Introduction
Coronary artery disease (CAD) is one of the most
common cardiovascular diseases in humans with a high incidence of
morbidity and mortality. It is well recognized that coronary artery
atherosclerosis is the vital cause of CAD, and CAD has become a
major public health issue worldwide. Since its first introduction
in 1977, percutaneous coronary intervention [PCI or percutaneous
transluminal coronary angioplasty (PTCA)] has been developed into
one of the main therapeutic strategies for CAD (1). PCI improves coronary blood flow,
reduces angina pectoris and improves the quality of life of
patients (2). Moreover, PCI is
relatively easy to perform and causes minimal injury. However, the
high incidence of restenosis (RS) following PCI usually limits its
clinical effect. The incidence of RS is approximately 20–50% at 6
months following PCI and with the implantation of the stent, the
incidence of RS is approximately 25–30% (3). Thus, vascular RS is a serious
complication of PCI, and thus the development of novel therapeutic
strategies for the prevention of RS is urgently required.
It has been well documented that the proliferation
and migration of vascular smooth muscle cells (VSMCs) is a key
factor in the development of RS (4). VSMCs in mature animals are highly
differentiated, and their principal function is contraction.
However, VSMCs can also proliferate and produce the matrix
components of the blood vessel wall during vasculogenesis (4). In addition, VSMCs retain remarkable
plasticity, and can undergo relatively rapid and reversible changes
in their phenotype in response to local environmental stress
(5). Vascular RS at an early
stage can be induced by vasospasm or decreased vessel elasticity
several hours or several days following PCI. Subsequently, the
damaged endothelial cells and macrophages invade the endothelial
sublayer and release cytokines, including endothelin, angiotensin
II, basic fibroblast growth factor, platelet-derived growth factor
and transforming growth factor. These cytokines stimulate the
migration and proliferation of VSMCs and induce the accumulation of
extracellular matrix components, which results in the remodeling of
the blood vessel wall and vascular RS (6). Although some intracellular signaling
pathways associated with the proliferation and migration of VSMCs
have been identified (2,7,8),
the role of direct intercellular communication pathways, gap
junctions (GJs), in the development of RS requires further
investigation.
GJs are plasma membrane spatial microdomains
constructed of assemblies of channel proteins termed connexins in
vertebrates and innexins in invertebrates (9). The channels provide direct
intercellular communication pathways allowing the rapid exchange of
ions, second messengers and small metabolites of up to 1 kDa in
molecular mass (10). In
vitro studies have demonstrated that the permeability,
conductance and other properties of GJ channels depend on the
precise make-up of their component connexins (11). In the major arteries, endothelial
GJs may simultaneously express 3 connexin isotypes, connexin
(Cx)40, Cx37 and Cx43, whereas VSMCs predominantly express Cx43
and, in some instances, Cx40 or Cx45 (12–14). It has been found that Cx43
expression is significantly increased during the alteration of the
VSMC phenotype (15).
Furthermore, the size, quantity, distribution and structure of Cx43
in vascular lesions may also be altered, which is known as Cx43
remodeling (16). It has been
demonstrated that Cx43 remodeling affects not only the conductivity
and permeability of the GJ itself, but also the electrical,
chemical and metabolic channels between adjacent cells (17–19). On the other hand, Cx43 remodeling
has also been shown to play a crucial role in the pathogenesis of
cardiovascular diseases (20).
In the present study, we established a model of
vascular RS by subjecting rat carotid arteries to angioplasty
balloon injury to mimic the development of RS following PCI. The
results revealed that the intimal area of the arteries gradually
increased following balloon injury. Simultaneously, the mRNA and
protein expression of Cx43 was also increased during the
development of RS. Importantly, the knockdown of Cx43 effectively
prevented the development of intimal hyperplasia and vascular RS
following balloon injury. Thus, our data indicate the vital role of
the GJ protein, Cx43, in the development of vascular RS, and may
thus provide a novel potential pharmacological target for the
prevention of vascular RS following PCI.
Materials and methods
Experimental animals
Male Sprague-Dawley rats (purchased from the
Department of Animal, Nanchang University, Nanchang, China)
weighing 300–400 g were maintained on a regular chow diet prior to
the study. All procedures for the animal experiments were carried
out in accordance with the National Institutes of Health
Guidelines, and were approved by the Ethics Committee for Animal
Axperiments of Nanchang University.
Establishment of model of vascular RS by
balloon injury
The rats were anesthetized with an intraperitoneal
injection of Hydral (10%, 3.5 ml/kg; Harbin Pharmaceutical Group
Co., Ltd., Harbin, China). To establish the model of vascular RS,
the angioplasty balloon (1.5×20 mm; Cordis Corp., Miami, FL, USA)
was inserted into the rat common carotid artery through an incision
in the left external carotid artery, as previously described
(21). The balloon was then
sufficiently inflated in the carotid artery and was drawn 3 times
consistently from the proximal area to the carotid bifurcation to
produce endothelial denudation. The external carotid was ligated
and blood flow in the common carotid was restored. In addition, the
rats were intramuscularly injected with benzylpenicillin sodium
(40×104 IU/day for 3 days; Harbin Pharmaceutical Group
Co., Ltd.) to prevent infection. The rats were euthanized by an
overdose of Hydral at 0, 7, 14 and 28 days (n=6/group) following
balloon injury. The injured common carotid arteries were collected
for hematoxylin and eosin (H&E) staining or western blot
analysis to evaluate vascular remodeling and the expression of Cx43
following balloon injury.
H&E staining
Three serial cryosections (at 5-μm-thick)
were prepared from the middle portion of the rat injured common
carotid arteries. The slices were stained with H&E (Sangon
Biotech, Shanghai, China) and histomorphological observation was
performed under a light microscope (Olympus, Tokyo, Japan) to
examine the structure of the blood vessel wall following balloon
injury. The intimal and medial area of the 3 serial cryosections in
each sample were measured using Image-Pro Plus 5.0 software (Media
Cybernetics, Inc., Houston, TX, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the rat carotid tissue
using TRIzol reagent (Tiangen, Beijing, China) and cDNA was
synthesized from the extracted total RNA using the SuperScript III
kit (Promega, Madison, WI, USA) following the manufacturer’s
instructions. The specific primers used for PCR were as follows:
Cx43 sense, 5′-AAAGGCGTTAAGG ATCGCGTG-3′ and antisense,
5′-GTCATCAGGCCGAGG CCT-3′ [as previously described (23)]; β-actin sense, 5′-CCCA
TCTATGAGGGTTACGC-3′ and antisense, 5′-TTTAATGT CACGCACGATTTC-3′.
All specific primers were chemically synthesized (Generay Biotech
Co. Ltd., Shanghai, China). The PCR reactions were performed using
the GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA,
USA). The amplification conditions for Cx43 was as follows: 94°C
for 2 min followed by 32 cycles at 94°C for 45 sec, 58°C for 45
sec, and 72°C for 90 sec, and a final extension at 72°C for 5 min.
The PCR conditions for β-actin was as follows: 94°C for 2 min
followed by 28 cycles at 94°C for 30 sec, 56°C for 30 sec, and 72°C
for 90 sec, and a final extension at 72°C for 5 min. The amplified
RT-PCR products were separated on 1.2% (w/v) agarose containing
ethidium bromide (both from Sangon Biotech) for 30 min. The results
of electro phoresis were photographed was using the Molecular
Imager® Chemi DOCTXRS+ system, and
the signal densities of the gels were analyzed using Quantity One
software (both from Bio-Rad, Hercules, CA, USA).
Western blot analysis
The carotid arteries were pulverized in
radioimmunoprecipitation assay (RIPA) lysis buffer (Sangon Biotech)
using a homogenizer. The whole lysate was centrifuged at 12,000 rpm
for 10 min at 4°C, and the pellets were resuspended in sample
buffer containing 4% sodium dodecyl sulfate (SDS; Sangon Biotech).
Proteins were separated by SDS-polyacrylamide gel electrophoresis
(PAGE) and transferred onto nitrocellulose membranes (Millipore,
Bedford, MA, USA). The membranes were blocked in 5% skim milk for 1
h at room temperature, and were incubated with rabbit polyclonal
anti-Cx43 antibody (zym-71-0700; 1:250 in 5% skim milk; Zymed
Laboratories, San Francisco, CA, USA) or rabbit monoclonal
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody
(MAB374; 1:1,000 in 5% skim milk; Chemicon, Temecula, CA, USA)
overnight at 4°C. The membranes were then washed 3 times with TBST,
then incubated with the relative HRP-conjugated IgG secondary
antibody (ZB-5301 and ZB-2305; Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) for 2 h at room
temperature and washed in TBST 3 times. Chemiluminescence were
carried out using Amersham ECL Prime Western Blotting Detection
reagents, and the immunobloting signal was detected using the
Molecular Imager Chemi DOCTXRS+ system
(Bio-Rad). The intensity of each Cx43 band was normalized to the
GAPDH band, and the relative expression of Cx43 following balloon
injury was normalized to the control.
Construction of GFP-Cx43-shRNA-lentiviral
vectors
In order to examine the role of Cx43 in the
development of vascular RS, the lentivirus expressing shRNA
targeting Cx43, Cx43-RNAi-LV, was constructed (GeneChem, Shanghai,
China). In brief, the shRNA sequence for Cx43
(5′-AGAGCACGGCAAGGTGAAA-3′) was designed using the manufacturer’s
RNA interference (RNAi) Designer program, and the negative control
construct (control shRNA) was created using a scrambled sequence
(5′-TTCTCCGAACGTGTCACGT-3′), as previously described (22). DNA oligos were chemically
synthesized (GeneChem, Shanghai, China), annealed and inserted into
the expression vector by double digestion with Age I and
Eco RI (New England Biolabs, Ipswich, MA, USA), and ligated
with T4 DNA ligase (Takara, Dalian, China) in accordance to the
manufacture’s instructions. The ligation was transformed into
competent E. coli cells and then confirmed by restriction
enzyme analysis and DNA sequencing. The sequences were then cloned
into pGCSIL-GFP to generate lentiviral vectors. The expression
vectors and package vectors were then transfected into HEK293T
cells (ATCC, Manassas, VA, USA) using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA). Following 48 h of culture, the
supernatants containing the lentiviruses, such as Cx43-RNAi-LV and
NC-GFP-LV (negative control) were harvested. Purification was then
performed using ultracentrifugation and the lentiviral titer was
determined.
Lentivirus-mediated RNAi knockdown of
Cx43
Firstly, the knockdown efficiency of Cx43 by the
lentiviral vector, Cx43-RNAi-LV, was confirmed in NRK-52E cells
(ATCC). In brief, NRK-52E cells at 30% confluence were infected
with Cx43-RNAi-LV and NC-GFP-LV, and the cells were then harvested
7 days following infection to obtain whole cell lysate. Following
electrophoresis of the cell lysate, the expression level of Cx43 in
each group was examined by western blot analysis. To determine the
effect of Cx43-RNAi-LV on the balloon injury-induced expression of
Cx43 in the arteries, the rats were randomly divided into 4 groups
(n=6 in each group) as follows: i) the control group (no balloon
injury); ii) the untreated group with ballon injury (balloon injury
only); iii) the group with ballon injury treated with Cx43-RNAi-LV
(balloon injury + Cx43-RNAi-LV); and iv) the group with ballon
injury treated with NC-GFP-LV (balloon injury + NC-GFP-LV). In
order to knockdown Cx43 in the VSMCs, the lentivirus
(5×108 TU/ml, 100 μl) expressing shRNA or the
scrambled RNA was injected into the balloon-injured rat carotid
arteries using a polyethylene catheter. The rats were euthanized by
an overdose of Hydral 28 days following balloon injury, and the
injured carotid arteries were collected for H&E staining or
western blot analysis to evaluate vascular remodeling and the
knockdown efficiency of Cx43.
Statistical analysis
The quantitative data are presented as the means ±
standard deviation (SD). Data were analyzed using either the
Student’s t-test to compare 2 conditions or ANOVA followed by
planned comparisons of multiple conditions. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
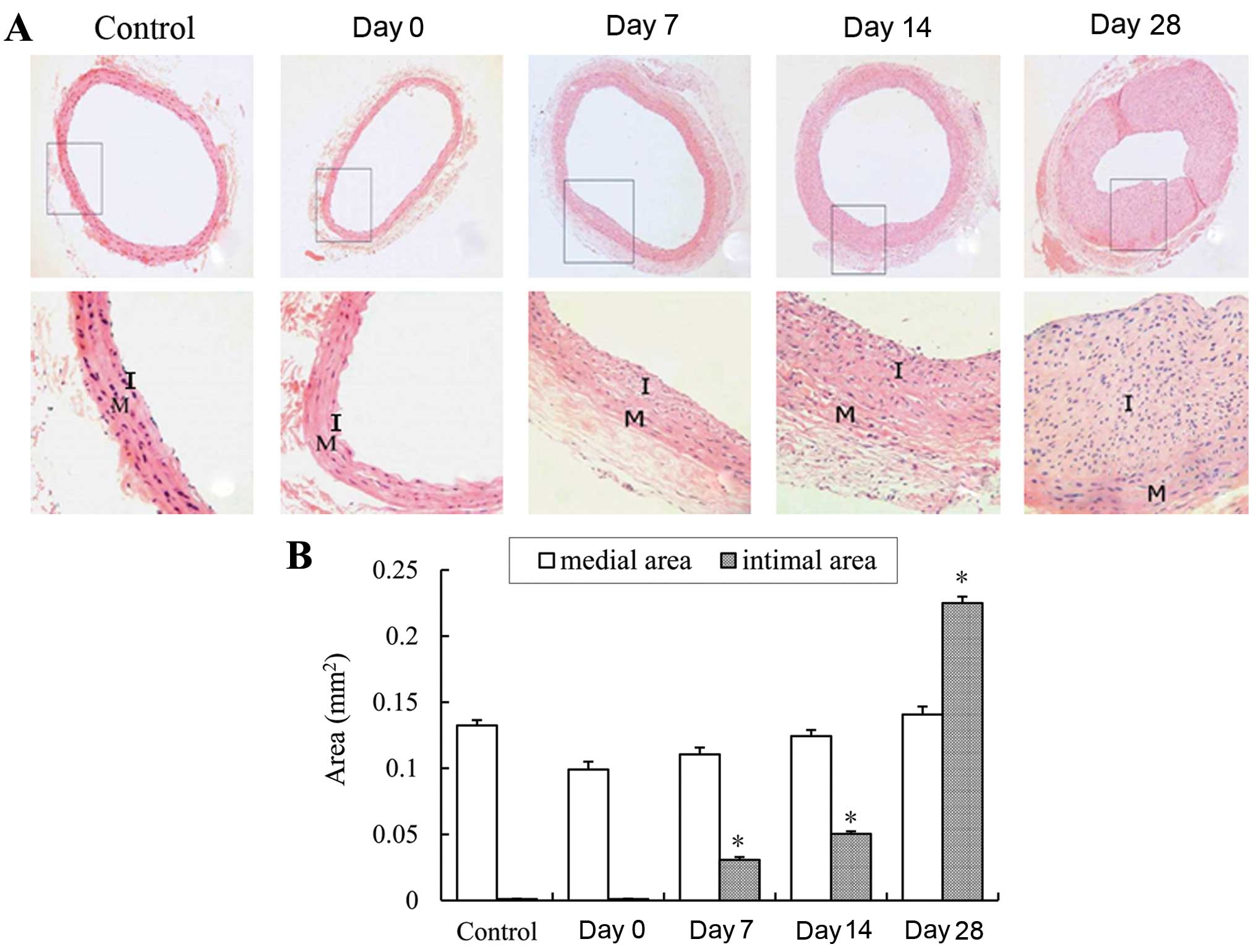
Confirmation of vascular RS following
balloon injury
Balloon injury was inflicted to the rat common
carotid arteries for the establishment of a model of vascular RS.
At 0, 7, 14 and 28 days following balloon injury, the rats were
sacrificed by an overdose of Hydral. The carotid arteries were
removed and subjected to cryosection at 5 μm. A
histomorphological observation of the arterial sections with
H&E staining was carried tou to evaluate RS at different time
points following balloon injury. Neointima formation occurred in
the injured arteries at 7 days (Fig.
1A). Intimal hyperplasia and RS were particularly evident at 14
and 28 days following balloon injury. Compared with the control
arteries, the intimal area of the arteries was significantly
increased at 14 and 28 days following balloon injury (Fig. 1B). These histomorphological
changes in the carotid arteries indicated that the model of
vascular RS induced by balloon injury was successful
established.
mRNA and protein expression of Cx43
during the development of RS following balloon injury
To investigate the role of the GJ protein, Cx43, in
the development of vascular RS, the mRNA and protein expression of
Cx43 in the arteries following balloon injury was examined by
RT-PCR and western blot analysis, respectively. Compared with the
controls, the mRNA expression of Cx43 was temporarily decreased at
7 days, and was subsequently increased at 14 and 28 days following
balloon injury (Fig. 2A and B).
Similarly, the protein expression of Cx43 was significantly
upregulated at 14 and 28 days following balloon injury, although a
transient decrease in Cx43 expression was detected at 7 days
(Fig. 2C and D). These results
suggest that the GJ protein, Cx43, is involved in the development
of intimal hyperplasia and vascular RS following balloon
injury.
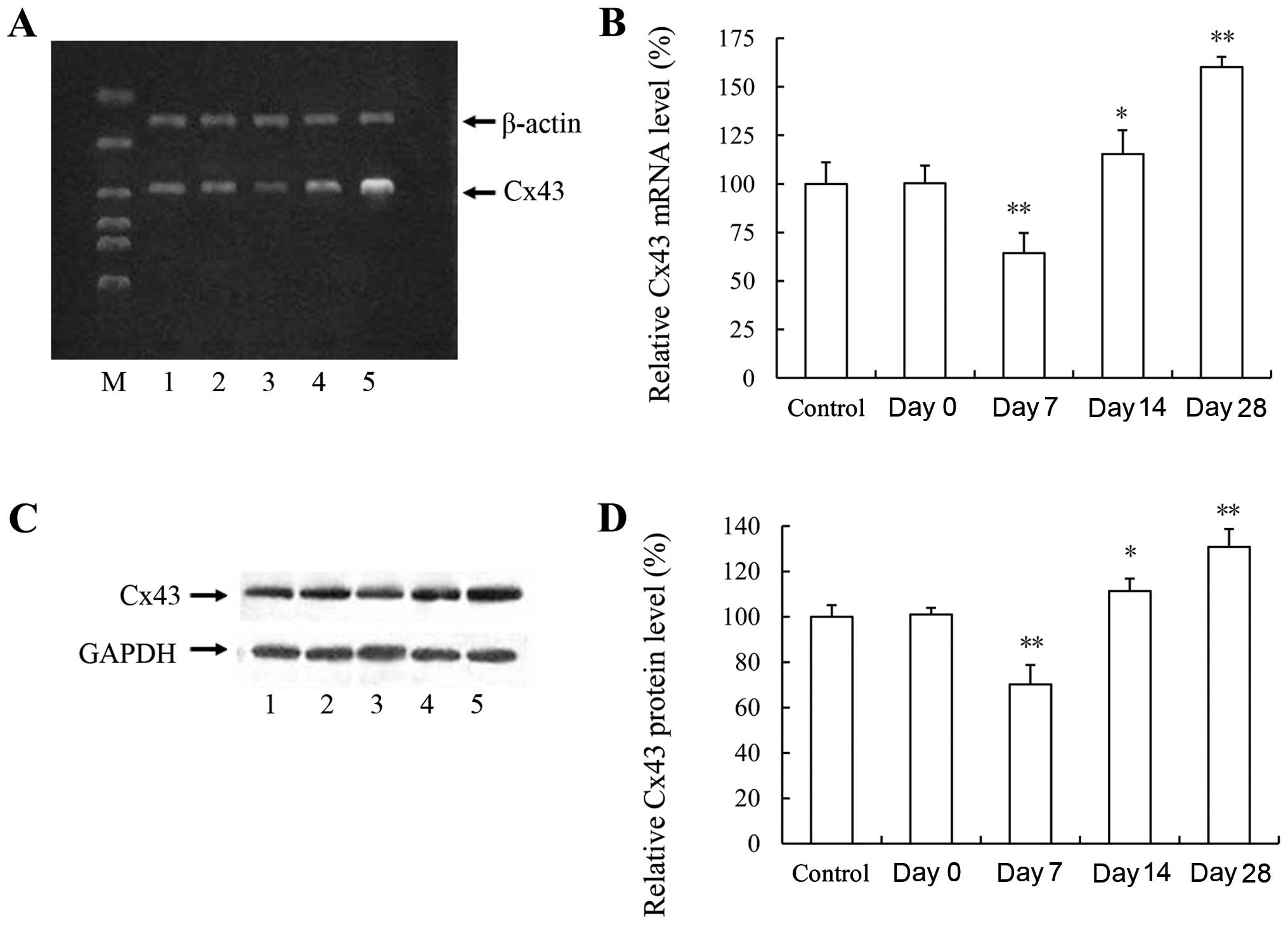 | Figure 2mRNA and protein expression of
connexin 43 (Cx43) during the development of restenosis (RS)
following balloon injury. (A) Electrophoresis of the RT-PCR product
of Cx43 and β-actin on an agarose gel. Lanes 1–5 represent samples
from the control, and days 0, 7, 14 and 28, respectively. Lane M
represents DNA Marker. β-actin was used as an endogenous reference.
DNA ladder was 2,000, 1,000, 750, 500, 250 and 100 bp from top to
bottom, respectively. (B) Quantification of relative Cx43 mRNA
level was indicated as the normalization of ratio of Cx43/β-actin
in each sample relative to the control. Data represent the means of
at least 3 independent experiments. *P<0.05 and
**P<0.01 vs. control. (C) Western blot analysis of
Cx43 protein expression during the development of RS following
balloon injury. Lanes 1–5 represent samples from the control, and
days 0, 7, 14 and 28, respectively. GAPDH was used as an endogenous
reference. (D) Quantification of relative Cx43 expression was
indicated as the normalization of ratio of Cx43/GAPDH in each
sample to relative to the control. Data represent the means of at
least 3 independent experiments. *P<0.05 and
**P<0.01 vs. control. |
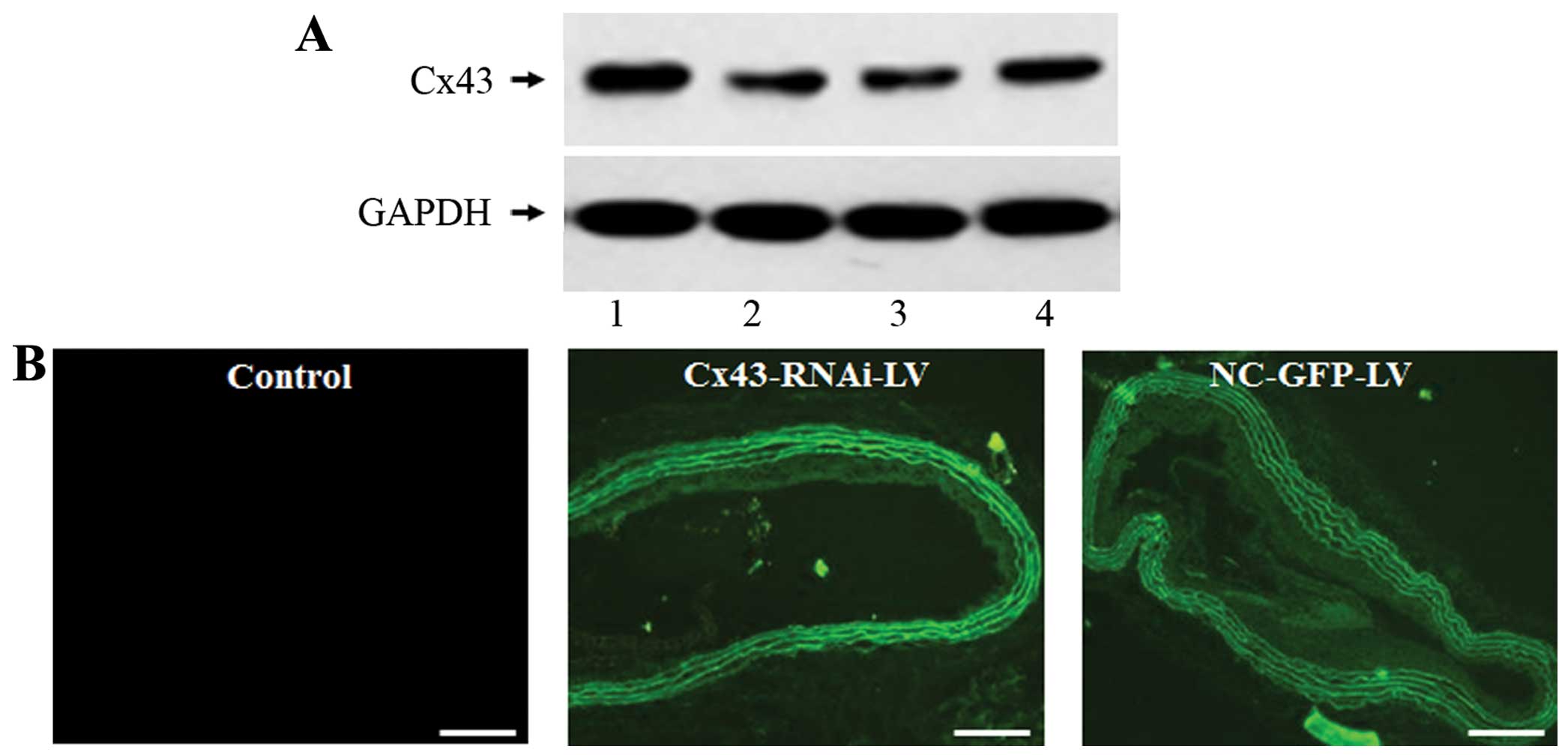
Lentivirus Cx43-RNAi-LV for the specific
knockdown of Cx43
In order to directly demonstrate the role of Cx43 in
the development of vascular RS following balloon injury, the
lentiviral vector expressing shRNA targeting Cx43 (Cx43-RNAi-LV)
and the negative control lentiviral vector (NC-GFP-LV) were
constructed. In vitro experiments revealed that the
expression of Cx43 was specifically decreased in the NRK-52E cells
infected with Cx43-RNAi-LV, but not in the NC-GFP-LV-infected cells
(Fig. 3A). These results
indicated that the lentiviral vector, Cx43-RNAi-LV, was effective
in silencing Cx43. For the knockdown of Cx43 in vivo, the
injured rat arteries were infected with Cx43-RNAi-LV or NC-GFP-LV,
and the infection efficiency in vivo was examined by
measuring the fluorescent signal of GFP. The green fluorescence was
strongly observed in the neointima and media in the
Cx43-RNAi-LV-treated and NC-GFP-LV-treated arteries, but not in the
controls (Fig. 3B). This
indicated that the balloon-injured rat carotid arteries were
successfully infected with the lentivirus.
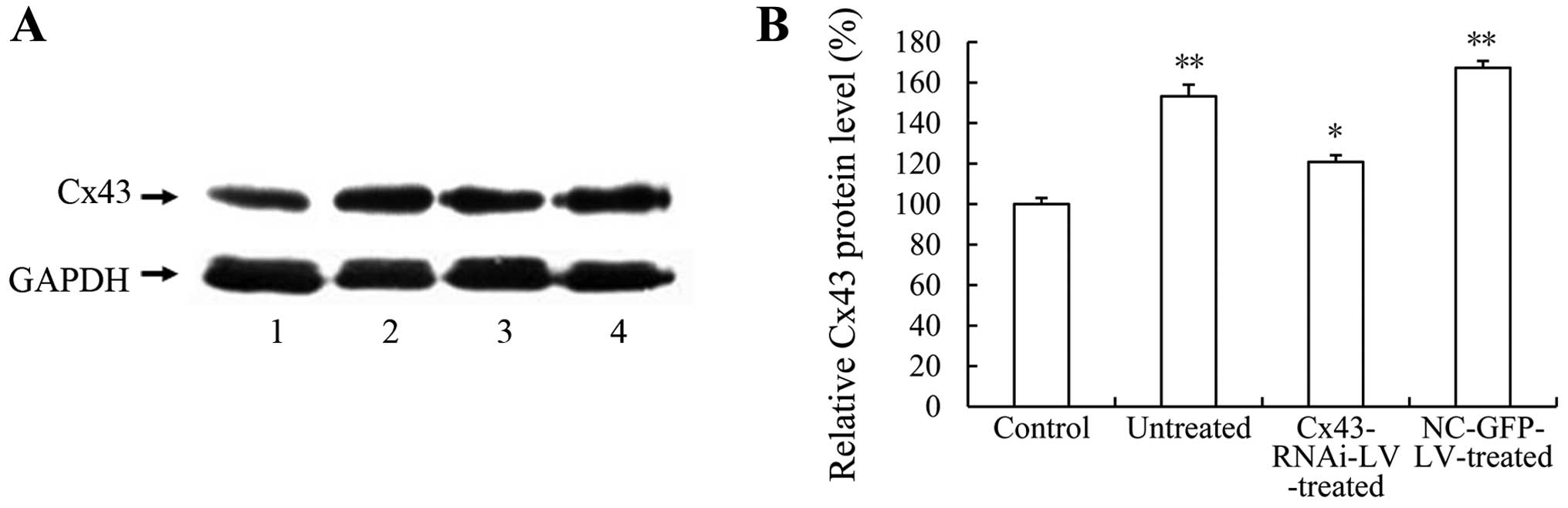
Lentivirus-mediated RNAi knockdown of
Cx43 attenuates the development of intimal hyperplasia and vascular
RS induced by balloon injury
Since the mRNA and protein expression of Cx43 was
upregulated during the development of vascular RS (Fig. 2), we firstly examined the effect
of Cx43-RNAi-LV on the balloon injury-induced expression of Cx43.
Compared with the untreated or NC-GFP-LV-treated arteries,
infection with Cx43-RNAi-LV significantly decreased the balloon
injury-induced expression of Cx43 (Fig. 4). In addition, the
histomorphological observation of the arterial sections (Fig. 5A) and statistical analysis of the
intimal/medial areas (Fig. 5B)
revealed that the lentivirus-mediated RNAi knockdown of Cx43
significantly attenuated balloon injury-induced intimal hyperplasia
and RS. These results directly demonstrate that the GJ protein,
Cx43, contributes to the development of vascular RS following
balloon injury, although the underlying mechanisms require further
investigation.
Discussion
PCI is one of the main therapeutic strategies used
for the treatment of CAD. However, the high incidence of vascular
RS limits its clinical effect, and the specific mechanisms
responsible for the development of RS following PCI have not yet
been fully elucidated. It is acknowledged that vascular RS is
mainly caused by endothelial denudation, as well as by the
proliferation and migration of VSMCs (1,2).
On the other hand, increasing evidence suggests that GJs, a basic
structure between cells, may be involved in the proliferation and
migration-related signaling pathways of VSMCs (22,24). In previous studies, it was found
that alterations in connexin expression correlated with the
development of certain vascular diseases, such as hypertension,
atherosclerosis and RS (25,26). Notably, the alteration of Cx43 in
size, quantity, distribution and structure strikingly influences
the conductivity and permeability of GJs, which changes the
electrical, chemical and metabolic channels between cells, and
subsequently causes ‘selective filtration’ in transmitting
information (17–19). In a previous study, in transgenic
mice, it was found that a decrease in Cx43 expression effectively
inhibited acute neointimal formation in mice with
hypercholesterolemia (27).
Therefore, the remodeling of Cx43 may play an important role in the
pathogenesis of vascular diseases, including hypertension,
atherosclerosis and RS. It should be of interest to investigate
whether the remodeling of Cx43 is also involved in the development
of intimal hyperplasia and vascular RS following PCI.
In the present study, we established a model of
vascular RS by subjecting rat common carotid arteries to balloon
injury to mimic the development of RS following PCI. Neointimal
formation was evident at 7 days following balloon injury (Fig. 1). The stenosis of the vessel lumen
was evident, and much more neointimal formation was observed at 14
days following injury. Notably, the lumen area was significantly
reduced, and neointimal hyperplasia was also markedly evident at 28
days following balloon injury. These results indicated that balloon
injury successfully reproduced RS, and mimicked the development of
RS following PCI. On the other hand, we examined the mRNA and
protein expression of Cx43 during the development of RS following
balloon injury. It was found that the mRNA and protein level of
Cx43 was temporarily decreased at 7 days following balloon injury,
which may be caused by tissue damage, stress response or a variety
of growth factors released from mechanically injured VSMCs at the
early stage of injury (6). By
contrast, the mRNA and protein expression of Cx43 was significantly
upregulated at 14 and 28 days following injury (Fig. 2). These results are consistent
with those of previous studies (28,29), and suggest the involvement of Cx43
in the development of intimal hyperplasia and RS following balloon
injury. To obtain direct evidence that Cx43 contributes to the
development of RS following balloon injury, a lentiviral vector
expressing shRNA was used to silence Cx43 in rat carotid arteries
(Fig. 3). Compared with the
control or NC-GFP-LV group, infection with Cx43-RNAi-LV was
effective for the specific knockdown of Cx43 in the NRK-52E cells
in vitro (Fig. 3A). In
addition, it was found that Cx43-RNAi-LV effectively decreased the
expression of Cx43 in the injured arteries in vivo (Fig. 4). Importantly, the
lentivirus-mediated RNAi knockdown of Cx43 effectively inhibited
the development of intimal hyperplasia and RS following balloon
injury (Fig. 5). Taken together,
these results indicate that Cx43 remodeling is involved in the
pathogenesis of RS in balloon-injured arteries, and strongly
suggests the vital role of Cx43 in the development of RS following
PCI. Although the specific mechanisms of action of Cx43 in the
development of RS remain unclear, it is hypothesized that the
renin-angiotensin-aldosterone system (RAAS) and the
mitogen-activated protein kinase (MAPK) signaling pathway mediate
the involvement of Cx43 in the development of intimal hyperplasia
and RS. It has previously been demonstrated that RAAS is activated
following balloon injury and that angiotensin Ⅱ significantly
induces neointimal hyperplasia (7). In human saphenous vein smooth muscle
cells (SMCs), angiotensin II has also been shown to significantly
induce the expression of Cx43 through the angiotensin II type I
receptor. Silencing Cx43 inhibits the angiotensin II-induced
proliferation and migration of SMCs. In addition, the inhibition of
the MAPK-AP-1 signaling pathway efficiently attenuates the
angiotensin II-induced Cx43 expression and proliferation of SMCs
(8). Therefore, the involvement
of Cx43 remodeling in the development of intimal hyperplasia and RS
following balloon injury may be mediated by the RAAS or MAPK-AP-1
signaling pathway.
In conclusion, to the very best of our knowledge,
our data demonstrate for the first time that the knockdown of the
GJ protein, Cx43, may be an effective strategy to prevent the
development of intimal hyperplasia and RS following acute injury to
rat carotid arteries. In the present study, we reproduced vascular
RS by balloon angioplasty injury. The mRNA and protein expression
levels of Cx43 were temporarily decreased, and subsequently
increased during the development of balloon injury-induced intimal
hyperplasia and RS. The knockdown of Cx43 with the lentiviral
vector, Cx43-RNAi-LV, significantly inhibited the balloon
injury-induced expression of Cx43, and also effectively attenuated
the development of intimal hyper-plasia and RS in the arteries
following balloon injury. These results indicate that the GJ
protein, Cx43, plays an important role in the pathogenesis of RS in
injured arteries. Therefore, our data suggest that Cx43 may be a
novel and promising pharmacological target for the prevention of
intimal hyperplasia and RS following PCI.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 30760286 and 81241125), the
Jiangxi Province Natural Science Foundation (grant nos.
20142BAB205022) and partially by the National Natural Science
Foundation of China (grant nos. 31360241 and 81472371), as well as
by the Postgraduate Student Foundation for New Teacher from the
Ministry of Education of China (20123601120001).
Abbreviations:
|
PCI
|
percutaneous coronary intervention
|
|
RS
|
restenosis
|
|
VSMCs
|
vascular smooth muscle cells
|
|
GJs
|
gap junctions
|
|
Cx43
|
connexin 43
|
|
RNAi
|
RNA interference
|
|
CAD
|
coronary artery disease
|
|
RAAS
|
renin-angiotensin-aldosterone
system
|
References
|
1
|
Trikalinos TA, Alsheikh-Ali AA, Tatsioni
A, Nallamothu BK and Kent DM: Percutaneous coronary interventions
for non-acute coronary artery disease: a quantitative 20-year
synopsis and a network meta-analysis. Lancet. 373:911–918. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meads C, Cummins C, Jolly K, Stevens A,
Burls A and Hyde C: Coronary artery stents in the treatment of
ischaemic heart disease: a rapid and systematic review. Health
Technol Assess. 4:1–153. 2000.PubMed/NCBI
|
|
3
|
Odell A, Grip L and Hallberg LR:
Restenosis after percutaneous coronary intervention (PCI):
experiences from the patients’ perspective. Eur J Cardiovasc Nurs.
5:150–157. 2006. View Article : Google Scholar
|
|
4
|
Zhang C, Chaturvedi D, Jaggar L, Magnuson
D, Lee JM and Patel TB: Regulation of vascular smooth muscle cell
proliferation and migration by human sprouty 2. Arterioscler Thromb
Vasc Biol. 25:533–538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owens GK: Regulation of differentiation of
vascular smooth muscle cells. Physiol Rev. 75:487–517.
1995.PubMed/NCBI
|
|
6
|
Crowley ST, Ray CJ, Nawaz D, Majack RA and
Horwitz LD: Multiple growth factors are released from mechanically
injured vascular smooth muscle cells. Am J Physiol.
269:H1641–H1647. 1995.PubMed/NCBI
|
|
7
|
Li F, Zhang C, Schaefer S, Estes A and
Malik KU: ANG II-induced neointimal growth is mediated via cPLA2-
and PLD2-activated Akt in balloon-injured rat carotid artery. Am J
Physiol Heart Circ Physiol. 289:H2592–H2601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia G, Cheng G, Gangahar DM and Agrawal
DK: Involvement of connexin 43 in angiotensin II-induced migration
and proliferation of saphenous vein smooth muscle cells via the
MAPK-AP-1 signaling pathway. J Mol Cell Cardiol. 44:882–890. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hervé JC, Phelan P, Bruzzone R and White
TW: Connexins, innexins and pannexins: bridging the communication
gap. Biochim Biophys Acta. 1719:3–5. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruzzone R, White TW and Paul DL:
Connections with connexins: the molecular basis of direct
intercellular signaling. Eur J Biochem. 238:1–27. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elfgang C, Eckert R, Lichtenberg-Fraté H,
et al: Specific permeability and selective formation of gap
junction channels in connexin -transfected HeLa cells. J Cell Biol.
129:805–817. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Kempen MJ and Jongsma HJ: Distribution
of connexin37, connexin40 and connexin43 in the aorta and coronary
artery of several mammals. Histochem Cell Biol. 112:479–486. 1999.
View Article : Google Scholar
|
|
13
|
Hong T and Hill CE: Ristricted expression
of the gap junctional protein connexin43 in the arterial system of
the rat. J Anat. 192:583–593. 1998. View Article : Google Scholar
|
|
14
|
Li X and Simard JM: Increase in Cx45 gap
junction channels in cerebral smooth muscle cells from SHR.
Hypertension. 40:940–946. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsushita T, Rama A, Charolidi N, Dupont
E and Severs NJ: Relationship of connexin43 expression to
phenotypic modulation in cultured human aortic smooth muscle cells.
Eur J Cell Biol. 86:617–628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ram R, Wescott AP, Varandas K, Dirksen RT
and Blaxall BC: Mena associates with Rac1 and modulates connexin 43
remodeling in cardiomyocytes. Am J Physiol Heart Circ Physiol.
306:H154–H159. 2014. View Article : Google Scholar :
|
|
17
|
Kieken F, Mutsaers N, Dolmatova E, et al:
Structural and molecular mechanisms of gap junction remodeling in
epicardial border zone myocytes following myocardial infarction.
Circ Res. 104:1103–1112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qu J, Volpicelli FM, Garcia LI, et al: Gap
junction remodeling and spironolactone-dependent reverse remodeling
in the hypertrophied heart. Circ Res. 104:365–371. 2009. View Article : Google Scholar :
|
|
19
|
Rucker-Martin C, Milliez P, Tan S, et al:
Chronic hemodynamic overload of the atria is an important factor
for gap junction remodeling in human and rat hearts. Cardiovasc
Res. 72:69–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sovari AA, Rutledge CA, Jeong EM, et al:
Mitochondria oxidative stress, connexin43 remodeling, and sudden
arrhythmic death. Circ Arrhythm Electrophysiol. 6:623–631. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng QH, Yang G, Yang W, Jiang B, Wu L and
Wang R: Protective effect of hydrogen sulfide on balloon
injury-induced neointima hyperplasia in rat carotid arteries. Am J
Pathol. 170:1406–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ai Z, Yin L, Zhou X, et al: Inhibition of
survivin reduces cell proliferation and induces apoptosis in human
endometrial cancer. Cancer. 107:746–756. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barac YD, Zeevi-Levin N, Yaniv G, et al:
The 1,4,5-inositol trisphosphate pathway is a key component in
Fas-mediated hypertrophy in neonatal rat ventricular myocytes.
Cardiovasc Res. 68:75–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chadjichristos CE, Morel S, Derouette JP,
et al: Targeting connexin 43 prevents platelet-derived growth
factor-BB-induced phenotypic change in porcine coronary artery
smooth muscle cells. Circ Res. 102:653–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Severs NJ, Rothery S, Dupont E, et al:
Immunocytochemical analysis of connexin expression in the healthy
and diseased cardiovascular system. Microsc Res Tech. 52:301–322.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brisset AC, Isakson BE and Kwak BR:
Connexins in vascular physiology and pathology. Antioxid Redox
Signal. 11:267–282. 2009. View Article : Google Scholar
|
|
27
|
Chadjichristos CE, Matter CM, Roth I, et
al: Reduced connexin43 expression limits neointima formation after
balloon distension injury in hypercholesterolemic mice.
Circulation. 113:2835–2843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Chen J, Sun Y, et al: Regulation
of connexin expression after balloon injury: possible mechanisms
for antiproliferative effect of statins. Am J Hypertens.
18:1146–1153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang LH, Chen JZ, Sun YL, et al: Statins
reduce connexin40 and connexin43 expression in atherosclerotic
aorta of rabbits. Int J Cardiol. 100:467–475. 2005. View Article : Google Scholar : PubMed/NCBI
|