Introduction
Skeletal muscle development is a strictly regulated
process involving the specification of mesodermal precursors into
myoblasts, following the differentiation and fusion of these cells
into multinucleated myotubes. Myogenic regulatory factors (MRFs)
play a specific role in muscle fusion during differentiation
(1,2). Among the MRFs, basic
helix-loop-helix (bHLH) transcription factors, myogenic
differentiation 1 (MyoD), myogenic factor 5 (Myf5), myogenin and
MRF4 are critical to muscle formation. MyoD and Myf5 are required
for the formation of skeletal muscle and play unique roles in the
development of epaxial and hypaxial muscle, respectively (3,4).
These two MRFs enable the differentiation of myogenic progenitors
into myoblasts, which differentiate into myotubes by myogenin.
Moreover, MyoD promotes myoblast differentiation by regulating the
cell cycle (5). MRF4 is important
for blocking the transcription of muscle-specific promoters, and
enabling the growth and proliferation of skeletal muscle
progenitors prior to differentiation. Myogenin activates MRF4
transcription independently, and synergistically stimulates the
MRF4 promoter (6).
Ursolic acid, a natural pentacyclic triterpenoid
carboxylic acid (7), is
abundantly found in a number of fruits and plants, including apples
(a major compound of apple peels), cranberries, basil, peppermint
and rosemary (8). It has been
used for pharmaceutical, cosmetic and food preparations. Of note,
ursolic acid has garnered much attention as a therapeutic compound
in a number of diseases, such as cancer (9), diabetes (10), Alzheimer’s disease (11) and obesity (12), due to its anti-inflammatory and
antitumor properties. Moreover, this triterpene has been reported
to increase the insulin-induced phosphorylation of Akt, a
serine/threonine-specific protein kinase, that plays a key role in
multiple cellular processes, such as glucose metabolism, apoptosis,
cell proliferation, transcription and cell migration (13). In skeletal muscle, Akt has been
reported to inhibit muscle atrophy and promote muscle hypertrophy,
which is linked to muscle protein synthesis. Recently, Kunkel et
al (14) demonstrated that
ursolic acid enhanced skeletal muscle insulin/insulin-like growth
factor 1 (IGF-I) signaling, leading to Akt activation, muscle
hypertrophy and reduced adiposity and blood glucose levels in mice.
Ursolic acid has also been shown to increase muscle mass through
Akt activation in fed a mice high-fat diet (15). In the same study, ursolic acid
exhibited a beneficial effect by not only increasing skeletal
muscle mass, but also increasing energy expenditure, leading to
reduced obesity, improved glucose tolerance and decreased hepatic
steatosis. These findings suggest the therapeutic potential for
ursolic acid in muscle atrophy caused by aging and illnesses,
including cancer and other metabolic diseases.
L-leucine is an essential amino acid that belongs to
a group of branched-chain amino acids (BCAAs). BCAAs, including
leucine, valine and isoleucine, are found mainly in skeletal
muscle, and are thus essential in supporting muscle growth,
comprising up to one-third of muscle (16). Among the BCAAs, leucine has been
shown to directly activate the mammalian target of rapamycin (mTOR)
signaling cascade which is closely associated with protein
synthesis in muscle (17).
Leucine has also been reported to stimulate muscle growth and
repair through the accumulation of glycogen, which is a muscular
energy source. β-hydroxy β-methylbutyrate (HMB), the most popular
muscle enhancing agent and a metabolite of leucine is known to
reinforce muscle (18). Several
lines of evidence indicate that dietary supplements, such as
leucine and HMB, promote body mass and strength (19,20). Due to their anabolic effects and
ability to promote muscle recovery following injury and in some
muscle diseases, the dietary supplementation of leucine or HMB is
considered an attractive therapeutic agent for reducing muscle loss
in aging populations (21,22).
The mTOR signaling pathway is one of the main
signaling pathways controling protein synthesis and cell
proliferation and is a serine/threonine protein kinase belonging to
the phosphatidylinositol 3-kinase (PI3K)-related kinase protein
family that regulates cell growth, cell proliferation, cell
motility and cell survival (20).
The mTOR signaling pathway is mediated by two complexes, mTOR
complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 and mTORC2
share several components and contain unique proteins. The activity
of mTORC1 is stimulated by insulin, growth factors, amino acids
(particularly leucine) and oxidative stress through the PI3K/Akt
pathway. Activated mTORC1 kinase upregulates protein synthesis
through the phosphorylation of key regulators of mRNA translation,
including eukaryotic translation initiation factor 4E binding
protein 1 (4E-BP1), a binding partner of the cap-binding protein,
eIF4E (23). 4E-BP1 is an
important regulator of overall translational levels in cells
(24). It has also been suggested
to control the growth rate of tissue during development as a growth
regulator (25). The ribosomal
protein S6 kinase 1 (S6K1) is one of two mammalian p70 proteins,
acting to converge growth factor, hormonal, nutrient and energy
signals in order to maintain cellular homeostasis. In addition, the
kinase activity of S6K1 leads to an increase in protein synthesis
and cell proliferation (26). The
aim of the present study was to investigate whether the combination
of ursolic acid and leucine promotes greater myogenic
differentiation compared to treatment with either agent alone in
C2C12 murine myoblasts.
Materials and methods
Reagents
Ursolic acid, L-leucine and monoclonal antibody
against β-actin were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Ursolic acid was dissolved in dimethyl sulfoxide (DMSO) and
stored at −20°C prior to the experiments and dilutions were made in
culture medium. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenytetrazolium
bromide (MTT) was obtained from Amresco (Solon, OH, USA).
Antibodies against myosin heavy chain (MHC; sc-20641), MyoD
(sc-760), myogenin (sc-576), phospho-Akt (Ser 473; sc-7985-R), and
Akt1/2/3 (sc-8312) were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Antibodies against 4E-BP1 (cat. no. 9452),
phospho-4E-BP1 (cat. no. 2855) and phospho-p70S6K (cat. no. 9234)
were purchased from Cell Signaling Technology (Danvers, MA, USA).
Polyclonal antibody against p70S6K (bs-6370R) was obtained from
Bioss (Woburn, MA, USA). Dulbecco’s modified Eagle’s medium (DMEM)
was purchased from Welgene Inc. (Daegu, Korea) and horse serum (HS)
was from Invitrogen (Grand Island, NY, USA). Fetal bovine serum
(FBS) and penicillin-streptomycin were purchased from HyClone
(Logan, UT, USA). The creatine kinase enzymatic assay kit
(MaxDiscovery™ Creatine Kinase Enzymatic Assay kit) was purchased
from Bioo Scientific Corp. (Austin, TX, USA).
Cell culture and cell viability
assay
The murine myoblast cell line, C2C1,2 was purchased
from the American Type Culture Collection (ATCC, Manassas, VA, USA)
and maintained at 37°C in humidified 95% air and 5% CO2
in growth medium (GM), containing DMEM supplemented with 10% FBS,
100 U/ml penicillin and 100 μg/ml streptomycin at up to
70–80% confluence. During proliferation, the cells were seeded at
2×105 cells/well in 6-well culture plates and grown in
GM. When the cells reached approximately 90–100% confluence, the GM
was removed and replaced with differentiation medium (DM),
containing DMEM supplemented with 2% horse serum (HS) and ursolic
acid, leucine or a combination of ursolic acid and leucine. The
medium was changed every 2 days until day 6. The effects of ursolic
acid, leucine or their combination on cell viability were
determined by MTT assay, which is based on the conversion of MTT to
MTT-formazan by mitochondrial enzymes.
Creatine kinase (CK) activity
The cells were washed with phosphate-buffered saline
(PBS) and then lysed with lysis buffer [40 mM Tris (pH 8.0), 120 mM
NaCl, 0.5% NP-40, 2 μg/ml aprotinin, 2 μg/ml
leupeptine and 100 μg/ml phenymethyl-sulfonyl fluoride
(PMSF)], and complete protease inhibitor and stored at −70°C until
use. CK activity was determined by a CK enzymatic assay kit (Bioo
Scientific Corp.), according to the manufacturer’s instructions.
Briefly, 250 μl of CK reagent were added to 5 μl of
cell lysate in a microplate. Subsequently, CK activity was
immediately measured 2 times with a 5-min time interval at 340 nm.
The assay was performed in duplicate. The average 5 min absorbance
increase was multiplied by 2,186 (conversion factor) to obtain the
CK activity (IU/l).
Western blot analysis
Following treatment, the cells were harvested and
washed with cold PBS and then lysed in lysis buffer. Following
centrifugation, the supernatant was collected and the protein
concentration was determined using protein assay reagents (Bio-Rad
Laboratories, Hercules, CA, USA). Equal amounts of protein were
denatured by boiling at 100°C for 5 min in 2X Laemmli sample buffer
(Bio-Rad Laboratories). The protein samples were separated on 6–15%
SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF)
membranes. The membranes were blocked with 5% non-fat dry milk in
Tris-buffered saline with Tween-20 buffer (TBS-T; 20 mM Tris, 100
mM NaCl, pH 7.5 and 0.1% Tween-20) for 1 h at room temperature. The
membranes were then incubated with various primary antibodies at
4°C overnight. After being thoroughly washed with TBS-T buffer, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (Santa Cruz Biotechnology). Antigen-antibody
complexes were detected by the Enhanced chemiluminescence (ECL)
detection system (GE Healthcare, Piscataway, NJ, USA).
Immunocytochemistry
For immunofluorescence analysis to evaluate myogenic
differentiation, the cells were cultured on gelatin-coated
coverslips. For this, the coverslips were soaked in 1 M HCl for 24
h, rinsed 3 times with distilled water and then rinsed 3 times with
95% ethanol. The coverslips were submerged in gelatin solution (0.1
mg/ml) for 5 min, air-dried and placed into 6-well culture plates.
Finally, the culture plates with coverslips were sterilized under a
UV lamp for at least 2 h. The cells (2×105/well) were
seeded and cultured overnight. The cells were then rinsed with PBS,
fixed with 4% formalin and 0.3% glutaraldehyde for 20 min, washed
with PBS, incubated in PBS supplemented with 0.2 % Triton X-100 and
2% normal goat serum for 1 h at 4°C. The cultures were then
incubated overnight with primary antibody against MHC (1:200),
followed by rhodamine-conjugated secondary antibody (Jackson
ImmunoResearch Laboratories, West Grove, PA, USA). The samples were
mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA)
to prevent fading. The samples were examined under a confocal laser
scanning microscope (Zeiss, Göettingen, Germany).
Fusion index
The images were randomly selected from at least 3
regions from each well. The fusion index was calculated based on
the scoring of random regions with stained nuclei and myotubes.
Reverese transcription-polymerase chain
reaction
Total RNA was prepared using TRIzol reagent
(Invitrogen). cDNA for the PCR template was synthesized using
TOPscript™ RT DryMIX(dT18) (Enzynomics, Daejeon, Korea). For PCR,
specific primers were used for the analysis of the expression for
the following molecules: MyoD forward, 5′-AGTGAATGAGGCCTTCGAGA-3′
and reverse, 5′-CTGGGTTCCCTGTTCTGTGT-3′ (514 bp); myogenin forward,
5′-ACCAGGAGCCCCACTTCTAT-3′ and reverse, 5′-ACGATGGACGTAAGGGAGTG-3′
(583 bp); GAPDH forward, 5′-ACTCCACTCACGGCAAATTC-3′ and reverse,
5′-CCTTCCACAATGCCAAAGTT-3′ (370 bp). Subsequently, the PCR
reactions were performed in a Mastercycler gradient unit
(Eppendorf, Hamburg, Germany) using PCR PreMix (Bioneer Corp.,
Daejeon, Korea). Each PCR cycle was 94°C for 30 sec, 60°C for 30
sec, and 72°C for 1 min. The numbers of cycles of PCR were 26 for
MyoD, 27 for myogenin and 25 for GAPDH. The amplified PCR products
(7 μl) were electrophoresed on 1.2% agarose gels and
visualized by ethidium bromide staining.
Statistical analysis
The results were expressed as the means ± SD of 3
separate experiments and analyzed using the Student’s t-test and
ANOVA. Values of P<0.05 or P<0.01 were considered to indicate
statistically significantly differences.
Results
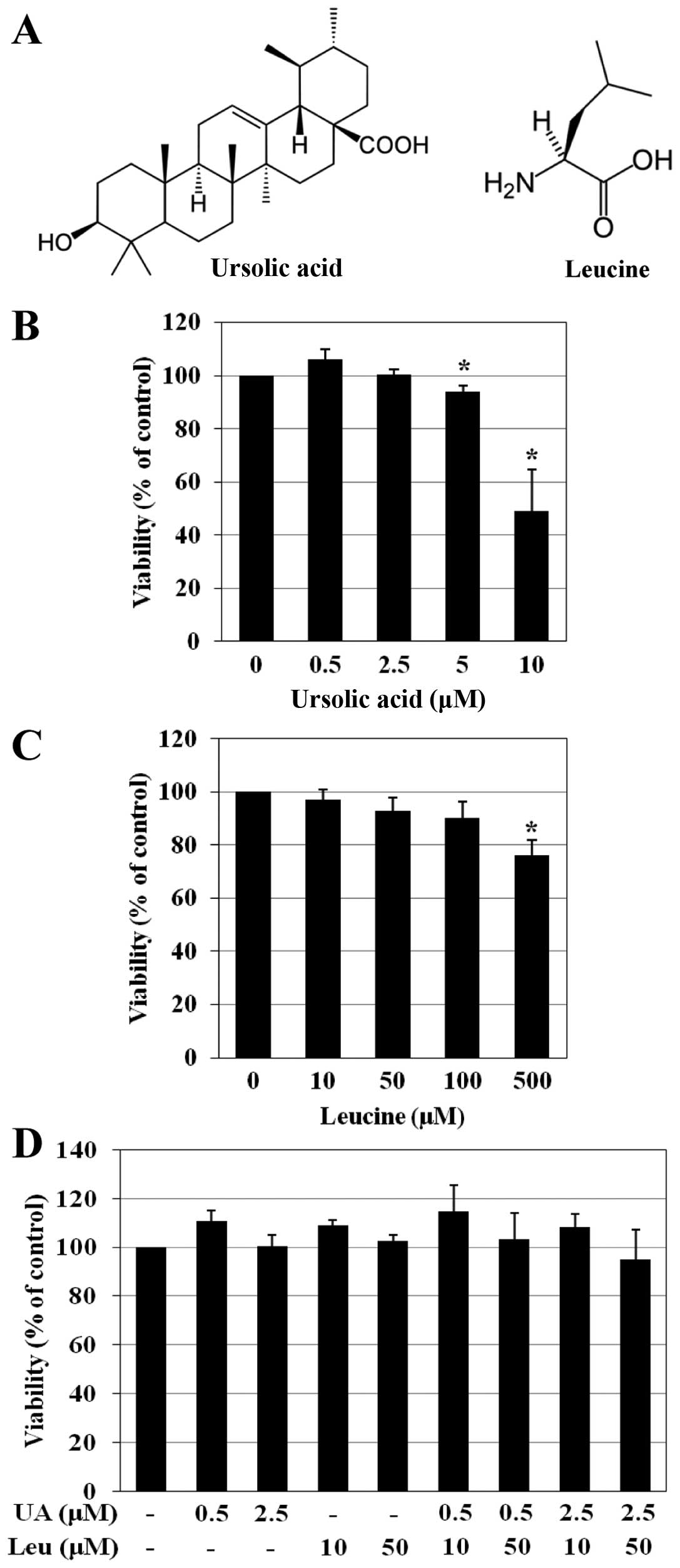
Ursolic acid and leucine have no effect
on C2C12 cell viability
To determine the effects of ursolic acid and leucine
on the viability of C2C12 cells, an MTT assay was performed. The
C2C12 cells were cultured in DM and treated with ursolic acid (0–10
μM) or leucine (0–500 μM) for 6 days. Cell viability
was not affected by treatment with 0–2.5 μM ursolic acid and
0–100 μM leucine. However, treatment with >2.5 μM
of ursolic acid and >100 μM of leucine exerted cytotoxic
effects (Fig. 1B and C). To
investigate whether the combination of ursolic acid and leucine
would promote the differentiation of C2C12 myoblasts, the cells
were treated with various concentrations of ursolic acid and
leucine (at concentrations that had not affected cell viability:
ursolic acid, 0.5 and 2.5 μM; leucine, 10 and 50 μM).
To confirm the effects of these combinations on cell viability, an
MTT assay was conducted again. Treatment with various combinations
of ursolic acid and leucine (Fig.
1D), similar to the treatments with either agent alone, had no
effect on viability of the C2C12 cells.
Ursolic acid, leucine, and the
combination of ursolic acid and leucine promote myotube
formation
Our first hypothesis in the present study was that
the combination of ursolic acid and leucine would promote greater
myogenic cell differentiation than either ursolic acid or leucine
alone. As shown in Fig. 2, both
ursolic acid and leucine induced morphological changes in the C2C12
cells. When the ursolic acid- or leucine-treated groups were
compared with the untreated groups (GM and DM), the cell shapes
differed greatly (Fig. 2 A–F).
After 3–4 days of differentiation, the cells seemed to lose their
typical triangular morphology and the cell shape gradually changed
into a new elongated shape. Furthermore, the
differentiation-promoting effects of the different combinations of
the agents were even more pronounced. The myotubes that formed in
the cells treated with combinations of ursolic acid and leucine
were more stretched and longer in shape than those in the cells
treated with each agent alone (Fig.
2G–J). In particular, in the cells that were treated with the
combination of 0.5 μM ursolic acid and 10 μM leucine,
the myotubes showed a spindly ring pattern, indicating muscle
hypertrophy and maturation (Fig.
2G).
The level of CK activity is increased by
the combination of ursolic acid and leucine
CK activity was used as an indicator of
differentiation. Compared to the GM control, CK activity levels in
the cells that were treated with ursolic acid or/and leucine for 6
days were increased (Fig. 3).
However, the CK activity level in the cells treated with either
agent alone (ursolic acid or leucine) was lower than that in the
cells treated with the various combinations of ursolic acid and
leucine. The level of CK activity in the cells treated with the
combination of 0.5 μM ursolic acid and 10 μM leucine
was significantly increased by 50% compared to the DM control
during differentiation, and this combination led to the most
significant increase in CK activity. The second most significant
increase in the level of CK activity was observed in the cells
treated with the combination of 2.5 μM ursolic acid and 10
μM leucine.
The combination of ursolic acid and
leucine enhances MHC protein expression
MHC is considered as a marker of the differentiated
state, particularly in the late differentiation of myoblasts.
First, western blot analysis was performed to detect the changes in
the protein expression levels of MHC over time. The protein levels
of MHC gradually increased over 6 days, with the highest levels
being observed on day 6 (Fig.
4A). Subsequently, in order to confirm whether the combination
of ursolic acid and leucine promotes the differentiation of C2C12
cells as opposed to treatment with each agent alone, the protein
level of MHC was evaluated. As expected, treatment with the
combination of 0.5 μM ursolic acid and 10 μM leucine
induced a significant increase in the expression level of MHC
(Fig. 4B). Compared to the DM
control, MHC expression increased by almost 2-fold. In addition,
compared to the cells treated with the agents alone (0.5 μM
ursolic acid or 10 μM leucine), the protein expression of
MHC increased by approximately 3- and 2-fold in the cells treated
with the combination of 0.5 μM ursolic acid and 10 μM
leucine, respectively (Fig.
4C).
The combination of ursolic acid and
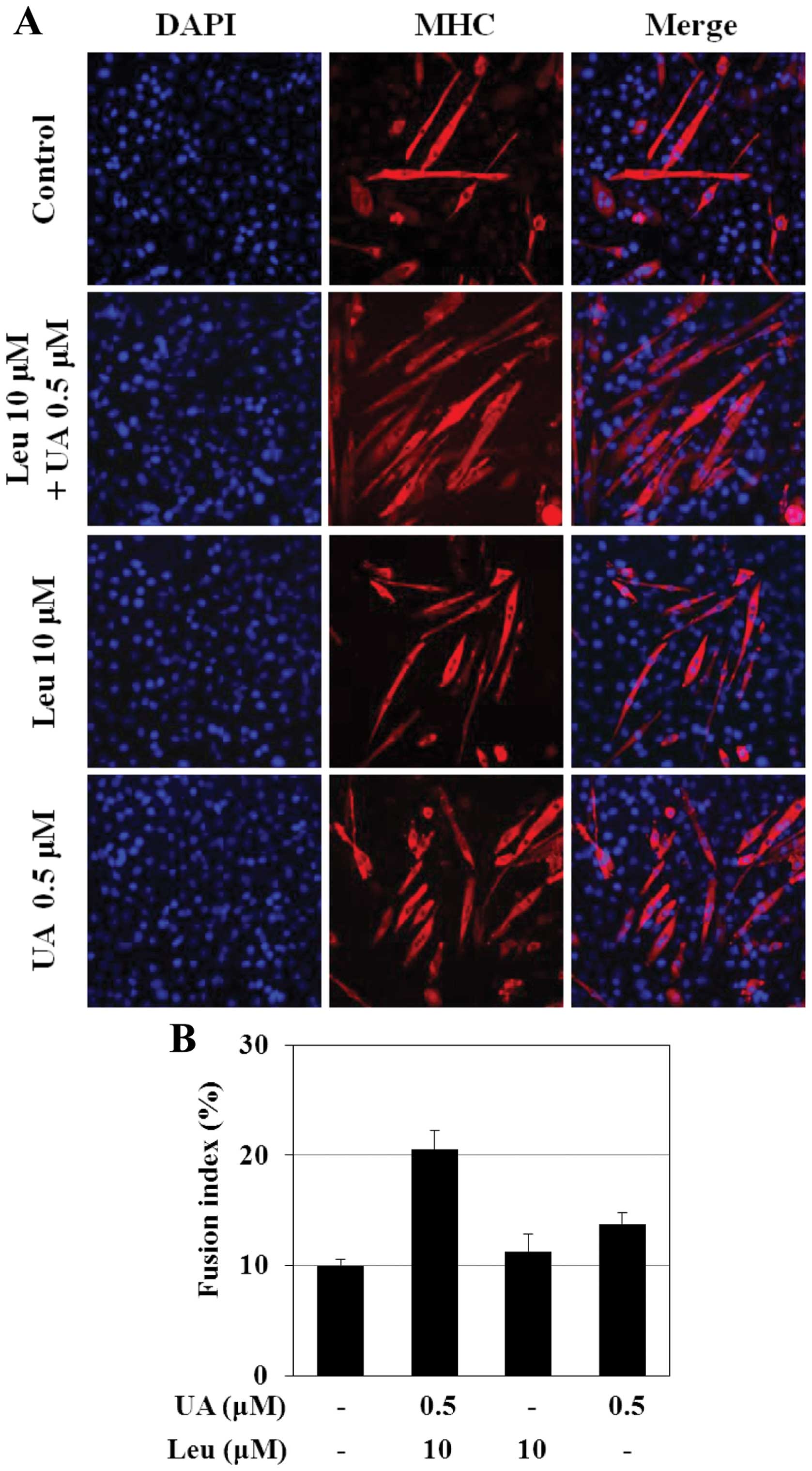
leucine increases the number of myotubes and the fusion index
To analyze the changes occurring in the nuclear
arrangement of myotubes during the late phase of differentiation,
immunofluorescence staining using antibody against MHC was
performed. As shown in Fig. 5A,
after 6 days of differentiation, most of the untreated cells
(controls) that expressed MHC were mononuclear; however, in the
presence of 0.5 μM ursolic acid or 10 μM leucine,
myotubes containing 2 and more nuclei were observed. The number of
myotubes containing 4 or more nuclei was highest in the cells
treated with the combination of 0.5 μM ursolic acid and 10
μM leucine. In the cells treated with the combination
treatment, the myotubes were characterized by a particular
arrangement of the nuclei, forming a ring, a morphological marker
of muscle maturation. With the combination treatment, the highest
density of MHC-expressing cells and a greater number of elongated
cells were observed, some of which fused into myotubes (Fig. 5A). The fusion index was also found
to be significantly higher (>2-fold) in the cells treated with
the combination of 0.5 μM ursolic acid and 10 μM
leucine, compared to the untreated cells (Fig. 5B).
The combination of ursolic acid and
leucine increases the expression levels of MyoD and myogenin
In our experiments, MHC was used as a marker of late
differentiation. In order to determine the effects of ursolic acid
and leucine on early differentiation, MyoD and myogenin were used
as biomarkers. First, quantitative analysis of the results from
western blot analysis further revealed time-dependent (on day 1, 2,
4 and 6) changes in the expression of the early differentiation
markers, MyoD and myogenin. As shown in Fig. 6A and B, MyoD was predominantly
expressed on day 1 (Fig. 6B),
while myogenin was predominantly expressed on day 2 (Fig. 6C). Compared with the untreated
controls, the protein expression of MyoD in the cells treated with
the combination of 0.5 μM ursolic acid and 10 μM
leucine increased by >2.5-fold (Fig. 6D and E). The epxression of
myogenin was also increased, although the increase was not as
notable as that of MyoD (Fig. 6D and
F). In addition, the mRNA levels of the muscle regulatory
factors, MyoD and myogenin, were elevated in response to the
combination treatment (Fig. 6G).
A sharp increase in the mRNA level of MyoD was observed in the
cells treated with the combination of ursolic acid and leucine
(Fig. 6H). The mRNA levels of
myogenin were also increased, although the increase was not as
notable as that of MyoD (Fig.
6I).
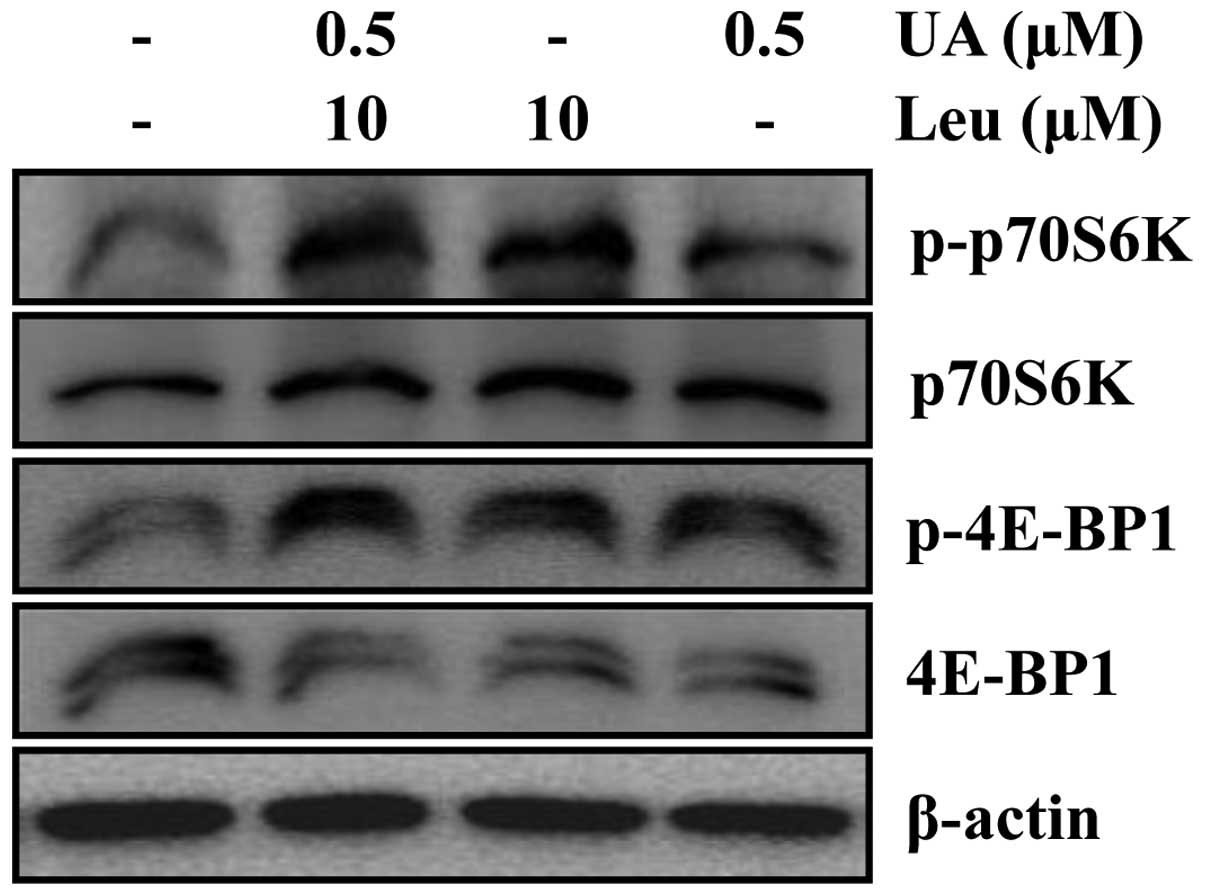
The combination of ursolic acid and
leucine promotes C2C12 myoblast differentiation through the mTOR
signaling pathway
Protein synthesis occurs during embryonic
development, cell growth, cell differentiation and aging. In
previous studies, signaling pathways, such as the IGF-1, PI3K/Akt,
and mTOR signaling cascades, have been shown to play a major role
in protein synthesis by targeting several components of the
translation machinery (27,28). To confirm the effects of the
combination of ursolic acid and leucine on the activation of the
mTOR signaling pathway, western blot analysis of S6K1 and 4E-BP1,
which are two key downstream targets of the mTOR signaling pathway
(29), was conducted. In
addition, the phosphorylation of 4E-BP1 and S6K1 are crucial
activation steps necessary for the downstream event (30). Treatment with the combination of
ursolic acid and leucine increased the phosphorylation of mTOR and
that of the downstream targets, 4E-BP1 and S6K1, as shown in
Fig. 7.
Discussion
Skeletal muscle plays important roles in initiating
movement, supporting respiration and maintaining homeostasis; the
loss of skeletal muscle mass or function is associated with aging
and a variety of diseases, such as cancer and diabetes (31). Several studies have assessed the
potential use of ursolic acid or leucine as an effective material
to stimulate myotube maturation and muscle differentiation in C2C12
cells (15,32,33). In the present study, we examined
whether the combination of ursolic acid and leucine is more
effective in inducing muscle cell differentiation when compared
with the use of either agent alone.
The process of the formation of myofibers is
controlled by a set of MRFs, such as MyoD and its relatives, Myf-5,
MRF4 and myogenin. MRFs have different roles during myogenesis, and
their expression levels also differ. While MyoD and Myf5 determine
the myogenic lineage of muscle progenitor cells (3,34),
myogenin and MRF4 drive the terminal differentiation and fusion of
myoblasts into myotubes, the developing myofibers (35,36). MyoD is regulated by several
biochemical modifications and interactions, such as ubiquitination
(37), acetylation (38), phosphorylation (39) and is negatively regulated by
methylation (40). In addition,
it can interact with cell cycle regulators, such as retinoblastoma
protein (pRB) (41) and cdk4
(42) to induce cell cycle
withdrawal directly during myogenic differentiation.
In this study, combination treatment with ursolic
acid and leucine exerted a stimulatory effect on myoblast
differentiation. The combination of ursolic acid and leucine
stimulated and enhanced the differentiation of myoblasts into
myotubes, as evidenced by the increased expression of myogenic
differentiation markers (protein levels of MHC, MyoD and myogenin)
and (mRNA levels of MyoD and myogenin). In addition, the
combination treatment enhanced myotube formation, as indicated by
the morphological analysis, CK analysis and the analysis of MHC
expression during the late differentiation phase. Combination
treatment stimulated differentiation, as evidenced by the increased
fusion rate and the increased average number of nuclei per myotube.
As evidenced by the number and size of myotubes formed, the
myoblasts treated with the combination treatment differentiated
more rapidly in culture than the control myoblasts and the
myoblasts treated with either agent alone (data not shown).
Secondly, we also found that the promoting effect of
the combination treatment on muscle differentiation, stimulating
muscle protein synthesis, was related to the activation of the mTOR
signaling pathway. This results was in accordance with the data of
previous studies indicating that the mTOR pathway mediates skeletal
muscle differentiation, eventually determining protein synthesis
and increasing muscle mass (20,43). The level of phosphorylation of
mTOR and its downstream targets, 4E-BP1 and p70S6K, was increased
by the combination treatment (Fig.
7). Based on the stimulatory effect observed on muscle protein
synthesis through this pathway, combination treatment with ursolic
acid and leucine may well have an additive or potentiating effect,
and not a synergistic effect, on the differentiation of muscle
cells.
In conclusion, our data demonstrate that combination
treatment with ursolic acid and leucine has the potential to
directly alter protein synthesis and enhance the differentiation of
murine C2C12 myoblasts. The cells treated with the combination of
ursolic acid and leucine exhibited a particular arrangement of the
nuclei, forming a ring pattern, which is a morphological marker of
muscle hypertrophy and maturation. In addition, the expression of
differentiation markers, such as MHC, MyoD and myogenin increased
at both the mRNA and protein level. The combination treatment
enhanced the differentiation of C2C12 cells through the mTOR
pathway. Overall, our data demonstrate that nutritional
interventions, such as the supplementation of ursolic acid in
combination with leucine, may prove to be beneficial in conditions
such as muscle atrophy (sarcopenia), where there is a deficiency in
muscle-building amino acids, such as BCAAs, and may help potentiate
muscle fiber protein synthesis.
Acknowledgments
The present study was supported by the R&D
Program of MOTIF/KEIT (10040391, the Development of Functional Food
Materials and Device for Prevention of Aging-associated Muscle
Function Decrease). This study was also supported by the National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MSIP; (no. 2009-0083538). The authors would also like
to thank the Aging Tissue Bank for providing research information
and Mr. Kyoung-Pil Lee (Pusan National University) for assisting
with confocal microscopy.
References
|
1
|
Dedieu S, Mazeres G, Cottin P and Brustis
JJ: Involvement of myogenic regulator factors during fusion in the
cell line C2C12. Int J Dev Biol. 46:235–241. 2002.PubMed/NCBI
|
|
2
|
Doherty JT, Lenhart KC, Cameron MV, Mack
CP, Conlon FL and Taylor JM: Skeletal muscle differentiation and
fusion are regulated by the BAR-containing Rho-GTPase-activating
protein (Rho-GAP), GRAF1. J Biol Chem. 286:25903–25921. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rudnicki MA, Schnegelsberg PN, Stead RH,
Braun T, Arnold HH and Jaenisch R: MyoD or Myf-5 is required for
the formation of skeletal muscle. Cell. 75:1351–1359. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kablar B, Krastel K, Ying C, Asakura A,
Tapscott SJ and Rudnicki MA: MyoD and Myf-5 differentially regulate
the development of limb versus trunk skeletal muscle. Development.
124:4729–4738. 1997.
|
|
5
|
Ishibashi J, Perry RL, Asakura A and
Rudnicki MA: MyoD induces myogenic differentiation through
cooperation of its NH2-and COOH-terminal regions. J Cell Biol.
171:471–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naidu PS, Ludolph DC, To RQ, Hinterberger
TJ and Konieczny SF: Myogenin and MEF2 function synergistically to
activate the MRF4 promoter during myogenesis. Mol Cell Biol.
15:2707–2718. 1995.PubMed/NCBI
|
|
7
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jager S, Trojan H, Kopp T, Laszczyk MN and
Scheffler A: Pentacyclic triterpene distribution in various plants
- rich sources for a new group of multi-potent plant extracts.
Molecules. 14:2016–2031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leng S, Hao Y, Du D, et al: Ursolic acid
promotes cancer cell death by inducing Atg5-dependent autophagy.
Int J Cancer. 133:2781–2790. 2013.PubMed/NCBI
|
|
10
|
Zhang W, Hong D, Zhou Y, et al: Ursolic
acid and its derivative inhibit protein tyrosine phosphatase 1B,
enhancing insulin receptor phosphorylation and stimulating glucose
uptake. Biochim Biophys Acta. 1760:1505–1512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilkinson K, Boyd JD, Glicksman M, Moore
KJ and El Khoury J: A high content drug screen identifies ursolic
acid as an inhibitor of amyloid beta protein interactions with its
receptor CD36. J Biol Chem. 286:34914–34922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He Y, Li Y, Zhao T, Wang Y and Sun C:
Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through
LKB1/AMPK pathway. PLoS One. 8:e701352013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung SH, Ha YJ, Shim EK, et al:
Insulin-mimetic and insulin-sensitizing activities of a pentacyclic
triterpenoid insulin receptor activator. Biochem J. 403:243–250.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kunkel SD, Suneja M, Ebert SM, et al: mRNA
expression signatures of human skeletal muscle atrophy identify a
natural compound that increases muscle mass. Cell Metab.
13:627–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunkel SD, Elmore CJ, Bongers KS, et al:
Ursolic acid increases skeletal muscle and brown fat and decreases
diet-induced obesity, glucose intolerance and fatty liver disease.
PLoS One. 7:e393322012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
She P, Olson KC, Kadota Y, et al: Leucine
and protein metabolism in obese Zucker rats. PLoS One.
8:e594432013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haegens A, Schols AM, van Essen AL, van
Loon LJ and Langen RC: Leucine induces myofibrillar protein
accretion in cultured skeletal muscle through mTOR dependent and
-independent control of myosin heavy chain mRNA levels. Mol Nutr
Food Res. 56:741–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fitschen PJ, Wilson GJ, Wilson JM and
Wilund KR: Efficacy of beta-hydroxy-beta-methylbutyrate
supplementation in elderly and clinical populations. Nutrition.
29:29–36. 2013. View Article : Google Scholar
|
|
19
|
Nissen S, Sharp RL, Panton L, Vukovich M,
Trappe S and Fuller JC Jr: beta-hydroxy-beta-methylbutyrate (HMB)
supplementation in humans is safe and may decrease cardiovascular
risk factors. J Nutr. 130:1937–1945. 2000.PubMed/NCBI
|
|
20
|
Salles J, Chanet A, Giraudet C, et al:
1,25(OH)2-vitamin D3 enhances the stimulating effect of
leucine and insulin on protein synthesis rate through Akt/PKB and
mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol Nutr
Food Res. 57:2137–2146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alway SE, Pereira SL, Edens NK, Hao Y and
Bennett BT: beta-Hydroxy-beta-methylbutyrate (HMB) enhances the
proliferation of satellite cells in fast muscles of aged rats
during recovery from disuse atrophy. Exp Gerontol. 48:973–984.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pereira MG, Baptista IL, Carlassara EO,
Moriscot AS, Aoki MS and Miyabara EH: Leucine supplementation
improves skeletal muscle regeneration after cryolesion in rats.
PLoS One. 9:e852832014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou H and Huang S: The complexes of
mammalian target of rapamycin. Curr Protein Pept Sci. 11:409–424.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Teleman AA, Chen YW and Cohen SM: 4E-BP
functions as a metabolic brake used under stress conditions but not
during normal growth. Genes Dev. 19:1844–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miron M, Verdú J, Lachance PE, Birnbaum
MJ, Lasko PF and Sonenberg N: The translational inhibitor 4E-BP is
an effector of PI(3)K/Akt signalling and cell growth in Drosophila.
Nat Cell Biol. 3:596–601. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding M, Xie Y, Wagner RJ, et al:
Adiponectin induces vascular smooth muscle cell differentiation via
repression of mammalian target of rapamycin complex 1 and FoxO4.
Arterioscler Thromb Vasc Biol. 31:1403–1410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hara K, Yonezawa K, Weng QP, Kozlowski MT,
Belham C and Avruch J: Amino acid sufficiency and mTOR regulate p70
S6 kinase and eIF-4E BP1 through a common effector mechanism. J
Biol Chem. 273:14484–14494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Proud CG: mTOR-mediated regulation of
translation factors by amino acids. Biochem Biophys Res Commun.
313:429–436. 2004. View Article : Google Scholar
|
|
29
|
Lian J, Yan XH, Peng J and Jiang SW: The
mammalian target of rapamycin pathway and its role in molecular
nutrition regulation. Mol Nutr Food Res. 52:393–399. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Burnett PE, Barrow RK, Cohen NA, Snyder SH
and Sabatini DM: RAFT1 phosphorylation of the translational
regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA.
95:1432–1437. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vinciguerra M, Musaro A and Rosenthal N:
Regulation of muscle atrophy in aging and disease. Adv Exp Med
Biol. 694:211–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Averous J, Gabillard JC, Seiliez I and
Dardevet D: Leucine limitation regulates myf5 and myoD expression
and inhibits myoblast differentiation. Exp Cell Res. 318:217–227.
2012. View Article : Google Scholar
|
|
33
|
Ogasawara R, Sato K, Higashida K, Nakazato
K and Fujita S: Ursolic acid stimulates mTORC1 signaling after
resistance exercise in rat skeletal muscle. Am J Physiol Endocrinol
Metab. 305:E760–E765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kablar B, Krastel K, Tajbakhsh S and
Rudnicki MA: Myf5 and MyoD activation define independent myogenic
compartments during embryonic development. Dev Biol. 258:307–318.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Myer A, Olson EN and Klein WH: MyoD cannot
compensate for the absence of myogenin during skeletal muscle
differentiation in murine embryonic stem cells. Dev Biol.
229:340–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meadows E, Cho JH, Flynn JM and Klein WH:
Myogenin regulates a distinct genetic program in adult muscle stem
cells. Dev Biol. 322:406–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Breitschopf K, Bengal E, Ziv T, Admon A
and Ciechanover A: A novel site for ubiquitination: the N-terminal
residue, and not internal lysines of MyoD, is essential for
conjugation and degradation of the protein. EMBO J. 17:5964–5973.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sartorelli V, Puri PL, Hamamori Y, et al:
Acetylation of MyoD directed by PCAF is necessary for the execution
of the muscle program. Mol Cell. 4:725–734. 1999. View Article : Google Scholar
|
|
39
|
Jo C, Cho SJ and Jo SA: Mitogen-activated
protein kinase kinase 1 (MEK1) stabilizes MyoD through direct
phosphorylation at tyrosine 156 during myogenic differentiation. J
Biol Chem. 286:18903–18913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ling BM, Bharathy N, Chung TK, et al:
Lysine methyltransferase G9a methylates the transcription factor
MyoD and regulates skeletal muscle differentiation. Proc Natl Acad
Sci USA. 109:841–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gu W, Schneider JW, Condorelli G, Kaushal
S, Mahdavi V and Nadal-Ginard B: Interaction of myogenic factors
and the retinoblastoma protein mediates muscle cell commitment and
differentiation. Cell. 72:309–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang JM, Wei Q, Zhao X and Paterson BM:
Coupling of the cell cycle and myogenesis through the cyclin
D1-dependent interaction of MyoD with cdk4. EMBO J. 18:926–933.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang H, Li F, Kong X, et al: Chemerin
regulates proliferation and differentiation of myoblast cells via
ERK1/2 and mTOR signaling pathways. Cytokine. 60:646–652. 2012.
View Article : Google Scholar : PubMed/NCBI
|