Introduction
Prenatal stress (PNS) is an important environmental
risk factor for the development of schizophrenia in adults, and the
second trimester of pregnancy in humans seems to be the most
vulnerable period for insults (1–6).
Additionally, various animal studies have demonstrated that PNS
during gestation elevates glucocorticoid levels and is related to
biochemical, physiological and behavioral changes in the offspring,
including reduced birth weight, cardiovascular and
neuroendocrinological abnormalities, attentional dysfunction,
enhanced anxiety-related behaviors and cognitive deficits (7–16).
For the study of PNS, pregnant female rats are commonly exposed to
stressful manipulations during the third week of pregnancy, which,
in terms of neural development, is approximately equivalent to the
second trimester of human gestation (13–15).
In animal studies, exposure to PNS has been shown to
result in a diminished number of hippocampal synapses and fewer
neurons in the brain (17).
Moreover, the hippocampi of adolescent and adult male rats exposed
to PNS exhibit decreases in dendritic length, spine density and the
number of neurons relative to non-stressed (NS) controls (18,19). PNS has also been shown to cause
variable changes in gene expression in the rat brain, including the
expression of genes associated with neuronal development, cell
differentiation and neurotransmitter function (14,20,21). As shown in our previous study,
dihydropyrimidinase like protein (Dpysl)2 and 3 were downregulated
in the prefrontal cortex of PNS rats and polymorphisms in the
DPYSL2 gene in humans may be associated with the development
of schizophrenia (22).
Ginseng, the root of Panax ginseng C.A. Meyer
(PG), has been used traditionally as an herbal medicine for over
1,000 years in East Asian countries, such as China, Japan and Korea
(23). PG is effective in the
treatment of various disorders, such as shallow respiration,
shortness of breath, cold limbs, lack of appetite, chest and
abdominal distension and profuse sweating (24). Researchers have focused on the
novel pharmacological effects of PG; some have attempted to explain
the pharmacological aspects of PG in various abnormalities in
humans, such as cancer, diabetes mellitus and neurodegenerative
disorders (25,26). PG has also been widely used to
enhance stamina and to address fatigue and physical stress for
thousands of years in Traditional Oriental medicine (27). A recent placebo-controlled study
indicated that the verbal working memory and visual working memory
of patients with schizophrenia was improved by treatment with a
ginseng extract (28).
The abovementioned findings have resulted in the
hypothesis that PG may affect the pathophysiology of schizophrenia.
Thus, in the present study, the behavioral patterns in the
offspring and changes in protein levels were examined in the
prefrontal cortex and hippocampus of rats exposed to PNS, and
whether treatment with PG reverses these changes which occur due to
PNS.
Materials and methods
Dried roots of PG were purchased from Yunpung,
Chungbuk, Korea, and the specimens were identified taxonomically by
an Oriental medicine physician at the National Institute of
Horticultural and Herbal Science, Rural Development Administration
(RDA), Suwon, Korea. The voucher specimen (HPR-207) was deposited
at the herbarium of the Herbal Crop Research Institute (Eumsung,
Korea).
We used water extraction as the majority of
traditional Oriental herbal materials have been decocted with
boiling water, and as ginsenosides are more soluble in water than
in organic solvents. The crushed plant materials (200 g each) were
extracted under reflux with distilled water 3 times. The combined
water extracts were lyophilized and the yield was 18.3% (wt/wt) for
PG in the dried state. They were stored at −20°C until use.
Powdered extracts of PG were dissolved in saline to a concentration
of 300 mg/ml. The animals were orally administered PG solution
during pregnancy.
The rat model of PNS was prepared as described in
previous studies with slight modifications (13,14). Pregnant Sprague-Dawley rats were
purchased from Central Laboratory Animal Inc. (Seoul, Korea) and
arrived at the animal facility on day 7 of gestation. The rats were
housed under standard conditions with a 12/12-h light/dark cycle
(lights on at 06:30) with free access to food and water. All animal
procedures were performed in accordance with the guidelines for the
care and use of laboratory animals of the US National Institutes of
Health.
Beginning on day 14 of gestation, exposure to PNS
was initiated and consisted of: i) restraint in well-ventilated
cylindrical plexiglas restrainers for 1 h; ii) exposure to a cold
environment (4°C) for 6 h; iii) overnight food deprivation; iv) 15
min of swim stress in room-temperature water; v) reversal of the
light-dark cycle; and/or vi) social stress induced by overcrowded
housing conditions during the dark phase of the cycle (13,14). Pregnant rats used as the controls
remained in the animal room during gestational days 14–21 and were
exposed to only normal animal-room husbandry procedures.
Following birth, the rats and their pups were left
undisturbed in their cages until weaning on postnatal day 23. At
this time, the male and female offspring were separated and
group-housed in cages with 1 or 2 littermates of the same gender
with free access to rat chow and water. The animals were exposed to
normal animal room conditions from that point forward until
experimental use on postnatal day 35 (14,15).
Modified behavioral tests, including a social
interaction test, the open-field test (OFT) and the forced-swim
test (FST), were carried out as previously described (13,29–31). The social interaction test was
adapted from previous studies and was conducted in a clear
plexiglas chamber (77×77×25 cm) (13,30,31). The room in which the chamber was
located was darkened during testing, and the chamber was
illuminated by a single 25 W red light bulb placed ~100 cm above
the base of the chamber (subject age, 30 days). Sessions were
filmed with a video camera placed 150 cm above the cage. The
experimenter remained outside the test room during testing, and the
test arena was cleaned after each session. Social interaction
partners were siblings of the same gender who resided in the same
cage after weaning and were of approximately equal body weight (in
the few cases where a sibling of the same gender was not available,
a playmate from similar conditions was used). Each session lasted
for 20 min and was scored in terms of the total duration of social
play and the numbers and types of interactions. Specifically, a
rater blinded to the treatment conditions scored behavioral
activity as aggressive [fighting (kicking, boxing and wrestling),
aggressive grooming and biting] or non-aggressive (sniffing,
following and grooming the partner) based on the video.
Experimental and target rats were not used in this paradigm more
than once, and the arena was cleaned with 70% ethanol after each
trial. The OFT was used to assess exploratory activity and
reactivity to a novel environment. On the test day, the subjects
were removed from their home cage (subject age, 32 days) and were
placed individually in the start box (15×15×20 cm) of the open
field arena (77×77×25 cm) for 5 min. The apparatus was composed of
black Polygal and no background noise was provided. The
experimenter exited the room, and the behavior of the subject was
recorded. Scoring included central boxes entered, line crossings,
runs, rears, grooming, cage sniffs and immobile behavior, as
previously described (29,30).
The modified FST was used (subject age, 34 days), as
described in previous studies (29,30). The rats were lowered individually
into a cylinder (height, 40 cm; diameter, 20 cm) filled with fresh,
warmed tap water (25±2°C). After 5 min, the rats were removed and
wiped with a clean towel to remove excess water before being
returned to their home cage. On the following day, each rat was
again placed in the cylinder for 15 min during which the time spent
swimming and climbing the time spent immobile were recorded with a
video camera and by an observer using a stopwatch. The predominant
behaviors were counted every 5 sec. Test scores were recorded and
included swiming activity (horizontal movement throughout the
chamber and crossing quadrants), climbing activity (upward-directed
movements up the side of the chamber and jump-ups from the bottom
of the chamber) and immobility (no additional activity other than
keeping the head above water or tiny whip kicks, as previously
described by Dulawa et al (29) and Schroeder et al (30).
The rats were anesthetized deeply with ethyl ether
and perfused with 4% paraformaldehyde. The fixed brains were
removed, frozen and cut into 30-μm sections. To detect Dpysl2
expression and neurofilament expression, frozen sections from the
rat prefrontal cortex and hippocampus were blocked with normal
horse serum, incubated with anti-Dpysl2 antibody (1:200; Cell
Signaling Technology, Danvers, MA, USA), NF200 antibody (1:160;
Sigma, St. Louis, MO, USA) or NeuN (1:100; EMD Millipore,
Billerica, MA, USA) and then incubated with a Cy3-conjugated
anti-rabbit (Dpysl2) and mouse (NF200) and a FITC-conjugated
anti-mouse (NeuN) secondary antibody secondary antibody (1:200,
1:500 and 1:500; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA). Fluoroshield™ with DAPI (Sigma) was used for the
nuclear staining of the brain tissues and mounting of the slides.
Fluorescence images were captured using a confocal laser-scanning
microscope (FV10-ASW; Olympus, Tokyo, Japan), and image
quantification was performed with ImageJ software using a protocol
previously described with slight modifications (33).
Prefrontal cortical and hippocampal tissues were
lysed in radioimmunoprecipitation assay (RIPA) buffer containing
protease inhibitors and then centrifuged at 14,000 rpm for 10 min
at 4°C. To identify Dpysl2 and neurofilament protein, 100 μg of the
lysed protein were placed on a 10 and 8% SDS gel and transferred
onto a polyvinylidene difluoride (PVDF) membranes (EMD Millipore).
After blocking with 5% skim milk, the membranes were probed with
anti-Dpysl2 (1:1,000; no. 9393; Cell Signaling Technology, Inc.),
anti-NF200 (1:1,000; N4142; Sigma), or anti-β-actin (Actb; 1:1,000;
sc-81178; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
antibodies overnight at 4°C and then with peroxidase-conjugated
secondary antibody (1:10,000; Sigma) for 1 h at room temperature.
Immunoreactive bands were detected using an enhanced
chemiluminescence kit (Elpis Biotech Inc., Daejeon, Korea), and
quantitative measurements of Dpysl2, NF200 and the Actb protein
were obtained using ImageJ software.
Difference between groups were analyzed using the
Student’s t-test. P-values <0.05 were considered to indicate a
statistically significant difference.
Results
To evaluate the extent to which treatment with PG
alters behavioral activity and protein expression changes that may
be related to the pathophysiology of schizophrenia during maternal
stress in pregnancy, we used a model of variable and unpredictable
stress in rats. Pregnant females were exposed to various stressors
from day 14 to 21 of gestation. We investigated the effects of
treatment with PG on the PNS-induced behavioral phenotypes using an
OFT, FST and a social interaction test.
We observed significant differences among the
control, PNS and PG-treated groups in the social interaction test
(Table I). In particular, one of
the non-aggressive behaviors, the number and duration of sniffs,
was decreased significantly in the PNS group (P<0.05; Table I) and was restored by oral
treatment with PG.
 | Table IAnalysis of behavior of rats in a
social interaction test and effects of PG. |
Table I
Analysis of behavior of rats in a
social interaction test and effects of PG.
| Behavior | Control | PNS | PG |
|---|
| Sniff (n)a,b | 38.43±3.32 | 24.14±2.21 | 43.00±4.78 |
| Sniff (s)a,b | 82.57±14.82 | 37.43±5.82 | 105.71±17.84 |
| Follow (n) | 12.57±3.47 | 9.00±2.30 | 4.29±1.63 |
| Follow (s) | 22.71±5.92 | 29.57±17.97 | 9.29±3.25 |
| Grooming partner
(n) | 4.43±0.95 | 2.43±0.72 | 3.29±0.72 |
| Grooming partner
(s) | 12.86±3.44 | 17.57±6.01 | 14.71±8.59 |
| Fight (n) | 2.71±2.23 | 2.43±1.25 | 1.43±0.57 |
| Fight (s) | 5.86±3.99 | 4.43±2.22 | 3.14±1.49 |
| Aggressive (n) | 6.00±1.83 | 3.86±1.42 | 2.14±0.86 |
| Aggressive (s) | 13.57±4.95 | 20.29±8.02 | 13.43±8.90 |
| Biting (n) | 0.57±0.37 | 0.29±0.29 | 0.00±0.00 |
| Biting (s) | 0.57±0.37 | 0.86±0.86 | 0.00±0.00 |
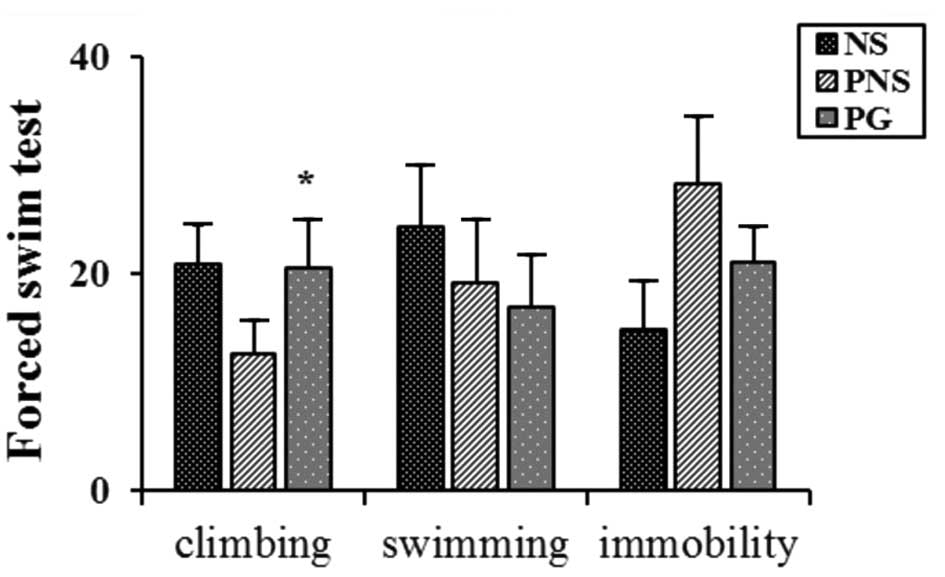
In the FST, the offspring of the rats exposed to PNS
exhibited decreased climbing activity and increased immobility
compared with the non-stressed (NS) rat offspring (P<0.05;
Fig. 1). The climbing activity
was recovered upon treatment with PG (P<0.05; Fig. 1).
The offspring of the NS rats and those exposed to
PNS were tested in the open field for 20 min. The PNS group showed
a significantly decreased number of central entries and line
crossings; these scores recovered following treatment with PG
(P<0.05; Table II). In
addition, the PNS group showed a significantly decreased number and
duration of runs and rear behavior; these scores recovered upon
treatment with PG (P<0.05; Table
II). Similarly, the number of times immobility occurred and the
duration of the immobility increased in the PNS group and returned
to the control levels upon treatment with PG (P<0.05; Table II).
 | Table IIBehavior of rats in an open-field
test and effects of PG. |
Table II
Behavior of rats in an open-field
test and effects of PG.
| Behavior | Control | PNS | PG |
|---|
| Central
entereda,b | 11.57±3.18 | 3.43±0.84 | 17.29±1.91 |
| Line
crossinga,b | 3.43±1.25 | 0.00±.00 | 4.86±0.80 |
| Run (n)b | 15.14±7.51 | 2.29±0.61 | 12.57±3.39 |
| Run (s)b | 22.43±13.58 | 3.14±0.91 | 15.57±5.26 |
| Rear (n)b | 77.29±18.97 | 33.86±9.08 | 95.00±6.67 |
| Rear (s)a,b | 194.29±28.77 | 59.14±15.38 | 203.00±19.26 |
| Grooming (n) | 21.00±2.74 | 14.43±3.80 | 18.43±2.50 |
| Grooming (s) | 241.71±56.06 | 199.86±42.57 | 241.14±25.80 |
| Cage sniff (n) | 33.43±6.50 | 31.00±7.28 | 49.86±6.54 |
| Cage sniff (s) | 129.71±21.60 | 170.86±37.31 | 148.57±23.76 |
| Immobile
(n)a,b | 1.14±0.34 | 12.43±4.91 | 0.86±0.40 |
| Immobile
(s)a,b | 3.43±1.60 | 71.43±26.51 | 2.00±1.23 |
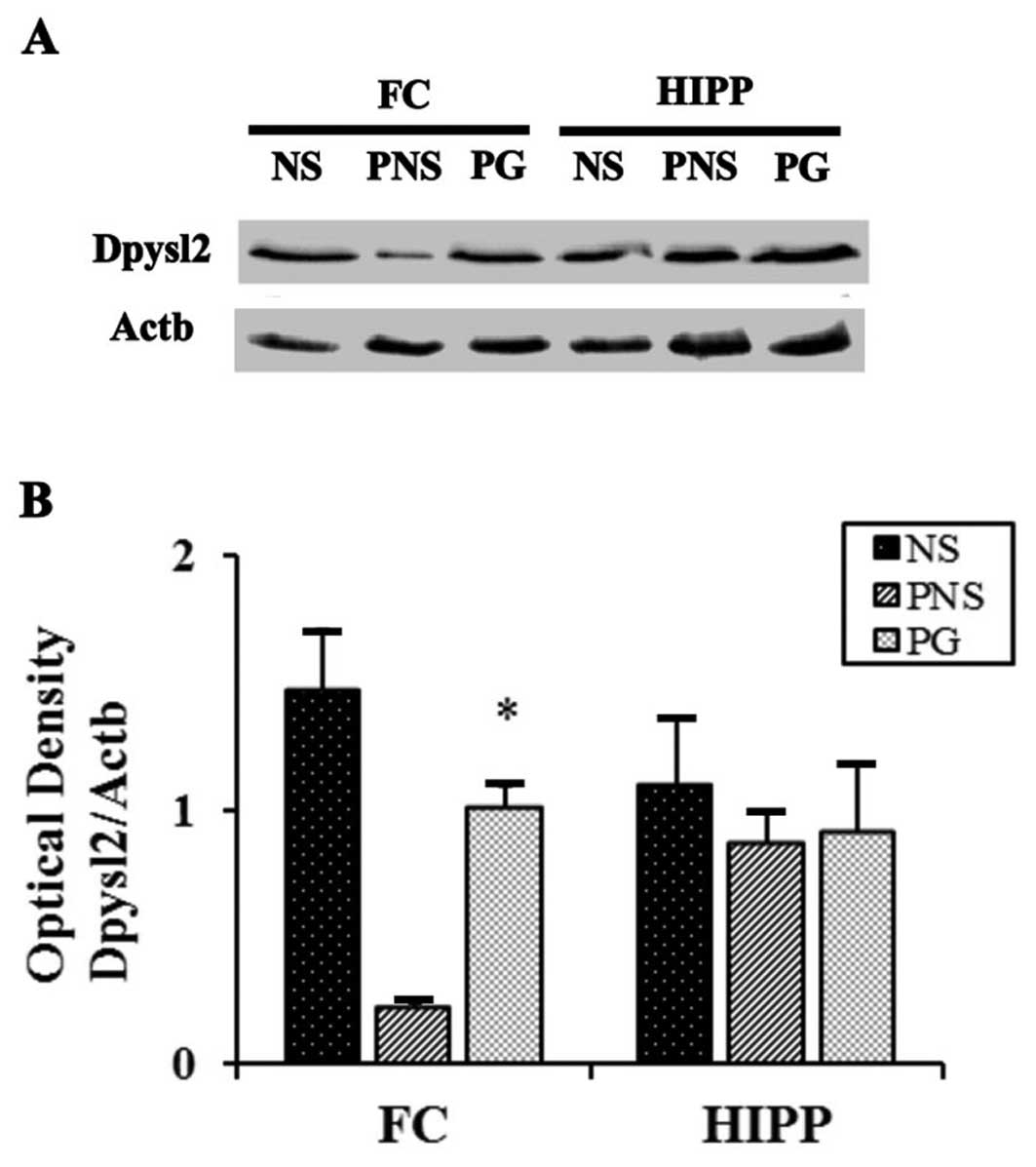
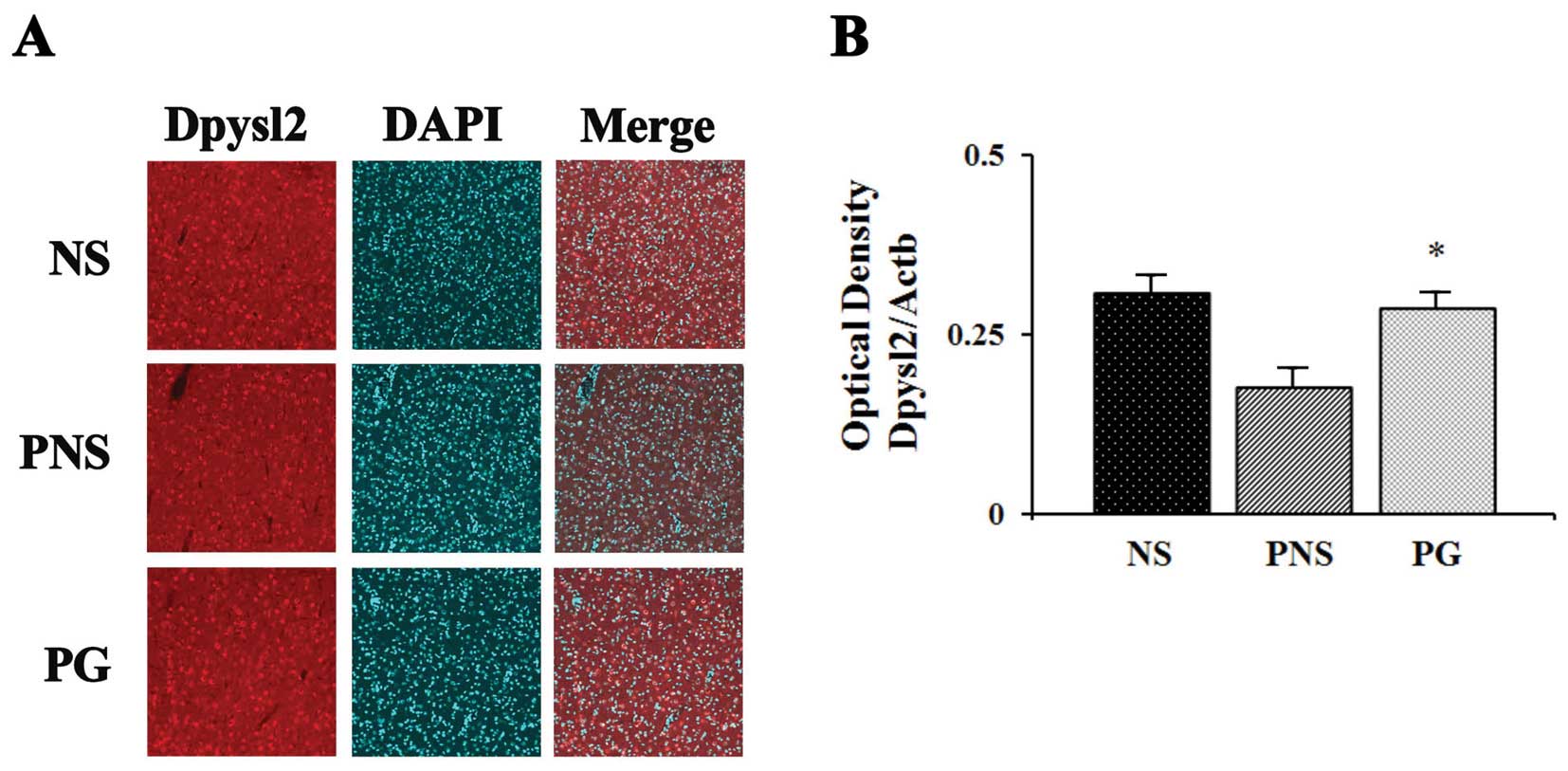
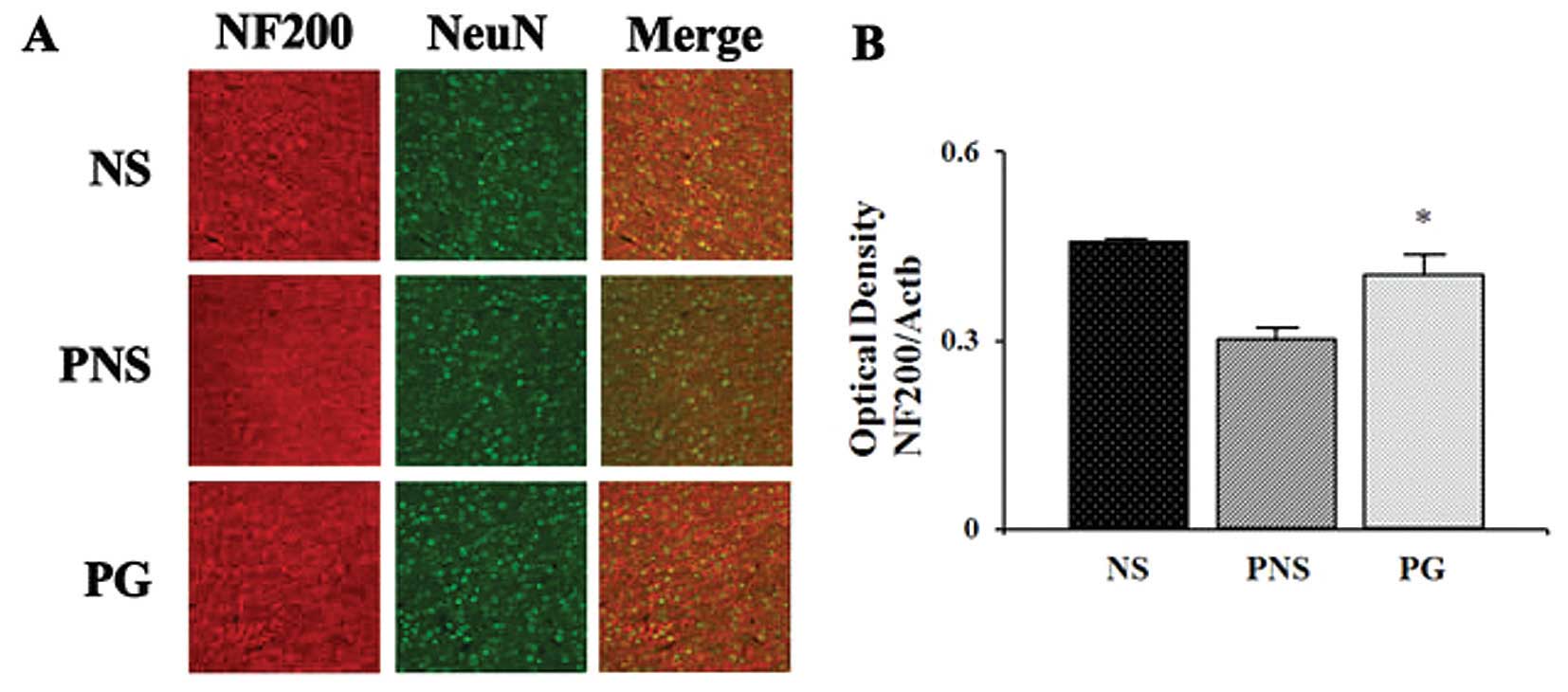
Dpysl2 is expressed in neurons of the central
nervous system and is concentrated at synaptic sites and in the
axons, where it may affect synaptic physiology (33). In our previous study, we
demonstrated that Dpysl2 was decreased in the offspring of rats
exposed to PNS (22). To
investigate the PNS-induced downregulation of Dpysl2 protein and
the effects of PG treatment, we performed western blot analysis
(Fig. 2) and immunohistochemical
anlaysis (Fig. 3) of the
prefrontal cortex areas of the brains of rats in the control, PNS
and PG group. Western blot analyses revealed that the expression
levels of Dpysl2 and neurofilament proteins in the prefrontal
cortex were significantly lower in the rats exposed to PNS than in
the control rats (all P-values <0.05; Figs. 2 and 3), whereas the expression levels of of
Actb in the prefrontal cortex were similar in all groups. This
differential expression of Dpysl2 and that of neurofilament
proteins was also evident in the immunofluorescence-stained images
of the brains of rats in the control, PNS and PG group and in the
measurements of immunohistochemical staining intensity (all
P-values <0.05; Figs. 4 and
5).
Discussion
In the present study, to examine the effects of PG
on the pathophysiology of stress-related psychiatric disorders,
such as schizophrenia, according to the neurodevelopmental theory,
we performed behavioral and protein expressional analyses in an
animal model of PNS.
Ginseng has been used in Asia for thousands of years
to improve vitality, wakefulness, respiration, angina, nausea,
attention span, memory, immune function and diminished libido, and
has more recently become one of the most popular herbal supplements
in the Western world (23,34–36).
Technological advances have led to the identification,
characterization and standardization of the active components in
ginseng extracts. Ginsenosides are unique triterpenoid saponins
found exclusively in PG, and to date more than 150 naturally
occurring ginsenosides have been isolated from ginseng (37–39). The biological funcionts of PG are
complex and include some effects that may be related to mental
disorders, such as affective and anxiety disorders, through the
modulation of the hypothalamic-pituitary-adrenal (HPA) axis and the
monoaminergic system (40,41).
In the context of novel theories related to
depression, the active ingredients of PG have been demonstrated to
exert neuroprotective effects and to increase neuronal survival.
In vitro, treatment with ginsenosides has been shown to
increase survival and promote neuronal plasticity and neurogenesis
in dopaminergic cells (42).
In vivo, ginsenosides have been shown to reduce hypoxic
brain injury in rats, and to protect against toxic interventions in
Parkinson’s disease (43–45). A previous study demonstrated the
antidepressant effects of total ginseng saponin, which contains
several ingredients (46).
However, it is important to identify the active ingredient(s) that
improve the depression-like behavior. Rg1 has a molecular structure
similar to that of ginsenoside Rb3, which possesses antidepressant
properties, and has been reported to increase brain-derived
neurotrophic factor (BDNF) expression following focal cerebral
ischemia (47,48).
The present study provides direct evidence that an
extract of PG can significantly recover PNS-induced psychiatric
effects in an animal model. Although some prenatal manipulations in
rats, such as immune challenge, viral infection and protein
malnutrition, also recapitulate sensory gating abnormalities and
cognitive disturbances, only unpredictable PNS, hippocampal
lesioning and prenatal immune challenge generate social impairment
in mice (49–51). Impaired social interaction
behavior was observed in rats exposed to PNS rats at 35 days of
age, as well as in young adult rats exposed to PNS. One of the
first clinical signs associated with human schizophrenia is social
withdrawal during adolescence (52–54). The emergence of social withdrawal
in adolescent rats exposed to PNS appears to be consistent with the
clinical schizophrenia literature and further supports the
relevance of this model to the schizophrenia phenotype. This
diminution in social interaction behaviors may reflect an increase
in anxiety in rats epxosed to PNS (55). In our study, the PNS-induced
decrease in non-aggressive behavior was restored by oral treatment
with PG. In addition, some behavioral patterns from FST and OFT,
tests for the analysis of depressive behaviors, were recovered by
treatment with PG. In the present study, we investigated the levels
of Dpysl2 and neurofilament proteins. In the developing brain,
Dpysl2 regulates axonal outgrowth by promoting microtubule
assembly, vesicle trafficking and synaptic physiology (56–59). The expression of DPYSL2 in
humans has been reported to be decreased in the brains of patients
with schizophrenia (60).
Neurofilaments form part of the axon skeleton and functionally
maintain neuronal caliber. They may also play a role in
intracellular transport to axons and dendrites (61). These findings suggest that the
application of a repeated variable PNS paradigm during the critical
periods of fetal brain development results in changes the
expression of neurodevelopmental proteins, such as neurofilament
proteins and Dpysl2, that may have enduring effects on axonal
outgrowth and synaptic function in the offspring later, during
adulthood. In the present study, the decrease in the expression of
neurofilament proteins and Dpysl2 following epxosure to PNS was
shown to be reversed by treatment with PG. PNS during gestation has
been implicated in the pathology of various psychiatric disorders,
such as schizophrenia and depression.
The present study provides valuable data regarding
an additional role of PG in addressing the pathogenesis of
psychiatric disorders, such as schizophrenia. However, further
research using cellular and animal model systems is required to
fully characterize the pharmacological functions of PG.
Acknowledgements
This study was performed with the support of the
Cooperative Research Program for Agriculture Science and Technology
Development (PJ009559), Rural Development Administration,
Korea.
References
|
1
|
Brown AS1, van Os JC, et al: Further
evidence of relation between prenatal famine and major affective
disorder. Am J Psychiatry. 157:190–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sullivan PF: The genetics of
schizophrenia. PLoS Med. 2:e2122005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huttunen MO and Niskanen P: Prenatal loss
of father and psychiatric disorder. Arch Gen Psychiatry.
35:429–431. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
King M, Nazroo J, Weich S, et al:
Psychotic symptoms in the general population of England: a
comparison of ethnic groups (The EMPIRIC study). Soc Psychiatry
Psychiatr Epidemiol. 40:375–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
King S, Laplante D and Joober R:
Understanding putative risk factors for schizophrenia:
retrospective and prospective studies. J Psychiatry Neurosci.
30:342–348. 2005.PubMed/NCBI
|
|
6
|
Lim C, Chong SA and Keefe R: Psychosocial
factors in the neurobiology of schizophrenia: a selective review.
Ann Acad Med Singapore. 38:402–406. 2009.PubMed/NCBI
|
|
7
|
Imamura Y, Nakane Y, Ohta Y and Kondo H:
Lifetime prevalence of schizophrenia among individuals prenatally
exposed to atomic bomb radiation in Nagasaki City. Acta Psychiatr
Scand. 100:344–349. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyer U and Feldon J: Epidemiology-driven
neurodevelopmental animal models of schizophrenia. Prog Neurobiol.
90:285–326. 2010. View Article : Google Scholar
|
|
9
|
Weinstock M: The long-term behavioural
consequences of prenatal stress. Neurosci Biobehav Rev.
32:1073–1086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seckl JR: Prenatal glucocorticoids and
long-term programming. Eur J Endocrinol. 151:U49–U62. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Kloet ER, Sibug RM, Helmerhorst FM and
Schmidt MV: Stress, genes and the mechanism of programming the
brain for later life. Neurosci Biobehav Rev. 29:271–281. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beydoun H and Saftlas AF: Physical and
mental health outcomes of prenatal maternal stress in human and
animal studies: a review of recent evidence. Paediatr Perinat
Epidemiol. 22:438–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee PR, Brady DL, Shapiro RA, et al:
Prenatal stress generates deficits in rat social behavior: reversal
by oxytocin. Brain Res. 1156:152–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinnunen AK, Koenig JI and Bilbe G:
Repeated variable prenatal stress alters pre- and postsynaptic gene
expression in the rat frontal pole. J Neurochem. 86:736–748. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koenig JI, Elmer GI, Shepard PD, et al:
Stress during gestation produces alterations in adult rat behavior:
relevance to schizophrenia. Soc Neurosci abs. 495.6. 2002.
|
|
16
|
Koenig JI, Elmer GI, Shepard PD, et al:
Prenatal exposure to a repeated variable stress paradigm elicits
behavioral and neuroendocrinological changes in the adult
offspring: potential relevance to schizophrenia. Behav Brain Res.
156:251–261. 2005. View Article : Google Scholar
|
|
17
|
Hayashi A, Nagaoka M, Yamada K, et al:
Maternal stress induces synaptic loss and developmental
disabilities of offspring. Int J Dev Neurosci. 16:209–216. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lemaire V, Koehl M, Moal LM and Abrous DN:
Prenatal stress produces learning deficits associated with an
inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci
USA. 97:11032–11037. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martínez-Téllez RI, Hernández-Torres E,
Gamboa C and Flores G: Prenatal stress alters spine density and
dendritic length of nucleus accumbens and hippocampus neurons in
rat offspring. Synapse. 63:794–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van den Hove DL, Kenis G, Brass A, et al:
Vulnerability versus resilience to prenatal stress in male and
female rats; implications from gene expression profiles in the
hippocampus and frontal cortex. Eur Neuropsychopharmacol.
23:1226–1246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mairesse J, Vercoutter-Edouart AS,
Marrocco J, et al: Proteomic characterization in the hippocampus of
prenatally stressed rats. J Proteomics. 75:1764–1770. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee HY, Joo J, Nah SS, et al: Changes in
Dpysl2 expression are associated with prenatally stressed rat
offspring and susceptibility to schizophrenia in humans. Int J Mol
Med. (In press).
|
|
23
|
Vogler BK, Pittler MH and Ernst E: The
efficacy of ginseng. A systematic review of randomised clinical
trials. Eur J Clin Pharmacol. 55:567–575. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dan B and Andrew G: Chinese Herbal
Medicine. 8th edition. Eastland Press; Seattle, WA: pp. 110–113.
1993
|
|
25
|
Helms S: Cancer prevention and
therapeutics: Panax ginseng. Altern Med Rev. 9:259–274.
2004.PubMed/NCBI
|
|
26
|
Park JD, Rhee DK and Lee YH: Biological
activities and chemistry of saponins from Panax ginseng C. A.
Meyer. Phytochem Rev. 4:159–175. 2005. View Article : Google Scholar
|
|
27
|
Qi LW, Wang CZ and Yuan CS: Isolation and
analysis of ginseng: advances and challenges. Nat Prod Rep.
28:467–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen EY and Hui CL: HT1001, a proprietary
North American ginseng extract, improves working memory in
schizophrenia: a double-blind, placebo-controlled study. Phytother
Res. 26:1166–1172. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dulawa SC, Holick KA, Gundersen B and Hen
R: Effects of chronic fluoxetine in animal models of anxiety and
depression. Neuropsychopharmacology. 29:1321–1330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schroeder M, Sultany T and Weller A:
Prenatal stress effects on emotion regulation differ by genotype
and sex in prepubertal rats. Dev Psychobiol. 55:176–192. 2013.
View Article : Google Scholar
|
|
31
|
Axel B, Brigitte P, Helmut S, et al:
Ketamin-induced changes in tar behavior: a possible animal model of
schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry.
27:687–700. 2003. View Article : Google Scholar
|
|
32
|
Joo J, Lee S, Nah SS, et al: Lasp1 is
down-regulated in NMDA receptor antagonist-treated mice and
implicated in human schizophrenia susceptibility. J Psychiatr Res.
47:105–112. 2013. View Article : Google Scholar
|
|
33
|
Goshima Y, Nakamura F, Strittmatter P and
Strittmatter SM: Collapsin-induced growth cone collapse mediated by
an intracellular protein related to UNC-33. Nature. 376:509–514.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bahrke MS and Morgan WR: Evaluation of the
ergogenic properties of ginseng: an update. Sports Med. 29:113–133.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Radad K, Gille G, Liu L and Rausch WD: Use
of ginseng in medicine with emphasis on neurodegenerative
disorders. J Pharmacol Sci. 100:175–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu CX and Xiao PG: Recent advances on
ginseng research in China. J Ethnopharmacol. 36:27–38. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baek NI, Kim DS, Lee YH, et al:
Ginsenoside Rh4, a genuine dammarane glycosidefrom Korean red
ginseng. Planta Med. 62:86–87. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Christensen LP: Ginsenosides chemistry,
biosynthesis, analysis and potential health effects. Adv Food Nutr
Res. 55:1–99. 2009. View Article : Google Scholar
|
|
40
|
Kim DH, Moon YS, Jung JS, et al: Effects
of ginseng saponin administered intraperitoneally on the
hypothalamo-pituitary-adrenal axis in mice. Neurosci Lett.
343:62–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fugh-Berman A and Cott JM: Dietary
supplements and natural products as psychotherapeutic agents.
Psychosom Med. 61:712–728. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Radad K, Gille G, Moldzio R, et al:
Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic
cells stressed with glutamate. Brain Res. 1021:41–53. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park EK, Choo MK, Oh JK, et al:
Ginsenoside Rh2 reduces ischemic brain injury in rats. Biol Pharm
Bull. 27:433–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ji YC, Kim YB, Park SW, et al:
Neuroprotective effect of ginseng total saponins in experimental
traumatic brain injury. J Korean Med Sci. 20:291–296. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Van Kampen J, Robertson H, Hagg T and
Drobitch R: Neuroprotective actions of the ginseng extract G115 in
two rodent models of Parkinson’s disease. Exp Neurol. 184:521–529.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dang H, Chen Y, Liu X, et al:
Antidepressant effects of ginseng total saponins in the forced
swimming test and chronic mild stress models of depression. Prog
Neuropsychopharmacol Biol Psychiatry. 33:1417–1424. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cui J, Jiang L and Xiang H: Ginsenoside
Rb3 exerts antidepressant-like effects in several animal models. J
Psychopharmacol. 697–713. 2011.PubMed/NCBI
|
|
48
|
Shen L and Zhang J: Ginsenoside Rg1
increases ischemia-induced cell proliferation and survival in the
dentategyrus of adult gerbils. Neurosci Lett. 344:1–4. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Borrell J, Vela JM, Arevalo-Martin A, et
al: Prenatal immune challenge disrupts sensorimotor gating in adult
rats. Implications for theetiopathogenesis of schizophrenia.
Neuropsychopharmacology. 26:204–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zuckerman L, Rehavi M, Nachman R and
Weiner I: Immune activation during pregnancy in rats leads to a
postpubertal emergence of disrupted latent inhibition, dopaminergic
hyperfunction, and altered limbic morphology in the offspring: a
novel neurodevelopmental model of schizophrenia.
Neuropsychopharmacology. 28:1778–1789. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Palmer AA, Printz DJ, Butler PD, et al:
Prenatal protein deprivation in rats induces changes in prepulse
inhibition and NMDA receptor binding. Brain Res. 996:193–201. 2004.
View Article : Google Scholar
|
|
52
|
Kelley ME, Gilbertson M, Mouton A and van
Kammen DP: Deterioration in premorbid functioning in schizophrenia:
a developmental model of negative symptoms in drug-free patients.
Am J Psychiatry. 149:1543–1548. 1992.PubMed/NCBI
|
|
53
|
Moller P and Husby R: The initial prodrome
in schizophrenia: searching for naturalistic core dimensions of
experience and behavior. Schizophr Bull. 26:217–232. 2000.
View Article : Google Scholar
|
|
54
|
Cornblatt BA: The New York high risk
project to the Hillside recognition and prevention (RAP) program.
Am J Med Genet. 114:956–966. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Weinstock M: Alterations induced by
gestational stress in brain morphology and behavior of the
offspring. Prog Neurobiol. 65:427–451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Arimura N, Menager C, Fukata Y and
Kaibuchi K: Role of CRMP-2 in neuronal polarity. J Neurobiol.
58:34–47. 2004. View Article : Google Scholar
|
|
57
|
Lin PC, Chan PM, Hall C and Manser E:
Collapsin response mediator proteins (CRMPs) are a new class of
microtubule-associated protein (MAP) that selectively interacts
with assembled microtubules via a taxol-sensitive binding
interaction. J Biol Chem. 286:41466–41478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Higurashi M, Iketani M, Takei K, et al:
Localized role of CRMP1 and CRMP2 in neurite outgrowth and growth
cone steering. Dev Neurobiol. 72:1528–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brittain JM, Piekarz AD, Wang Y, et al: An
atypical role for collapsin response mediator protein 2 (CRMP-2) in
neurotransmitter release via interaction with presynaptic
voltage-gated calcium channels. J Biol Chem. 284:31375–1390. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Johnston-Wilson NL, Sims CD, Hofmann JP,
et al: Disease-specific alterations in frontal cortex brain
proteins in schizophrenia, bipolar disorder, and major depressive
disorder. The Stanley Neuropathology Consortium. Mol Psychiatry.
5:142–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cassereau J, Nicolas G, Lonchampt P, et
al: Axonal regeneration is compromised in NFH-LacZ transgenic mice
but not in NFH-GFP mice. Neuroscience. 228:101–108. 2013.
View Article : Google Scholar
|