Introduction
The hair follicle (HF) is the most prominent mini
organ of the skin and is remarkable for its dynamic structure. The
fundamental characteristic of hair biology involves the production
of a hair shaft (anagen), apoptosis driven by regression (catagen),
and the relative resting (telogen) phase. The HF undergoes repeated
cycles of regression and regeneration throughout the lifetime of an
organism. Each phase of the hair cycle is characterized by the
distinctive, strictly co-coordinated progression of tissue
proliferation, differentiation and apoptosis, thus maintaining
hairy phenotype of an organism (1,2).
The growth and development of HFs are activated by a
variety of growth factors, hormones and cytokines on different hair
growth phases. Molecules, such as fibroblast growth factor 5
(FGF5), brain-derived neurotrophic factor (BDNF), p75, p53 and
transforming growth factor (TGF)-β1 promote the induction of the
catagen phase (3). Among these
molecules, the overexpression of TGF-β1 in the epidermis of
transgenic mice has been shown to lead to the inhibition of normal
skin development (4). Therefore,
recently, TGF-β1 has been reported to control murine HF regression
(catagen) in vivo (5).
The most striking characteristic of nude mice is the
complete lack of fur development; these mice have been established
as a valuable biomedical tool since their discovery in 1966
(6). Although the nude mouse
phenotype appears hairless at the skin, its dermis contains a
substantial number of active HFs. However, these follicles are
aberrant and undeveloped (6,7).
The impaired differentiation of nude follicles exhibits structural
imperfections of the cortex, hair cuticle and inner root sheath
(8). As a result, a hair shaft
bend and coil at the sebaceous gland and failure to penetrate the
epidermis, is responsible for the lack of external fur coat in nude
mice (9,10).
Various research groups have used nude mice as a
model for hair biology and have reported that cyclosporin A (CsA)
(11), keratinocyte growth factor
(KGF) (12) and AS101 (13) are potential therapeutic tools.
However, chemically synthesized drugs are known for their adverse
side-effects. On the other hand, natural products provide
tremendous opportunities to discover novel therapeutic agents to
replace synthetic drugs; thus, research has focused on
ethnopharmacognosy.
The medicinal plant, Eclipta alba (L.) Hassk
(E. alba) has been reported to exert numerous therapeutic
effects, such as as antitumor (14), anti-hepatic (hepatitics C virus)
(15), and anticancer effects
(16). It is an excellent source
of secondary metabolites, such as flavonoids, phytosterols and
coumestans. Phytochemical coumestans, including wedelolactone,
demethylwedelolactone and saponins are responsible for the main
medicinal effects of E. alba. This medical herb has been
reported to posses hair growth-promoting activities, and
wedelolactone and demethylwedelolactone have been identified as the
major molecules (17,18).
In the present study, we investigated the effects of
different extracts of this medicinal plant on nude mouse skin with
inherited hair follicular abnormalities. The unique findings may
provide new insight for better control of hair loss and demonstrate
the roles of the major regulating molecules in the development of
nude mouse HFs.
Materials and methods
Plant sample, extraction and
fractionation
Dried aerial parts of E. alba were collected
from the Jecheon Medicinal Herb Association, Korea and
authenticated by Dr Ki Hwan Bae (College of Pharmacy, Chungnam
National University, Daejeon, Korea) where the voucher specimens
were deposited. The sample was ground into powder and extracted 3
times with petroleum ether at 40°C for 4 h under reflux, then
filtered and concentrated under a vacuum evaporator (Serial No.
41440910; EYELA, N-N Series, Rikakikai Co. Ltd. Tokyo, Japan) to
yield the corresponding petroleum ether extract (PEE; yield 0.89%
w/w). The resulting residue was then extracted 3 times with
methanol at 70°C for 4 h then filtered and evaporated. The dried
MeOH extract residue was suspended in distilled water and the
resulting aqueous suspension was fractionated sequentially with the
hexane fraction (HeF) and n-butanol fraction (BuF) at 1:1 (v/v)
ratio 3 times at room temperature. The resulting 2 fractions and
remaining water fraction (WaF) were evaporated under a vacuum
(extraction yield, HeF 6.19%, BuF 1.12% and WaF 6.74% w/w).
Experimental animals
Athymic male nude (nu/nu) mice of BALB/c origin at 7
weeks of age, were purchased from Dae-Han Biolink, Inc. (Eumseong,
Korea). They were kept in autoclaved cages with filter bonnets in a
laminar flow unit under 12 h light:dark periods at 24±2°C in a
humidified atmosphere and were fed sterilized food and distilled
water. The experiments were performed in the Animal Center of
Chungnam National University under aseptic conditions in accordance
with the NIH guidelines for the care and use of laboratory animals.
The authorization code number is CNU-00244 (Chungnam National
University).
Administration of PEE and fractions of E.
alba
The mice were divided in to 6 groups; 5 males were
allocated to each of the 6 groups. The animals in group 1 received
0.4 ml of the vehicle mixture (propylene glycol:ethanol:dimethyl
sulfoxide, 67:30:3% v/v) (Sigma, St. Louis, MO, USA), and the
animals in group 2 received minoxidil 2% (Mino). The animals in
groups 3, 4, 5 and 6 received a 5 mg sample of PEE, HeF, BuF and
WaF of E. alba with the vehicle formulation. Treatment was
performed by topical application once per day on the backs of nude
mouse skin for 20 consecutive days.
Evaluation of hair coverage area and
density
The mice were evaluated for hair coverage area by a
score of 0 to 8 as described in Table
I. Hair scores were taken on day 0, 5, 7, 12, 16 and 20. To
evaluate the change in hair density, digital images of each mouse
were randomly acquired on experimental days 8 and 16 in the same
region (3.6 mm2) of interscapular skin. The change in
hair density was evaluated by analyzing the images (x200
magnification; actual area, 3.6 mm2) using Kong,
Bom-Viewer Plus software (Bomtech Electronics Co., Ltd., Seoul,
Korea).
 | Table IScale for the evaluation of hair
coverage area in BALB/c athymic nude mice. |
Table I
Scale for the evaluation of hair
coverage area in BALB/c athymic nude mice.
| Explanation | Scale |
|---|
| Skin pink, no
hair | 0 |
| Skin thick, no
hair | 1 |
| Skin thick scattered
hair | 2 |
| Hair 1–10% | 3 |
| Hair 10–25% | 4 |
| Hair 25–50% | 5 |
| Hair 50–75% | 6 |
| Hair >75% | 7 |
| Full body coat | 8 |
Histological assessment of hair
growth
Skin samples were fixed in 10% neutral buffered
formalin for histological analysis. Paraffin-embedded 4 μm
sections were stained with Mayer’s hematoxylin and eosin (H&E;
Sigma). The morphology and structure of the HFs of the nude mice
were evaluated microscopically in the H&E-stained sections of
dorsal skin at a magnification of x1,000. Five fields per section
(magnification, x100) were used for counting the number of dermal
and subcutaneous HFs with respect to the total number of HFs.
Histological processing and digital photomicrographs were acquired
using the Leica application suite, version 4.0.0 (Leica
Microsystems Ltd., Heerbrugg, Switzerland).
Assessment of keratinocyte proliferation
with anti-BrdU
Keratinocyte proliferation was evaluated by an
intraperitoneal injection of BrdU (50 mg/kg body weight; Sigma) 1 h
before the mice were sacrificed. Dorsal skin from both the treated
and control animals was collected on experimental day 16 and fixed
with 4% paraformaldehyde, dehydrated and embedded in paraffin.
After sectioning, the sections were dewaxed and denatured in 1.5
mol/l of HCl for 30 min and neutralized with phosphate-buffered
saline for 1 h. BrdU incorporation was detected by
immunohistochemical (IHC) staining of the paraffin-embedded
sections with mouse anti-BrdU primary antibody (1:200; Cat. no.
SC-32323) in a moist chamber, at room temperature for 3 h (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After washing 3
times, the sections were incubated with secondary antibodies
[Biotinylated secondary antibody (Life Technologies, Carlsbad, CA,
USA)] for 15 min. The assessment of follicular and epidermal
keratinocyte and sebaceous gland epithelial cell BrdU labeling was
performed by an observer blinded to the treatments using the
original magnification of x400.
Western blot analysis for TGF-β1
Western blot analysis was performed to evaluate the
expression level of TGF-β1 during follicular morphogenesis. The
skin samples were homogenized and lysed in protein extraction
buffer (Pro-Prep; Intron Biotechnology, Inc., Seongnam, Korea). The
protein concentration was measured by Bradford assay (Bio-Rad,
Boston, MA, USA); equal amounts of protein (20 mg/sample) were
separated by 15% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE; Mini Protean II; Bio-Rad) and then
transferred onto a PVDF membrane (Immobilon). The membrane was
blocked for 12 h at 4°C with 5% skimmed milk (Uppsala, Sweden) in
1X TBS (10 Mm Tris pH 7.5, 100 mM NaCl and 0.5% Tween-20).
Immunodetection was performed by incubation at appropriate
dilutions (1:500, TGF-β1 polyclonal antibody) at 4°C overnight then
incubated with the secondary antibodies goat anti-rabbit IgG-HRP
(both from Santa Cruz Biotechnology, Inc.) conjugate for 1 h at
room temperature. After washing, the blots were detected by ECL
western blotting detection reagents (Santa Cruz Biotechnology,
Inc.). The band intensities were quantified using NIH ImageJ
software.
Statistical analysis
The experimental data are expressed as the means ±
standard deviation (SD). The Student’s t-test or one-way ANOVA were
used for the assessment of significance between the different
treatment groups. Statistical analysis was performed using SAS 9.2
software. A value of p<0.05 was considered to indicate a
statistically significant difference.
Results
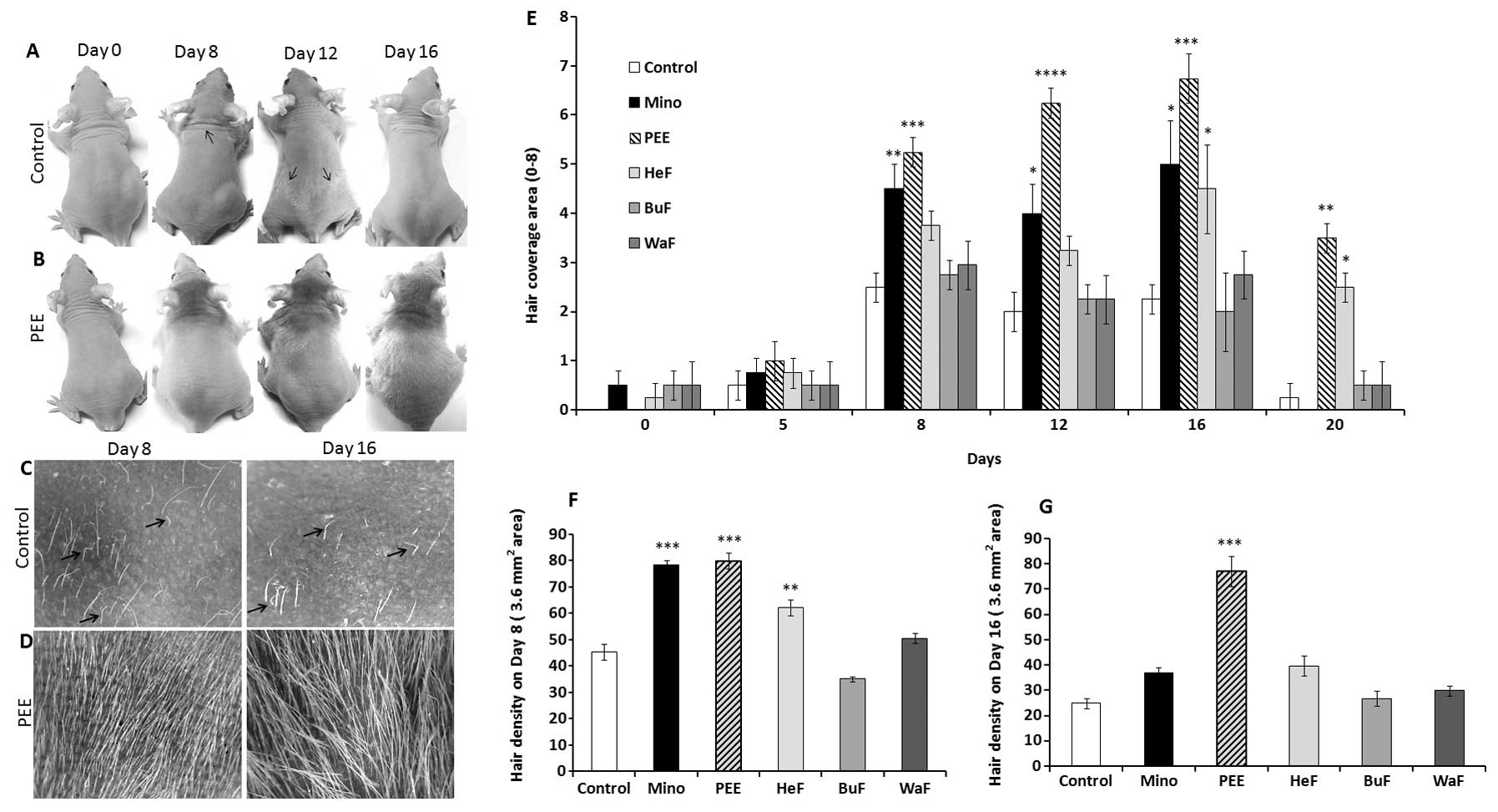
Stimulatory effect of PEE of E. alba on
hair growth patterns in nude mice
The changes occurring in hair growth patterns in
athymic nude mice were documented after the initiation of topical
application for 20 consecutive days. The effects of PEE of E.
alba were evident as early as 7 days following the commencement
of treatment, and became obvious on the dorsal body surface. Hair
growth on the PEE-treated mice first became evidient on the heads
then consistently extended to the caudal region of the tail on
experimental day 16 (Fig. 1B). On
the other hand, the control mice exhibited relatively sparse and
shorter hair growth that was roughly distributed on different
regions of the body on day 10; this phenomena noticeably
corresponds to the ‘wave like pattern’ of the nude phenotype
(Fig. 1A) (19). Eventually, the control mice became
nearly complete ‘nude’ on experimental day 16 (Fig. 1A and C), while PEE induced a
distinctly smoother, thicker hair shaft, leading to dense and
marked hair coverage on days 8 and 16 (Fig. 1B and D).
Effects of PEE of E. alba on hair
coverage area and density
The stimulatory effects of PEE of E. alba on
the hair coverage area of the nude mice were evaluated as they
received specific concentrations of PEE, HeF, BuF, WaF vs. the
vehicle and or 2% minoxidil. The effects of PEE on the hair
coverage area of the mice in all treatment groups were precisely
estimated for each mouse by giving them a score from 0 to 8
(Table I). The maximum hair
growth score was significantly (p<0.001) increased in the mice
treated with PEE of E. alba than in the mice in the other
groups from day 8 and consistently covered the maximum area of the
body on day 16 (Fig. 1E). By
contrast, in the nude mice, rapid hair loss was observed in the
minoxidil and other treatment groups, which then returned to
baseline levels. In terms of hair density, on days 8 and 16
following the initiation of treatment, the mice in the PEE-treated
group exhibited a significant increase (p<0.0001) in hair
density compared to the other groups (Fig. 1G). Although, minoxidil had a
significant effect (p<0.001) on sustaining hair density on day
8, progressive hair loss decreased and hair density thus also
decreased on day 16. This is a characteristic pattern observed in
the hair growth patterns of athymic nude mice.
Stimulatory effects of PEE of E. alba on
distorted HFs of athymic nude mice
The skin specimens of the control mice exhibited
numerous dystrophic HFs, that twisted and coiled within the
follicular infundibulum (Fig. 2C)
and failed to penetrate the epidermis. However, the hair shafts
that penetrated the epidermis were heavily twisted and frequently
fractured before achieving a substantial length (Fig. 2A), as is normally evident in nude
mouse skin. This striking characteristic of nude mice indicates
that the hair fibers suffer from abnormal keratinization (20). On the other hand, the skins of the
PEE-treated nude mice had relatively normal follicles containing
well differentiated straight hair shafts which continued through
the follicular ostia to the skin’s surface (Fig. 2B and D). In nude mice, the
structure of the HFs, inner root sheath and hair shaft exhibit
abnormalities (8), the most
striking of which is the cuticle of the HF which is either
discontinuous or, more often, totally absent (Fig. 2E). By contrast, the HFs of the
PEE-treated mice were regularly formed and intact, coated by a
clearly discernible hair cuticle (Fig. 2F). Furthermore, the control mice
exhibited abortive HFs which revealed histological signs of the
late catagen stage (Fig. 2A and
G). On the other hand, treatment with PEE of E. alba
resulted in a histological pattern identical to that observed in
the late anagen phase of cycling hair and induced a marked increase
in the number of HFs compared with the control mice (Fig. 2H).
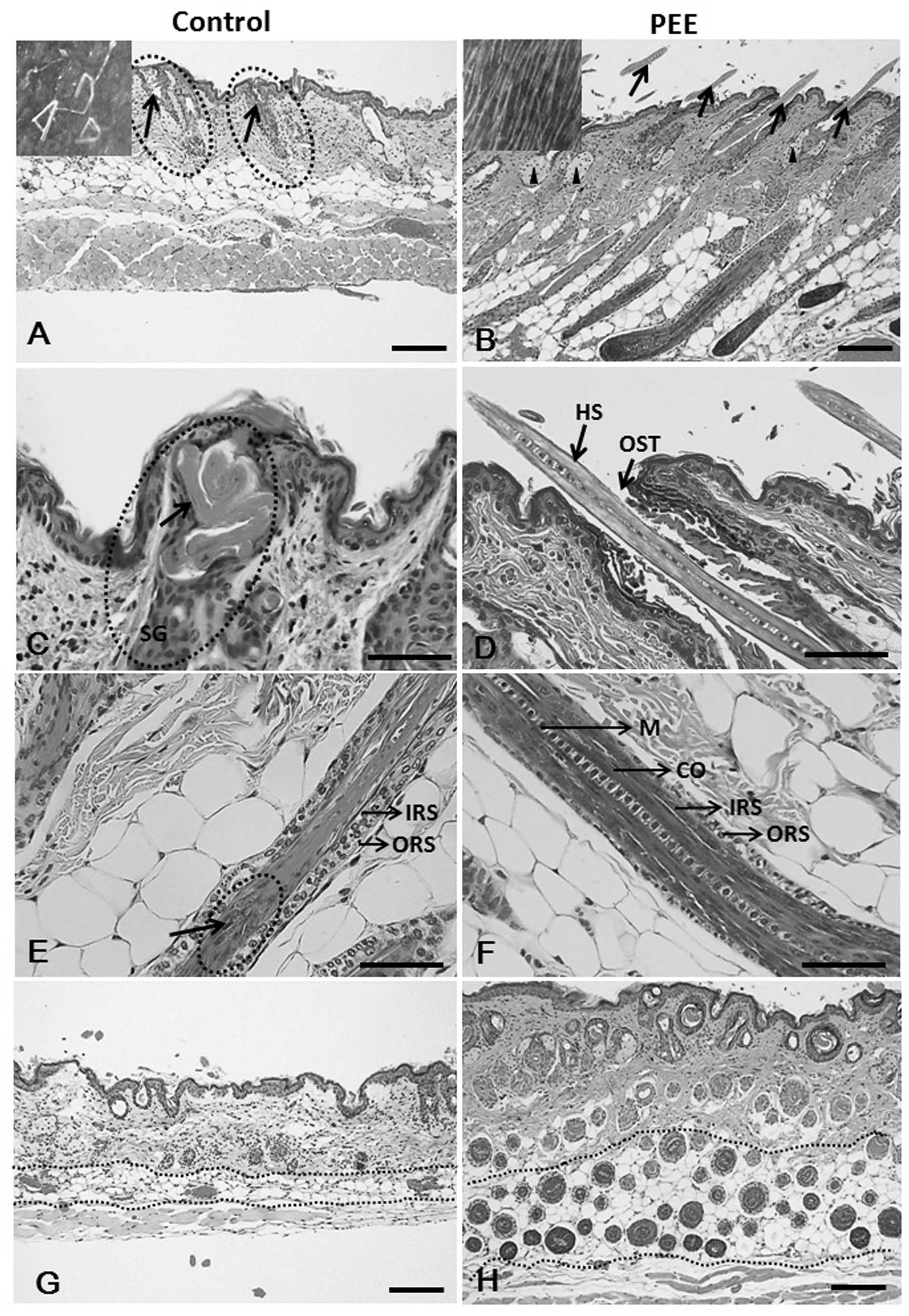 | Figure 2Hematoxylin and eosin
(H&E)-stained skin sections of the vehicle- and petroleum ether
extract (PEE)-treated mice. (A, arrows and inset) Fragmented hair
shafts with keratinized debris that do penetrate the epidermis are
heavily twisted. Note that vehicle-treated nude mouse HFs reveal
histological signs of the late catagen phase on day 16. (B)
PEE-treated mouse skins exhibit prominent follicles (arrows), as
well as moderate sebaceous gland hypertrophy (arrowheads) and the
presence of HFs above the skin surface (arrows and inset). Note
that the HFs of the PEE-treated mice were uniformly in the late
anagen phase of the hair cycle. (C) Hair shaft twist and coil at
the level of sebaceous gland in the vehicle-treated mice skin
specimen. (D) PEE-treated mouse skin specimen shows straight hair
shaft penetrating the skin surfac. (E) Vehicle-treated nude
follicle, cortex formation is severely injured. (F) Hair shaft
regularly formed, intact and coated by a clearly discernible hair
cuticle in PEE-treated mice. (G) Control mouse specimen with
abortive HFs (arrows) and lower number of HFs. (H) PEE-treated nude
mice reveal normal HFs and an increase in the number of hair
follicles. HS, hair shaft; IRS, inner root sheath; ORS, outer root
sheath; M, medulla; CO, cortex; SG, sebaceous gland; HF, hair
follicle; OST, ostium. Scale bars: (A, B, G and H) 100 μm;
(C–F) 50 μm. |
PEE of E. alba enhances keratinocyte
proliferation in the follicular matrix
To determine the difference in the follicular
keratinocyte proliferation rate, we labeled the proliferating cells
with BrdU in vivo on day 16 and subsequently stained them
with an antibody to BrdU. Our results revealed that in the
PEE-treated mice, the number of BrdU-labeled keratinocytes per
anagen follicle increased significantly, particularly in the
follicular matrix and outer root sheath compared to the control
mice (Fig. 3A and B). The mean
number of BrdU-positive cells per anagen follicle was 31.2±2 in the
PEE-treated mice as compared with 14.9±1.7 in the control mice.
This increase was statistically significant (p<0.001). Moreover,
the PEE-treated nude mice also exhibited a significant increase in
the number of BrdU-labeled proliferating epidermal keratinocytes
(p<0.001) and BrdU-positive epithelial cells per sebaceous gland
(p<0.01) (Fig. 3D, F and
G).
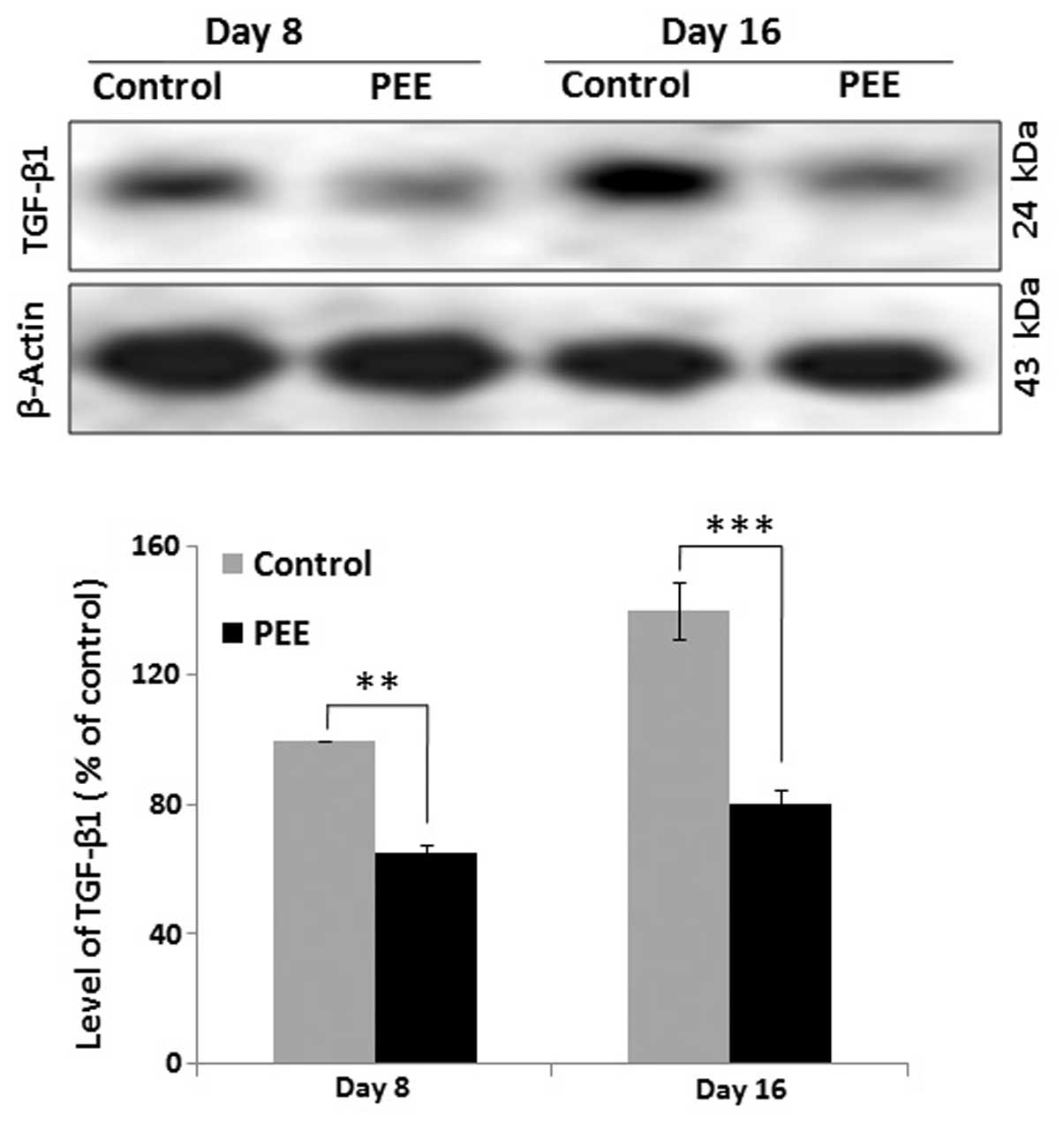
Expression of TGF-β1 during follicular
morphogenesis
The expression level of TGF-β1 was quantified by
western blot analysis at different time point (on days 8 and 16) to
determine the comparative expression of TGF-β1 in the vehicle- and
PEE-treated mouse skins. Quantitative analysis of the western blots
revealed a significant (p<0.001) decrease in the expression
levels of TGF-β1 in the PEE-treated mice during early anagen (day
8) and during the late anagen or anagen-catagen transition (day 16)
compared with the control mice (Fig.
4). Quantitative analysis indicated that the delayed hair
regression in the PEE-treated mouse skins was associated with an
altered expression of TGF-β1.
Discussion
In this study, we investigated the hair growth
stimulatory effects of PEE. Treatment involved the topical
application of PEE and different solvent fractions of E.
alba on the skins of nude mice. Among the treatment groups, PEE
had an outstanding effect on hair growth in the nude mice. In the
PEE-treated nude mice, the skin surface exhibited a large number of
HFs penetrating the epidermis. This evidence, together with
previous data has raised the possibility that PEE of E. alba
may have profound effects on HFs in nude mice (21). This unique finding also
establishes the hypothesis that nude mice are not hairless and that
the development and differentiation of HFs are injured by severe
disturbances of the keratinization process (8,21,22).
Normal hair growth requires a balance between
keratinocyte growth and differentiation in the HF (23). However, in nude mice, the
keratinization processes is markedly impaired, and as a result, HFs
in nude mice exhibit structural abnormalities in the cortex and
inner root sheath (8). The
results from our histological specimens revealed that PEE of E.
alba may modulate the structural defects of HFs in nude mice.
Moreover, IHC staining also revealed that PEE of E. alba
stimulated follicular proliferation in hair matrix cells and
induced to neutralized defects in nude epidermal keratin
differentiation. Therefore, it has a directly effect on HFs in nude
mice by compensating for inherent genetic defects.
The anagen-to-catagen transition is known to be
driven by factors, such as TGF-β1 and TGF-β2 and characterized by
apoptotic cell death in hair bulb epithelial cells and outer root
sheath (ORS) cells (5,24–28). In our study, the topical
application of PEE of E. alba led to a decrease in TGF-β1
expression in nude mice, leading to enhanced keratinocyte
proliferation and thereby prolonging the anagen stage in the
PEE-treated mice. On the other hand, the vehicle-treated mice
exhibited premature catagen development and a reduced number of
proliferating keratinocytes in the HFs. Notably, the normal
expression of TGF-β1 controls follicular regression in both mice
and humans in vivo (5,27).
More importantly, the high expression of TGF-β1 in the epidermis
leads to the suppression of epithelial cell proliferation and the
eventual inhibition of normal skin development (4). Thus, the alteration of TGF-β1
signaling in PEE-treated mice may be associated with the enhanced
keratinocyte proliferation and subsequently delayed hair regression
phase.
In conclusion, the present study demonstrates the
precise biological mechanisms and underlying effects of PEE of
E. alba on hair growth, as well as the anti-apoptotic
effects of this standardized extract. Thus, PEE of E. alba
may be considered an effective standardized extract that modulates
defects in keratinocyte differentiation in the HFs of nude mice by
promoting the proliferation of epidermal basal cells and cells in
the hair matrix. Based on these fruitful findings, the use of such
a stimulatory agent may provide a novel strategy for the management
of various forms of alopecia and may have clinical implications for
hair loss.
References
|
1
|
Paus R: Control of the hair cycle and hair
diseases as cycling disorders. Curr Opin Dermatol. 3:248–258.
1996.
|
|
2
|
Paus R: Principles of hair cycle control.
J Dermatol. 25:793–802. 1998.
|
|
3
|
Rishikaysh P, Dev K, Diaz D, Qureshi WM,
Filip S and Mokry J: Signaling involved in hair follicle
morphogenesis and development. Int J Mol Sci. 15:1647–1670. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sellheyer K, Bickenbach JR, Rothnagel JA,
Bundman D, Longley MA, Krieg T, Roche NS, Roberts AB and Roop DR:
Inhibition of skin development by over expression of transforming
growth factor beta 1 in the epidermis of transgenic mice. Proc Natl
Acad Sci USA. 90:5237–5241. 1993. View Article : Google Scholar
|
|
5
|
Foitzik K, Lindner G, Mueller-Roever S,
Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino
T, Soma T, Dotto GP and Paus R: Control of murine hair follicle
regression (catagen) by TGF-beta1 in vivo. FASEB J. 14:752–760.
2000.PubMed/NCBI
|
|
6
|
Flanagan SP: ‘Nude’ a new hairless gene
with pleiotropic effects in the mouse. Genet Res. 8:295–309. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pantelouris EM: Athymic development in the
mouse. Differentiation. 1:437–450. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Köpf-Maier P, Mboneko VF and Merker HJ:
Nude mice are not hairless. A morphological study. Acta Anat
(Basel). 139:178–190. 1990. View Article : Google Scholar
|
|
9
|
Mecklenburg L, Nakamura M, Sundberg JP and
Paus R: The nude mouse skin phenotype: the role of Foxn1 in hair
follicle development and cycling. Exp Mol Pathol. 71:171–178. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mecklenburg L, Tychsen B and Paus R:
Learning from nudity: lessons from the nude phenotype. Exp
Dermatol. 14:797–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gafter-Gvili A, Sredni B, Gal R, Gafter U
and Kalechman Y: Cyclosporin A-induced hair growth in mice is
associated with inhibition of calcineurin-dependent activation of
NFAT in follicular keratinocytes. Am J Physiol Cell Physiol.
284:C1593–C1603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Danilenko DM, Ring BD, Yanagihara D,
Benson W, Wiemann B, Starnes CO and Pierce GF: Keratinocyte growth
factor is an important endogenous mediator of hair follicle growth,
development, and differentiation. Normalization of the nu/nu
follicular differentiation defect and amelioration of
chemotherapy-induced alopecia. Am J Pathol. 147:145–154.
1995.PubMed/NCBI
|
|
13
|
Sredni B, Gal R, Cohen IJ, Dazard JE,
Givol D, Gafter U, Motro B, Eliyahu S, Albeck M, Lander HM and
Kalechman Y: Hair growth induction by the Tellurium immunomodulator
AS101: association with delayed terminal differentiation of
follicular keratinocytes and ras-dependent up-regulation of KGF
expression. FASEB J. 18:400–402. 2004.
|
|
14
|
Liu QM, Zhao HY, Zhong XK and Jiang JG:
Eclipta prostrata L. phytochemicals: isolation, structure
elucidation, and their antitumor activity. Food Chem Toxicol.
50:4016–4022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manvar D, Mishra M, Kumar S and Pandey VN:
Identification and evaluation of anti Hepatitis C virus
phytochemicals from Eclipta alba. J Ethnopharmacol. 144:545–554.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaudhary H, Dhuna V, Singh J, Kamboj SS
and Seshadri S: Evaluation of hydro-alcoholic extract of Eclipta
alba for its anticancer potential: an in vitro study. J
Ethnopharmacol. 136:363–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roy RK, Thakur M and Dixit VK: Hair growth
promoting activity of Eclipta alba in male albino rats. Arch
Dermatol Res. 300:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Datta K, Singh AT, Mukherjee A, Bhat B,
Ramesh B and Burman AC: Eclipta alba extract with potential for
hair growth promoting activity. J Ethnopharmacol. 124:450–456.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Militzer K: Hair Growth pattern in nude
mice. Cells Tissues Organs. 168:285–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schlake T, Schorpp M, Maul-Pavicic A,
Malashenko AM and Boehm T: Forkhead/winged-helix transcription
factor Whn regulates hair keratin gene expression: molecular
analysis of the nude skin phenotype. Dev Dyn. 217:368–376. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hozumi Y, Imaizumi T and Kondo S: Effect
of cyclosporin on hair-existing area of nude mice. J Dermatol Sci.
7(Suppl): S33–S38. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Panteleyev AA, Paus R, Ahmad W, Sundberg
JP and Christiano AM: Molecular and functional aspects of the
hairless (hr) gene in laboratory rodents and humans. Exp Dermatol.
7:249–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuchs E: Epidermal differentiation and
keratin gene expression. J Cell Sci Suppl. 17:197–208. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paus R, Foitzik K, Welker P, Bulfone-Paus
S and Eichmüller S: Transforming growth factor-beta receptor type I
and type II expression during murine hair follicle development and
cycling. J Invest Dermatol. 109:518–526. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hardy MH: The secret life of the hair
follicle. Trends Genet. 8:55–61. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soma T, Dohrmann CE, Hibino T and Raftery
LA: Profile of transforming growth factor-beta responses during the
murine hair cycle. J Invest Dermatol. 121:969–975. 2003. View Article : Google Scholar
|
|
27
|
Soma T, Tsuji Y and Hibino T: Involvement
of transforming growth factor beta2 in catagen induction during the
human hair cycle. J Invest Dermatol. 118:993–997. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuji Y, Denda S, Soma T, Raftery L, Momoi
T and Hibino T: A potential suppressor of TGF-beta delays catagen
progression in hair follicles. J Investig Dermatol Symp Proc.
8:65–68. 2003. View Article : Google Scholar : PubMed/NCBI
|