Introduction
Chronic hyperglycemia increases advanced glycation
end-products (AGE) that bind to the receptor for AGE (RAGE). The
AGE-RAGE axis induces transforming growth factor-β (TGF-β), cell
hypertrophy and increases extracellular matrices that are
implicated in the pathogenesis of diabetic nephropathy (DN)
(1–4). RAGE engagement by the specific
ligands triggers DN-related signaling pathways, including
extracellular regulated kinases (ERK1/2), p38 kinase and c-Jun
N-terminal kinase (JNK) (5).
High glucose increases RAGE expression in mesangial
cells (6) and the pivotal role of
RAGE in DN can be illustrated by the finding that glomerular RAGE
expression is increased in diabetic mice (7). Additionally, RAGE overexpression
exacerbates, whereas RAGE deletion attenuates experimental DN
(3,8). TGF-β is another central player in DN
as it is pivotal in cell hypertrophy and increased extracellular
matrices in DN (1,4). Diabetic renal hypertrophy is
associated with cell cycle arrest and increased glomerular
p21WAF1 (cyclin-dependent kinase inhibitor) expression
(9,10), whereas p21WAF1-knockout
mice are protected from glomerular hypertrophy despite increased
TGF-β levels (11).
RAGE binds to a number of ligands besides AGE,
including S100B (a member of the S100/calgranulin family) (12). S100B is mainly expressed and
secreted by the neural cells (12), whereas cerebral levels of S100B
are increased in the diabetic rats (13). Notably, in two previous studies,
it was found that S100B increases TGF-βl and fibronectin
expression, while activating ERK1/2 and p38 kinase pathways in
mesangial cells (14,15). However, the roles of endogenous
S100B in high glucose-induced effects are not known.
Therefore, the roles of S100B in high
glucose-induced p21WAF1, extracellular matrices, TGF-βl
and cell hypertrophy in mouse mesangial (MES13) cells were
investigated in the present study. Additionally, the molecular
mechanisms of S100B-induced TGF-β activity were assessed in terms
of the ERK1/2, p38 kinase and JNK pathways.
Materials and methods
Materials
Dimethyl sulfoxide, PD98059 (ERK1/2 inhibitor),
SB203580 (p38 kinase inhibitor), SP600125 (JNK inhibitor) and
SB431542 (type I TGF-β receptor inhibitor) were purchased from the
Sigma-Aldrich Co. (St. Louis, MO, USA). S100B was purchased from
the Abcam Co. (Cambridge, MA, USA).
Cells
The mouse mesangial cell line (MES13 cells,
BCRC-60366) was purchased from the Bioresource Collection and
Research Center (Hsinchu, Taiwan), which obtained the cells
(CRL-1927) from the American Type Culture Collection (Manassas, VA,
USA). Cells were used within 30 passages of the original passage
and were cultured in a 3:1 mixture of Dulbecco’s modified Eagle’s
medium and Ham’s F12 medium (final glucose 6.67 mM), 14 mM HEPES,
1% penicillin/streptomycin (Gibco, Grand Island, NY, USA) and 5%
fetal bovine serum (FBS; Gibco) in 5% CO2 at 37°C. MES13
cells were serum-starved for 16 h before treatment with S100B (1
μM) or the signaling pathway inhibitors.
Immunoblotting
Total cell lysates were harvested, resolved by 10%
SDS-polyacrylamide gel electrophoresis, and were subsequently
transferred to Protran membranes (0.45 mm; Schieicher &
Schuell, Keene, NH, USA). The membranes were blocked in blocking
solution and subsequently probed with the following primary
antibodies: S100B (ab41548, rabbit anti-rat) and col4α1 (ab6586,
rabbit anti-human; Abcam Co.), p21WAF1 (sc-397, goat
anti-human) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
(sc25778, rabbit anti-human; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), fibro-nectin (AB1954, rabbit anti-rat; Chemicon
International, Inc., Temecula, CA, USA), α-tubulin (MS-581-P0,
mouse anti-chicken monoclonal antibody; Thermo Fisher Scientific,
Inc., Fremont, CA, USA), ERK1/2 (9102, rabbit anti-rat), p-ERK1/2
(4370, rabbit anti-human; Thr202/Tyr204), p38 kinase (9212, rabbit
anti-human), p-p38 kinase (4631, rabbit anti-human; Thr180/Try182),
JNK (9258, rabbit anti-human) and p-JNK (9251, rabbit anti-human;
Thr183/Tyr185) (Cell Signaling Technology, Inc., Beverly, MA, USA).
The membrane was subsequently incubated in 4,000x diluted
horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse
secondary antibody. The protein bands were detected using the
enhanced chemiluminescence system. The intensity of the immunoblot
bands was quantified by densitometric analysis. Results are
expressed as the ratio of intensity of the protein of interest to
that of α-tubulin or the indicated protein from the same
sample.
Reverse transcriptase-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the REzol reagent
(PT-KP200CT) according to the manufacturer’s instructions (Protech
Technologies, Inc., Taipei, Taiwan). Briefly, cDNA was synthesized
from 1 μg RNA with the reverse transcriptase system (Promega
Corp., Madison, WI, USA). RT-qPCR of the obtained cDNA was
performed in triplicate on an ABI 7900 Fast RT-PCR System (Applied
Biosystems, Warrington, Cheshire, UK) using SYBR Green for qPCR
(Protech Corp.). The primers used were: mouse S100B forward,
5′-TGGTTGCCCTCATTGATGTCT-3′ and reverse,
5′-CTCGTTGTTGATAAGCTCCTTCAG-3′; and mouse 18S rRNA forward,
5′-CCAGTAAGTGCGGGTCATAAGC-3′ and reverse,
5′-CCTCACTAAACCATCCAATCGG-3′.
Enzyme-linked immunosorbent assay
(ELISA)
Medium S100B was measured using the S100B ELISA kit
(Millipore Corp., Billerica, MA, USA) according to the
manufacturer’s instructions. The absorbance at 450 nm was recorded
by the ELISA reader. The level of secreted S100B was calculated for
one million cells.
Luciferase reporter plasmid
transfection
The p21 promoter reporter construct p21P was a gift
from Dr X.F. Wang (Department of Pharmacology, Duke University
Medical Center, Durham, NC, USA) (16). The human TGF-β1
promoter-luciferase construct phTG5-luc was a gift from Dr J.L.
Virelizier (Unité d’Immunologie Virale, Institut Pasteur, Paris,
France) (17). The TGF-β
bioactivity reporter p3TP-lux was a gift from Dr J. Massagué
(Memorial Sloan-Kettering Cancer Center, New York, NY, USA)
(18). Plasmids were transiently
transfected into the MES13 cells in 6-well plates (1×104
cells/well) using the TurboFect reagent (Fermentas Inc., Israel).
Medium containing 5% FBS was added 24 h later and cells were
treated with high glucose or 1 μM S100B for the indicated
times. Cells were lysed and luciferase activity was measured by the
Dynatech ML1000 luminometer (Dynatech Laboratories, Inc.,
Chantilly, VA, USA).
Cell hypertrophy
Cells were grown in 6-well plates until
approximately 50% confluent and were subsequently made quiescent
for 16 h in the medium containing 0.1% FBS. The cultures were
treated with HG (30 mM) and S100B (1 μM) for the indicated
times, following which the cells were trypsinized, washed twice
with phosphate-buffered saline (PBS) and counted using a
hemocytometer with trypan blue staining. Equal numbers of cells
were lysed in buffer [0.1% (wt/vol) SDS, 0.5% (wt/vol) sodium
deoxycholate and 1.0% (wt/vol) Nonidet P-40, in PBS]. The total
protein content was measured by the Bio-Rad protein assay kit
(Bio-Rad, Hercules, CA, USA). Cell hypertrophy was assessed by
steady-state total protein (μg)/104 cells.
Small interfering RNA (siRNA)
transfection
S100B siRNA (sense, 5′-GCC CUC AUU GAU GUC UUC CAC
CAG U-3′ and anitsense, 5′-ACU GGU GGA AGA CAU CAA UGA GGG C-3′)
were purchased from the Invitrogen Corp. (Grand Island, NY, USA).
Negative control siRNA (Trilencer-27 siRNA) was from the OriGene
Technologies Inc. (Rockville, MD, USA). We transfected 50 nM siRNA
into cells in 6-well plates (10×104 cells/well) by using
the TurboFect reagent (Fermentas Inc.). Cells were exchanged with
fresh 5% FBS-containing medium 24 h after transfection.
Statistics
Data are expressed as the means ± standard error of
the mean. In vitro experimental data were collected from ≥3
repeated experiments. One-way analysis of variance followed by
post-hoc Dunnett tests was used for the comparison between the
treated and control groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
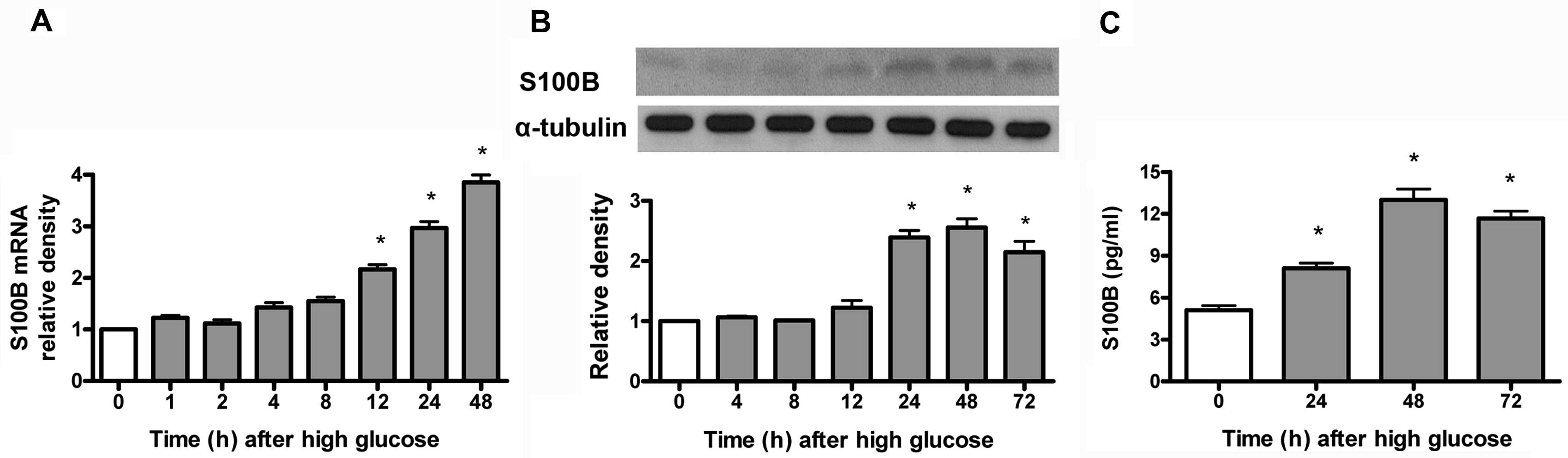
High glucose increases S100B protein and
mRNA expression in mes13 cells
S100B mRNA was measured by RT-qPCR, while
S100B protein was measured by immunoblotting and ELISA. As shown in
Fig. 1, high glucose (30 mM)
time-dependently (12–48 h) increased S100B mRNA (Fig. 1A) and time-dependently (24–72 h)
increased S100B protein (Fig. 1B)
expression and medium S100B protein levels (Fig. 1C).
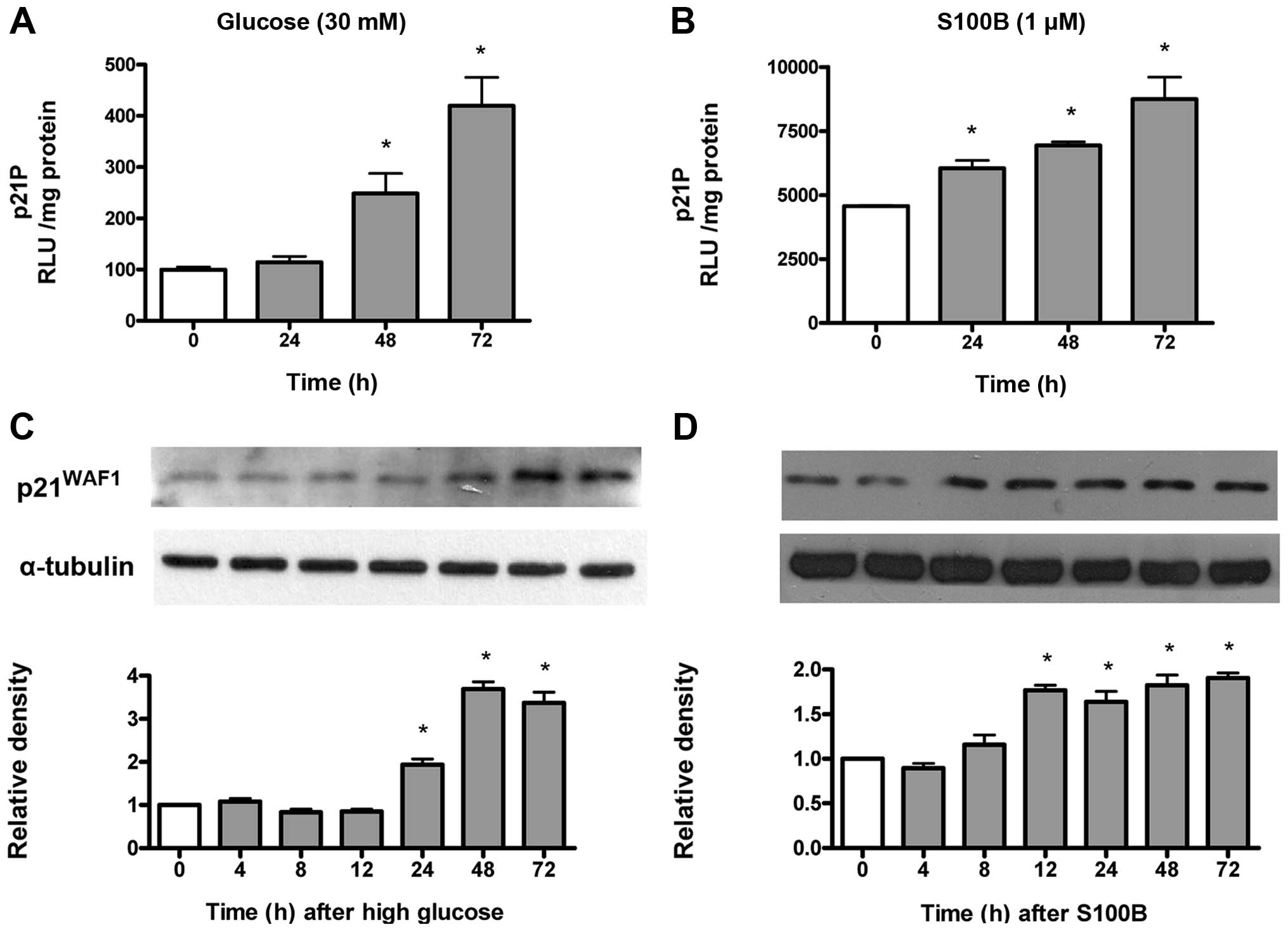
High glucose and S100B time-dependently
increase p21WAF1 gene transcription and protein
expression
High glucose-induced cell hypertrophy is associated
with increased p21WAF1 expression in mesangial cells
(10). Gene transcriptional
activity of the p21WAF1 gene was measured by
p21P, while protein expression was measured by immunoblotting. As
shown in Fig. 2A, high glucose
time-dependently (48–72 h) increased the p21WAF1
gene transcriptional activity. Similarly, S100B (1 μM)
time-dependently (24–72 h) increased the p21WAF1
gene transcriptional activity (Fig.
2B). As shown in Fig. 2C,
high glucose time-dependently (48–72 h) increased
p21WAF1 protein expression. Similarly, S100B (1
μM) time-dependently (12–72 h) increased p21WAF1
protein expression (Fig. 2D).
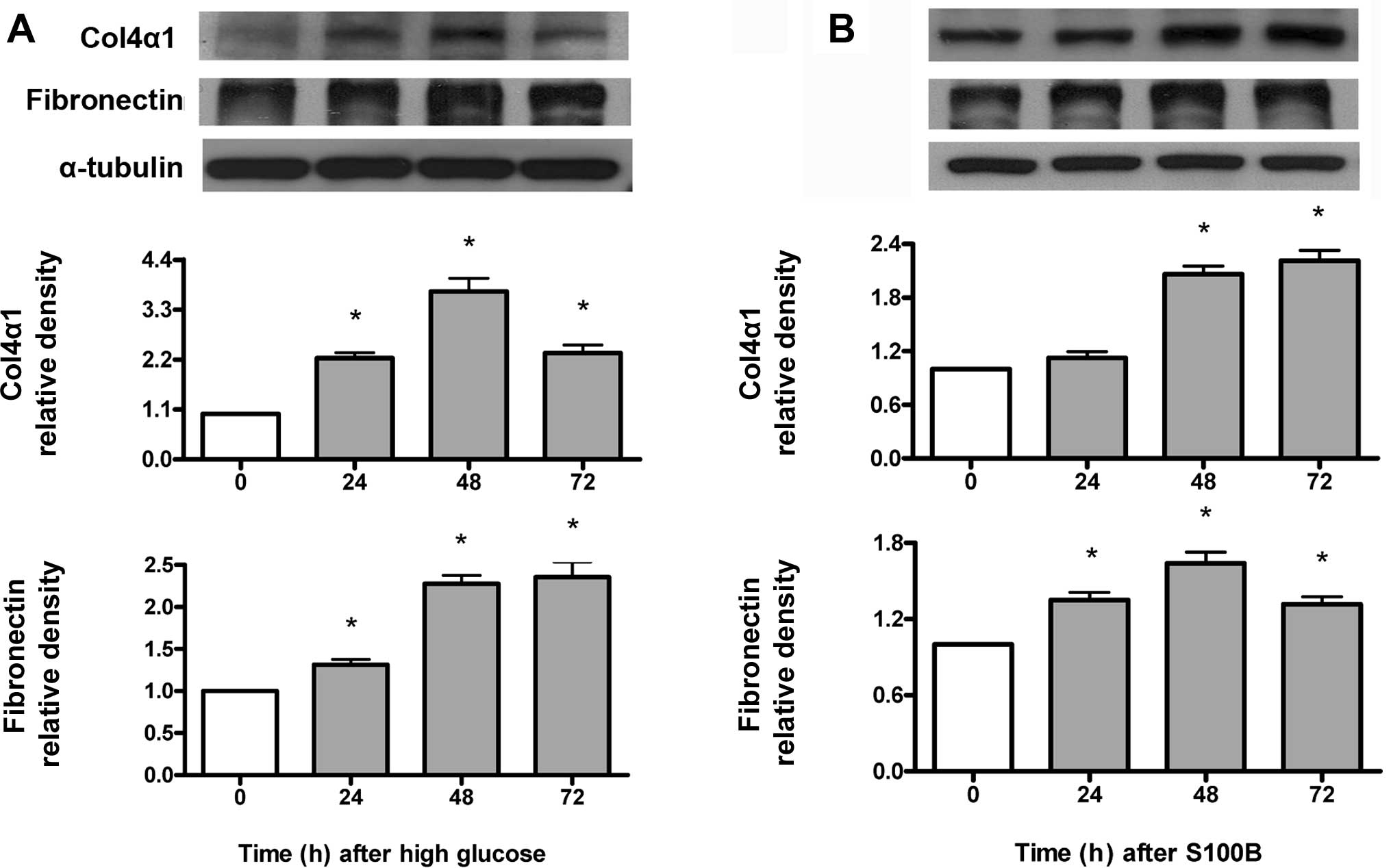
High glucose and S100B time-dependently
increase type IV collagen and fibronectin protein expression
Type IV collagen and fibronectin protein expression
was measured by immunoblotting. As shown in Fig. 3A, high glucose (30 mM) increased
type IV collagen and fibronectin protein expression at 24–72 h.
Similarly, S100B (1 μM) increased type IV collagen protein
expression at 48–72 h, but increased fibronectin protein expression
at 24–72 h (Fig. 3B).
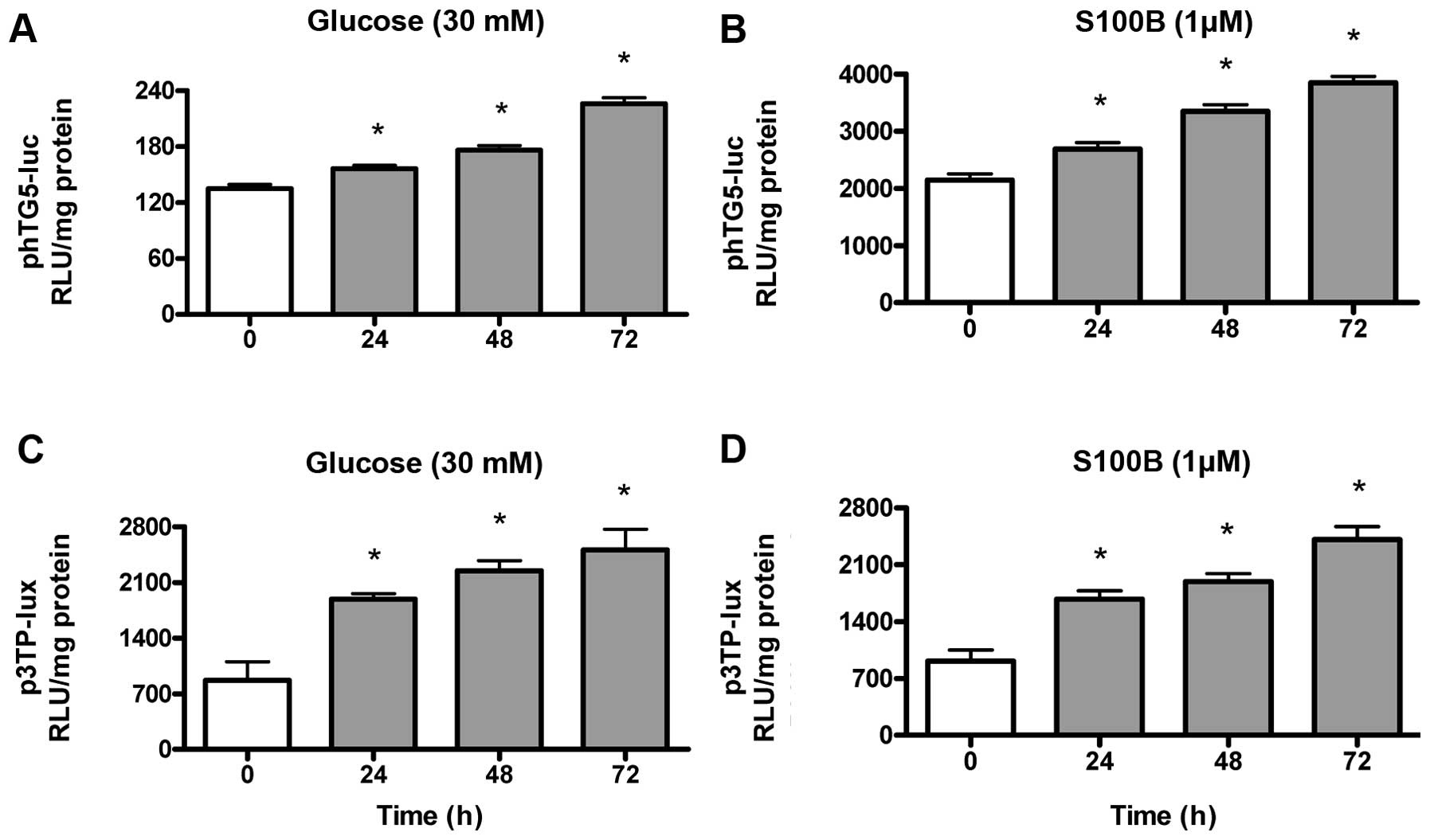
High glucose and S100B time-dependently
induce TGF-β gene transcription and bioactivity
Gene transcriptional activity of the TGF-β
gene was measured by transient transfection of phTG5-luc, while
TGF-β bioactivity was measured by transient transfection of
p3TP-lux. As shown in Fig. 4A and
B, high glucose (30 mM) and S100B (1 μM)
time-dependently (24–72 h) increased the TGF-β gene
transcriptional activity. As shown in Fig. 4C and D, high glucose (30 mM) and
S100B (1 μM) time-dependently (24–72 h) increased TGF-β
bioactivity.
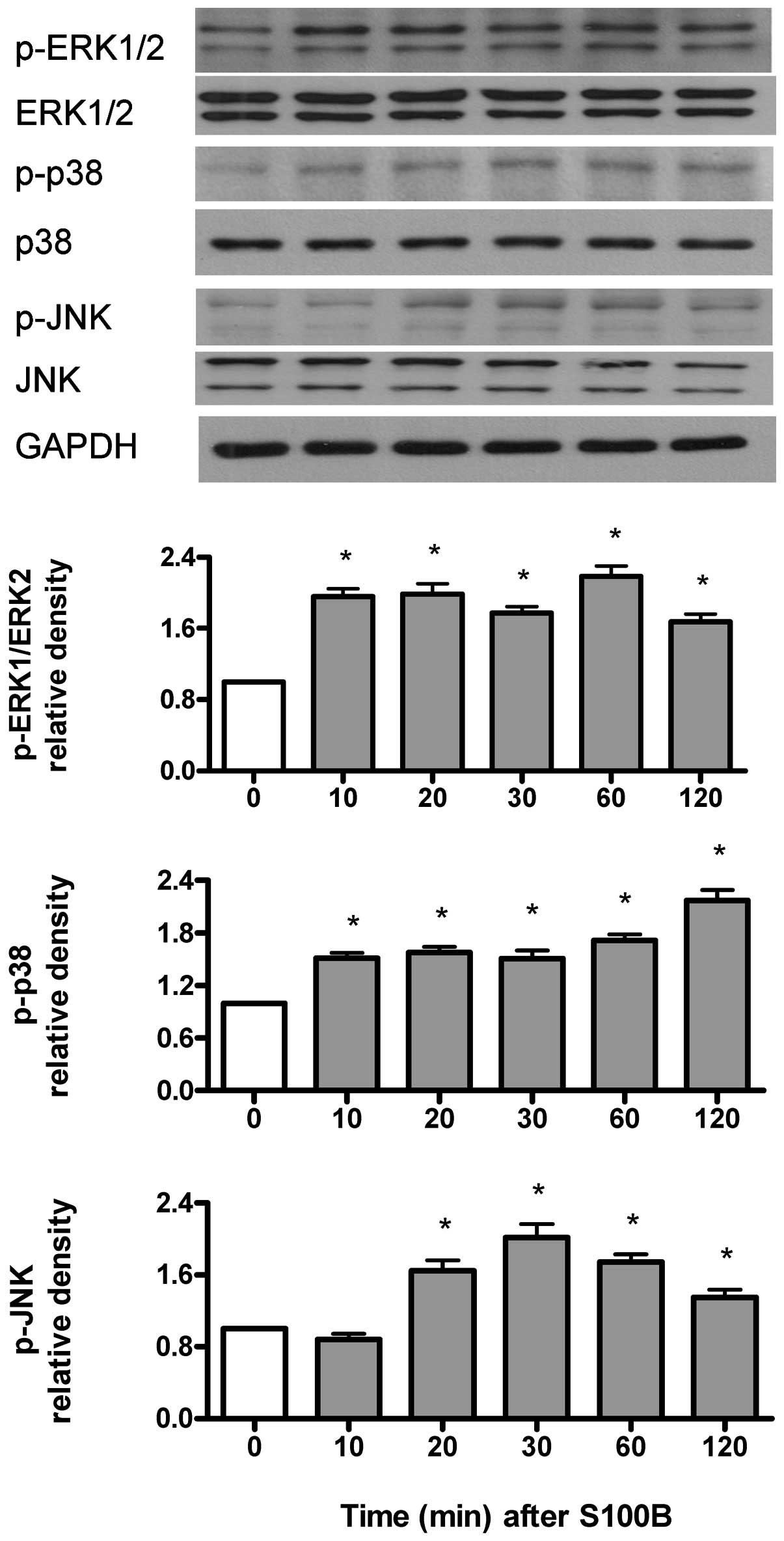
S100B time-dependently activates ERK1/2,
p38 and JNK kinases
p-ERK1/2, p-p38 kinase and p-JNK were measured by
immunoblotting and were normalized to their respective
unphosphorylated forms. As shown in Fig. 5, S100B (1 μM) increased
p-ERK1/2 and p-p38 kinase at 10–120 min, but increased p-JNK at
20–120 min.
S100B-induced TGF-β gene transcription
and TGF-β bioactivity are dependent on ERK1/2, p38 and TGF-β
receptors
Gene transcriptional activity of the TGF-β
gene was measured by phTG5-luc, while TGF-β bioactivity was
measured by p3TP-lux. SB203580 (p38 kinase inhibitor), PD98059
(ERK1/2 inhibitor) and SB431542 (type I TGF-β receptor inhibitor)
attenuated S100B (1 μM)-induced TGF-β gene
transcriptional activity (Fig.
6A) and TGF-β bioactivity (Fig.
6B) at 24 h. By contrast, SP600125 (JNK inhibitor) only
attenuated S100B (1 μM)-induced TGF-β bioactivity (Fig. 6B), but not TGF-β gene
transcriptional activity (Fig.
6A) at 24 h.
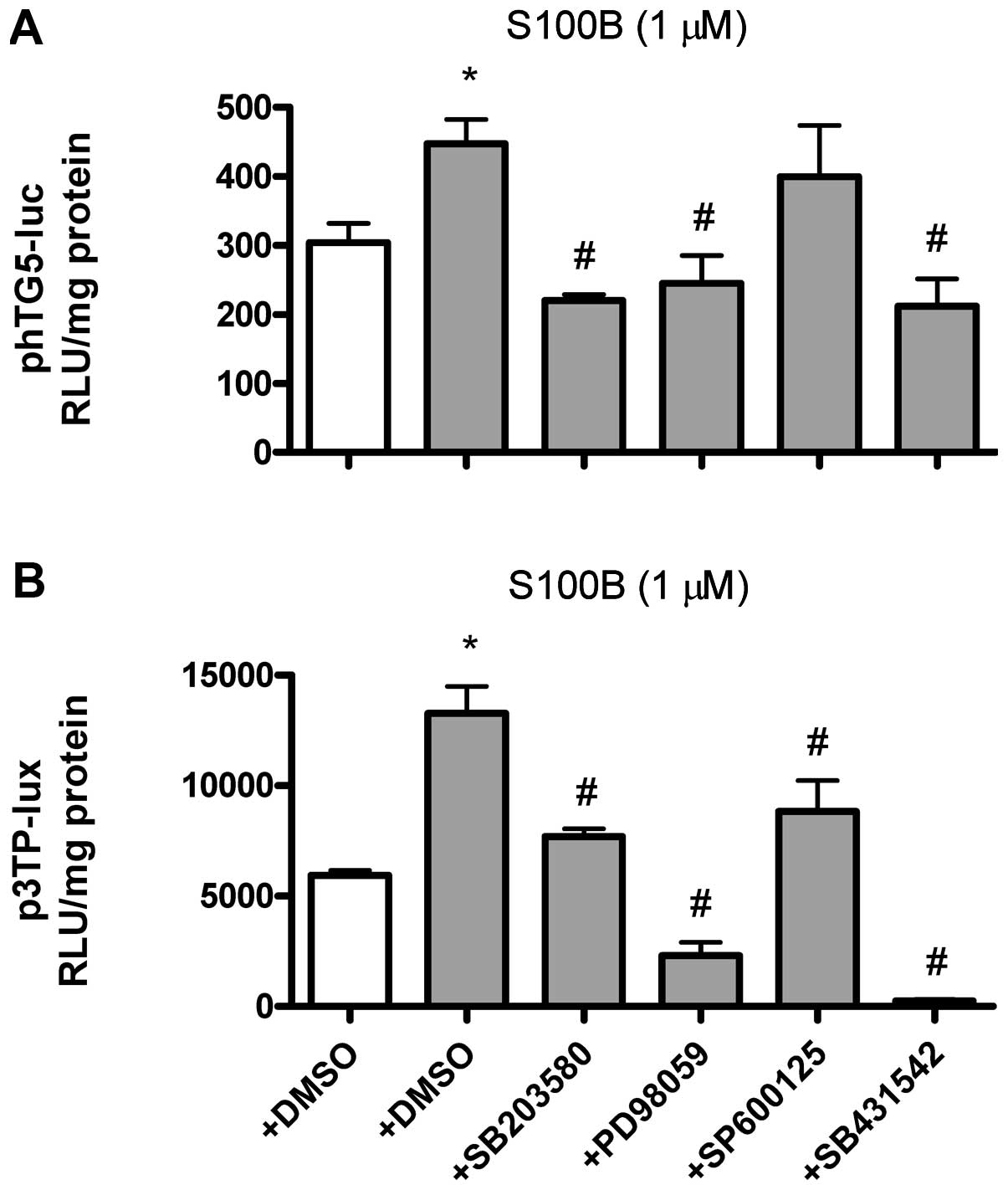 | Figure 6S100B-induced TGF-β gene
transcription and bioactivity are dependent on ERK1/2, p38 and
TGF-β receptor. TGF-β transcriptional activity or bioactivity
(expressed as the relative light units, RLU) were measured by
transient transfection of phTG5-luc (TGF-β promoter plasmid) or
p3TP-lux (TGF-β bioactivity reporter plasmid), respectively.
SB203580, PD98059, SP600125 and SB431542 were dissolved in dimethyl
sulfoxide (DMSO) (final concentration 0.1%). (A) Effects of
SB203580 (10 μM, p38 inhibitor, pre-treated for 1 h),
PD98059 (10 μM, ERK1/2 inhibitor, pre-treated for 1 h),
SP600125 (10 μM, JNK inhibitor, pre-treated for 1 h) and
SB431542 (10 μM, TGF-β receptor type I inhibitor,
pre-treated for 1 h) on S100B (1 μM, closed bars)-induced
TGF-β transcription at 24 h. (B) Effects of SB203580,
PD98059, SP600125 and SB431542 on S100B (1 μM, closed
bars)-induced TGF-β bioactivity at 24 h. Data are expressed as the
means ± standard error of the mean of three independent
experiments. *P<0.05 vs. lane 1.
#P<0.05 vs. lane 2. TGF-β, transforming growth
factor-β; ERK1/2, extracellular regulated kinases; JNK, c-Jun
N-terminal kinase. |
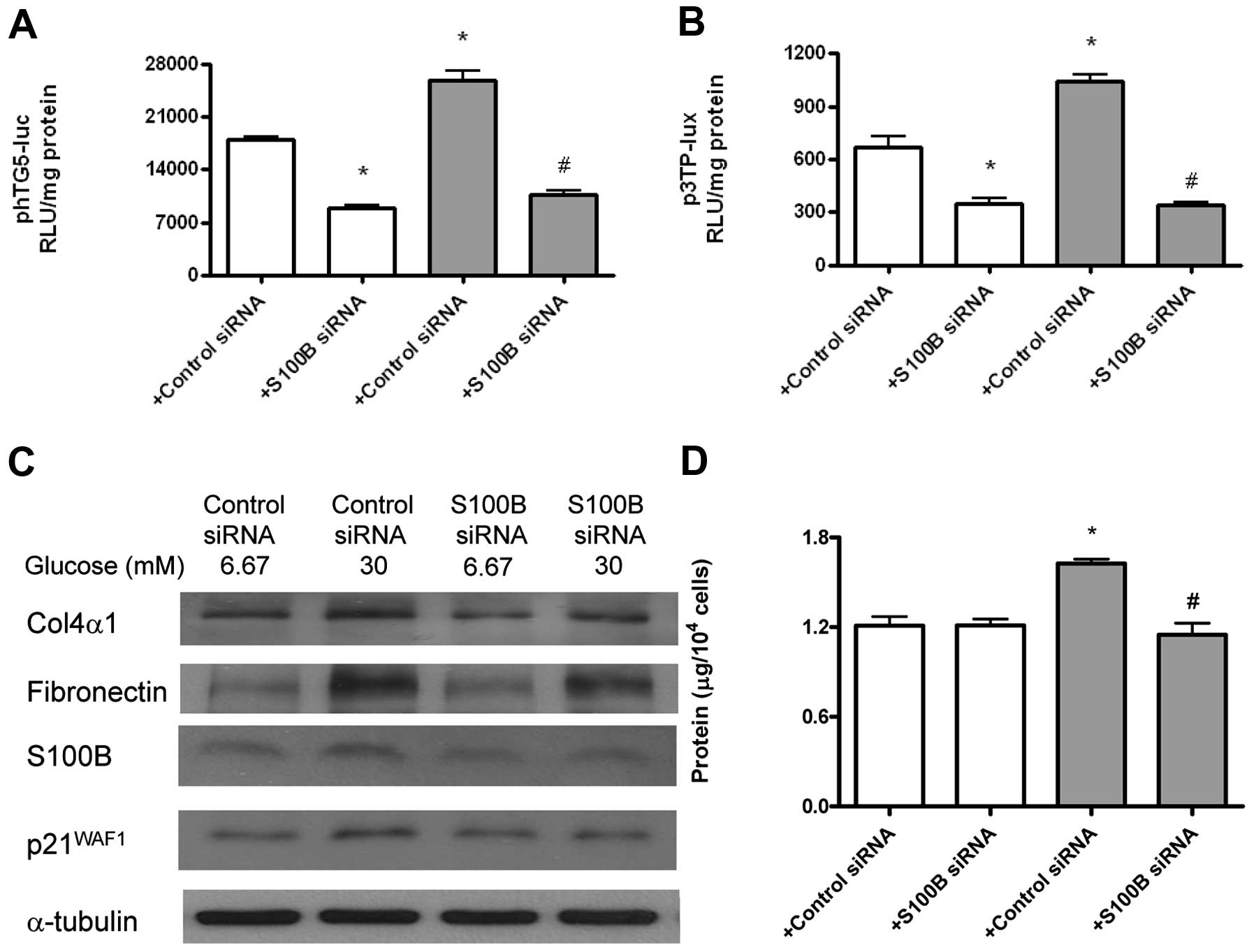
High glucose-induced TGF-β and
pro-fibrotic genes are dependent on S100B
Gene transcriptional activity of the TGF-β
gene was measured by transient tranfection of phTG5-luc, while
TGF-β bioactivity was measured by p3TP-lux. Type IV collagen,
fibronectin, S100B and p21WAF1 proteins were measured by
immunoblotting. The role of S100B was measured by S100B
siRNA. S100B (but not the scrambled) siRNA attenuated high
glucose-induced TGF-β gene transcriptional activity
(Fig. 7A) and bioactivity
(Fig. 7B) at 24 h. Additionally,
S100B (but not the scrambled) siRNA increased high
glucose-induced type IV collagen, fibronectin, S100B and
p21WAF1 protein expression at 48 h (Fig. 7C). Finally, S100B (but not
the scrambled) siRNA attenuated high glucose-induced cell
hypertrophy (Fig. 7D) at 48
h.
Discussion
To the best of our knowledge, this is the first
study to demonstrate that high glucose increased S100B in mesangial
cells and that high glucose and S100B increased p21WAF1,
type 4 collagen, fibronectin and TGF-β activity. Additionally,
S100B-induced TGF-β is dependent on ERK1/2 and p38 kinase, whereas
high glucose-induced expression of p21WAF1, type IV
collagen, fibronectin and TGF-β and cell hypertrophy are dependent
on S100B. These findings shed light on the roles of S100B in
DN.
The finding that high glucose increased S100B
expression in mesangial cells is similar to two previous studies
showing increased S100B mRNA levels in diabetic human
glomeruli (19) or diabetic rat
renal cortex (20) in the Gene
Expression Omnibus microarray database (21), although the original studies did
not mention it. High glucose and exogenous S100B were also found to
induce the p21WAF1 gene transcription and protein
expression, type IV collagen and fibronectin expression and
TGF-β gene transcription and bioactivity. These findings
prompted us to further elucidate the roles of S100B in high
glucose-induced effects.
High glucose-induced TGF-β gene transcription
and bioactivity, p21WAF1 gene transcription and
protein expression, and type IV collagen and fibronectin expression
are dependent on S100B. In addition, high glucose-induced cell
hypertrophy is also dependent on S100B. As S100B is a specific
ligand for RAGE (3), it is likely
that the above high glucose-induced effects are dependent on RAGE.
However, it is also likely that intracellular S100B acts without
engaging RAGE (22).
ERK1/2, p38 kinase and JNK pathways have been
indicated in the pathogenesis of DN (1,5).
These pathways are also the downstream targets of the RAGE
signaling (5). S100B activated
the ERK1/2, p38 kinase and JNK pathways in the present study.
Additionally, S100B-induced TGF-β transcription and
bioactivity are dependent on the TGF-β receptor and the ERK1/2 and
p38 kinase pathways. These findings are compatible with the notion
of the auto-induction of TGF-β (23) and also corroborate two previous
findings that TGF-β1 induction is dependent on ERK1/2 and p38
kinase in mesangial cells (24,25). By contrast, S100B-induced TGF-β
bioactivity (but not gene transcription) is dependent on the JNK
pathway. This finding is compatible with the notion that JNK is a
part of the TGF-β non-Smad signaling pathways (26).
In conclusion, S100B induced TGF-β via the ERK1/2
and p38 kinase pathways. The significance of high glucose-induced
S100B is demonstrated by the findings that high glucose-induced
pro-fibrotic genes (TGF-β, type IV collagen and fibronectin) and
cell hypertrophy-related p21WAF1 are dependent on S100B.
Thus, S100B may be a novel target for the treatment of DN.
Acknowledgments
This study was supported in part by grants from the
National Science Council of Taiwan (101-2314-B-037-036-MY3 to L.-Y.
C. and 102-2314-B-037-011-MY3 to J.-Y. G.) and the Center for Lipid
and Glycomedicine Research (KMU-TP103D16 to L.-Y. C.). The authors
would like to thank Dr X.F. Wang, Dr J.L. Virelizier and Dr J.
Massagué for the gifts of the plasmids.
References
|
1
|
Gnudi L: Cellular and molecular mechanisms
of diabetic glomerulopathy. Nephrol Dial Transplant. 27:2642–2649.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forbes JM and Cooper ME: Glycation in
diabetic nephropathy. Amino Acids. 42:1185–1192. 2012. View Article : Google Scholar
|
|
3
|
Ramasamy R, Yan SF and Schmidt AM: The
diverse ligand repertoire of the receptor for advanced glycation
endproducts and pathways to the complications of diabetes. Vasc
Pharmacol. 57:160–167. 2012. View Article : Google Scholar
|
|
4
|
Loeffler I and Wolf G: Transforming growth
factor‑beta and the progression of renal disease. Nephrol Dial
Transplant. 29(Suppl 1): i37–i45. 2014. View Article : Google Scholar
|
|
5
|
Xie J, Méndez JD, Méndez-Valenzuela V and
Aguilar-Hernández MM: Cellular signalling of the receptor for
advanced glycation end products (RAGE). Cell Signal. 25:2185–2197.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meek RL, LeBoeuf RC, Saha SA, et al:
Glomerular cell death and inflammation with high-protein diet and
diabetes. Nephrol Dial Transplant. 28:1711–1720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reddy MA, Sumanth P, Lanting L, et al:
Losartan reverses permissive epigenetic changes in renal glomeruli
of diabetic db/db mice. Kidney Int. In press. 85:362–373. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forbes JM and Cooper ME: Mechanisms of
diabetic complications. Physiol Rev. 93:137–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wolf G: Cell cycle regulation in diabetic
nephropathy. Kidney Int. 58:S59–S66. 2000. View Article : Google Scholar
|
|
10
|
Kuan CJ, al-Douahji M and Shankland SJ:
The cyclin kinase inhibitor p21WAF1, CIP1 is increased in
experimental diabetic nephropathy: potential role in glomerular
hypertrophy. J Am Soc Nephrol. 9:986–993. 1998.PubMed/NCBI
|
|
11
|
Al-Douahji M, Brugarolas J, Brown PA,
Stehman-Breen CO, Alpers CE and Shankland SJ: The cyclin kinase
inhibitor p21WAF1/CIP1 is required for glomerular hypertrophy in
experimental diabetic nephropathy. Kidney Int. 56:1691–1699. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leclerc E, Fritz G, Vetter SW and Heizmann
C: Binding of S100 proteins to RAGE: an update. Biochim Biophys
Acta. 1793:993–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zimmer DB, Chessher J, Wilson GL and
Zimmer WE: S100A1 and S100B expression and target proteins in type
I diabetes. Endocrinol. 138:5176–5183. 1997.
|
|
14
|
Jung DH, Kim YS and Kim JS: KIOM-79
prevents S100b-induced TGF-beta1 and fibronectin expression in
mouse mesangial cells. J Ethnopharmacol. 125:374–379. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung DH, Kim YS, Kim NH, Lee J, Jang DS
and Kim JS: Extract of Cassiae Semen and its major compound inhibit
S100b-induced TGF-beta1 and fibronectin expression in mouse
glomerular mesangial cells. Eur J Pharmacol. 641:7–14. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Datto MB, Yu Y and Wang XF: Functional
analysis of the transforming growth factor beta responsive elements
in the WAF1/Cip1/p21 promoter. J Biol Chem. 270:28623–28628. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michelson S, Alcami J, Kim SJ, et al:
Human cytomegalovirus infection induces transcription and secretion
of transforming growth factor beta 1. J Virol. 68:5730–5737.
1994.PubMed/NCBI
|
|
18
|
Wrana JL, Attisano L, Cárcamo J, et al:
TGF beta signals through a heteromeric protein kinase receptor
complex. Cell. 71:1003–1014. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baelde HJ, Eikmans M, Doran PP, Lappin DW,
de Heer E and Bruijn JA: Gene expression profiling in glomeruli
from human kidneys with diabetic nephropathy. Am J Kidney Dis.
43:636–650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Langer WJ, Devish K, Carmines PK and Lane
PH: Prepubertal onset of diabetes prevents expression of renal
cortical connective tissue growth factor. Pediatr Nephrol.
23:275–283. 2008. View Article : Google Scholar
|
|
21
|
Barrett T, Wilhite SE, Ledoux P, et al:
NCBI GEO: archive for functional genomics data sets-update. Nucleic
Acids Res. 41:D991–D995. 2013. View Article : Google Scholar
|
|
22
|
Donato R, Sorci G, Riuzzi F, et al:
S100B’s double life: intracellular regulator and extracellular
signal. Biochim Biophys Acta. 1793:1008–1022. 2009. View Article : Google Scholar
|
|
23
|
Kim SJ, Angel P, Lafyatis R, et al:
Autoinduction of transforming growth factor beta 1 is mediated by
the AP-1 complex. Mol Cell Biol. 10:1492–1497. 1990.PubMed/NCBI
|
|
24
|
Isono M, Cruz MC, Chen S, Hong SW and
Ziyadeh FN: Extracellular signal-regulated kinase mediates
stimulation of TGF-beta1 and matrix by high glucose in mesangial
cells. J Am Soc Nephrol. 11:2222–2230. 2000.PubMed/NCBI
|
|
25
|
Burt DJ, Gruden G, Thomas SM, et al: P38
mitogen-activated protein kinase mediates hexosamine-induced
TGFbeta1 mRNA expression in human mesangial cells. Diabetologia.
46:531–537. 2003.PubMed/NCBI
|
|
26
|
Mu Y, Gudey SK and Landstrom M: Non-Smad
signaling pathways. Cell Tissue Res. 347:11–20. 2012. View Article : Google Scholar
|