Introduction
Solid tumors contain many different cellular
components in addition to tumor cells, including fibroblasts,
lymphocytes, dendritic cells, macrophages and other myeloid cells.
Complex interactions between the stromal cells in this
microenvironment regulate tumor development and progression
(1). The stromal cells and
mediators of inflammation form a major part of the epithelial tumor
microenvironment (2,3). Gastric cancer is a classic model of
chronic inflammation preceding malignancy, and the tumor-promoting
inflammatory microenvironment promotes the malignant transformation
process (4).
The cancer-related inflammatory microenvironment
covers several types of stromal cells, which include macrophages,
carcinoma-associated fibroblasts (CAFs), leukocytes and mesenchymal
stem cells (MSCs). Among these stromal cell types, MSCs have been
strongly associated with the progression of cancer (5,6).
We have previously isolated MSC-like cells from gastric cancer
tissue (GC-MSCs) and adjacent normal gastric tissue (GCN-MSCs)
(7,8). We have also previously found that
the ability of GC-MSCs to promote gastric cancer was stronger than
that of GCN-MSCs (9). GC-MSCs
secreted higher levels of inflammatory cytokines than the GCN-MSCs.
This finding suggests that GC-MSCs and GCN-MSCs are representative
of different stages of cancer-related inflammatory conditions. The
inflammatory microenvironment plays an important role in the
conversion of MSCs into tumor-supporting cells.
Accumulating evidence indicates that MSCs
co-cultured with cancer cells or treated with cancer-cell
culture-conditioned medium can be activated to assume the
tumor-promoting phenotype (10).
Recently, Ren et al (11)
reported that tumor stromal cells can endow normal stromal cells
with tumor-promoting properties. In a previous study of ours, we
treated human umbilical cord-derived MSCs (hUC-MSCs with gastric
cancer cell-derived exosomes and found that the hUC-MSCs
differentiated into CAFs (12).
In order to mimic gastritis infection microenvironment better, we
infected hucMSC (hUC-MSCs with Helicobacter pylori (H.
pylori) and found that the hUC-MSCs also differentiated into
CAFs and promoted epithelial-mesenchymal transition in gastric
epithelial cells (13). We have
also previously found that the hUC-MSCs activated by inflammatory
macrophages contribute to human gastric carcinogenesis through
nuclear factor (NF)-κB activation (14). These findings suggest that
hUC-MSCs can be activated to acquire the cancer-promoting
phenotype.
Gastric cancer cell-derived exosomes, H.
pylori and macrophages are important constituents of
cancer-related inflammation. Notably, inflammatory cytokines are
mediators that regulate a broad range of processes involved in the
pathogenesis of cancer (15).
Among these cytokines, interleukin (IL)-6 has been proven to be a
key growth-promoting and anti-apoptotic inflammatory cytokine and
is also one of the effector signals in the promotion of
carcinogenesis (16–18). Furthermore, IL-6 acts as an
essential factor mediating the interaction between MSCs and cancer
cells (18–20). Recently, Sung et al
(21) revealed that the
upregulation of IL-6 in bone marrow-derived MSCs triggered a
reactive stromal response to prostate cancer. Whether IL-6 in an
inflammatory microenvironment acts on MSCs and induces them to
acquire the cancer-promoting phenotype remains unknown.
In the present study, we pre-treated hUC-MSCs with
IL-6 and investigated the phenotype and function in gastric cancer
development in vitro and in vivo. The present study
provides new evidence on whether the inflammatory cytokine, IL-6,
can ‘educate’ hUC-MSCs to support the development of gastric
cancer.
Materials and methods
hUC-MSC isolation and culture
hUC-MSCs were obtained and the characteristics of
the isolated hUC-MSCs were investigated as previously described
(22). Briefly, the hUC-MSCs were
photographed for the analysis of their morphological appearance.
Surface antigens, including FITC-CD34, CD71, HLA-DR, PE-CD29, CD38,
CD44, CD105 and HLA-I, were detected and analyzed by flow
cytometry. Von Kossa staining and Oil Red O staining were used to
evaluate their osteogenic differentiation and adipogenic
differentiation potential, respectively. hUC-MSCs at passage 3 were
used for the experiments. All experimental protocols were approved
by the Ethics Committee of Jiangsu University, Zhenjiang,
China.
Cell lines and culture
The GES-1 gastric epithelial cell line and the
SGC-7901 gastric cancer cell line were maintained and cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Grand
Island, NY, USA) containing 4,500 mg/l glucose (HG-DMEM),
L-glutamine and 110 mg/l sodium pyruvate supplemented with 10%
fetal bovine serum (FBS; Life Technologies). The cells were
incubated at 37°C in humidified air with 5% CO2. The
GES-1 cells were treated with 2×10−5 mol/l of
N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) (Sigma-Aldrich Co.,
LLC., St. Louis, MO, USA) for 24 h and most of the cells died in
the following days. One week later, colonies of the transformed
cells were formed and were used as an in vitro model of
gastric precancerous lesions.
Pre-treatment of hUC-MSCs with IL-6
One day before treatment, the hUC-MSCs were
trypsinized and counted. The hUC-MSCs (4×104) were
plated in a 6-well plate (Corning Inc., Corning, NY, USA) and
allowed to adhere overnight. The culture medium of the hUC-MSCs was
discarded and replaced with fresh culture medium containing 50
ng/ml of human recombinant IL-6 (R&D Systems Inc., Minneapolis,
MN, USA). After 48 h, the hUC-MSCs were used for the following
experiments.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cells using
TRIzol® reagent (Life Technologies) according to the
manufacturer’s instructions, and an equal amount of RNA was reverse
transcribed using the RevertAid First Strand cDNA Synthesis kit
(Fermentas, Glen Burnie, MD, USA). RT-qPCR was performed to detect
the changes in mRNA expression using the SYBR-Green I Real-Time PCR
kit (Vazyme Biotech Co., Ltd., Nanjing, China) and the Bio-Rad
fluorescence thermal cycler (Bio-Rad Laboratories, Hercules, CA,
USA). The relative mRNA expression was normalized to the insert
control gene, β-actin, according to the manufacturer’s
instructions. The primers used in the present study were produced
by Invitrogen (Life Technologies). All primer sequences and RT-qPCR
conditions are listed in Table
I.
 | Table ISequences of primers used for RT-qPCR
and the conditions for amplification. |
Table I
Sequences of primers used for RT-qPCR
and the conditions for amplification.
| Genes | Primers sequences
(5′-3′) | Annealing
temperature (˚C) | Product length
(bp) |
|---|
| MCP-1 | F:
GAACCGAGAGGCTGAGACTA | | |
| R:
GCCTCTGCACTGAGATCTTC | 59 | 259 |
| CCL5 | F:
GGATTCCTGCAGAGGATCAA | | |
| R:
GTGGTGTCCGAGGAATATGG | 62 | 154 |
| IL-6 | F:
TACATCCTCGACGGCATCTC | | |
| R:
AGCTCTGGCTTGTTCCTCAC | 61 | 252 |
| IL-8 | F:
GCTCTGTGTGAAGGTGCAGTTT | | |
| R:
TTCTGTGTTGGCGCAGTGT | 62 | 144 |
| β-actin | F:
CACGAAACTACTCCCAACTCC | | |
| R: CATACTCC
TGCTTGAGCTGATC | 56 | 265 |
Luminex assay
The human Cytokine and Chemokine Magnetic Bead Panel
kit (#HCYTOMAG-60K) (Merck Millipore, Darmstadt, Germany) was
designed to detect granulocyte colony stimulating factor (G-CSF),
IL-10, platelet-derived growth factor-BB (PDGF-BB), IL-6, IL-8,
monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor α
(TNFα) and vascular endothelial growth factor (VEGF) in the
supernatant from hUC-MSCs and IL-6-pre-treated hUC-MSCs. All
procedures were processed according to the manufacturer’s
instructions. The signal was detected and analyzed using the
Luminex 200 System (Merck Millipore).
Western blot analysis
The primary antibodies used for western blot
analysis were as follows: rabbit antibodies against phosphorylated
(p-)signal transducer and activator of transcription 3 (STAT3; Cat.
no. 11045), STAT3 (Cat. no.23220), p-NF-κB (Cat. no. 11014), NF-κB
(Cat. no. 21012; all obtained from Signalway Antibody Co., Ltd.,
Baltimore, MD, USA), and α-smooth muscle actin (α-SMA; Cat. no.
BS7000; Bioworld Technology, Louis Park, MN, USA). Following
incubation with the secondary antibodies (Cat. no. 13012-2;
Signalway Antibody Co., Ltd.), the signal was visualized using HRP
substrate (Millipore, Billerica, MA, USA) and analyzed using MD
Image Quant Software. GAPDH was used as the loading control.
Co-culture model
The MNNG-transformed GES-1 cells or SGC-7901 gastric
cancer cells were plated into the lower chamber of a 6-well plate
for 8 h. The hUC-MSCs and the IL-6-pre-treated hUC-MSCs were then
placed into the top chamber of Transwell plates (0.4-μm pore
size; Corning Inc.). The MSCs were seeded at a density of
1×105 cells/well in 1.6 ml of complete DMEM medium. The
GES-1 and SGC-7901 cells were seeded at a density of
1×105 cells/well in 2.5 ml of HG-DMEM medium. The GES-1
and SGC-7901 cells were collected for analysis following indirect
co-culture with the MSCs for 48 h. Cells cultured in medium only
were used as the controls.
Cell colony formation
Following co-culture for 48 h, the GES-1 and
SGC-7901 cells were trypsinized and resuspended to a concentration
of 1,000 cells/2 ml HG-DMEM with 10% FBS and were then incubated
for 10 days. Colonies were fixed with methanol, stained with
crystal violet and counted.
Transwell migration assay
Following co-culture for 48 h, the GES-1 and
SGC-7901 cells (1×105 cells/well) were plated into the
top chamber, and medium containing 10% FBS was placed into the
bottom chamber of Transwell plates (8-μm pore size; Corning
Inc.). Following incubation at 37°C in 5% CO2 for 10 h,
the cells remaining on the upper surface of the membrane were
removed with a cotton swab. The top chamber cells were incubated
for 10 h, and cells that did not migrate through the pores were
removed using a cotton swab. Cells on the lower surface of the
membrane were fixed with methanol and stained with crystal violet.
The migration ability of the cells was determined by counting the
cells under a microscope (SN:9G15626; Olympus, Tokyo, Japan) in at
least 6 fields for each assay.
Immunofluorescence staining
Following co-cultrure for 48 h, the SGC-7901 cells
were washed 3 times with cold PBS, fixed with 4% paraformaldehyde
for 20 min, permeabilized with 0.1% Triton X-100 for 5 min, blocked
with 5% BSA and incubated with proliferating cell nuclear antigen
(PCNA) primary antibody (Cat. no. BS6438; Bioworld Technology) at
4°C over night and followed by Cy3-conjugated anti-rabbit secondary
antibody (Cat. no. C2306; Sigma-Aldrich). The cells were then
stained with DAPI for nuclear staining, and images were acquired
using a Nikon Eclipse Ti-S microscope.
Animal model
BALB/c nude mice (4–5 weeks old) were purchased from
the SLAC Laboratory Animal Center (Shanghai, China) and were
randomly divided into 3 groups. The animals were maintained in
accordance with institutional policies, and all experiments were
performed with approval of the University Committee on the Use and
Care of Animals of Jiangsu University. The animals were injected
subcutaneously with untreated SGC-7901 cells alone, SGC-7901 cells
together with hUC-MSCs or IL-6-pre-treated hUC-MSCs into the
backside of mice. Tumors were surgically removed, photographed and
weighed 4 weeks after injection.
Immunohistochemistry
Formalin-fixed paraffin-embedded mouse tumor tissue
sections were first deparaffinized in xylene and rehydrated through
graded ethanol. Subsequently, the sections were boiled for 10 min
in citrate buffer (pH 6.0, 10 mM) for antigen retrieval. Endogenous
peroxidase activity was then inhibited by exposure to 3% hydrogen
peroxide for 10 min. The sections were then blocked with 5% BSA
(Boster Bioengineering, Co., Ltd., Wuhan, China) and incubated with
properly diluted PCNA primary antibody (Bioworld Technology) at
37°C for 1 h. After the sections were washed with PBS, they were
then incubated with diluted secondary antibody for 20 min. Finally,
the sections were visualized with 3,3′-diaminobenzidine (DAB) and
then counterstained with hematoxylin for examination under a light
microscope (×100, SN:9G15626; Olympus). The terminal
deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
(TUNEL) assay was conducted to measure cell apoptosis according to
the manufacturer’s instructions (Boster Bioengineering, Co.,
Ltd.).
Statistical analysis
All experiments were conducted at least in
triplicate. Data were presented as the means ± standard error.
Statistical analysis was performed using SPSS 11.0 software.
Potential differences between groups with different treatments were
determined using one-way ANOVA or an independent-sample t-test. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Phenotype of hUC-MSCs pre-treated with
IL-6
The hUC-MSCs cultured at the third passage were
treated with IL-6 for 48 h. The morphology of the hUC-MSCs
pre-treated with IL-6 (IL-6-hUC-MSCs) did not differ from the
spindle shape of the parental hUC-MSCs (Fig. 1A). In order to determine whether
the hUC-MSCs can be activated by IL-6, the protein levels of
phosphorylated (p-)STAT3, STAT3, p-NF-κB, NF-κB and α-SMA were
determined by western blot analysis. The results revaled that STAT3
protein was significantly activated and phosphorylated by IL-6,
accompanied by the increased levels of α-SMA protein (Fig. 1B). However, the phosphorylation
levels of NF-κB, which is an important inflammatory transcription
factor, were markedly decreased (Fig.
1B).
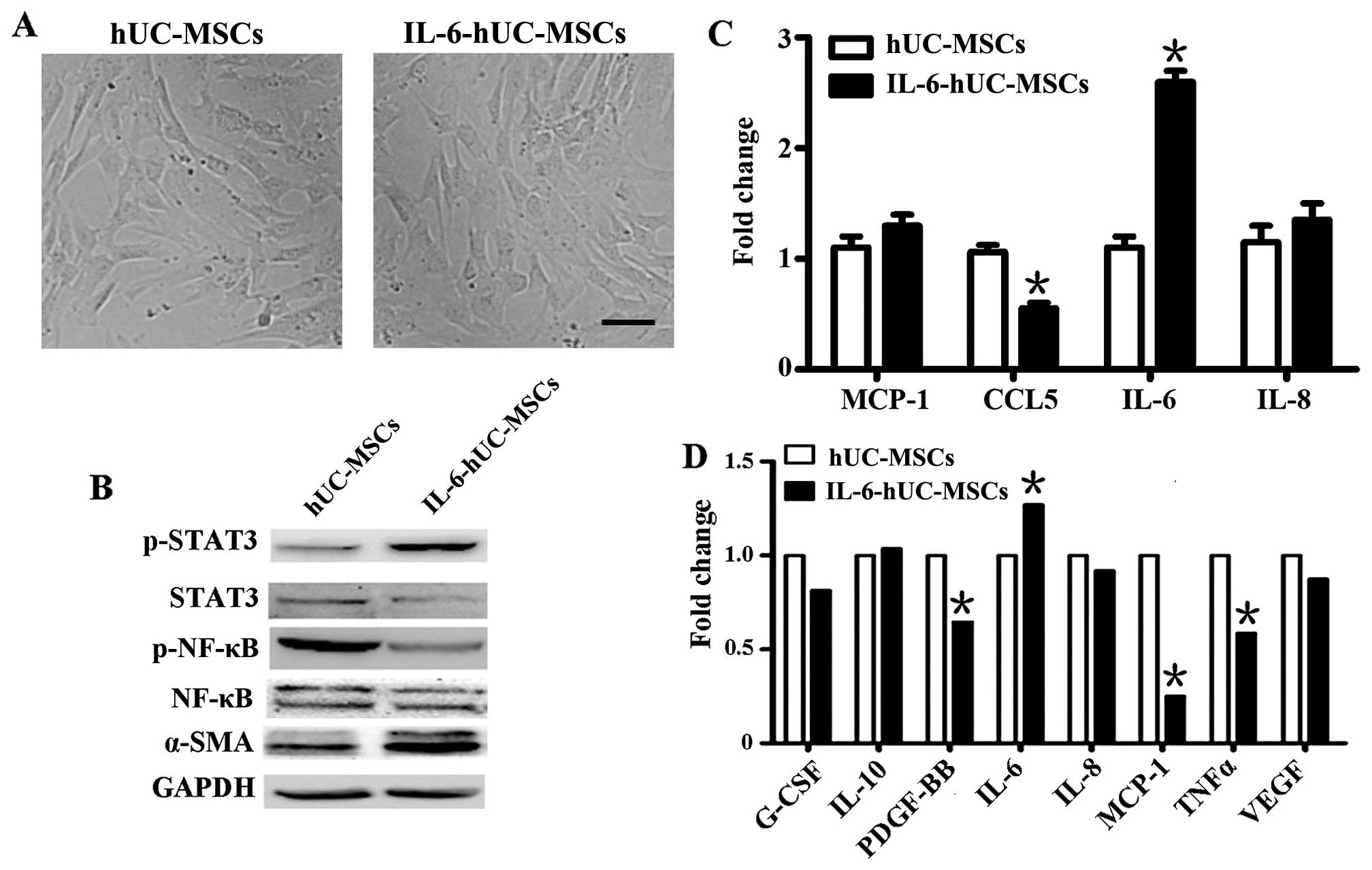 | Figure 1Phenotype human umbilical cord-derived
mesenchymal stem cells (hUC-MSCs) treated with interleukin (IL)0-6
and inflammatory cytokines expression. (A) The morphology of
hUC-MSCs and IL-6-hUC-MSCs; magnification, ×100; scale bar, 50
μm. (B) Western blot analysis of protein expression of
phosphorylated (p-)signal transducer and activator of transcription
3 (STAT3), STAT3, p-nuclear factor (NF)-κB, NF-κB and α-smooth
muscle actin (α-SMA) in hUC-MSCs and IL-6-hUC-MSCs. (C) RT-qPCR of
monocyte chemoattractant protein-1 (MCP-1), chemokine (C-C motif)
ligand 5 (CCL5), IL-6 and IL-8 mRNA levels in the 2 aforememtioned
types of cells. (D) Luminex assy of granulocyte colony stimulating
factor (G-CSF), IL-10, platelet-derived growth factor-BB (PDGF-BB),
IL-6, IL-8, MCP-1, tumor necsoris factor α (TNFα) and vascular
endothelial growth factor (VEGF) levels in the supernatant from
hUC-MSCs and IL-6-hUC-MSCs. IL-6-hUC-MSCs, hUC-MSCs pre-treated
with IL-6 for 48 h. The values are expressed as the means and
standard errors or the mean from at least 3 independent
experiments. *P<0.05. |
Inflammatory cytokines secreted by
hucMSCs pre-treated with IL-6
To evaluate whether NF-κB inactivation affects
inflammatory cytokine secretion by IL-6-hUC-MSCs, we initially
performed RT-qPCR to determine the mRNA levels of MCP-1, chemokine
(C-C motif) ligand 5 (CCL5), IL-6 and IL-8 in the hUC-MSCs
pre-treated with IL-6. We found that the mRNA levels of IL-6 were
significantly increased, whereas the mRNA levels of CCL5 were
significantly decreased. The other mRNA levels of the other 2
cytokines (MCP-1 and IL-8) did not exhibit any marked differences
(Fig. 1C). To further analyze the
cytokine profiles in the hUC-MSCs pre-treated with IL-6, the
luminex analysis system was used to determine the content of
several inflammation- and cancer-related cytokines in the cell
culture supernatant, which included G-SCF, IL-10, PDGF-BB, IL-6,
IL-8, MCP-1, TNFα and VEGF. We observed that the levels of PDGF-BB,
MCP-1 and TNFα were markedly down-regulated in the supernatant of
IL-6-hUC-MSCs. The level of IL-6 in the supernatant was
upregulated, which was similar to the IL-6 mRNA level. The levels
of the other cytokines (G-CSF, IL-10, IL-8, and VEGF) did not
differ significantly between the hUC-MSCs and IL-6-hUC-MSCs
(Fig. 1D).
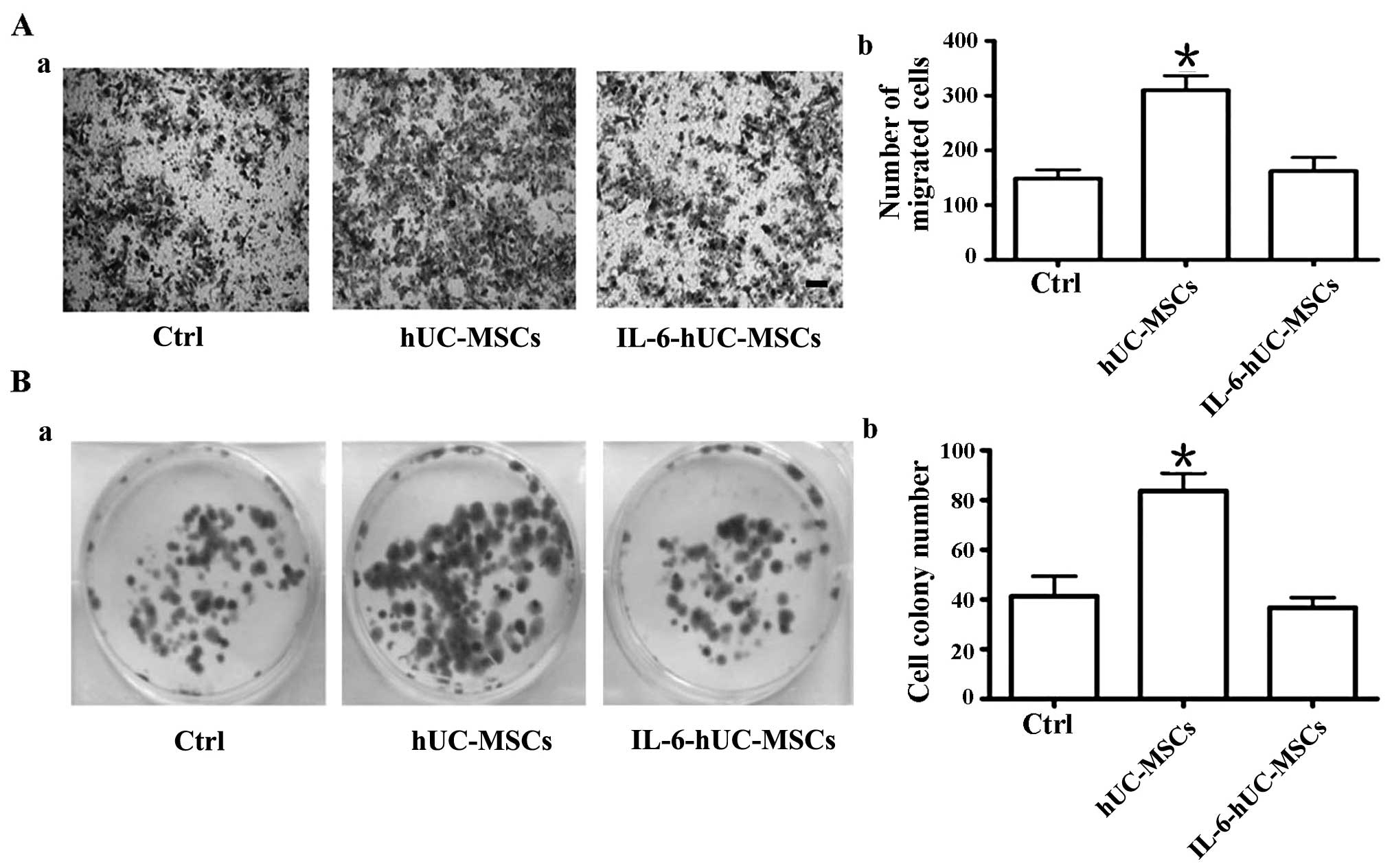
Pre-treatment of hUC-MSCs with IL-6
abolishes their growth-promoting effect on MNNG-transformed gastric
epithelial cell in vitro
MSCs have been strongly associated with cancer
development and progression (5,6).
Our results demonstrated that pre-treatment with IL-6 significantly
inhibited inflammatory cytokines secretion by hUC-MSCs. In order to
elucidate the role of IL-6-hUC-MSCs in gastric cancinogenesis, we
treated GES-1 gastric epithelial cells with the carcinogenic agent,
MNNG, and established MNNG-transformed GES-1 gastric epithelial
cells (MNNG-GES-1). MNNG-GES-1 cells are representative of
precancerous epithelial cells. We co-cultured the MNNG-GES-1 cells
with the hUC-MSCs or IL-6-hUC-MSCs in Transwell plates for 48 h.
Subsequently, we analyzed the migration and proliferation ability
of the MNNG-GES-1 cells. Transwell assay revealed that the number
of migrated cells in the IL-6-hUC-MSC group was smaller than that
of the hUC-MSC group (Fig. 2A).
Cell colony formation assay revealed that the size and number of
the cell colonies in the IL-6-hUC-MSC group was smaller than that
in the hUC-MSC group (Fig. 2B).
There was no difference observed in the migration and proliferation
ability of the MNNG-GES-1 cells between the control group and the
IL-6-hUC-MSC group. The data indicated that the hUC-MSCs
significantly promoted MNNG-GES-1 cell migration and proliferation.
However, pretreatment with IL-6 abolished the growth-promoting
effect of hUC-MSCs on MNNG-GES-1 cells.
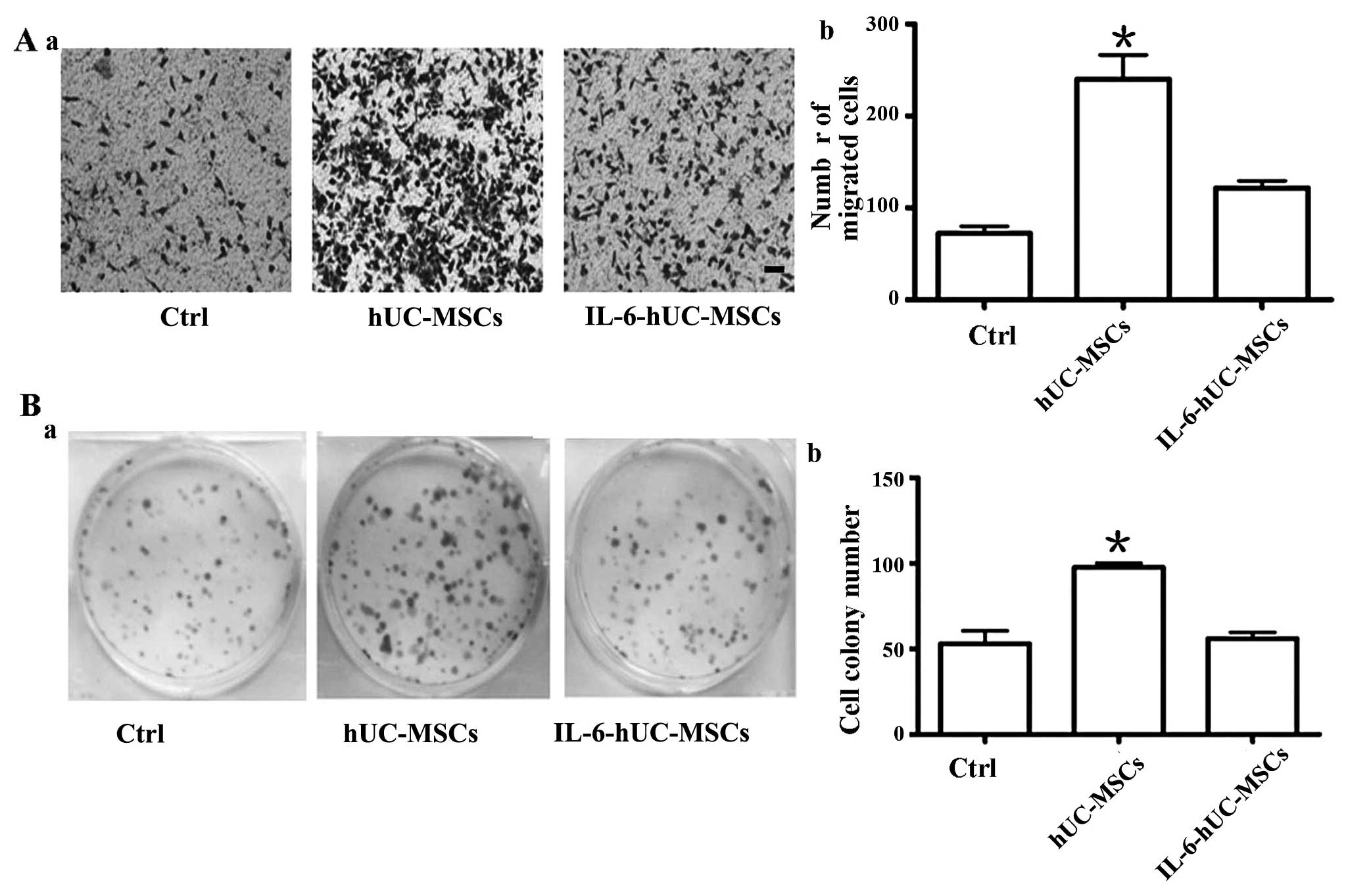
Pre-treatment with IL-6 strips hUC-MSCs
of their growth promoting effect on gastric cancer cells through
the inhibition of cell proliferation in vitro
In order to determine the effect of IL-6-hucMSC on
gastric cancer cells, we co-cultured the SGC-7901 gastric cancer
cells with hUC-MSCs or IL-6-hUC-MSCs in a Transwell plate for 48 h.
As shown in Fig. 3, the number of
migrated cells in the IL-6-hUC-MSC group was smaller than that
observed in the hUC-MSC group (Fig.
3A). The size and number of cell colonies in the IL-6-hUC-MSC
group were smaller than those in the hUC-MSC group (Fig. 3B). The migration and proliferation
ability of the SGC-7901 cells in the IL-6-hUC-MSC group was similar
to that in the control group.
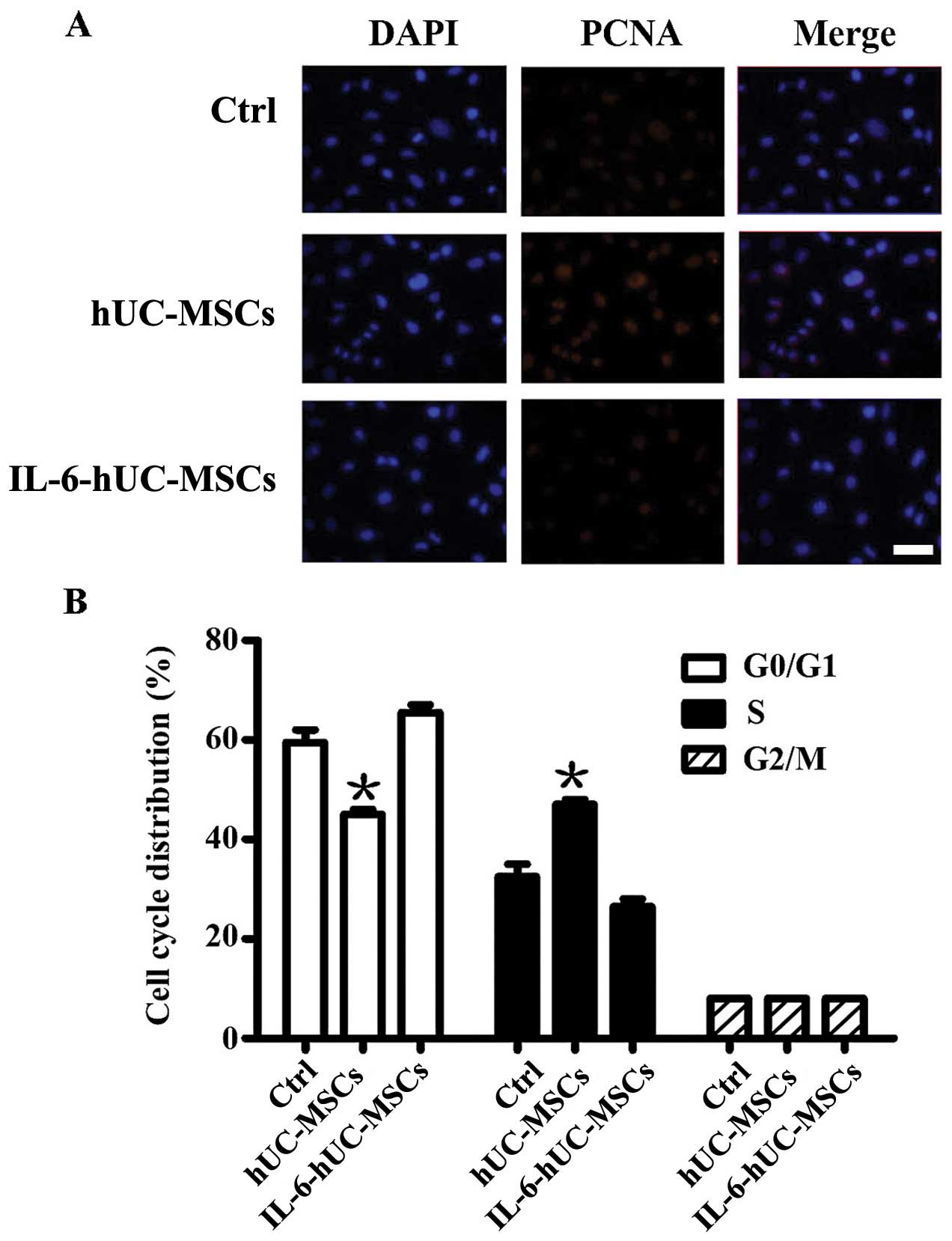
To elucidate the effects of IL-6-hUC-MSCson the
proliferation ability and cell cycle distribution of SGC-7901
cells, we determined PCNA expression and cell cycle progression by
immunofluorescence staining and flow cytometry, respectively. The
results revealed that PCNA expression was upregulated in the
hUC-MSC group, wherease its expression level in the IL-6-hUC-MSC
group was similar to that in the control group (Fig. 4A). Cell cycle analysis revealed
that, compared to the control group, the percentage of SGC-7901
cells in the S phase was evidently increased in the hUC-MSC group
and slightly decreased in the IL-6-hUC-MSC group (Fig. 4B). These data suggest that the
hUC-MSCs significantly promote gastric cancer cell migration and
proliferation. Pre-treatment with IL-6 stripped the hUC-MSCs of
their growth-promoting effect on gastric cancer cells.
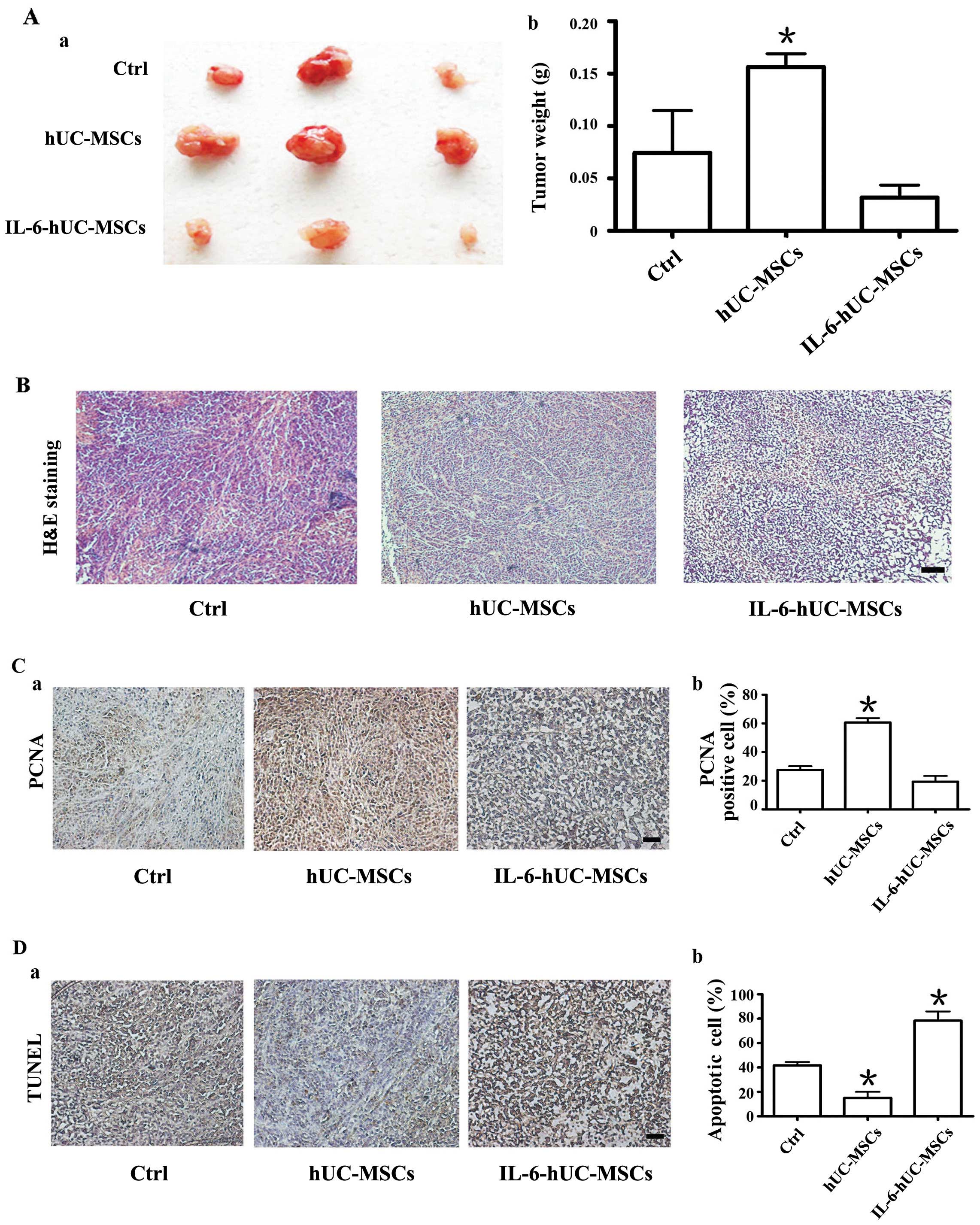
Pre-treatment of hUC-MSCs with IL-6
abolishes their growth-promoting effect on SGC-7901 gastric cancer
cell-derived tumor xenografts in vivo
The above data indicated that pre-treatment with
IL-6 eliminated the promoting effect of hUC-MSCs on the migration
and proliferation of gastric cancer cells in vitro. To
confirm the role of IL-6-hUC-MSCs in gastric cancer cells in
vivo, we co-injected SGC-7901 gastric cancer cells with
hUC-MSCs or IL-6-hUC-MSCs into BALB/c nude mice to establish a
subcutaneous tumor xenograft model of gastric cancer. SGC-7901
cells alone were transplanted as a control. Four weeks later, the
xenograft tumors were removed, photographed and weighed. As shown
in Fig. 5A, the size and weight
of the tumors from the group co-injected with hUC-MSCs were
evidently greater compared to the other 2 groups. The size and
weight of the tumors in the group co-injected with IL-6-hUC-MSCs
were similar to those in the control group (Fig. 5A-a and -b). H&E staining of
the tumor tissue in each group revealed that the tissue structure
was orderly and tightly organized in the control and hucMSC groups,
whereas it was obviously loose and messy in the IL-6-hUC-MSC group
(Fig. 5B).
Based on the above phenomenon, we wished to
determine whether pre-treatment with IL-6 abolishes the
growth-promoting effect of HUC-MSCs in gastric cancer through the
inhibition of cell proliferation and the induction of cell
apoptosis. We performed PCNA and TUNEL-based immunohistochemical
staining on tissue sections from each group. The results revealed
that the percentage of PCNA-positive cells in the IL-6-hUC-MSC
group was markedly lower than that in the hucMSC group, wherease it
was approximately equal to that in the control group (Fig. 5C). Apoptosis assay revealed that
the percentage of apoptotic cells in the hUC-MSC group was the
lowest (10%) among the 3 groups. However, the percentage of
apoptotic cells in the IL-6-hUC-MSC group was reached 75% which was
much higher than that in the control group (Fig. 5D). These data indicate that
pre-treatment with IL-6 abolishes the growth-promoting effect of
hUC-MSCs on gastric cancer cells.
Discussion
Inflammatory conditions affecting the stomach are
associated with an increased risk of cancer and progression. The
hallmarks of cancer-associated inflammation mainly include the
infiltration of cells of the microenvironment, cytokines,
chemokines, growth factors and matrix-degrading enzymes (4). MSCs, which are considered to be the
origin of tumor-associated fibroblasts, are emerging as one of the
major components of the tumor microenvironment (23,24). Previous studies have demonstrated
that MSCs are capable of migrating to primary tumor sites, where
the tumor-associated inflammatory environment converts newly
arrived MSCs into tumor-resident MSCs that display distinct
properties, particularly a strong tumor-promoting activity
(25,26). Our group firstly isolated GC-MSCs
and GCN-MSCs from gastric tissues with different inflammatory
conditions and found that GC-MSCs exhibited a greater capacity to
promote the growth of gastric cancer (9). The abovementioned data suggest that
cancer-related inflammation is an important factor contributing to
the of MSCs to be ‘educated’ as tumor stromal cells.
IL-6, as an inflammatory cytokine, does not only
participate in the communication between cells, but is also
involved in carcinogenesis (27).
Based on the function of IL-6 in inflammation-related
carcinogenesis, we focused on the role of IL-6 in the conversion of
hUC-MSCs into tumor-supporting cells.
CAFs, as classic stromal cells in the tumor
microenvironment, have been extensively investigated and may
originate from MSCs (29). The
overexpression of α-SMA and cancer-promoting inflammatory cytokines
is normally used to define the CAF-like phenotype (6). In order to analyze the phenotype of
hUC-MSCs pre-treated with IL-6, we focused on α-SMA and
inflammatory cytokines. Our results revealed that α-SMA expression
was significantly induced in the hUC-MSCs by pretreatment with
IL-6. STAT3, as a downstream effector of IL-6, was phosphorylated
(its expression was analyzed to confirm the role of treatment with
IL-6). The phenotype of CAFs suggests that hUC-MSCs may be
activated by IL-6 superficially. However, when we examined the mRNA
levels and supernatant content of inflammatory cytokines in
hUC-MSCs and IL-6-hUC-MSCs, we found that the levels of several
cancer-promoting cytokines, including CCL5, PDGF-BB, MCP-1 and
TNFα, were markedly downregulated in the IL-6-hUC-MSCs. CCL5 is
involved in the cross-talk between breast cancer cells and MSCs.
Breast cancer cells stimulate CCL5 secretion by MSCs, and CCL5 in
turn mediates MSC-induced cancer cell migration and invasion
(30,31). Furthermore, ovarian cancer cells
have been shown to reprogram normal fibroblasts into becoming CAFs
through the downregulation of miR-214, which increased the
production of CCL5 and endowed fibroblasts with tumor-promoting
properties (32). Ren et
al (25) compared the
cytokine profiles between MSCs isolated from spontaneous lymphomas
(L-MSCs) and bone marrow-derived MSCs (BM-MSCs) and found that
MCP-1 expression was significantly increased in the supernatant of
L-MSCs. MCP-1 is important for the recruitment of macrophages or
monocytes by tumor-educated MSCs in promoting tumor development
(25,34). TNFα is the prototypical
pro-inflammatory cytokine. Inflammatory cells in the tumor
microenvironment can produce TNFα. TNFα signaling can promote cell
survival, invasion and angiogenesis (33). PDGF-BB has been demonstrated to
modulate endothelial cell proliferation and tumor angiogenesis
(34). The downregulation of the
above cytokines indicates that pre-treatment with IL-6 impairs the
cross-talk of hUC-MSCs with tumor cells or other cells of the tumor
microenvironment.
In this study, the secretion of cytokines by
IL-6-hUC-MSCs was suppressed, whereas only the secretion of IL-6
was induced. We confirmed that the increase in IL-6 secretion was
not due to the contamination of recombinant IL-6 protein. We
inferred that pre-treatment with IL-6 may induce hUC-MSCs to
secrete IL-6. We also wished to determine whether hUC-MSCs
pretreated with IL-6 promote the development of gastric cancer. In
order to reveal the role of IL-6-pre-treated hUC-MSCs in gastric
cancer, we co-cultured MNNG-transformed precancerous GES-1 cells or
SGC-7901 gastric cancer cells with hUC-MSCs or IL-6-hUC-MSCs. We
found that the hUC-MSCs evidently promoted the proliferation and
migration of GES-1 and SGC-7901 cells. However, following treatment
with IL-6, the hUC-MSCs did not have a growth-promoting effect on
gastric epithelial and cancer cells. To further evaluate the
altered function of hUC-MSCs pre-treated with IL-6 in vivo,
we co-transplanted the MSCs with SGC-7901 gastric cancer cells into
nude mice. Consistent with our in vitro results, the
hUC-MSCs significantly promoted gastric cancer growth. There were
no obvious differences between the control and the IL-6-hUC-MSC
group. Pathological and histochemical analysis indicated that the
hUC-MSCs promoted gastric cancer growth by enhancing the
proliferation ability of the cancer cells and inhibiting apoptosis.
On the contrary, pre-treatment with IL-6 endowed the hUC-MSCs with
the functional properties of the inhibition of cell proliferation
and the induction of cell apoptosis.
These data indicate that hUC-MSCs pre-treated with
IL-6 do not promote gastric cancer progression and that their
growth-promoting effect on gastric cancer is abolished. Analysis of
the expression profiles of cytokines revealed that, regardless of
the IL-6 levels being increased in the IL-6-hUC-MSCs, this did not
induce a growth-promoting effect on gastric cancer. We inferred
that hUC-MSCs promote gastric cancer progression through the
synergistic action of inflammatory cytokines. It has been suggested
that the NF-κB pathway plays a key role in tumor-infiltrating
inflammatory cells (1). NF-κB
activation in these cells leads to the secretion of
pro-inflammatory cytokines. In the present study, NF-κB, as a key
inflammatory transcriptional factor, was found to be inactivated in
hUC-MSCs stimulated with IL-6; this provides a possible explanation
for the fact that several cancer-promoting cytokines were
suppressed in the supernatant from hUC-MSCs pre-treated with IL-6.
Moreover, in a previous study of ours, we demonstrated that NF-κB
activation was necessary for H. pylori to induce the
differentiation of hUC-MSCs into CAFs (13). A previous study demonstrated that
the inhibition of NF-κB activation in the tumor microenvironment
represents a potentially effective strategy for arresting tumor
growth (35). This finding
suggests that NF-κB inactivation blocks the conversion of hUC-MSCs
by IL-6 into tumor-supporting cells.
Although we found that stimulation with IL-6 altered
the growth-promoting effect of hUC-MSCs on gastric cancer cells
in vitro and in vivo, the mechanism through which
this process occurs remains unclear. Future research is required to
clarify the mechanism and study the role of IL-6-pretreated
hUC-MSCs in other types of cancer.
The present study focused on the phenotype of
IL-6-pretreated hUC-MSCs and their effects on gastric epithelial
cells. We found that pre-treatment with IL-6 significantly
abolished the promoting effect of hUC-MSCs on the proliferation and
migration of gastric epithelial cells. The findings of this study
provide new insight into the role of the inflammatory cytokine,
IL-6, in the tumor-promoting effects of MSCs and its function in
gastric cancer.
Acknowledgments
The present study was supported by the Major
Research Plan of the National Natural Science Foundation of China
(grant no. 91129718), the National Natural Science Foundation of
China (grant no. 81302119), the Natural Science Foundation of the
Jiangsu Province (grant nos. BK2012709 and BK20130540), the
Doctoral Program Foundation of State Education Ministry (grant no.
20113227110011), the Jiangsu Province for Natural Science Research
in Colleges and Universities (grant no. 13KJB320001), the Jiangsu
University Excellent Young Teacher Project and the Scientific
Research Foundation of Jiangsu University for Senior Professional
Talents (grant no. 13JDG088).
References
|
1
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33:S79–S84. 2013. View Article : Google Scholar
|
|
3
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014(149185): 2014
|
|
4
|
Allavena P, Garlanda C, Borrello MG, Sica
A and Mantovani A: Pathways connecting inflammation and cancer.
Curr Opin Genet Dev. 18:3–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Hou J, Han Z, Wang Y, Hao C, Wei L
and Shi Y: One cell, multiple roles: contribution of mesenchymal
stem cells to tumor development in tumor microenvironment. Cell
Biosci. 3(5): 2013
|
|
6
|
Barcellos-de-Souza P, Gori V, Bambi F and
Chiarugi P: Tumor microenvironment: bone marrow-mesenchymal stem
cells as key players. Biochim Biophys Acta. 1836.321–335. 2013.
|
|
7
|
Cao H, Xu W, Qian H, et al: Mesenchymal
stem cell-like cells derived from human gastric cancer tissues.
Cancer Lett. 274:61–71. 2009. View Article : Google Scholar
|
|
8
|
Xu X, Zhang X, Wang S, et al: Isolation
and comparison of mesenchymal stem-like cells from human gastric
cancer and adjacent non-cancerous tissues. J Cancer Res Clin Oncol.
137:495–504. 2011. View Article : Google Scholar
|
|
9
|
Wang M, Zhao C, Shi H, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-toub M, Almusa A, Almajed M, Al-Nbaheen
M, Kassem M, Aldahmash A and Alajez NM: Pleiotropic effects of
cancer cells’ secreted factors on human stromal mesenchymal stem
cells. Stem Cell Res Ther. 4(114): 2013
|
|
11
|
Ren G, Liu Y, Zhao X, et al: Tumor
resident mesenchymal stromal cells endow naïve stromal cells with
tumor-promoting properties. Oncogene. 33:4016–4020. 2013.
View Article : Google Scholar
|
|
12
|
Gu J, Qian H, Shen L, et al: Gastric
cancer exosomes trigger differentiation of umbilical cord derived
mesenchymal stem cells to carcinoma-associated fibroblasts through
TGF-beta/Smad pathway. PLoS One. 7:e524652012. View Article : Google Scholar
|
|
13
|
Zhang Q, Wang M, Huang F, et al: H. pylori
infection-induced MSC differentiation into CAFs promotes
epithelial-mesenchymal transition in gastric epithelial cells. Int
J Mol Med. 32:1465–1473. 2013.PubMed/NCBI
|
|
14
|
Yang T, Zhang X, Wang M, et al: Activation
of mesenchymal stem cells by macrophages prompts human gastric
cancer growth through NF-κB pathway. PLoS One. 9:e975692014.
View Article : Google Scholar
|
|
15
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi Y, Zhang M, Li H, et al: Autophagy
inhibition by sustained over-production of IL-6 contributes to
arsenic-induced carcinogenesis. Cancer Res. 74:3740–3752. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naugler WE, Sakurai T, Kim S, Maeda S, Kim
K, Elsharkawy AM and Karin M: Gender disparity in liver cancer due
to sex differences in MyD88-dependent IL-6 production. Science.
317:121–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kinoshita H, Hirata Y, Nakagawa H, et al:
Interleukin-6 mediates epithelial-stromal interactions and promotes
gastric tumorigenesis. PLoS One. 8:e609142013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai KS, Yang SH, Lei YP, et al:
Mesenchymal stem cells promote formation of colorectal tumors in
mice. Gastroenterology. 141:1046–1056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rattigan Y, Hsu JM, Mishra PJ, Glod J and
Banerjee D: Interleukin 6 mediated recruitment of mesenchymal stem
cells to the hypoxic tumor milieu. Exp Cell Res. 316:3417–3424.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sung SY, Liao CH, Wu HP, et al: Loss of
let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal
stem cells triggering a reactive stromal response to prostate
cancer. PLoS One. 8:e716372013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiao C, Xu W, Zhu W, et al: Human
mesenchymal stem cells isolated from the umbilical cord. Cell Biol
Int. 32:8–15. 2008. View Article : Google Scholar
|
|
23
|
Studeny M, Marini FC, Dembinski JL, et al:
Mesenchymal stem cells: potential precursors for tumor stroma and
targeteddelivery vehicles for anticancer agents. J Natl Cancer
Inst. 96:1593–1603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spaeth EL, Dembinski JL, Sasser AK, et al:
Mesenchymal stem cell transition to tumor-associated fibroblasts
contributes to fibrovascular network expansion and tumor
progression. PLoS One. 4:e49922009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren G, Zhao X, Wang Y, et al:
CCR2-dependent recruitment of macrophages by tumor-educated
mesenchymal stromal cells promotes tumor development and is
mimicked by TNFα. Cell Stem Cell. 11:812–824. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goldstein RH, Reagan MR, Anderson K,
Kaplan DL and Rosenblatt M: Human bone marrow-derived MSCs can home
to orthotopic breast cancer tumors and promote bone metastasis.
Cancer Res. 70:10044–10050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brighenti E, Calabrese C, Liguori G,
Giannone FA, Trerè D, Montanaro L and Derenzini M: Interleukin 6
downregulates p53 expression and activity by stimulating ribosome
biogenesis: a new pathway connecting inflammation to cancer.
Oncogene. Feb 17–2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polanska UM and Orimo A:
Carcinoma-associated fibroblasts: non-neoplastic tumour-promoting
mesenchymal cells. J Cell Physiol. 228:1651–1657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mi Z, Bhattacharya SD, Kim VM, Guo H,
Talbot LJ and Kuo PC: Osteopontin promotes CCL5-mesenchymal stromal
cell-mediated breast cancer metastasis. Carcinogenesis. 32:477–487.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mitra AK, Zillhardt M, Hua Y, Tiwari P,
Murmann AE, Peter ME and Lengyel E: MicroRNAs reprogram normal
fibroblasts into cancer-associated fibroblasts in ovarian cancer.
Cancer Discov. 2:1100–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guilloton F, Caron G, Ménard C, et al:
Mesenchymal stromal cells orchestrate follicular lymphoma cell
niche through the CCL2-dependent recruitment and polarization of
monocytes. Blood. 119:2556–2567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kulbe H, Thompson R, Wilson JL, et al: The
inflammatory cytokine tumor necrosis factor-alpha generates an
autocrine tumor-promoting network in epithelial ovarian cancer
cells. Cancer Res. 67:585–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xue Y, Lim S, Yang Y, et al: PDGF-BB
modulates hematopoiesis and tumor angiogenesis by inducing
erythropoietin production in stromal cells. Nat Med. 18:100–110.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karin M and Greten FR: NF-kappaB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|