Introduction
Endocrine disruption has become a critical issue in
environmental science, particularly after the endocrine-disrupting
chemical pollution accident in Taiwan which attracted public
attention worldwide (1).
Consequently, over the last few years, multi-disciplinary research
programmes regarding this issue have been conducted in several
countries. Several environmental chemicals with anti-androgenic
activities that have the potential to disrupt normal male sexual
differentiation in utero have recently been identified
(2,3). The detection of chemicals with the
potential to disrupt normal androgen function is critical for the
protection of human and ecological health.
The androgen receptor (AR), a member of the nuclear
receptor superfamily, is composed of 919 amino acids (4). It contains an N-terminal
transactivation domain, a central DNA-binding domain and a
C-terminal ligand-binding domain (5). AR may form a dimer and interact with
many co-regulators to modulate androgen target genes (6). Previous studies have demonstrated
that AR is involved in a series of developmental and physiological
functions, particularly in male sexual differentiation (7), the maintenance of adolescent sexual
maturation (8), sperm production
(9) and male sex hormone
regulation (10). The function of
androgens is mediated by the AR (11). Research has indicated that, during
the process of sexual development, the number and activity of ARs
has direct effects on target organs, and thus plays a crucial role
in the onset of hypospadias (12). Male sexual differentiation is the
result of complex mechanisms involving developmental genetics and
endocrinology. Hormone function is mediated through specific
receptors, functioning as transcriptional regulators. The
disruption of these genetic events leads to abnormal sexual
dimorphism involving both external and internal genitalia, and may
also interfere with the development of other organs (13).
Transgenic and knockout mice have long been used as
animal models to study the function of genes in vivo.
Testicular feminization in male mice and androgen insensitivity
syndrome in human male patients are the common models used for the
study of the loss of androgen function (14). To generate tissue-specific AR
knockout (ARKO) mice or female ARKO mice, a Cre-loxP strategy for
conditional knockout is required. The Cre-loxP system utilizes the
expression of P1 phage cre recombinase (Cre) to catalyze the
expression of DNA located between flanking loxP sites (15). This strategy differs from the
standard targeted gene disruption procedure in that the embryonic
stem (ES) cells are generated in which the targeted segment is not
disrupted but flanked by loxP sites (floxed). Thus, the target gene
functions normally and mice can be bred which are homozygous at the
targeted locus.
In the present study, we describe the generation and
characterization of ARKO mice. The potential in vivo
application of this mouse model for the study of the effects of
environmental endocrine disruptors (EEDs) on sexual differentiation
in AR−/−, AR+/− and AR+/+ male
mice is also discussed. We aimed to examine the role of EDDs in the
third stage of sexual differentiation through the regulation of the
expression of Wilms tumor 1 (WT1), lutropin/choriogonadotropin
receptor (LHR), 17-β-hydroxysteroid dehydrogenase type 3 (17βHSD3)
and steroid-5-alpha-reductase, alpha polypeptide 2 (SRD5A2) genes
in AR−/−, AR+/− and AR+/+ male
mice. The results of the present study may provide a theoretical
basis for the development of future preventive strategies to reduce
EED contact.
Materials and methods
All experiments were approved by the Ruijin Hospital
Ethics Committee and were performed in accordance with ethical
standards.
Materials
C57BL/6 mice, 35–40 days old, weighing 18–22 g, were
supplied by the Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). The mice were raised in an air-conditioned room
under controlled lighting and were fed standard laboratory chow and
water ad libitum. DNA polymerase was purchased from Toyobo
Co. (Osaka, Japan), proteinase K was from Merck (Billerica, MA,
USA) and kits for the detection of testosterone and estradiol were
purchased from Amersham Biosciences Corp. (Piscataway, NJ, USA).
Immunohistochemical staining kits were purchased from CapitalBio
Corp. (Beijing, China) and bisphenol A (BPA) and D binding protein
(DBP) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies to LHR, 17βHSD3 and SRD5A2 were from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Construction of targeting vector
Amhr2-Cre
We designed the PCR primer 1 according to the
Amhr2 promoter sequence previously reported (16). Genomic DNA from blood collected
from the C57BL/6 mice was isolated as a template for PCR
amplification. The amplified fragment has an expected size of 5 kb
and was subcloned to the pMD18-T plasmid, and named Amhr5K-P-TV for
further sequencing. The universal primer 2 was used to perform the
amplification of bands of approximately 1 kb from Amhr5K-P-TV, and
universal primer 3 was used to yield amplification bands of Cre
approximately1 kb from the plasmid Aluminum-cre; both fragments
were recycled. Recycling DNA was used as a template, and the
reverse sequence of primer 2 and the forward sequence of primer 3
were used to perform the amplification of the 2 kb strip. The
recycled 2 kb strip was digested with the HindIII and
XbaI enzymes, and ligated with the pGL3-Basic plasmid
digested with the same enzymes for further transfection. The
identification of positive clones was carried out by PCR and the
clone was named Amhr-1k CRE4. A fragment of 3 kb was amplified from
plasmid Amhr5K-P-TV with primer 4, digested with the MluI
and EcoRI enzymes and subcloned into the Amhr-1k-CRE4 clone
(primer information is presented in Table I). Identification of positive
clones was carried out by PCR and the clone was named Amhr-4k-CRE6
(8.2 KB). Following digestion with the EcoR, BamHⅠ,
EcoRV and ScaⅠ enzymes, a promoter of 7.1 Kb was
obtained which was connected to plasmid pRCH. The identification of
the plasmid containing leydig cells expressing Cre recombinant
enzyme and the specificity of the genetically modified (gm)
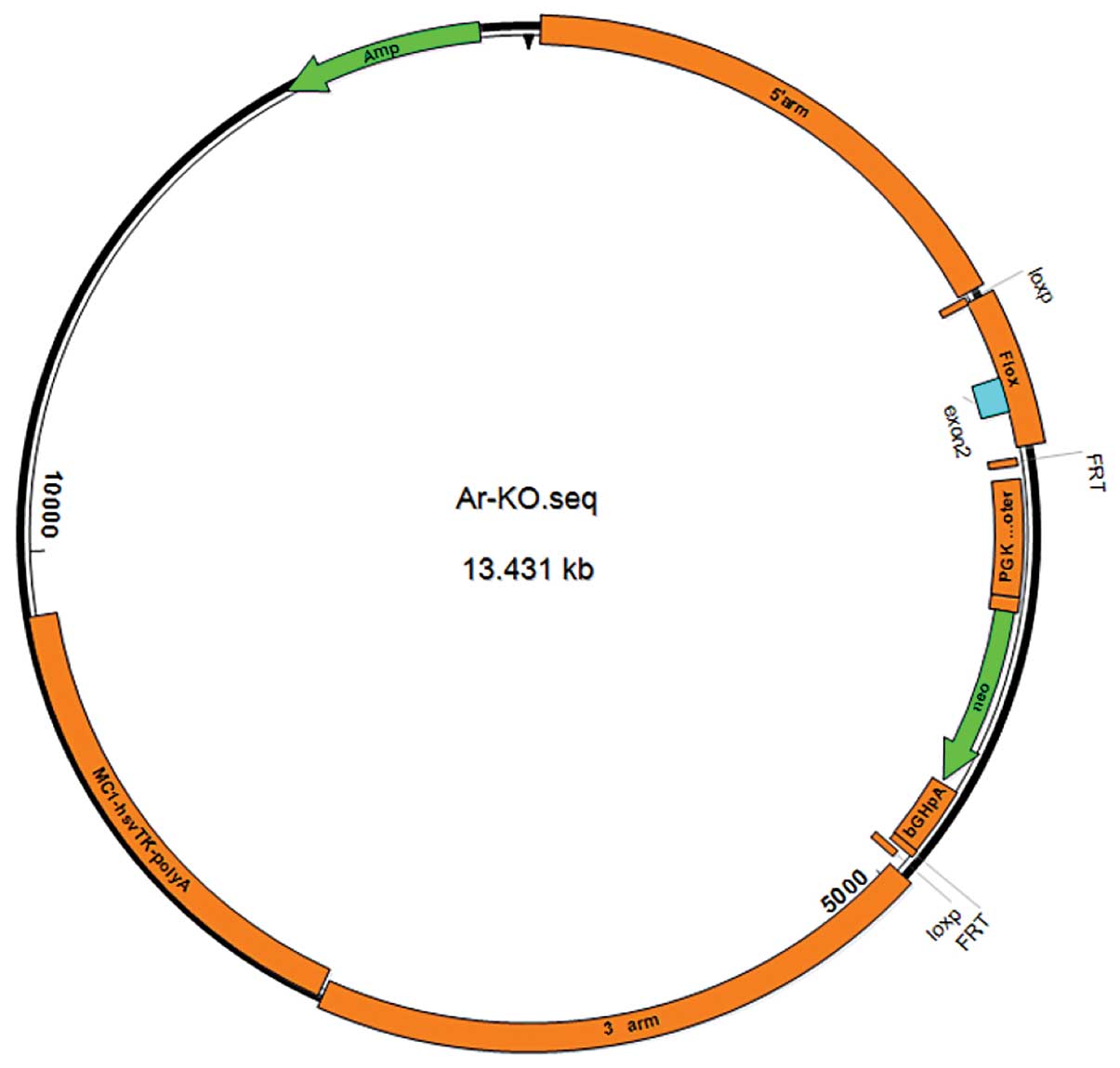
sequence [Amhr2-Cre (7.1)] are shown in Fig. 1.
 | Table IPrimers used for the construction of
target vectors, Amhr2-Cre. |
Table I
Primers used for the construction of
target vectors, Amhr2-Cre.
| Primer | Sequence |
|---|
| Primer 1 | Forward:
5′-AAAAGGACATTAAGACCACATAAT-3′
Reverse: 5′-GAAGCAGTGTCCAAAGCCCCCATG-3′ |
| Primer 2 | Forward:
5′-CTCCAAGCTTCCTCTGCCTCTTGAGT-3′
Reverse: 5′-TGTACGGTCAGTAAATTGGACATAAACCAGCAAAAACCAG-3′ |
| Primer 3 | Forward:
5′-CTGGTTTTTGCTGGTTTATGTCCAATTTACTGACCGTACA-3′
Reverse: 5′-AATCTCTAGACTAATCGCCATCTTCCAGCA-3′ |
| Primer 4 | Forward:
5′-TACGACGCGTGCATCTGCCACTGTGCCTGG-3′
Reverse: 5′-CAGCCCGGACCGACGATGAA-3′ |
The microinjection of fertilized
eggs
With the use of KpnI and SacI double
enzyme digestion to construct the transgenic plasmid, the injection
fragments were recycled. According to the conventional method, we
performed microinjection and transplantation of the fertilized
eggs.
Genotypic identification of genetically
modified mice
Genomic DNA was extracted from the gonadal tissue of
mice for PCR analysis. According to the gene sequence upstream and
downstream of the Cre enzyme gene, we designed a pair of primers
(primer 5: forward, TCTGTAGACTCTAGGCAGTTCCTGT and reverse,
CAGCCCGGACCGACGATGAA). We employed this primer set to propagate the
Cre enzyme gene of approximately 2,115 bp. The PCR reaction
conditions were as follows: 95°C for 3 min; followed by 26 cycles
of 95°C for 15 sec (denaturation) and 58°C (annealing temperature),
72°C for 2 min (elongation) and 72°C for 10 min.
Heterozygous female mice mating with
Amhr2-Cre transgenic mice, the experimental groups and exposure to
EEDs
Heterozygous female mice mated with Amhr2-Cre
transgenic mice bred heterozygous mice of the F1 generation. The F1
generation was then backcrossed to C57BL/6 mice for 2 generations,
which then produced pure strains of heterozygous mice. F1
generation transgenic mice were selfed, producing homozygous mice
with a clear genetic background. Heterozygous female mice were
mated with homozygous male mice and became pregnant. The mice were
divided into the following groups: the control group (no
intervention), the BPA group (100 mg/l/day, by gavage) and the DBP
group (100 mg/kg/day, by gavage). The pregnant heterozygous female
mice were exposed to EEDs (mice were administered either DBP at 100
mg/kg/day or BPA at 100 mg/l/day).
Reverse transcription-quantitative
(real-time) PCR (RT-qPCR)
Total cellular RNA was prepared using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and the
expression levels of WT1, LHR, 17βHSD3 and SRD5A2 were determined
by real-time PCR using SYBR-Green. The data were normalized to
GAPDH expression and represent the average of 3 independent
experiments. The primer sequences were as follows: WT1
forward, 5′-CAAATGACATCCCAGCTTGA-3′ and reverse
5′-GACACCGTGCGTGTGTATTC-3′; LHR forward,
5′-ATATTCAAGAGATGCACTGTGCAG-3′ and reverse,
5′-AAGCAGAGTGTCAATGGGAAATAG-3′.
Western blot analysis
Western blot analysis was performed as previously
described (17). Cell lysates
were subjected to SDS-polyacrylamide gel electrophoresis and
immunoblot analysis with antibodies to WT1, LHR, 17βHSD3 and SRD5A2
(all from Santa Cruz Biotechnology). Radioiodinated Staphylococcus
protein A (IPA) was used as the antibody and β-actin was used as a
control for normalization.
Results
Construction of transgenic mouse model
(Amhr2-Cre)
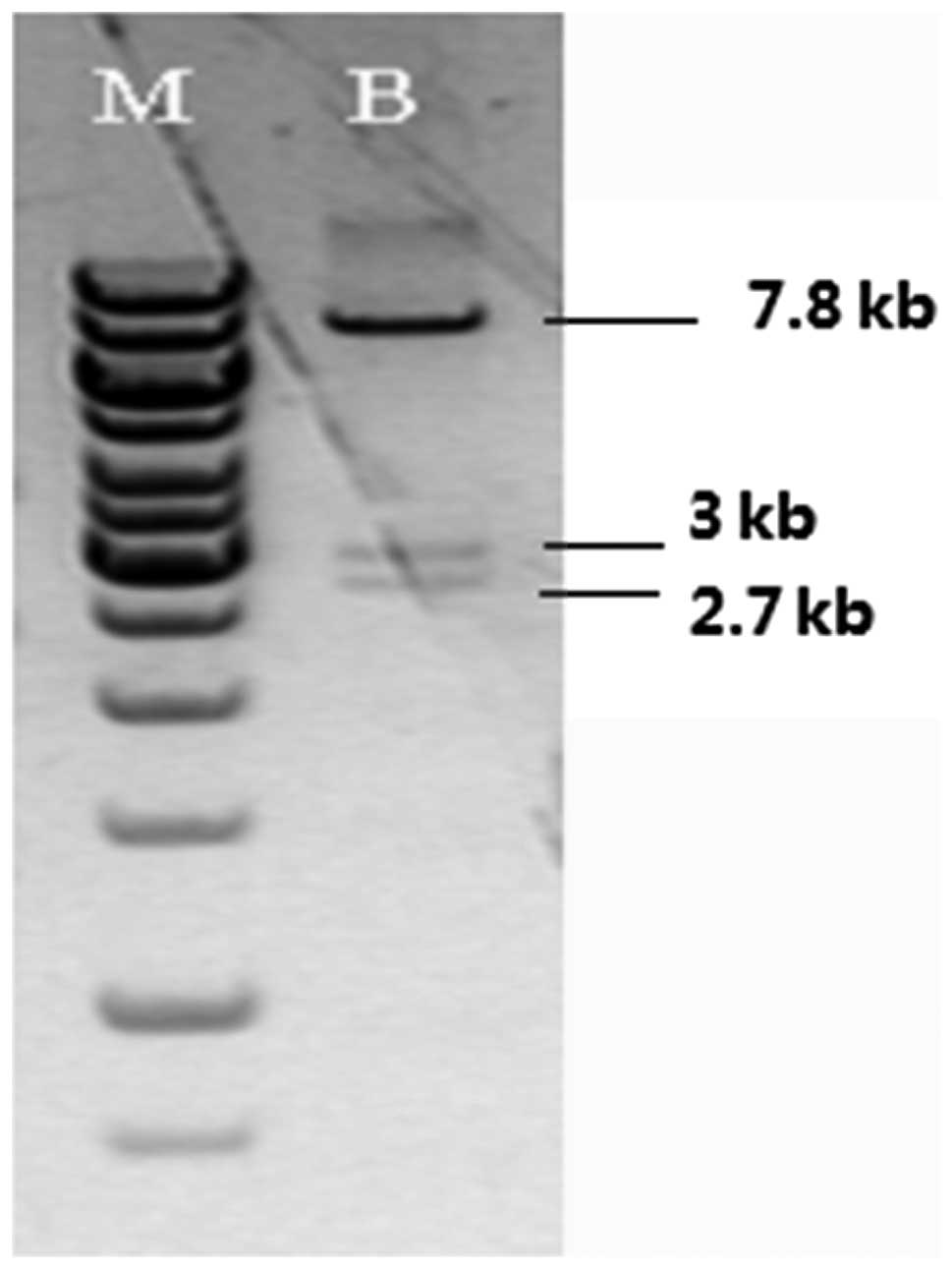
The AR gene condition knockout plasmid was
identified and confirmed by enzyme digestion with BamHI.
Fig. 2 illustrates that 3
fragments with a size of 2.7, 3 and 2.7 kb were visible by agarose
gel electrophoresis.
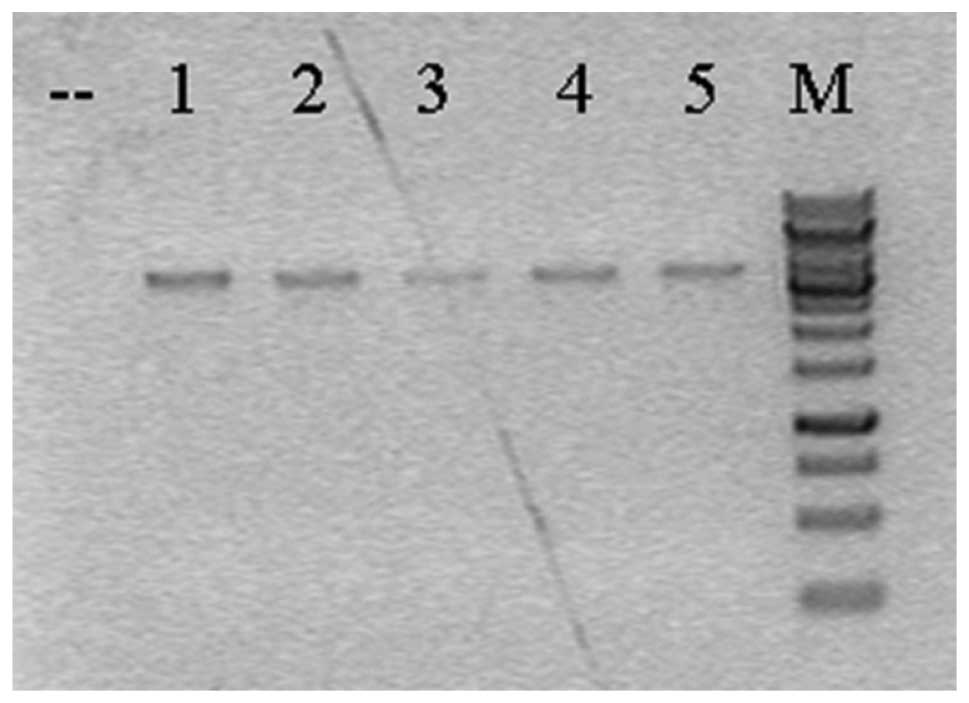
Breeding of chimeric mice
A total of 96 drug-resistant ES cell clones was
identified by PCR; homologous recombination arms occurred in 5 ES
clones (Fig. 3). PCR products
confirmed by DNA sequencing further pointed out that there were
only 2 positive clones, and these 2 positive ES clone blastocysts
were injected and implanted in the uterus of pseudo-pregnant mice.
The mice with a chimeric rate >50% were mated with C57BL/6 mice;
thus, 6 F1 generation female mice with 2 ‘positive arms’ were
produced.
Comparison between AR−/− male
mice and normal mice
Maldevelopment, i.e. enlargement of the prostate,
the seminal vesicles, the epididymis and the sponge balls was
observed in the knockout mice. By contrast, in the control group,
the prostate, the seminal vesicles, the epididymis and the ball
sponges were normally developed. Comparisons regarding the
anogenital distance, testicular weight, blood testosterone and
estradiol concentrations were made between the control group and
the knockout group as shown in Table
II. There was no statistically significant difference in weight
between these 2 groups (P>0.05). Compared with the control
group, in the knockout group, the anogenital distance was
significantly shortened, the testicular weight was significantly
reduced, the testosterone levels were decreased and the estradiol
levels were elevated; the differences were statistically
significant (P<0.05).
 | Table IIComparison of anogenital distance,
testicular weight, blood testosterone levels and estradiol
concentration between the 2 groups. |
Table II
Comparison of anogenital distance,
testicular weight, blood testosterone levels and estradiol
concentration between the 2 groups.
| Index | Control group | Knockout group |
|---|
| Weight (g) | 22.3±2.1 | 21.2±1.3 |
| Anogenital distance
(cm) | 1.2±0.1 | 0.5±0.1 |
| Testicular weight
(g) | 0.087±0.002 | 0.005±0.001 |
| Blood testosterone
(nmol/l) | 0.87±0.533 | 0.054±0.043 |
| Estradiol
concentration (nmol/l) | 796±130 | 1386±280 |
Heterozygoous mice mated with Amhr2-Cre
transgenic mice, the experimental groups and exposure to EEDs
In the group of AR+/− male mice
administered BPA (100 mg/l/day, by gavage) and DBP (100 mg/kg/day,
by gavage) hypospadias was successfully induced, while the
AR−/− mice did not have hypospadias. The detailed
information is presented in Table
III.
 | Table IIIIncidence of hypospadias in
AR+/− and AR−/− male mice exposed to
EEDs. |
Table III
Incidence of hypospadias in
AR+/− and AR−/− male mice exposed to
EEDs.
| Exposure to
EEDs | AR+/−
male mice/cases of hypospadias | AR−/−
male mice/cases of hypospadias |
|---|
| BPA group | 32/30 | 31/0 |
| DBP group | 29/26 | 33/0 |
Disorders of sexual development in male
mice following exposure to EEDs
The 10-week-old offspring mice were euthanized by
anesthesia (in mice, testicular descent into the scrotum occurs at
45–55 days, sexual maturity is reached in approximatley 70 days).
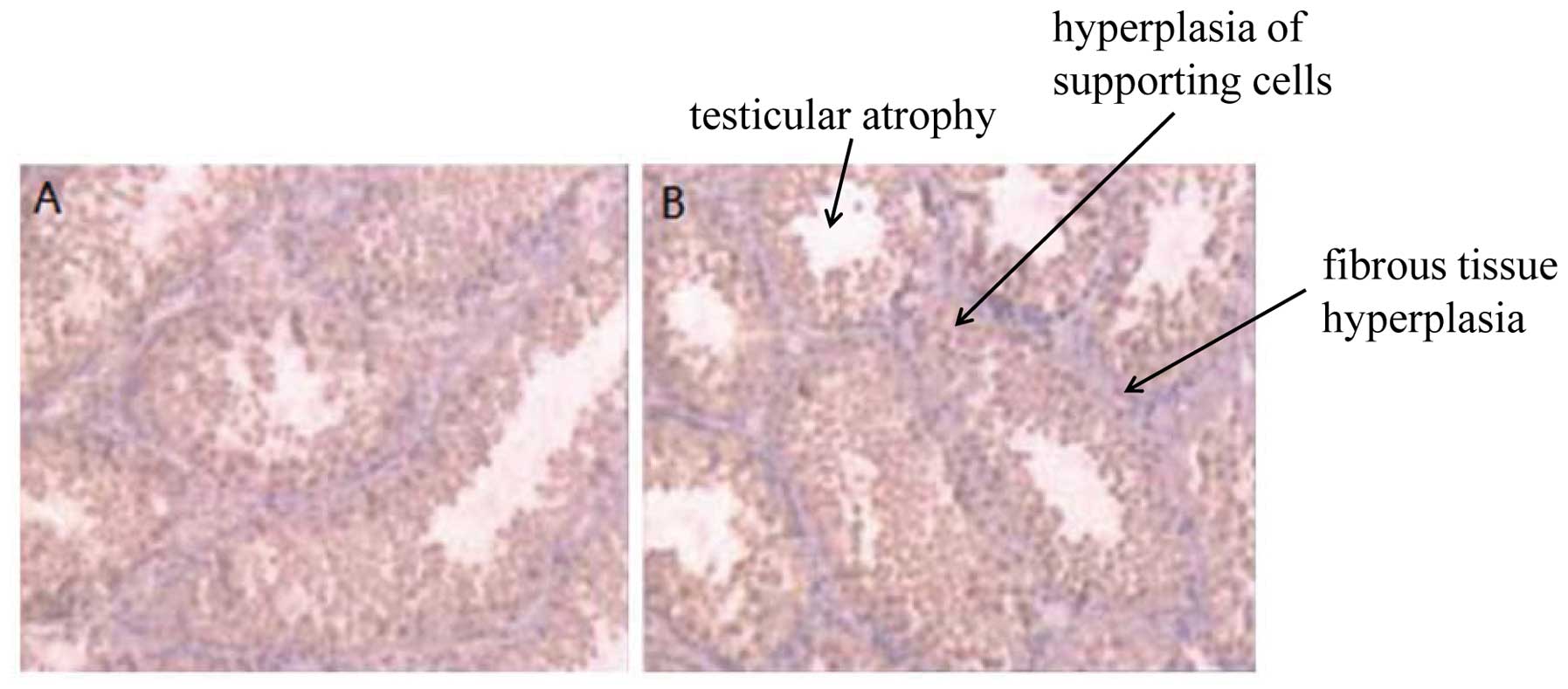
Secondary sexual differentiation, cryptorchidism, genital
malformation, testicular atrophy, hyperplasia of supporting cells
and fibrous tissue hyperplasia were observed in the male
heterozygous mice exposed to EEDs (Fig. 4). These results suggest that EEDs
are involved in the embryonic stage of the sexual development of
male mice and contribute to the occurrence of disorders of sexual
developmental during the embryonic stage.
Exposure to EEDs downregulates gene
expression in mice
Testicular tissue-specific expression was analyzed
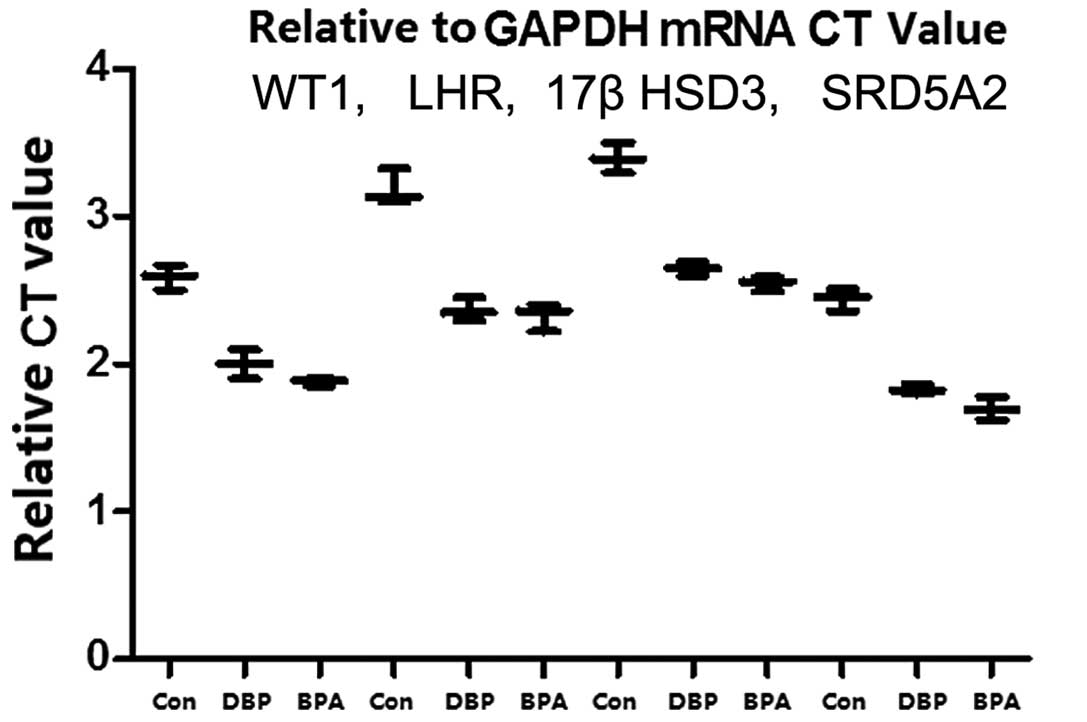
by RT-qPCR. The quantitative detection of WT1, LHR, 17βHSD3 and
SRD5A2 gene expression in the mice exposed to EEDs (BPA and DBP)
demonstrated that the expression of the aforementioned 4 genes was
lower than that observed in the control group (non-exposed mice).
The mRNA expression levels of WT1, LHR, 17βHSD3 and SRD5A2 in
testicular tissue were the lowest in the BPA group and the highest
in the control group (Fig. 5).
These results suggest that EEDs are involved in the embryonic stage
of the sexual development of male mice and contribute to the
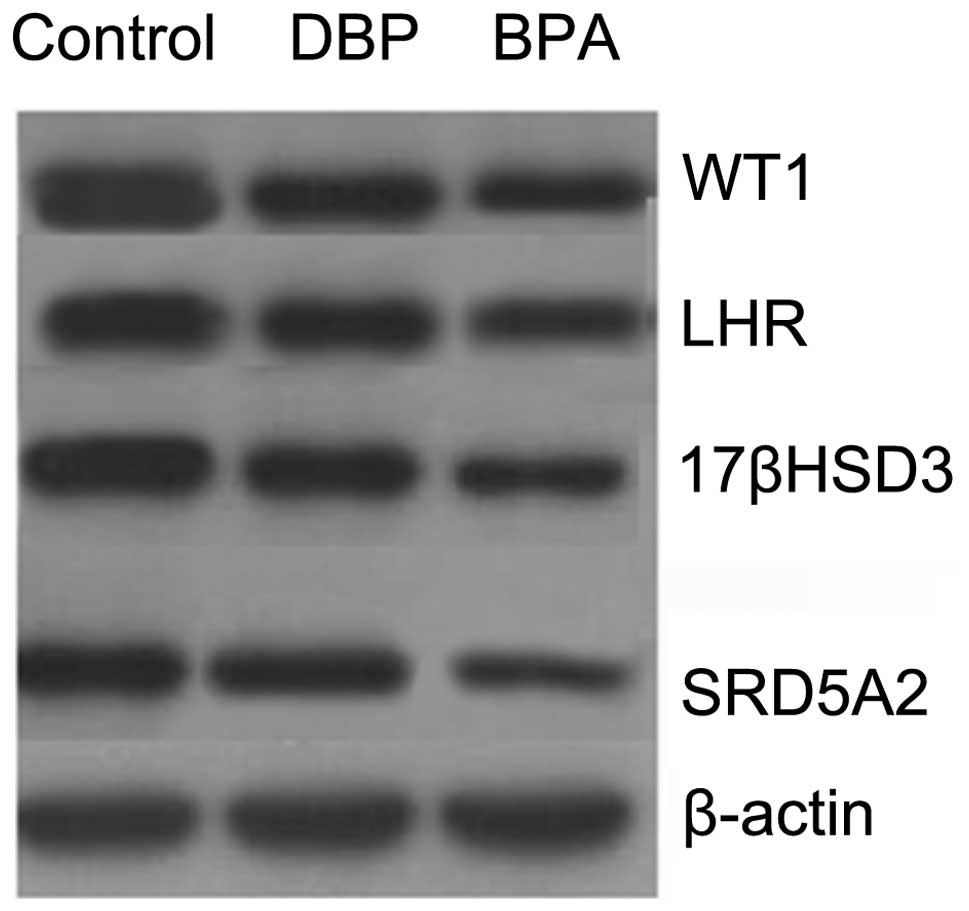
occurrence of disorders of sexual development. Western blot
analysis was used for the detection of the protein expression of
WT1, LHR, 17βHSD3 and SRD5A2 in testicular tissue. The results
revealed that in the BPA and DBP groups, the expression levels of
of the aforementioned 4 genes were lower than those in the control
group. The protein expression levels of WT1, LHR, 17βHSD3 and
SRD5A2 in testicular tissue were the lowest in the BPA group and
the highest in the control group (Fig. 6).
Discussion
In recent years, the incidence of hypospadias has
been gradually increasing. A great amount of research on a wide
range of aspects, from the epidemiological to the genetic aspects
of hypospadias, has been carried out (18,19). However, no consensus has yet been
reached on this issue. Previous studies have suggested that the
understanding of the normal development of the male external
urethral orifice is an important step towards the understanding of
the development of hypospadias (20,21). Research focusing on the normal
embryonic development of the penile urethra has formed a basis for
understanding the development and pathogenesis of hypospadias
(22,23). By comparing the development of the
urethra between humans and mice, scientists have found that the
growth and development of the urethra are very similar between the
two species during urethral seam formation (24). However, further studies are
required to determine the association between AR expression and
androgen signaling in males.
An increase in the incidence of hypospadias was
reported in 1975 by the then newly established Norwegian Birth
Defects Monitoring System (25).
While the number of cases in that study was small (25), a continuation of this initial
increase was subsequently reported by another study (26). Czeizel examined the rates of
isolated hypospadia cases in Hungary between 1971 and 1983 and
found that the incidence had increased significantly during this
time period (P<0.01) (27).
Matlai and Beral (28) examined
the rates of malformations reported at birth and found a
significant increase in cases of cryptorchidism, hypospadias and
hydrocele between 1969 and 1983 in England and Wales (all P-values
<0.001). Owing to the aggravating environmental pollution, the
issue of developmental deformities has attracted public attention.
However, the damage or defects that EEDs cause to the male
reproductive system require further investigation. A number of
scholars believe that the incidence of hypospadias in recent
decades in males is strongly associated with the significant
increase in exposure to EEDs, such as BPA and DBP, which exist in
high levels in the environment (29,30). Existing research indicates that
the male reproductive system is the target organ of EEDs, and that
exposure to EEDs may result in significantly lower testosterone
levels and can cause hypospadias, cryptorchidism, dysplasia of the
epididymis and other male urogenital disorders (31).
Exposure to EEDs during the third stage of sexual
development negatively affects the expression of genes related to
male sexual development, such as WT1, LHR, 17βHSD3 and SRD5A2 at
the transcriptional and translational level. The transcription
factors, SF-1 and WT1, play a pivotal role in mammalian gonadal
development and sexual differentiation. In human embryos, both SF-1
and WT1 are expressed when the gonadal ridge first forms at 32 days
post-ovulation (32). As the sex
cords develop, providing morphological evidence of testis
differentiation, SF-1 localizes predominantly to developing Sertoli
cells in the sex cords, whereas WT1 retains a broader pattern of
expression (33). At later
stages, SF-1 predominantly localizes to steroidogenic Leydig cells,
and WT1 localizes to the sex cords (33). In the ovaries, SF-1 and WT1
transcripts persist in the gonadal ridge from the earliest
developmental stages throughout the critical period of sex
determination (33). Human male
sexual development is regulated by chorionic gonadotropin and
luteinizing hormone. The aberrant sexual development caused by both
activating and inactivating mutations of human luteinizing hormone
receptor (LHR) has been described. Constitutive activity of the LHR
causes LH releasing hormone-independent precocious puberty in boys
and the autosomal dominant disorder, familial male-limited
precocious puberty (34). The
17βHSD3 gene on chromosome 9q22 contains 11 exons. Defects in the
conversion of androstenedione to testosterone in the fetal testes
by the enzyme, 17beta-hydroxysteroid dehydrogenase (17β-HSD), give
rise to instances of individuals having female external genitalia
despite being genetically male (35). Androgen production increases to
normal progressively, so that testosterone and dihydrotestosterone
concentrations are sufficiently high to gradually induce somatic
and genital civilization, thus enabling an adequate male gender
function (36). Prospective
studies have suggested that the risk of developing prostate cancer
may be increased in association with high serum concentrations of
free testosterone and androstanediol glucuronide (Adiol G)
(37,38). Polymorphisms have been identified
in the 17-hydroxylase cytochrome P450 gene (CYP17) and SRD5A2, two
genes that are involved in the biosynthesis and metabolism of
androgens in males (39).
In conclusion, we successfully developed Amhr2-Cre
genetically modified mice. Recombination between the LoxP loci in
the genome for the application of the Cre-LoxP system was used to
generate the knockout mice. Gene expression in testicular tissue
was examined to determine its association with the development of
hypospadias, cryptorchidism, small penis and testicles and other
pediatric urological disorders. Our data indicate that the Cre
recombinase is expressed in testicular tissue in Amhr2-Cre
transgenic mice. These mice may serve as a useful tool for
generating testis-specific gene knockout mice. The role of Cre
recombinase in the development of disorders of the reproductive
system may provide the ideal genetic tools for future research.
Acknowledgments
The present study was supported in part by grants
from the National Natural Science Foundation of China (30700830),
the Shanghai Hospital Science and Technology Resource Sharing
Program Funded by the Shanghai Shenkang Hospital Development Center
(SHDC12007708) and the Science and Technology Fund of Shanghai
JiaoTong University School of Medicine (grant no. 06XJ21022). We
thank the Shanghai Institute of Biochemistry and Cell Biology
(SIBCB), the Shanghai Institutes for Biological Sciences (SIBS),
the Chinese Academy of Sciences (CAS) and the Shanghai Research
Center For Model Organisms. We are grateful to Yuan-Chang Yan,
Yunbin Zhang, Jinjin Wang, Weijue Xu and Su Yan for their
assistance in biochemical analysis and the animal experiments.
References
|
1
|
Yang J, Hauser R and Goldman RH: Taiwan
food scandal: the illegal use of phthalates as a clouding agent and
their contribution to maternal exposure. Food Chem Toxicol.
58:362–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Ye L, Wang X, et al: In silico
investigations of anti-androgen activity of polychlorinated
biphenyls. Chemosphere. 92:795–802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan C, Wang Q, Liu YP, et al:
Anti-androgen effects of the pyrethroid pesticide cypermethrin on
interactions of androgen receptor with corepressors. Toxicology.
311:178–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steketee K, Timmerman L, Ziel-van der Made
AC, Doesburg P, Brinkmann AO and Trapman J: Broadened ligand
responsiveness of androgen receptor mutants obtained by random
amino acid substitution of H874 and mutation hot spot T877 in
prostate cancer. Int J Cancer. 100:309–317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jenster G, van der Korput HA, Trapman J
and Brinkmann AO: Identification of two transcription activation
units in the N-terminal domain of the human androgen receptor. J
Biol Chem. 270:7341–7346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J and Al-Azzawi F: Mechanism of
androgen receptor action. Maturitas. 63:142–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bao AM and Swaab DF: Sexual
differentiation of the human brain: relation to gender identity,
sexual orientation and neuro-psychiatric disorders. Front
Neuroendocrinol. 32:214–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raznahan A, Lee Y, Stidd R, et al:
Longitudinally mapping the influence of sex and androgen signaling
on the dynamics of human cortical maturation in adolescence. Proc
Natl Acad Sci USA. 107:16988–16993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lazaros L, Xita N, Takenaka A, Sofikitis
N, Makrydimas G, Stefos T, Kosmas I, Zikopoulos K, Hatzi E and
Georgiou I: Semen quality is influenced by androgen receptor and
aromatase gene synergism. Hum Reprod. 27:3385–3392. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarachana T, Xu M, Wu RC and Hu VW: Sex
hormones in autism: androgens and estrogens differentially and
reciprocally regulate RORA, a novel candidate gene for autism. PloS
One. 6:e171162011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heinlein CA and Chang C: Androgen receptor
(AR) coregulators: an overview. Endocr Rev. 23:175–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilhelm D and Koopman P: The makings of
maleness: towards an integrated view of male sexual development.
Nat Rev Genet. 7:620–631. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiort O and Holterhus PM: The molecular
basis of male sexual differentiation. Eur J Endocrinol.
142:101–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeh S, Tsai MY, Xu Q, et al: Generation
and characterization of androgen receptor knockout (ARKO) mice: an
in vivo model for the study of androgen functions in selective
tissues. Proc Natl Acad Sci USA. 99:13498–13503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holt CL and May GS: A novel phage λ
replacement Cre-lox vector that has automatic subcloning
capabilities. Gene. 133:95–97. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teixeira J, Kehas DJ, Antun R and Donahoe
PK: Transcriptional regulation of the rat Müllerian inhibiting
substance type II receptor in rodent Leydig cells. Proc Natl Acad
Sci USA. 96:13831–13838. 1999. View Article : Google Scholar
|
|
17
|
Burnette WN: ‘Western blotting’:
electrophoretic transfer of proteins from sodium dodecyl
sulfate-polyacrylamide gels to unmodified nitrocellulose and
radiographic detection with antibody and radioiodinated protein A.
Anal Biochem. 112:195–203. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carmichael SL, Shaw GM, Nelson V, Selvin
S, Torfs CP and Curry CJ: Hypospadias in California: trends and
descriptive epidemiology. Epidemiology. 14:701–706. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalfa N, Philibert P and Sultan C: Is
hypospadias a genetic, endocrine or environmental disease, or still
an unexplained malformation? Int J Androl. 32:187–197. 2009.
View Article : Google Scholar
|
|
20
|
Rey RA and Grinspon RP: Normal male sexual
differentiation and aetiology of disorders of sex development. Best
Pract Res Clin Endocrinol Metab. 25:221–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ching ST, Cunha GR, Baskin LS, Basson MA
and Klein OD: Coordinated activity of Spry1 and Spry2 is required
for normal development of the external genitalia. Dev Biol.
386:1–11. 2014. View Article : Google Scholar
|
|
22
|
Qiao L, Tasian GE, Zhang H, et al:
Androgen receptor is overexpressed in boys with severe hypospadias,
and ZEB1 regulates androgen receptor expression in human foreskin
cells. Pediatr Res. 71:393–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vottero A, Minari R, Viani I, et al:
Evidence for epigenetic abnormalities of the androgen receptor gene
in foreskin from children with hypospadias. J Clin Endocrinol
Metab. 96:E1953–E1962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhoj EJ, Ramos P, Baker LA, et al: Human
balanced translocation and mouse gene inactivation implicate
Basonuclin 2 in distal urethral development. Eur J Hum Genet.
19:540–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller JR: Some epidemiological aspects of
teratogen detection. Mutat Res. 33:45–54. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parker A, Newell KW, Torfs M and Israel E:
Appropriate tools for health care: developing a technology for
primary health care and rural development. WHO Chron. 31:131–137.
1977.PubMed/NCBI
|
|
27
|
Czeizel A, Toth J and Czvenits E:
Increased birth prevalence of isolated hypospadias in Hungary. Acta
Paediatr Hung. 27:329–337. 1985.
|
|
28
|
Matlai P and Beral V: Trends in congenital
malformations of external genitalia. Lancet. 325:1081985.
View Article : Google Scholar
|
|
29
|
Baskin LS, Himes K and Colborn T:
Hypospadias and endocrine disruption: is there a connection?
Environ Health Perspect. 109:11752001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang MH and Baskin LS: Endocrine:
disruptors, genital development, and hypospadias. J Androl.
29:499–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dalsenter P, Santana G, Grande S, Andrade
AJ and Araujo S: Phthalate affect the reproductive function and
sexual behavior of male Wistar rats. Hum Exp Toxicol. 25:297–303.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nachtigal MW, Hirokawa Y,
Enyeart-VanHouten DL, Flanagan JN, Hammer GD and Ingraham HA:
Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1
in sex-specific gene expression. Cell. 93:445–454. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanley NA, Ball SG, Clement-Jones M, et
al: Expression of steroidogenic factor 1 and Wilms’ tumour 1 during
early human gonadal development and sex determination. Mech Dev.
87:175–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu SM, Leschek EW, Rennert OM and Chan WY:
Luteinizing hormone receptor mutations in disorders of sexual
development and cancer. Fetal Pediatr Pathol. 19:21–40. 2000.
View Article : Google Scholar
|
|
35
|
Geissler WM, Davis DL, Wu L, et al: Male
pseudohermaphroditism caused by mutations of testicular
17β-hydroxysteroid dehydrogenase 3. Nat Genet. 7:34–39. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rosler A: Steroid 17β-hydroxysteroid
dehydrogenase deficiency in man: an inherited form of male
pseudohermaphroditism. J Steroid Biochem Mol Biol. 43:989–1002.
1992. View Article : Google Scholar
|
|
37
|
Parsons JK, Carter HB, Platz EA, Wright
EJ, Landis P and Metter EJ: Serum testosterone and the risk of
prostate cancer: potential implications for testosterone therapy.
Cancer Epidemiol Biomarkers Prev. 14:2257–2260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schatzl G, Madersbacher S, Thurridl T,
Waldmueller J, Kramer G, Haitel A and Marberger M: High-grade
prostate cancer is associated with low serum testosterone levels.
Prostate. 47:52–58. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi JH, Kim GH, Seo EJ, Kim KS, Kim SH
and Yoo HW: Molecular analysis of the AR and SRD5A2 genes in
patients with 46, XY disorders of sex development. J Pediatr
Endocrinol Metab. 21:545–553. 2008.PubMed/NCBI
|