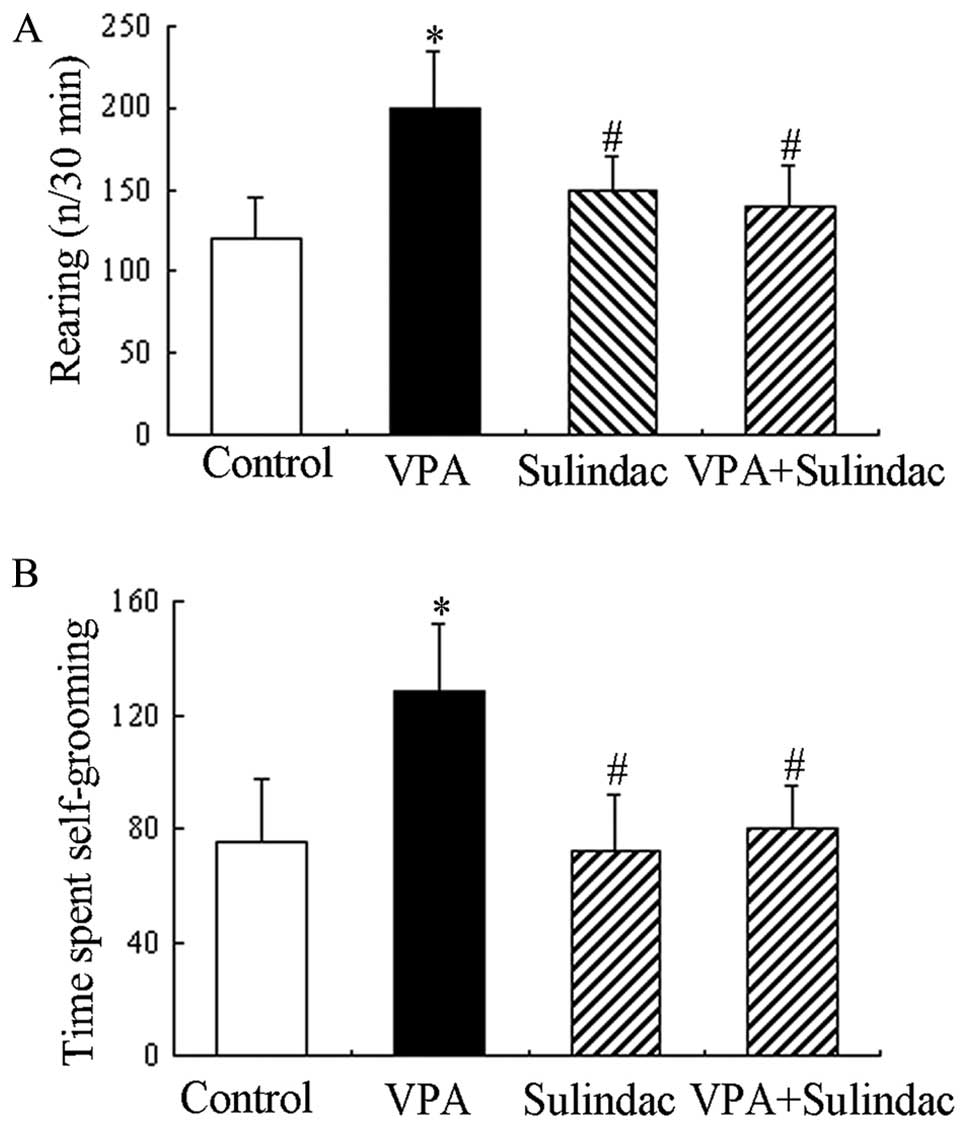
C). Additionally, self-grooming and rearing behaviors were also
scored during the open field locomotor activity task. The
VPA-treated animals spent a significantly longer period of time in
self-grooming activities compared to the controls (Fig. 5B). There was also a marked
increase observed in the number of rearing episodes in the
VPA-treated animals compared to the control animals (Fig. 5A). In comparison to the
VPA-treated rats, the VPA plus sulindac-treated rats were
hypoactive with a decreased number of self-grooming and rearing
behavioral activities (Fig. 5A and
B). There were no differences observed between the VPA plus
sulindac-treated rats and the controls in the total activity across
the 30-min testing session.
Discussion
The present study demonstrates the antioxidant
properties of the NSAID, sulindac, based on a decrease in
VPA-induced 4-HNE expression and ROS production following treatment
with sulindac. The anti-inflammatory agent also protected rat
cortical neurons against VPA-induced neurotoxicity, and ameliotared
the repetitive/stereotypic behavioral abnormalities in Wistar rats
prenatally exposed to VPA as a rat model of autism.
VPA, an anticonvulsant and mood-stabilizing drug, is
widely used for the treatment of different types of seizures and
myoclonic epilepsy (19). Several
mechanisms have been suggested for VPA hepatotoxicity, and most of
them are associated with oxidative stress. VPA has been shown to
induce ROS production and lipid peroxidation formation (20), induce DNA damage (21) and decrease cell viability in
hippocampal neurons (22). In
agreement with previous studies, this study demonstrated that VPA
increased ROS production and 4-HNE expression, thus inducing
oxidative stress, and subsequently reducing neuronal viability.
However, the co-incubation of the neurons with VPA and sulindac
reduced the VPA-induced oxidative stress and increased neuronal
viability. It is possible that the antioxidant and free radical
scavenging properties of sulindac (23), are responsible for these
neuroprotective effects. According to our previous results
(4), the mechanisms through which
sulindac exerts its antioxidant and free radical scavenging
properties involve the upregulation of the β-catenin
phosphorylation to suppress the activation of the canonical Wnt
signaling pathway, leading to the downregulation of the downstream
target genes, cyclin D1 and engrailed 2; these results are in
accordance with those of previous studies demonstrating that
sulindac is a specific inhibitor of the Wnt/β-catenin pathway
(12,13).
Motor stereotypies are defined as patterned,
repetitive, purposeless movements. These stigmatizing motor
behaviors represent a manifestation of the third core criterion
required for the diagnosis of autism, and are being viewed as
potential early markers suggestive of autism (24), and hamper normal brain
development, learning and social adaption. Moreover, motor
stereotypies may be a tangible expression of the underlying
neurobiology of this neurodevelopmental disorder. Furthermore,
given the limited advances made towards unraveling the neurobiology
of autism through the study of cardinal non-motor manifestations,
such as language disorder and social impairments, a growing number
of studies have turned their attention to motor impairments and
stereotypies as a potential source of additional insight into the
pathophysiology of autism (15,25,26).
The present study demonstrated that VPA-treated rats
showed a significant increase in the number of
repetitive/stereotypic-like behavioral activities, which is largely
in accordance with previous behavioral data (27,28). This may be due to the reason that
VPA-induced brain dysplasia, or increased glutamine transport leads
to excessively excited brain neurons (29,30). However, treatment of the animals
with the NSAID, sulindac, improved the behavioral response, with
the VPA plus sulindac-treated rats performing better than the
VPA-treated animals, perhaps due to the reason that sulindac
reduces the glutamate concentration or glutamine transport
(31). Although sulindac improved
performance in the open field test, it failed to completely
suppress the VPA-induced motor deficits, as these rats showed more
repetitive/stereotypic-like behavioral activities at certain time
points compared to the control animals.
It has been shown that N-methyl D-aspartate
NMDA receptor antagonists result into stereotypic movement disorder
(32,33), while sulindac increases the NR1
and NR2B NMDA receptor subunits in aged Fischer 344 rats (34). NMDA receptors are vital for brain
function and these receptors are central to many of the
activity-dependent changes occurring in synaptic strength and
connectivity (35). It has been
demonstrated that VPA reduces NMDA signaling and NMDA
receptor-interacting protein expression in the rat brain (36). Sulindac prevents the age-related
increase in the expression of the pro-inflammatory cytokine,
interleukin-1β (1L-1β), in the hippocampus (34). It is possible that sulindac
suppresses the inflammatory response in the brain, as well as
scavenging ROS and protects the cortical neurons from VPA-induced
neurotoxicity. Sulindac possibly reduces the loss of NMDA receptors
and prevents the decrease in the number of cortical neurons,
thereby averting VPA-induced stereotypic movement disorder.
The principal defense systems against oxygen free
radicals are superoxide dismutase (SOD), glutathione (GSH), GSH
peroxidases, GSH reductase, catalase and antioxidant nutrients. GSH
and the related enzymes are present in the majority of cells.
Imbalances in the expression of GSH and associated enzymes have
been implicated in the pathogenesis of autism (2,37).
It has been demonstrated that VPA decreases GSH reductase activity
with a subsequent decrease in GSH levels (38,39), and increases glutathione
peroxidase activity (40). The
ratio of GSH/GSSG is a good measure of the oxidative stress of an
organism. This study demonstrated that VPA enhanced 4-HNE and ROS
levels, suggesting the generation of oxidative stress. However,
treatment with the NSAID, sulindac, attenuated the increase in
4-HNE levels. It has been demonstrated that antioxidants prevent
NMDA receptor depletion (41),
suggesting that these NSAIDs also serve as antioxidants and prevent
the depletion of NMDA receptors. This is another possible mechanism
through which these NSAIDs improve performance in the open field
test since it has been suggested that NMDA receptors are important
during the acquisition of normal stereotypic movements (42).
In conclusion, the results from the present study
led us to hypothesize that the canonical Wnt signaling pathway
induces oxidative stress and therefore contributes to the
susceptibility to autism. Sulindac, with antioxidant properties
associated with the inhibition of the activition of the canonical
Wnt pathway, protects primary cortical neurons, as well as rats
against VPA-induced oxidative stress. Sulindac also improves
stereotypic movement disorders that are adversely affected by VPA.
The findings of the present study further support the argument that
sulindac may play a beneficial role in neurodevelopmental
disorders, such as autism.
Acknowledgements
This study was financially supported by grants from
the National Natural Science Foundation of China (nos. 81301174,
81260211 and 81271466) and the Doctoral Scientific Research
Foundation of Xinxiang Medical University.
References
|
1
|
Ghanizadeh A and Derakhshan N:
N-acetylcysteine for treatment of autism, a case report. J Res Med
Sci. 17:985–987. 2012.
|
|
2
|
Al-Ayadhi LY and Elamin NE: Camel milk as
a potential therapy as an antioxidant in autism spectrum disorder
(ASD). Evid Based Complement Alternat Med. 2013:6028342013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Xu L, Zhu X, et al: Demethylation
of specific Wnt/β-catenin pathway genes and its upregulation in rat
brain induced by prenatal valproate exposure. Anat Rec.
293:1947–1953. 2010. View
Article : Google Scholar
|
|
4
|
Zhang Y, Sun Y, Wang F, Wang Z, Peng Y and
Li R: Downregulating the canonical Wnt/β-catenin signaling pathway
attenuates the susceptibility to autism-like phenotypes by
decreasing oxidative stress. Neurochem Res. 13:1409–1419. 2012.
View Article : Google Scholar
|
|
5
|
Hall AC, Lucas FR and Salinas PC: Axonal
remodeling and synaptic differentiation in the cerebellum is
regulated by WNT-7a signaling. Cell. 100:525–535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chenn A and Walsh CA: Regulation of
cerebral cortical size by control of cell cycle exit in neural
precursors. Science. 297:365–369. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brault V, Moore R, Kutsch S, et al:
Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion
results in dramatic brain malformation and failure of craniofacial
development. Development. 128:1253–1264. 2001.PubMed/NCBI
|
|
8
|
Martin PM, Yang X, Robin N, et al: A rare
WNT1 missense variant overrepresented in ASD leads to increased Wnt
signal pathway activation. Transl Psychiatry. 3:e3012013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atkin TA, MacAskill AF, Brandon NJ and
Kittler JT: Disrupted in Schizophrenia-1 regulates intracellular
trafficking of mitochondria in neurons. Mol Psychiatry. 16:122–124.
1212011. View Article : Google Scholar
|
|
10
|
Bošković M, Grabnar I, Terzič T, Kores
Plesničar B and Vovk T: Oxidative stress in schizophrenia patients
treated with long-acting haloperidol decanoate. Psychiatry Res.
210:761–768. 2013. View Article : Google Scholar
|
|
11
|
Crespi B, Stead P and Elliot M: Evolution
in health and medicine Sackler colloquium: Comparative genomics of
autism and schizophrenia. Proc Natl Acad Sci USA. 107(Suppl 1):
1736–1741. 2010. View Article : Google Scholar :
|
|
12
|
Lemjabbar-Alaoui H, Dasari V, Sidhu SS, et
al: Wnt and Hedgehog are critical mediators of cigarette
smoke-induced lung cancer. PLoS One. 1:e932006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Chang X, Lee J, et al: Cigarette
smoke induces promoter methylation of single-stranded DNA-binding
protein 2 in human esophageal squamous cell carcinoma. Int J
Cancer. 128:2261–2273. 2011. View Article : Google Scholar :
|
|
14
|
Hansen MB, Nielsen SE and Berg K:
Re-examination and further development of a precise and rapid dye
method for measuring cell growth/cell kill. J Immunol Methods.
119:203–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pearson BL, Pobbe RL, Defensor EB, et al:
Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of
autism. Genes Brain Behav. 10:228–235. 2011. View Article : Google Scholar :
|
|
16
|
Orner GA, Dashwood WM, Blum CA, Diaz GD,
Li Q and Dashwood RH: Suppression of tumorigenesis in the Apc(min)
mouse: down-regulation of beta-catenin signaling by a combination
of tea plus sulindac. Carcinogenesis. 24:263–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rice PL, Kelloff J, Sullivan H, et al:
Sulindac metabolites induce caspase- and proteasome-dependent
degradation of beta-catenin protein in human colon cancer cells.
Mol Cancer Ther. 2:885–892. 2003.PubMed/NCBI
|
|
18
|
Toledo EM, Colombres M and Inestrosa NC:
Wnt signaling in neuroprotection and stem cell differentiation.
Prog Neurobiol. 86:281–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Löscher W: Basic pharmacology of
valproate: a review after 35 years of clinical use for the
treatment of epilepsy. CNS Drugs. 16:669–694. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jafarian I, Eskandari MR, Mashayekhi V,
Ahadpour M and Hosseini MJ: Toxicity of valproic acid in isolated
rat liver mitochondria. Toxicol Mech Methods. 23:617–623. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan S, Ahmad T, Parekh CV, Trivedi PP,
Kushwaha S and Jena G: Investigation on sodium valproate induced
germ cell damage, oxidative stress and genotoxicity in male Swiss
mice. Reprod Toxicol. 32:385–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang C, Luan Z, Yang Y, Wang Z, Cui Y and
Gu G: Valproic acid induces apoptosis in differentiating
hippocampal neurons by the release of tumor necrosis factor-alpha
from activated astrocytes. Neurosci Lett. 497:122–127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santos F, Teixeira L, Lúcio M, et al:
Interactions of sulindac and its metabolites with phospholipid
membranes: an explanation for the peroxidation protective effect of
the bioactive metabolite. Free Radic Res. 42:639–650. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldman S, O’Brien LM, Filipek PA, Rapin I
and Herbert MR: Motor stereotypies and volumetric brain alterations
in children with Autistic Disorder. Res Autism Spectr Disord.
7:82–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lemonda BC, Holtzer R and Goldman S:
Relationship between executive functions and motor stereotypies in
children with Autistic Disorder. Res Autism Spectr Disord.
6:1099–1106. 2012. View Article : Google Scholar
|
|
26
|
Hattier MA, Matson JL, Macmillan K and
Williams L: Stereotyped behaviours in children with autism spectrum
disorders and atypical development as measured by the BPI-01. Dev
Neurorehabil. 16:291–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schneider T and Przewlocki R: Behavioral
alterations in rats prenatally exposed to valproic acid: animal
model of autism. Neuropsychopharmacology. 30:80–89. 2005.
View Article : Google Scholar
|
|
28
|
Schneider T, Turczak J and Przewlocki R:
Environmental enrichment reverses behavioral alterations in rats
prenatally exposed to valproic acid: issues for a therapeutic
approach in autism. Neuropsychopharmacology. 31:36–46. 2006.
|
|
29
|
Markram K, Rinaldi T, La Mendola D, Sandi
C and Markram H: Abnormal fear conditioning and amygdala processing
in an animal model of autism. Neuropsychopharmacology. 33:901–912.
2008. View Article : Google Scholar
|
|
30
|
Rinaldi T, Silberberg G and Markram H:
Hyperconnectivity of local neocortical microcircuitry induced by
prenatal exposure to valproic acid. Cereb Cortex. 18:763–770. 2008.
View Article : Google Scholar
|
|
31
|
Bass SE, Sienkiewicz P, Macdonald CJ, et
al: Novel dithiolethione-modified nonsteroidal anti-inflammatory
drugs in human hepatoma HepG2 and colon LS180 cells. Clin Cancer
Res. 15:1964–1972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pitsikas N, Zisopoulou S and Sakellaridis
N: Nitric oxide donor molsidomine attenuates psychotomimetic
effects of the NMDA receptor antagonist MK-801. J Neurosci Res.
84:299–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pitsikas N, Zisopoulou S, Pappas I and
Sakellaridis N: The selective 5-HT(6) receptor antagonist Ro
04–6790 attenuates psychotomimetic effects of the NMDA receptor
antagonist MK-801. Behav Brain Res. 188:304–309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mesches MH, Gemma C, Veng LM, et al:
Sulindac improves memory and increases NMDA receptor subunits in
aged Fischer 344 rats. Neurobiol Aging. 25:315–324. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bliss TV and Collingridge GL: A synaptic
model of memory: long-term potentiation in the hippocampus. Nature.
361:31–39. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Basselin M, Chang L, Chen M, Bell JM and
Rapoport SI: Chronic administration of valproic acid reduces brain
NMDA signaling via arachidonic acid in unanesthetized rats.
Neurochem Res. 33:2229–2240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kern JK, Haley BE, Geier DA, Sykes LK,
King PG and Geier MR: Thimerosal exposure and the role of sulfation
chemistry and thiol availability in autism. Int J Environ Res
Public Health. 10:3771–3800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsieh CL, Wang HE, Tsai WJ, Peng CC and
Peng RY: Multiple point action mechanism of valproic
acid-teratogenicity alleviated by folic acid, vitamin C, and
N-acetylcysteine in chicken embryo model. Toxicology. 291:32–42.
2012. View Article : Google Scholar
|
|
39
|
Tunali S: The effects of vitamin B6 on
lens antioxidant system in valproic acid-administered rats. Hum Exp
Toxicol. 33:623–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Z, Qin X, Zhao X, et al: Valproic
acid regulates antioxidant enzymes and prevents
ischemia/reperfusion injury in the rat retina. Curr Eye Res.
37:429–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patel AP, John MA, Handy RD and Robert SJ:
Carbon monoxide exposure in rat heart: glutathione depletion is
prevented by antioxidants. Biochem Biophys Res Commun. 302:392–396.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deutsch SI, Pepe GJ, Burket JA, Winebarger
EE, Herndon AL and Benson AD: D-cycloserine improves sociability
and spontaneous stereotypic behaviors in 4-week old mice. Brain
Res. 1439:96–107. 2012. View Article : Google Scholar : PubMed/NCBI
|