Introduction
Bone marrow stromal cells (BMSCs), a population of
cells that have the capability of self-renewal and plasticity in
vitro, can differentiate into multiple cell lineages in
specific conditions, such as osteocytes, chondrocytes, fibroblasts
and adipocytes (1–3). BMSCs are characterized by positive
expression of surface markers, including cluster of differentiation
105 (CD105), CD44, CD73, CD90, CD29 and CD56, and negative
expression of hematopoietic stem cell surface markers, such as
CD45, CD34 and CD14 (4).
Recently, BMSCs have attracted significant attention as an
alternative to autologous chondrocytes for the repair of articular
cartilage within the field of cartilage tissue engineering
(5,6). For successful chondrogenic
differentiation of BMSCs, the cells should be induced by various
factors, and among these Chinese herbs are a potential. Previous
studies reported that an effective component of Chinese herbs was
an inductive agent for chondrogenic differentiation of mesenchymal
stem cells (7–9).
In particular, Indian hedgehog (Ihh), a member of
the vertebrate hedgehog gene family to control the cartilage
hypertrophy (10), plays a key
role in the regulation of the chondrocyte proliferation and
differentiation during endochondral bone formation (11,12). Ihh is mainly produced by
pre-hypertrophic and hypertrophic chondrocytes and binds to
membrane receptor Patched (Ptc), which acts sub-stoichiometrically
to suppress Smoothened (Smo) activity in the absence of Ihh signal.
Upon Ihh binding to Ptc, Smo is activated to transmit signals
downstream, which results in transcription regulation of the target
genes of the hedgehog signaling pathway (13–15).
Tougu Xiaotong formula (TXF), a hospital preparation
of the Second People’s Hospital of Fujian Province (Fuzhou, China)
consisting of 4 component herbs, including Morindae
officinalis, Radix paeoniae alba, Ligusticum
wallichii and Herba sarcandrae glabrae, has been proven
to ameliorate the progress of cartilage degeneration by the
regulation of chondrocyte autophagy and apoptosis (16,17). However, the exact molecular
mechanisms of the therapeutic effects of TXF remain unclear. Since
the cell cycle plays an important role in the proliferation of
BMSCs (18,19), the present study results showed
that TXF promoted BMSC proliferation by inducing the
G1/S transition. TXF was also found to mediate BMSCs to
chondrogenic differentiation by activating the Ihh signaling
pathway.
Materials and methods
Animals
Four-week-old male Sprague Dawley (SD) rats were
purchased from the Shanghai Slack Laboratory Animal Co. (Shanghai,
China); animal permit number: SCXK (Shanghai, China) 2007-0005. All
the experiments involving the animals complied with the Guidance
Suggestions for the Care and Use of Laboratory Animals (2006)
administered by the Ministry of Science and Technology of the
People’s Republic of China.
Herbal preparation
All the herbs of TXF were purchased from the Third
People’s Hospital of Fujian Province (Fuzhou, China). First, the
original herbs were dried for 24 h in an air-circulating oven
(model SFG-02.600; Hengfeng, Huangshi, China) at 50°C. They were
subsequently shredded further and then crushed to the appropriate
particle size in a high-speed rotary cutting mill (model ZN-400A;
Zhongnan, Changsha, China). According to the proportion of the TXF
formula (2:2:1:1), 108 g of herbal powder was extracted with 1,500
ml distilled water by refluxing twice, for 2 h each time. The
undissolved materials were removed by filtration with Whatman
filter paper, and the filtrate was evaporated on a rotary
evaporator (model RE-2000; Yarong, Shanghai, China). The
concentrated solution was dried to a constant weight in a vacuum
drying oven (model DZF-300; Yiheng, Shanghai, China) after the
filtrate was concentrated to a relative density of 1.25. The mother
liquor of the TXF extracts was prepared by dissolving the extract
powder in Dulbecco’s modified Eagle’s medium (DMEM; HyClone,
Carlsbad, CA, USA) to a concentration of 20 mg/ml.
High-performance liquid chromatography
(HPLC) analysis
The study established a method of fingerprint
analysis for quality control of TXF through detecting the HPLC
fingerprint of the extracts of 10 TXF herb batches by an Agilent
1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA), and
the common peak was analyzed by the ‘Similarity Evaluation System
for Chromatographic Fingerprint of TCM’ software (version 2004A).
HPLC was performed using an Ultimate™ XB-C18 column (250×4.6 mm, 5
μm). Methanol (solvent A) and 0.1% phosphoric acid (solvent
B) were the mobile phase and the detection wavelength was 277 nm,
the flow rate was 1 ml/min and the column temperature was 30°C. The
gradient procedure was as follows: 5% A at 0–5 min, 5–20% A at 5–10
min, 20–42% A at 15–25 min, 42–65% A at 25–40 min, 65–80% A at
40–55 min and 80–100% A at 55–70 min.
Isolation, culture and identification of
rat BMSCs
Extraction and separation between BMSCs was
performed using a complete marrow direct culture method. The SD
rats were sacrificed by breaking the neck. The femurs and tibias
were separated under sterile conditions and immersed in 75% alcohol
for 20 min. Following the separation of the muscles and tendons
from the femurs and tibias, the marrow cavity was rinsed repeatedly
with DMEM into 15 ml centrifuge tubes and centrifuged at 160 × g
for 5 min to obtain a cell pellet. The supernatant fluid was
removed and the cells were resuspended in DMEM with 15% fetal
bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin
(all from HyClone). The primary cells were seeded in culture flasks
and cultured at 37°C in a 5% CO2 incubator (termed P0).
The cells were observed under a phase-contrast microscope (Olympus,
Tokyo, Japan) and subcultured when they reached 80% confluency
after 8 days (termed P1). The 3rd generation of BMSCs was
identified using flow cytometry (Becton-Dickinson, San Jose, CA,
USA), which measured the expression of BMSC surface marker, CD90,
and hematopoietic stem cell surface marker, CD45.
Experimental design
The 3rd generation of BMSCs were collected and
stimulated with various concentrations of TXF (0–1,600
μg/ml) for 24, 48 and 72 h, and the changes of the cell
cycle were analyzed.
For chondrogenic differentiation, the cells were
incubated in DMEM with 50 μg/ml vitamin C and
10−7 mol/ml dexamethasone (Sigma, St. Louis, MO, USA)
[auxiliary-induced medium (AIM)]. BMSCs were divided into 6 groups;
control, 200 μg/ml TXF, 200 μg/ml TXF + AIM, 10 ng/ml
transforming growth factor-β1 (TGF-β1) (PeproTech, Rocky Hill, NJ,
USA) + AIM, 10 ng/ml TGF-β1 + 200 μg/ml TXF + AIM and AIM
groups. All the groups were treated once every 2 days for 2
weeks.
Cell viability analysis
Following treatment with TXF, 100 μl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
[0.1 mg/ml in phosphate-buffered saline (PBS)] was added to each
well, and the samples were incubated at 37°C for 4 h. The
purple-blue MTT formazan precipitate was dissolved in 150 μl
dimethyl sulfoxide and the 96-well plate was agitated for 10 min.
The absorbance was measured at 490 nm using an enzyme-linked
immunosorbent assay reader (model EXL800; BioTek, Winooski, VT,
USA).
Cell cycle analysis
The cell cycle of BMSCs was determined by flow
cytometric analysis using a fluorescence-activated cell sorting
(FACS) caliber. Propidium iodide (PI) staining was performed
according to the manufacturer’s instructions for the cell assay kit
(KeyGen, Nanjing, China). The percentage of cells in the different
phases was calculated by the ModFit software (Verity Software
House, Topsham, ME, USA), and the cell numbers in the
G0/G1, S and G2/M phases were
obtained.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated with TRIzol reagent (Thermo
Fisher Scientific, Waltham, MA, USA). RNA (2 μg) was reverse
transcribed into cDNA using a First Strand cDNA Synthesis kit
(Thermo Fisher Scientific). The obtained cDNA was used to determine
the mRNA levels of cyclin D1, cyclin-dependent kinase 4
(CDK4) and CDK6. β-actin was used as an internal
control. The sequences of the primers used for amplification of
cyclin D1, CDK4, CDK6 and β-actin (Sangon Biotech,
Shanghai, China) were as follows: Cyclin D1 forward, 5′-AAT GCC AGA
GGC GGA TGA GA-3′ and reverse, 5′-GCT TGT GCG GTA GCA GGA GA-3′,
189 base pairs (bp); CDK4 forward, 5′-GAA GAC GAC TGG CCT
CGA GA-3′ and reverse, 5′-ACT GCG CTC CAG ATT CCT CC-3′, 109 bp;
CDK6 forward, 5′-TTG TGA CAG ACA TCG ACG AG-3′ and reverse,
5′-GAC AGG TGA GAA TGC AGG TT-3′, 151 bp; and β-actin forward,
5′-GAG AGG GAA ATC GTG CGT GAC-3′ and reverse, 5′-CAT CTG CTG GAA
GGT GGA CA-3′, 453 bp.
Immunohistochemistry (IHC) analysis
Chondrogenic differentiation of BMSCs was induced on
the coverslips (Cosmobrand, Beijing, China) after 2 weeks. IHC was
applied to identify that BMSCs differentiated to chondrocytes by
detecting the collagen II expression. IHC was performed according
to the manufacturer’s instructions for the Polink-2
plus® polymer horseradish peroxidase (HRP) detection
system (GBI Labs, Mukilteo, WA, USA). The cells on the coverslips
were fixed for 30 min by 4% paraformaldehyde (4°C), treated in 3%
H2O2 for 10 min (room temperature), blocked
with 10% sheep serum for 30 min (room temperature) and incubated in
primary antibody solution collagen II (BS1071; Bioworld Technology,
St. Louis Park, MN, USA) overnight (4°C). The cells on the
coverslips were treated with Polymer Helper, poly-HRP anti-rabbit
immunoglobulin G and 3,3′-diaminobenzidine for 20, 30 and 10 min,
respectively. Finally, Hematoxylin (Sigma) was redyed in each
coverslips. To exclude any non-specific staining, PBS was used to
replace the collagen II as the negative control. Images were
captured using a phase-contrast microscope (magnification, x100;
Olympus).
Western blot analysis
The cells were suspended in western blotting lysis
buffer for 30 min. After centrifugation at 20,000 × g at 4°C for 15
min, the supernatant was collected. The protein concentration was
determined by the bicinchoninic acid protein assay (Beyotime,
Shanghai, China). Equal amounts of protein (20 μg) were
separated by electrophoresis on 12% SDS-polyacrylamide gels and
transferred onto PVDF membranes (Millipore, Billerica, MA, USA).
The membranes were blocked with blocking solution (5% skimmed milk
powder) for 2 h at room temperature and incubated in primary
antibody solution of cyclin D1 (BS2436; Bioworld Technology), CDK4
(sc-260; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), CDK6
(14052-1-AP; Proteintech Group, Inc., Chicago, IL, USA), Ihh
(sc-1196) and Ptc (sc-6149; both from Santa Cruz Biotechnology),
cartilage oligomeric matrix protein (COMP) (5641-1; Epitomics,
Burlingame, CA, USA) Smo (BS3428; Bioworld Technology), collagen II
and β-actin (AP0060; Bioworld Technology) overnight at 4°C, and
subsequently with appropriate HRP-conjugated secondary antibody
followed by enhanced chemiluminescence detection. Finally, protein
images were captured and analyzed by a motored molecular imaging
system (model GEL DOC 2000; Bio-Rad, Hercules, CA, USA).
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for all the statistical analysis. All the data represented at least
three independent experiments and statistical analysis of the data
was performed with analysis of variance. Differences with P<0.05
were considered to indicate a statistically significant
difference.
Results
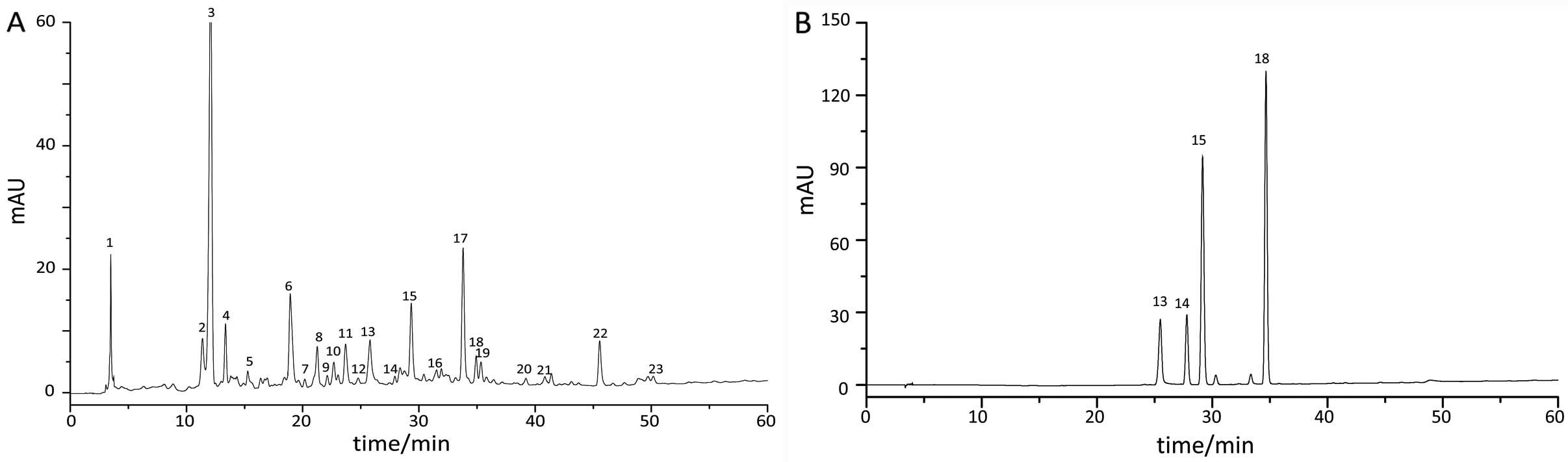
Fingerprint chromatography of TXF
The samples and reference substance were diluted in
methanol and were determined by an Agilent 1200 HPLC system.
Results found that 23 common peaks were analyzed in the fingerprint
chromatography of 10 TXF batches using the ‘Similarity Evaluation
System for Chromatographic Fingerprint of TCM’ software (version
2004A), and 4 compositions were identified from common peaks by
comparing the retention time of the chromatographic peak between
the sample and reference substance (Fig. 1). The reference substance was
composed of paeoniflorin (peak 13), isofraxidin (peak 14), ferulic
acid (peak 15) and rosmarinic acid (peak 18).
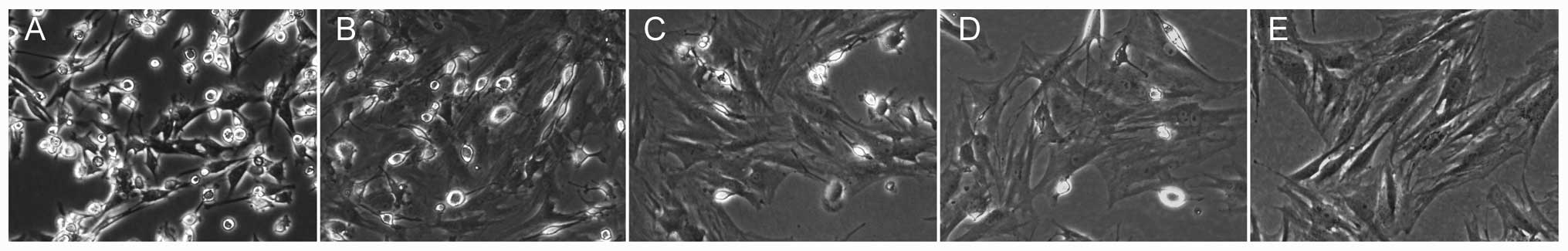
Morphological observation and
identification of BMSCs
BMSCs were easily isolated from bone marrow and
expanded in culture medium by the whole bone marrow adherent
culture method (20,21). The P0 cells had small amounts of
adherence after day 1 and formed round or polygon shapes, and
subsequently had a large number of adherent cells accompanied by
short spindle cells on day 3 (Fig.
2A). The cells were subcultured when they reached 80%
confluence after 8 days (Fig.
2B). The size and shape of the P0 cells were different,
however, becoming increasingly more uniform with increasing
passages (Fig. 2C–E). CD90 and
CD45 expression was detected for the identification of BMSCs by the
flow cytometry assay. The P3 cells exhibited a positive expression
of the BMSC surface marker, CD90, and negative expression of the
hematopoietic stem cell surface marker, CD45 (Fig. 2G and H), and demonstrated
significant reproductive activity (Fig. 2F). Therefore, the third generation
of BMSCs were used in the subsequent experiments.
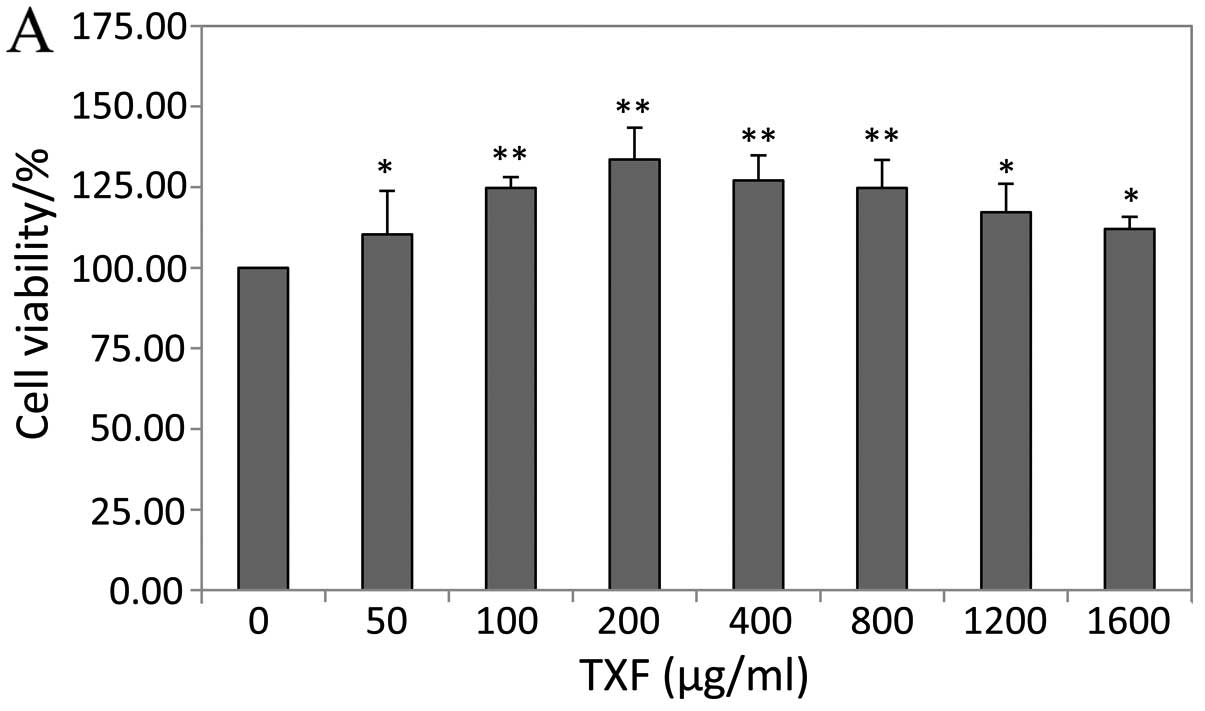
TXF enhances BMSC viability
The effect of TXF on the viability of BMSCs was
determined by the MTT assay. Treatment with 50–1,600 μg/ml
TXF for 24 h increased cell viability by 10–34%, and treatment with
200 μg/ml TXF for 24, 48 and 72 h increased cell viability
by 33.59±2.47, 14.31±1.53 and 12.74±1.03%, respectively (P<0.01
or P<0.05 vs. untreated cells), suggesting that TXF effects the
viability in a dose- and time-dependent manner (Fig. 3).
TXF promotes the G1/S
transition of BMSCs
G1/S transition is one of the two main
checkpoints that regulate the progression of the cell cycle
(22). To determine the mechanism
of the proliferative activity of TXF, its effect on the
G1 to S phase transition in BMSCs was analyzed via PI
staining followed by FACS analysis. After simulation for 24 h, the
percentage of G0/G1 phase cells treated with
50, 100 and 200 μg/ml TXF (52.82±2.56, 34.27±3.12 and
30.16±2.93%) was significantly lower than that of the untreated
control cells (70.68±3.97%; P<0.01), while the S phase cells in
the treated cells were higher than that of the untreated cells
(Fig. 4). The results showed that
TXF induced the proliferation of BMSCs by stimulating the
G1/S transition.
TXF promotes cyclin D1, CDK4 and CDK6
expression
Cyclin D1, CDK4 and CDK6 are key regulators of the
G1/S transition (23).
The mRNA and protein expression of cyclin D1, CDK4 and CDK6 were
analyzed by RT-PCR and western blotting, and the cyclin D1,
CDK4 and CDK6 mRNA expression in BMSCs treated with
TXF was higher compared to the untreated cells (P<0.01 or
P<0.05) (Fig. 5A); and the
protein expression patterns of cyclin D1, CDK4 and CDK6 were
similar to their respective mRNA expression, respectively (Fig. 5B). The results indicated that TXF
promoted BMSCs from the G1 to the S phase by
upregulating the expression of cyclin D1, CDK4 and CDK6.
Immunohistochemistry detection of
chondroblast-like cells
Collagen II is produced by chondrocytes (24), and increased collagen II
expression is an indicator for differentiation of BMSCs into
chondrocytes. Collagen II in the extracellular matrix (ECM) was
determined by IHC. There were no collagen II positive cells in the
basal medium (control) (Fig. 6).
Following culture in AIM, BMSCs began to synthesize a small amount
of collagen II after 2 weeks, and after TXF, TGF-β1 or TGF-β1 + TXF
were added to AIM, collagen II positive cells clearly increased.
Conversely, only a small increase of collagen II positive cells was
found following the independent application of TXF. The TXF + AIM
group increased the amount of collagen II positive cells in a
dose-dependent manner, with the highest level of collagen II
positive cells at 200 μg/ml TXF (Fig. 6). Taken together, the results
indicated that TXF promoted chondrogenic differentiation of BMSCs
in AIM, but independent application of TXF did not induce
differentiation of BMSCs into chondrocytes.
TXF induces chondrogenic differentiation
of BMSCs in association with TGF-β1
COMP, the main composition of articular cartilage,
plays an important role in maintaining the stability of joint
structure (25). For chondrogenic
differentiation of BMSCs, COMP is another important indicator. The
protein expression of collagen II and COMP was analyzed by western
blotting. Data from the western blot assay showed that the protein
expression of collagen II and COMP in the AIM, TXF + AIM, TGF-β1 +
AIM and TGF-β1 + TXF + AIM groups were significantly higher
compared to the control group (P<0.05), and the highest levels
of collagen II and COMP expression were in the TGF-β1 + TXF + AIM
group (P<0.05, significant vs. TGF-β1 + AIM group) (Fig. 7). Taken together, the results
indicated that TXF induced chondrogenic differentiation of BMSCs in
association with TGF-β1.
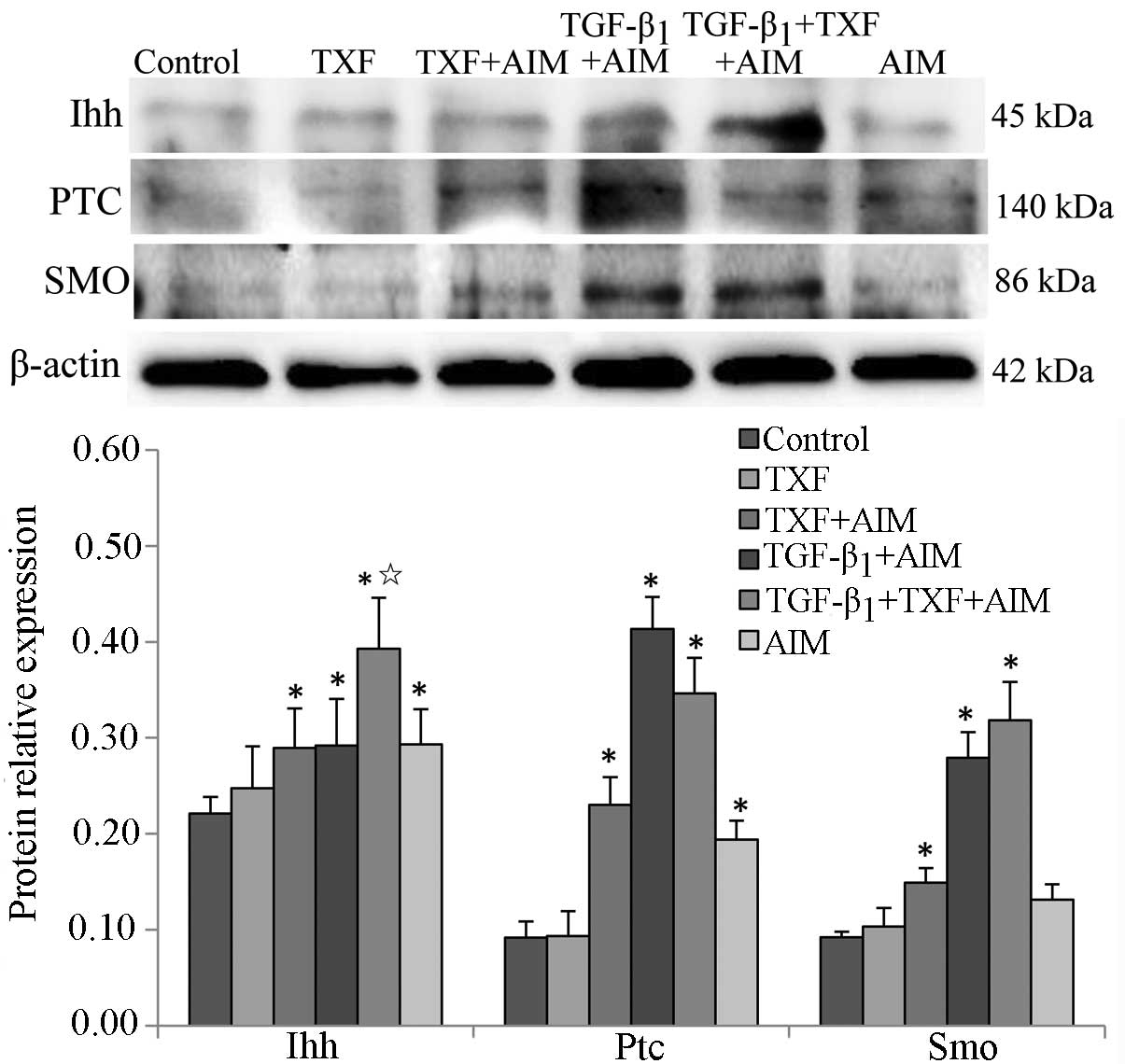
Ihh signaling pathway regulates the
chondrogenic differentiation of BMSCs
A previous study showed that Ihh enhanced the
expression of type II collagen in the process of human mesenchymal
stem cells promoting meniscal regeneration (26). In the present study, the influence
of TXF on the protein expression of the key regulators of Ihh
signaling pathway was assessed in the BMSCs undergoing chondrogenic
differentiation by western blotting. The protein expression levels
of Ihh, Ptc and Smo in the TXF + AIM, TGF-β1 + AIM, TGF-β1 + TXF +
AIM and AIM groups were significantly increased compared to the
control group (P<0.05) (Fig.
8). In addition, the protein level of Ihh in the TGF-β1 + TXF +
AIM group was higher than that of the TGF-β1 + AIM group
(P<0.05). There was no significant difference of the protein
levels of Smo and Ptc between the TGF-β1 + TXF + AIM and TGF-β1 +
AIM groups (P>0.05). Taken together, the results indicated that
differentiation of BMSCs into chondroblasts is associated with the
Ihh pathway, and TXF-induced chondrogenic differentiation of BMSCs
is associated with TGF-β1 by activating the Ihh signaling
pathway.
Discussion
Previously, cell-based cartilage tissue engineering
provided a challenge for the treatment of cartilage injury
(27), among which BMSCs are seed
cells with the highest potential for articular cartilage repair in
tissue engineering due to the extensive expansion capacity and
multipotential differentiation. A number of studies demonstrated
that certain effective components of Chinese herbs induced the
differentiation of BMSCs into chondrocytes (7–9).
In the present study, we hypothesized that TXF promoted
chondrogenic differentiation of BMSCs. The data suggested that TXF
induced chondrogenic differentiation of BMSCs in association with
TGF-β1 via activating the Ihh signaling pathway. In addition, TXF
also promoted BMSCs proliferation by upregulating the expression of
cyclin D1, CDK4 and CDK6.
To evaluate the side-effect of TXF on the BMSCs, the
cell viability was determined by the MTT assay. The data exhibited
that treatment with TXF promoted BMSCs viability in a dose- and
time-dependent, indicating that TXF was not cytotoxic to BMSCs. To
further explore the mechanism, its effect was examined for the
G1 to S phase transition in BMSCs via PI staining
followed by FACS analysis. The results exhibited that the
percentage of the proportion of BMSCs in the
G0/G1 and S phases was significantly reduced
and increased, respectively, following TXF treatment, indicating
that TXF promotes the proliferation by promoting BMSCs from the
G1 to the S phase in vitro. In addition, cyclin
D1 forms complexes with CDK4 or CDK6 that may regulate the
G1/S transition, which is one of the two main
checkpoints used by a cell to control the progression of the cell
cycle, and subsequently promote cell proliferation (22,23,28). Treatment with TXF enhanced the
mRNA and protein expression of cyclin D1, CDK4 and CDK6 in BMSCs.
Taken together, the results indicated that TXF promoted BMSCs
proliferation by upregulating the expression of cyclin D1, CDK4 and
CDK6.
Collagen II is synthesized and secreted into the
cartilage ECM (29). To further
study the effect of TXF on the chondrogenic differentiation of
BMSCs, collagen II in the ECM was determined by IHC in the present
study. The TXF + AIM group increased the amount of collagen II in
positive cells in a dose-dependent manner, while the independent
application of TXF did not induce BMSCs into chondroblast-like
cells. The results showed that TXF promoted chondrogenic
differentiation of BMSCs in the special culture conditions
containing AIM. Additionally, the study analyzed the expression
levels of collagen II and COMP by western blotting. In cartilage,
COMP acts as a molecular bridge in maintaining the interstitial
collagen II network (30). The
TXF + TGF-β1 + AIM group significantly enhanced the protein
expression of collagen II and COMP compared to the TGF-β1 + AIM
group. TGF-β1, which can promote BMSCs proliferation and
differentiation, is one member of the TGF-β superfamily (31). The results indicated that
TXF-induced BMSC chondrogenic differentiation in association with
TGF-β1 in vitro.
Hedgehog mainly has three types of homologous
proteins in mammals, known as Ihh, Sonic hedgehog and Desert
hedgehog (32). Ihh was reported
to play an essential role in regulating chondrocyte maturation,
hypertrophy and differentiation (11,33). The previous study also showed that
Ihh protein has the ability to promote differentiation of
chondrogenic precursor cells (34), and inactivation of its membrane
receptor, Ptc, in the mouse limb has novel inhibitory effects of
cell autonomously-activated hedgehog signaling on chondrogenesis
(35). In the present study, the
TXF + AIM, TGF-β1 + AIM, TGF-β1 + TXF + AIM and AIM groups
upregulated the protein expression of Ihh, Ptc and Smo compared to
the control group, and the protein level of Ihh in the TGF-β1 + TXF
+ AIM group was higher than that of the TGF-β1 group. The results
demonstrated that the chondrogenic differentiation of BMSCs was
associated with the Ihh signaling pathway, and TXF induced BMSC
chondrogenic differentiation in association with TGF-β1 by
increasing the expression of Ihh, Ptc and Smo.
In conclusion, the present study demonstrates that
TXF promotes the proliferation by upregulating the expression of
cyclin D1, CDK4 and CDK6, and induces BMSC chondrogenic
differentiation in association with TGF-β1 via activating the Ihh
signaling pathways, suggesting that TXF is a potential therapeutic
agent for bone and joint disease by promoting chondrogenic
differentiation of BMSCs. As a result of the lack of empirical
evidence, future studies focusing on investigating the influence of
TXF on the chondrogenic differentiation of BMSCs in vivo
when combined with the cartilage tissue engineering are
necessary.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81373818 and
81102609), the Key Project of Fujian Provincial Department of
Science and Technology (grant no. 2014Y0064), the Natural Science
Foundation of Fujian Province (grant no. 2014J01357), the
Developmental Fund of Chen Keji Integrative Medicine (CKJ2014001)
and the Special Research Fund for Doctor Discipline in College
(20123519110001).
References
|
1
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charbord P: Bone marrow mesenchymal stem
cells: historical overview and concepts. Hum Gene Ther.
21:1045–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal cells
The International Society for Cellular Therapy position statement.
Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
5
|
Li WJ, Tuli R, Okafor C, et al: A
three-dimensional nanofibrous scaffold for cartilage tissue
engineering using human mesenchymal stem cells. Biomaterials.
26:599–609. 2005. View Article : Google Scholar
|
|
6
|
Ochi M, Uchio Y, Tobita M and Kuriwaka M:
Current concepts in tissue engineering technique for repair of
cartilage defect. Artif Organs. 25:172–179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Wei G, Wang X, et al: Targeting of
the Sonic Hedgehog pathway by atractylenolides promotes
chondrogenic differentiation of mesenchymal stem cells. Biol Pharm
Bull. 35:1328–1335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin J, Xiu Z and Wu Z: Effect of pilose
antler polypeptides on the apoptosis of rabbit marrow mesenchymal
stem cells differentiated into chondrogenic phenotype in vitro.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 20:427–430. 2006.(In
Chinese). PubMed/NCBI
|
|
9
|
Huh JE, Park YC, Seo BK, et al: Cartilage
protective and chondrogenic capacity of WIN-34B, a new herbal
agent, in the collagenase-induced osteoarthritis rabbit model and
in progenitor cells from subchondral none. Evid Based Complement
Alternat Med. 2013:5275612013. View Article : Google Scholar
|
|
10
|
Mak KK, Kronenberg HM, Chuang PT, Mackem S
and Yang Y: Indian hedgehog signals independently of PTHrP to
promote chondrocyte hypertrophy. Development. 135:1947–1956. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
St-Jacques B, Hammerschmidt M and McMahon
AP: Indian hedgehog signaling regulates proliferation and
differentiation of chondrocytes and is essential for bone
formation. Genes Dev. 13:2072–2086. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Q, Zhang Y and Chen Q: Indian hedgehog
is an essential component of mechanotransduction complex to
stimulate chondrocyte proliferation. J Biol Chem. 276:35290–35296.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hooper JE and Scott MP: The Drosophila
patched gene encodes a putative membrane protein required for
segmental patterning. Cell. 59:751–765. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakano Y, Guerrero I, Hidalgo A, Taylor A,
Whittle JR and Ingham PW: A protein with several possible
membranespanning domains encoded by the Drosophila segment polarity
gene patched. Nature. 341:508–513. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taipale J, Cooper MK, Maiti T and Beachy
PA: Patched acts catalytically to suppress the activity of
Smoothened. Nature. 418:892–897. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Lang W, Ye H, et al: Tougu Xiaotong
capsule inhibits the tidemark replication and cartilage degradation
of papain-induced osteoarthritis by the regulation of chondrocyte
autophagy. Int J Mol Med. 31:1349–1356. 2013.PubMed/NCBI
|
|
17
|
Li XH, Wu MX, Ye HZ, et al: Experimental
study on the suppression of sodium nitroprussiate-induced
chondrocyte apoptosis by Tougu Xiaotong Capsule-containing serum.
Chin J Integr Med. 17:436–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Xie R, Hou W, et al: PTHrP
prevents chondrocyte premature hypertrophy by inducing
cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation
and proteasomal degradation. J Cell Sci. 122:1382–1389. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang SG, Song SM, Kim JR, Park CS, Song
WK and Chun JS: Regulation of type II collagen expression by
cyclin-dependent kinase 6, cyclin D1, and p21 in articular
chondrocytes. IUBMB Life. 59:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neuhuber B, Swanger SA, Howard L, Howard
L, Mackay A and Fischer I: Effects of plating density and culture
time on bone marrow stromal cell characteristics. Exp Hematol.
36:1176–1185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen ZZ, Van Bockstaele DR, Buyssens N, et
al: Stromal populations and fibrosis in human long-term bone marrow
cultures. Leukemia. 5:772–781. 1991.PubMed/NCBI
|
|
22
|
Nurse P: Ordering S phase and M phase in
the cell cycle. Cell. 79:547–550. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen R, Wang X, Drissi H, Liu F, O’Keefe
RJ and Chen D: Cyclin D1-cdk4 induce runx2 ubiquitination and
degradation. J Biol Chem. 281:16347–16353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pufe T, Petersen W, Fändrich F, et al:
Programmable cells of monocytic origin (PCMO): a source of
peripheral blood stem cells that generate collagen type
II-producing chondrocytes. J Orthop Res. 26:304–313. 2008.
View Article : Google Scholar
|
|
25
|
Oldberg A, Antonsson P, Lindblom K and
Heinegård D: COMP (cartilage oligomeric matrix protein) is
structurally related to the thrombospondins. J Biol Chem.
267:22346–22350. 1992.PubMed/NCBI
|
|
26
|
Horie M, Choi H, Lee RH, et al:
Intra-articular injection of human mesenchymal stem cells (MSCs)
promote rat meniscal regeneration by being activated to express
Indian hedgehog that enhances expression of type II collagen.
Osteoarthritis Cartilage. 20:1197–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hettrich CM, Crawford D and Rodeo SA:
Cartilage repair: third-generation cell-based technologies - basic
science, surgical techniques, clinical outcomes. Sports Med
Arthrosc. 16:230–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beier F: Cell-cycle control and the
cartilage growth plate. J Cell Physiol. 202:1–8. 2005. View Article : Google Scholar
|
|
29
|
Ito H, Rucker E, Steplewski A, et al:
Guilty by association: some collagen II mutants alter the formation
of ECM as a result of atypical interaction with fibronectin. J Mol
Biol. 352:382–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agarwal P, Schulz JN, Blumbach K, et al:
Enhanced deposition of cartilage oligomeric matrix protein is a
common feature in fibrotic skin pathologies. Matrix Biol.
32:325–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miura Y, Parvizi J, Fitzsimmons JS and
O’Driscoll SW: Brief exposure to high-dose transforming growth
factor-beta1 enhances periosteal chondrogenesis in vitro: a
preliminary report. J Bone Joint Surg Am. 84-A:793–799.
2002.PubMed/NCBI
|
|
32
|
McMahon AP, Ingham PW and Tabin CJ:
Developmental roles and clinical significance of hedgehog
signaling. Curr Top Dev Biol. 53:1–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi SW, Jeong DU, Kim JA, et al: Indian
Hedgehog signalling triggers Nkx3.2 protein degradation during
chondrocyte maturation. Biochem J. 443:789–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Enomoto-Iwamoto M, Nakamura T, Aikawa T,
et al: Hedgehog proteins stimulate chondrogenic cell
differentiation and cartilage formation. J Bone Miner Res.
15:1659–1668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bruce SJ, Butterfield NC, Metzis V, Town
L, McGlinn E and Wicking C: Inactivation of Patched1 in the mouse
limb has novel inhibitory effects on the chondrogenic program. J
Biol Chem. 285:27967–27981. 2010. View Article : Google Scholar : PubMed/NCBI
|