Introduction
Vascular inflammation is a complex and
multifactorial pathophysiological process that plays a key role in
the development and progression of various cardiovascular diseases,
including atherosclerosis and congestive heart failure (1,2).
There are various risk factors involved, such as oxidative stress
and modified low-density lipoprotein (LDL) cholesterol that may
contribute to the onset and progression of vascular inflammation
and result in chronic inflammation (3). This process is predominantly
mediated by a diverse group of cell adhesion molecules (CAMs),
which are expressed on the surface of vascular endothelial cells
and smooth muscle cells in response to several inflammatory stimuli
(4). The interaction between
leukocytes and vascular cells is considered a hallmark of vascular
inflammation (4,5). Indeed, clinical studies have
demonstrated that the increased expression of CAMs, such as
intercellular adhesion molecule-1 (ICAM-1) and vascular cell
adhesion molecule-1 (VCAM-1) contributes to vascular dysfunction
through the recruitment of inflammatory cells and their
transmigration into target sites. Therefore, the functional
inhibition of CAMs may be a critical therapeutic strategy for the
treatment of vascular diseases.
During vascular inflammation, pro-inflammatory
cytokines, such as tumor necrosis factor (TNF)-α, C-reactive
protein and interleukin (IL)-6 appear to accelerate vascular
dysfunction by inducing the expression of CAMs, which leads to the
alternation of cell-cell and cell-matrix interactions (6). In particular, TNF-α has been
implicated as a central mediator of vascular inflammation (7). TNF-α causes vascular oxidative
stress, vascular remodeling, thrombosis, cell infiltration and
apoptosis and leads to vascular damage (8,9).
Therefore, in the present study, we used TNF-α to induce vascular
inflammation in human aortic smooth muscle cells (HASMCs).
The root of Cynanchum wilfordii (C.
wilfordii) has been used widely as a traditional herbal
medicine in Asia for the treatment of insomnia, anxiety, anemia,
senescence and various geriatric diseases. The biological effects
of the root of C. wilfordii against tumors, antioxidants,
diabetes mellitus, gastric disorders, neuronal damage and
hypercholesterolemia have been reported (10–15).
However, there is little information available on
the molecular mechanisms responsible for the anti-inflammatory
effects of the extract and bioactive components of the root of
C. wilfordii on vascular-type cells. It is known that the
root of C. wilfordii contains several active compounds,
including gagaminine, pregnane glycosides, cynanchone, various
wilfosides and cynauricuosides, sarcotine, penupogenin, cynandione
A (Cyn A) and anthraquinones (16). Recently, Yang et al
(17) reported that Cyn A from
the root of C. wilfordii exerts anti-inflammatory effects on
lipopolysaccharide-treated brain macrophages/BV2 microglial
cells.
In the present study, we investigated the
anti-inflammatory effects of a root extract of C. wilfordii
under optimal extraction conditions in order to elucidate the
molecular mechanisms of action of the vascular protective
properties of the root of C. wilfordii and identify its
major active components.
Materials and methods
Materials and reagents
The chemicals used in the present study were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies
against ICAM-1 (Cat. no. 4915), p65 (Cat. no. 8242), lamin A/C
(Cat. no. 2032) and β-actin (Cat. no. 4967) were obtained from Cell
Signaling Technology (Beverly, MA, USA). Anti-VCAM-1 antibody
(sc-8304) was purchased from Santa Cruz Biotechnology (Santa Cruz,
CA, USA).
Cell culture
Primary HASMCs were obtained from ScienCell Research
Laboratories (San Diego, CA, USA). The cells were cultured as
monolayers in smooth muscle cell (SMC) medium (ScienCell)
containing essential and non-essential amino acids, vitamins,
organic and inorganic compounds, hormones, growth factors, trace
minerals and 2% fetal bovine serum (FBS) at 37°C in a humidified
atmosphere of 95% air and 5% CO2. For subcultures, the
cells were detached using 0.125% trypsin containing 0.01 M
ethylenediaminetetraacetic acid (EDTA). The cells used in the
present study were from the early passages (passages 2–6). THP-1
cells were from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and weres used for the cell adhesion assay with
the HASMCs. These cells were cultured in RPMI-1640, and
supplemented with 2 mM L-glutamine, 100 mg/ml streptomycin, 100
IU/ml penicillin and 10% FBS.
Preparation and characterization of an
ethanol root extract of C. wilfordii (CWE)
The root of C. wilfordii used in the present
study was collected through KNRRC (Medicinal Plants Resources Bank
NRF-2010-0005790) supported by the Korea Research Foundation (the
resources of which were provided by the Ministry of Education,
Science and Technology of Korea) in 2014. A voucher specimen (no.
MPRBP00962) was deposited in the herbarium of Gachon University
(Seongnam, Korea). The powder from the root of C. wilfordii
(2,500 g) was extracted twice with 0–100% ethanol for 48 h at room
temperature and the extract was concentrated under reduced
pressure. The decoction was filtered, lyophilized and stored at 4°C
until use.
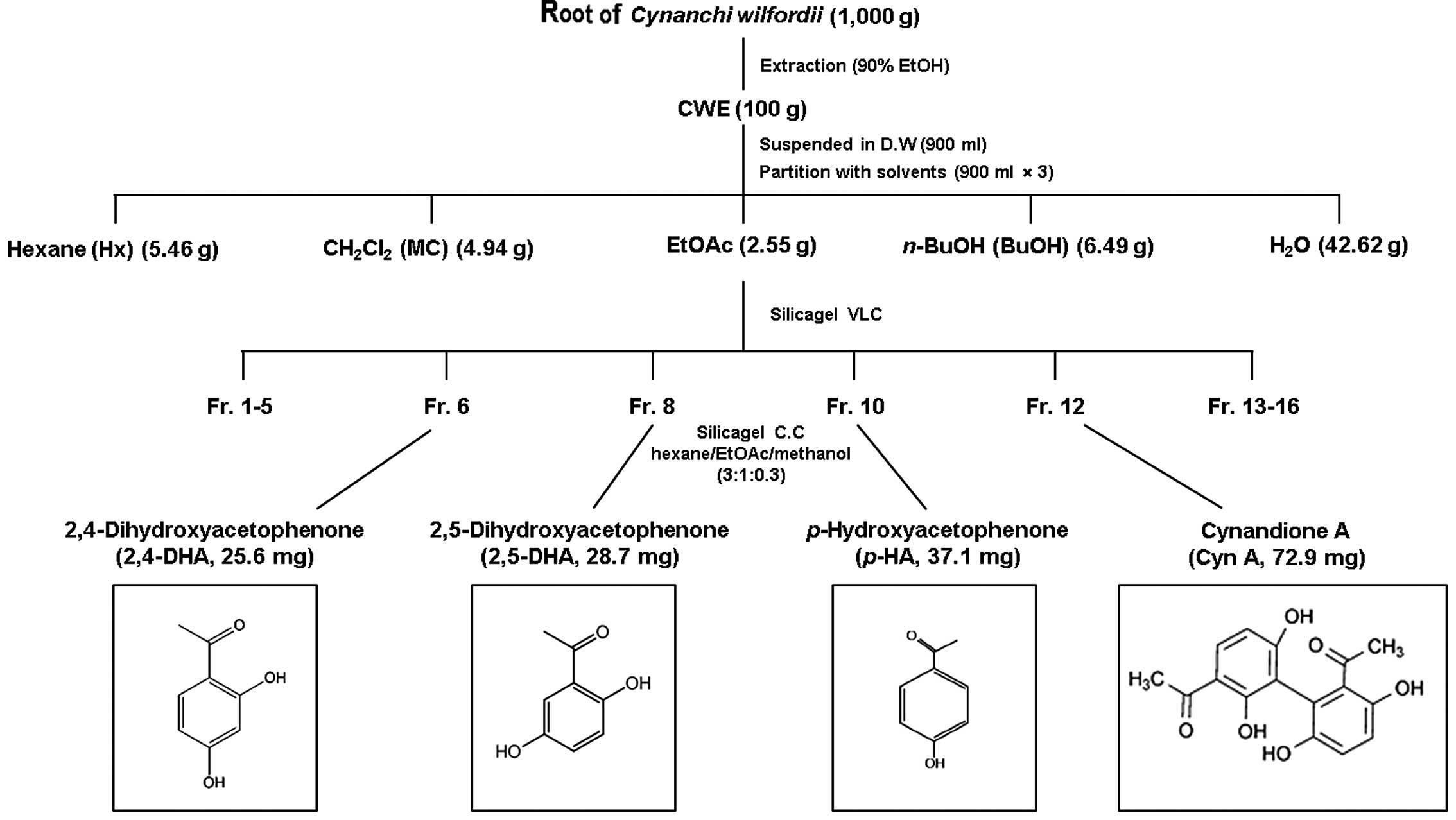
Isolation and structural identification
of components from CWE
CWE (100 g) was dissolved in distilled water and
partitioned with n-hexane (Hx fraction), dichloromethane
(CH2Cl2, MC fraction), ethyl acetate (EtOAc
fraction), n-butanol (n-BuOH fraction) and water
(H2O fraction). The yield of dried extract from the
starting crude materials was approximately 30.8% (wt/wt). The EtOAc
fraction has a potent suppressive effect on the expression of the
adhesion molecules, VCAM-1 and ICAM-1. Hence, the EtOAc fraction
(2.55 g) was fixed on Celite, fractionated by vacuum liquid
chromatography (VLC) on a silica gel, and eluted to 16
sub-fractions. Fractions 6, 8, 10 and 12 were subjected to silica
gel column chromatography (CC) (150 g, 50×120 mm) and eluted with
hexane/EtOAc/methanol (3:1:0.3) to yield 2,4-dihydroxyacetophenone
(2,4-DHA), 2,5-dihydroxyacetophenone (2,5-DHA),
p-hydroxyacetophenone (p-HA) and (Cyn A):
2,4-DHA: colorless solid; 1H-NMR (500
MHz, CDCl3): δ 7.72 (1H, d, J=7.04 Hz),
6.35 (1H, dd, J=2.5 and 7.04 Hz), 6.24 (1H, d, J=2.0
Hz), 2.51 (3H, s); 13C-NMR (125 MHz, CDCl3):
δ 114.5, 166.6, 103.6, 166.4, 109.2, 134.6, 204.3, 26.3. ii)
2,5-DHA: yellow powder; 1H-NMR (500 MHz,
CDCl3): δ 7.21 (1H, d, J=2.24 Hz), 7.01
(1H, dd, J=2.5 and 7.04 Hz), 6.78 (1H, d, J=7.3 Hz),
2.58 (3H, s); 13C-NMR (125 MHz, CDCl3):
δ 120.8, 156.7, 119.7, 166.4, 126.0, 116.5, 206.0, 27.0.
p-HA: colorless powder; 1H-NMR
(500 MHz, CDCl3): δ 7.89 (2H, d, J=8.92
Hz), 6.84 (2H, d, J=10.12 Hz), 2.51 (3H, s);
13C-NMR (125 MHz, CDCl3): δ 130.2,
132.2, 116.2, 164.0, 199.5, 26.3.
Cyn A: yellow needles; 1H-NMR (500 MHz,
CDCl3): δ 6.94 (1H, d, J=8.92 Hz), 6.80
(1H, d, J=8.92 Hz), 6.50 (1H, d, J=8.96 Hz), 2.57
(3H, s), 2.17 (3H, s); 13C-NMR (125 MHz,
CDCl3): δ 127.8, 120.4, 152.4, 118.2, 121.8,
149.1, 207.4, 31.0, 114.5, 163.8, 113.2, 134.0, 108.8, 163.7,
204.6, 26.4.
Experimental animals
The experimental animal facility and study protocols
(GIACUC-R2013017) were approved by the Animal Care and Use
Committee of Gachon University. All experimental procedures were
undertaken in compliance with the Guide for the Care and Use of
Laboratory Animals (National Institutes of Health, Bethesda, MD,
USA) and the National Animal Welfare Law of the Republic of
Korea.
Four-week-old male C57BL/6 mice were obtained from
Japan SLC Inc. (Shizuoka, Japan) and maintained in a controlled
environment of 22±1°C and a humidity of 50±10% with a 12-h
light-dark cycle for 1 week prior to the commencement of the
experiments. Mice had access to sterile standard mouse chow and
water ad libitum. At the start of the study, the diet was
changed to an atherogenic (ATH) diet [1.25% (w/w) cholesterol, 0.5%
(w/w) cholic acid and 16% (w/w) fats in the form of soybean oil,
cocoa butter and coconut oil] or a normal control (NC) chow [0.3%
(w/w) cholesterol, no cholic acid and 5% (w/w) fats]. Both diets
were obtained from Research Diets Inc. (New Brunswick, NJ,
USA).
The mice were divided randomly into 6 groups of 5
mice as follows: i) mice fed a normal control chow diet plus the
vehicle (PBS; NC group); ii) mice fed an ATH diet plus the vehicle
(PBS; ATH group); iii) mice fed an ATH diet plus 50 mg/kg body
weight (bw)/day of CWE; iv) mice fed an ATH diet plus 100 mg/kg
bw/day of CWE; v) mice fed an ATH diet plus 200 mg/kg bw/day of
CWE; and vi) mice fed an ATH diet plus 10 mg/kg bw/day of
simvastatin (Simv; Sigma-Aldrich) via oral gavage for 12 weeks. At
the end of the treatments, each mouse was anesthetized and the
thorax was opened. The aorta was dissected following perfusion with
phosphate-buffered saline (PBS) and stored at −80°C until RNA
isolation.
Cell viability
The HASMCs were seeded in 96-well flat-bottom plates
(2×104 cells/well) and then treated with CWE (2, 20 and
200 μg/ml) for 16 h. The cells were incubated with 100
μl of 5 mg/ml MTT
[3-(4,5-dimethylthiazolyl)-2,5-diphenyl-tetrazolium bromide]
(Sigma-Aldrich) for a further 2–4 h. After the supernatant was
removed, 100 μl of DMSO per well was added to the cells and
mixed on a shaker for 15 min to dissolve the formazan crystals
formed. The optical density (OD) colored solution was quantified at
a 570 nm wavelength using an enzyme-linked immunoabsorbent assay
(ELISA) reader (model 550 microplate reader, Bio-Rad Laboratories,
Hercules, CA, USA).
Monocyte adhesion assay
The adhesion of THP-1 cells to the HASMCs was
measured as previously described (18). Briefly, the HASMCs (which were
grown in 96-well plates) were pre-treated with CWE (2, 20 and 200
μg/ml) for 2 h at 37°C. The cells were washed with medium
and incubated with fresh growth medium containing TNF-α (10 ng/ml)
for 8 h. The medium was removed from the wells and calcein
AM-labeled THP-1 cells (2×105 cells/ml) in 0.2 ml of the
medium were added to each well. The test and control samples were
used in triplicate in each experiment. Following incubation for 1 h
in 5% CO2 at 37°C, micro-wells were washed twice with
0.2 ml of warm medium. The number of adherent cells was detected
using a fluorescence microscope (IX71; Olympus, Tokyo, Japan)
equipped with a digital camera (DP71; Olympus) and processed using
ImageJ software version 1.45s (National Institutes of Health). The
increase in the adhesion of THP-1 cells upon stimulation of the
HASMCs with TNF-α was calculated in relation to the basal adhesion
of THP-1 cells to the unstimulated HASMCs (which was set to 1).
Western blot analysis
The cells were pretreated with CWE (2, 20 and 200
μg/ml) or dexamethasone (50 ng/ml) for 2 h. The cells were
washed with medium and incubated with fresh growth medium
containing TNF-α (10 ng/ml) for 30 min or 8 h. Following treatment,
the cells were washed twice with PBS and lysed in ice-cold lysis
buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.5% (v/v)
NP-40, 0.1% (w/v) sodium dodecyl sulfate (SDS)] containing protease
inhibitor cocktail (Roche Diagnostics Corp., Indianapolis, IN, USA)
for 1 h. The lysates were then collected after centrifugation at
1500 × g for 10 min at 4°C. Cytosolic and nuclear extracts were
prepared using a Nuclear Extract kit (Active Motif, Carlsbad, CA,
USA) according to the manufacturer’s instructions. The protein
concentration was determined using a protein assay kit (Bio-Rad
Laboratories) with bovine serum albumin (BSA) as the standard.
Protein lysates (20 μg) were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred by
electrophoretic means to an Immobilon®-P Polyvinylidene
difluoride membrane (Amersham, Arlington Heights, IL, USA) and
probed with appropriate antibodies. The blots were developed using
an enhanced chemoluminescence (ECL) kit (Amersham). In all the
westernt blotting experiments, the blots were re-probed with
anti-β-actin antibody as a control for protein loading.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using a single-step
guanidinium thiocyanate-phenol-chloroform method. The yield and
purity of the RNA were confirmed by measuring the ratio of the
absorbance values at 260 and 280 nm. PCR was undertaken using
ICAM-1- and VCAM-1-specific primers to identify their respective
specific cDNA. The following sequence-specific primers were
synthesized: 5′-ATTTTCTGG GGCAGGAAGTT-3′ and 5′-ACGTCAGAACAACCGAAT
CC-3′ for human VCAM-1; 5′-AGCACCTCCCCACCTAC TTT-3′ and
5′-AGCTTGCACGACCCTTCTAA-3′ for human ICAM-1. The following pair of
oligonucleotides was used as the internal control:
5′-AACTTTGGCATTGTGGAAGG-3′ and 5′-ACACATGGGGGTAGGAACA-3′ for human
glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The absence of
contaminants was routinely checked by an RT-PCR assay of negative
control samples without the addition of a primer. Following
amplification, the samples were stored at −20°C.
High-performance liquid chromatography
(HPLC)
An Alliance 2695 system (Waters Corp., Milford, MA,
USA) coupled with a Waters 2998 photodiode array detector was used
for the quantitative chromatographic analysis of CWE. The
analytical column was a Sunfire™ 4.6 × 150 mm C18 column (particle
size, 5 μm; Waters Corp.). The mobile phase consisted of (A)
acetic acid (0.5% v/v) and (B) acetonitrile using a gradient
elution of A/B = 90/10 (0 min) → A/B = 65/35 (10 min) → A/B = 0/100
(30 min). The flow rate was 1.0 ml/min and the injection volume was
10 μl; ultraviolet (UV) detection was conducted at 254 nm.
CWE was dissolved in ethanol at 10 mg/ml, and p-HA (purity
99%), 2,4-DHA (purity 99%) and 2,5-DHA (purity 97%) were used as
standard solutions.
Statistical analysis
Each result is reported as the mean ± SEM. One-way
analysis of variance was used to determine significance among
groups, after which the modified Student’s t-test with the
Bonferroni correction was used for the comparison between
individual groups. A value of P<0.05 was considered to indicate
a statistically significant difference.
Results
Optimal ethanol concentration for the
root extract of C. wilfordii for the inhibition of the expression
of adhesion molecules
We investigated the effects of the ethanol
concentration for the root extract of C. wilfordii on the
inhibition of the expression of adhesion molecules in the
TNF-α-stimulated HASMCs. Ten different concentrations of ethanol
(10, 20, 30, 40, 50, 60, 70, 80, 90 and 100%, v/v) were used by
adjusting the composition of ethanol and water in the extraction
solvent. The cells were pre-treated with 100 μg/ml of each
ethanol extract and then incubated with fresh growth medium
containing TNF-α (10 ng/ml) for 8 h. We found that the ethanol
extracts obtained with various concentrations had suppressive
effects on TNF-α-induced VCAM-1 expression in the HASMCs (Fig. 1). Among these, the most marked
inhibitory effect on VCAM-1 expression was observed by treatment
with an ethanol concentration of 90% (Fig. 1). The extracts obtained at low or
high ethanol concentrations (10, 20 and 100%) had lower efficacy,
but they were comparable with the cells treated with dexamethasone
(50 ng/ml).
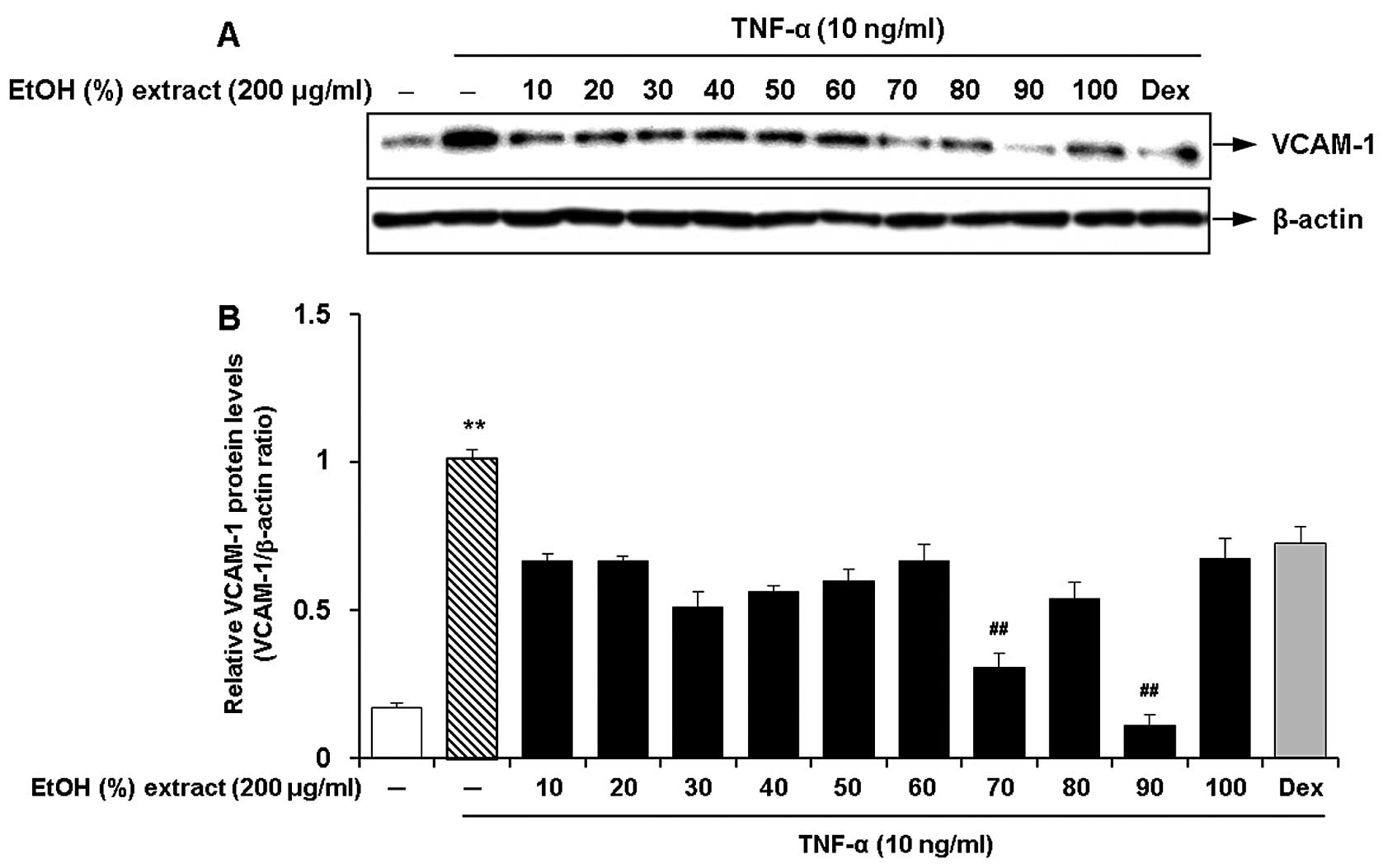 | Figure 1Effects of the ethanol concentration
(10, 20, 30, 40, 50, 60, 70, 80, 90 and 100%, v/v) for the
Cynanchum wilfordii extract (CWE) on the expression of
vascular cell adhesion molecule (VCAM)-1 in tumor necrosis factor
(TNF)-α-stimulated human aortic smooth muscle cells (HASMCs). (A)
The cells were pre-treated with samples of each extract (200
μg/ml) or dexamethasone (Dex; 50 ng/ml) for 2 h and then
stimulated with TNF-α (10 ng/ml) for 12 h. The protein levels of
VCAM-1 were determined by western blot analysis. (B) Densitometric
analysis of western blots is represented as the mean band density
normalized to β-actin. Results are the means ± SEM (n=3).
Significantly different values are represented by a symbols
(**P<0.01 compared to untreated control,
##P<0.01 compared to treatment with TNF-α alone). |
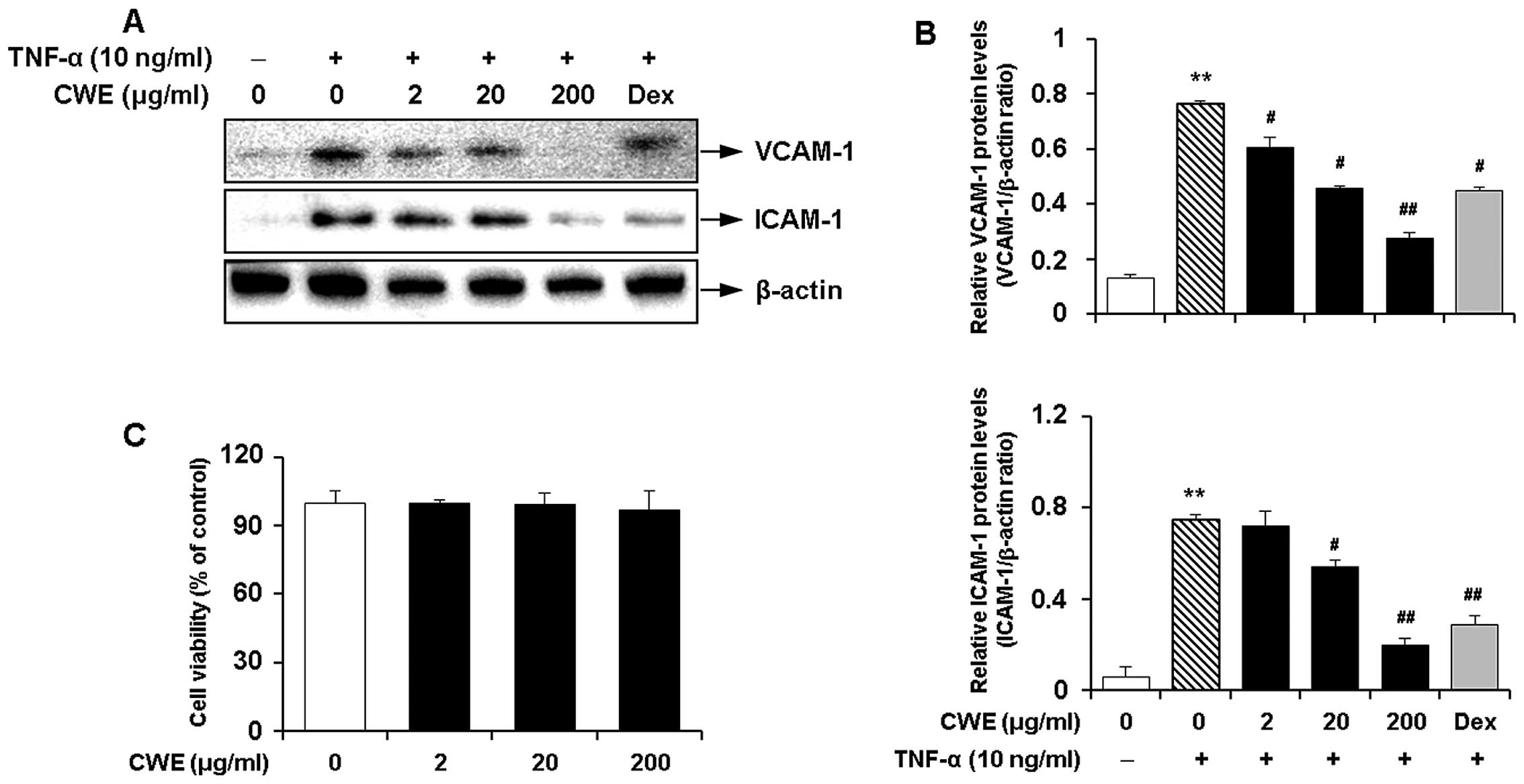
Inhibitory effect of CWE on the
TNF-α-induced expression of adhesion molecules in HASMCs
We determined the effects of CWE on the
TNF-α-induced expression of adhesion molecules in HASMCs. Western
blot analysis produced the following results i) TNF-α significantly
induced the expression of VCAM-1 and ICAM-1; and ii) CWE
downregulated the TNF-α-induced expression of the adhesion
molecules in a dose-dependent manner (Fig. 2A and B).
Moreover, MTT assay revealed that CWE did not affect
cell viability and was not cytotoxic to the cells at the
concentrations used (Fig.
2C).
Effect of CWE on the TNF-α-induced
adhesion of THP-1 monocytes to HASMCs
We determined the effects of CWE on the adherence of
THP-1 monocytes to TNF-α-stimulated HASMCs. The HASMCs were treated
without or with various concentrations (2, 20 and 200 μg/ml)
of CWE for 2 h prior to stimulation with TNF-α (10 ng/ml).
Stimulation with TNF-α elicited a significant increase in the
adhesion of THP-1 monocytes to the HASMCs (P<0.01). Treatment
with CWE significantly inhibited the adhesion of the THP-1
monocytes to the HASMCs in a dose-dependent manner (Fig. 3).
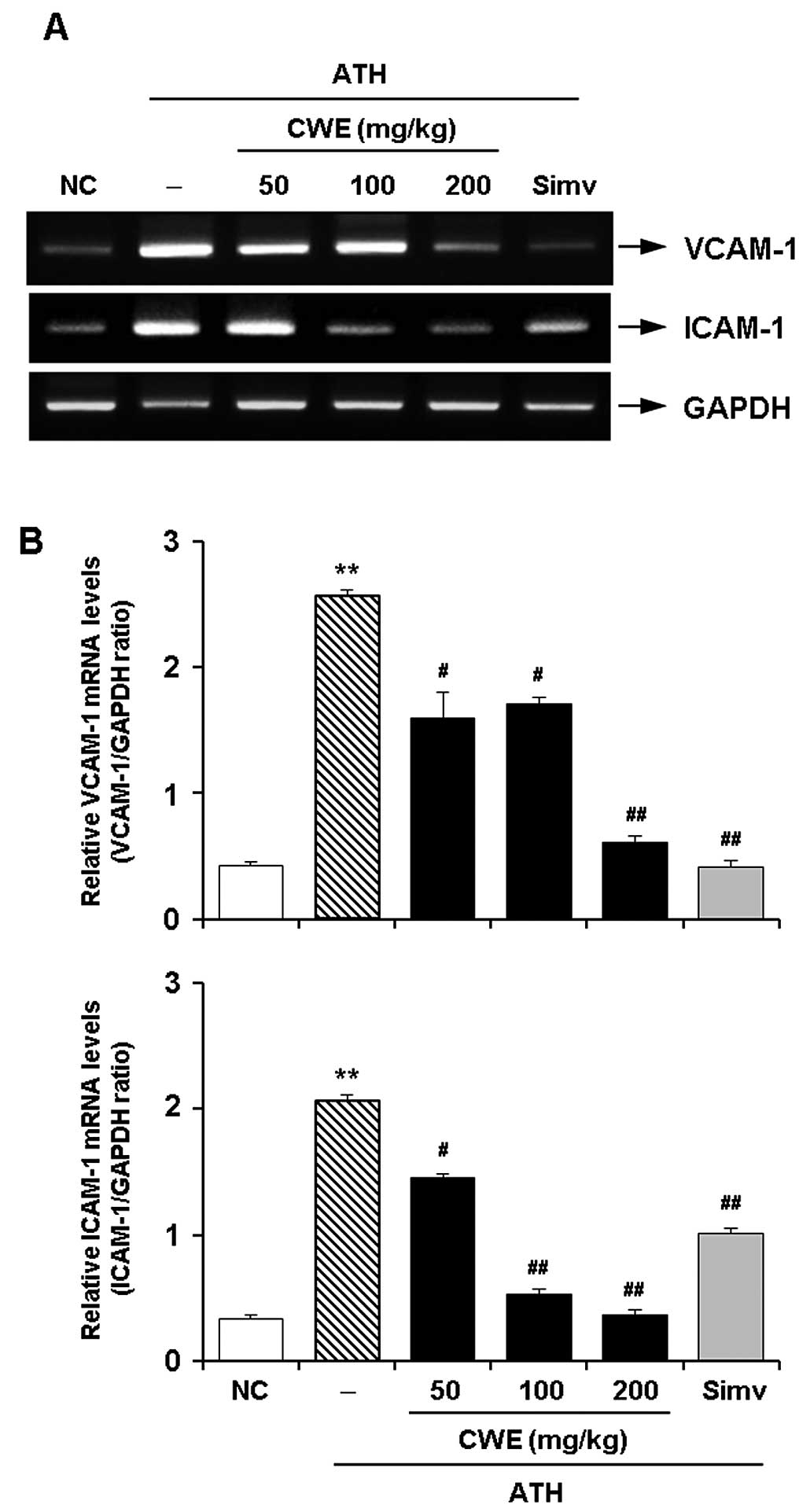
Effect of CWE on the expression of
adhesion molecules in the aorta in vivo
To verify the in vitro effects of CWE, an
in vivo experiment was undertaken using a mouse model of ATH
diet-induced hypercholesterolemia. RT-PCR revealed the expected
significant increase in the mRNA expression of VCAM-1 and ICAM-1 in
the aortas of the hypercholesterolemic mice. The administration of
CWE for 12 weeks dose-dependently reduced the expression of VCAM-1
and ICAM-1 in the aortaso the hypercholesterolemic mice. The
suppressive effects of CWE (100 and 200 mg/kg) on the expression of
CAMs were comparable to those observed by treatment with Simv
(Fig. 4).
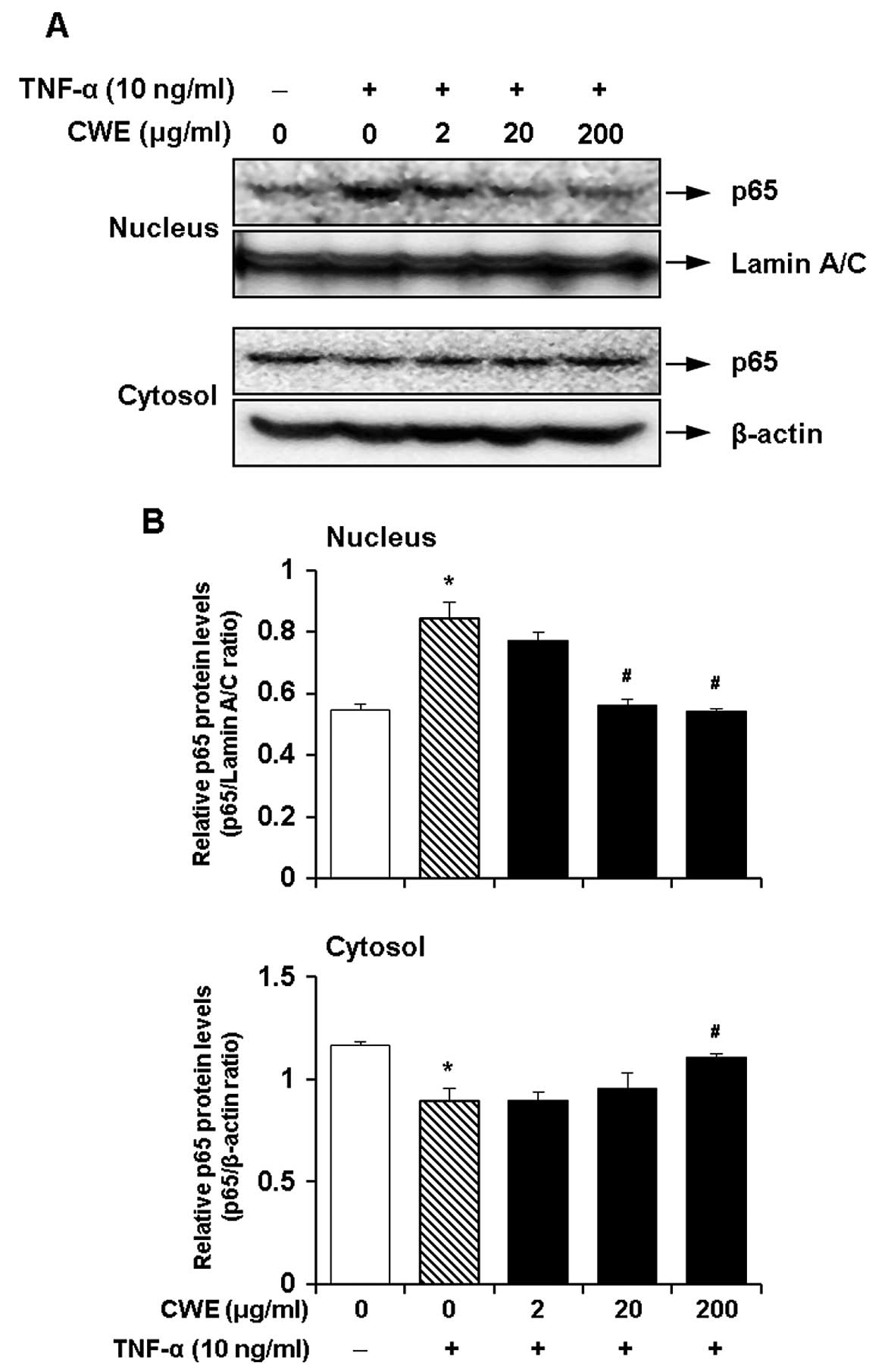
Effect of CWE on the TNF-α-induced
nuclear translocation of nuclear factor-κB (NF-κB)
NF-κB is a crucial transcription factor for the
induction of the expression of adhesion molecules by TNF-α
(19,20). Therefore, we investigated whether
the inhibitory effects of CWE on the TNF-α-induced expression of
ICAM-1 and VCAM-1 are mediated by the activation of NF-κB. The
cells were treated with CWE (2, 20 and 200 μg/ml) for 2 h
prior to stimulation with TNF-α for 30 min. CWE decreased the
translocation of NF-κB p65 to the nuclear fraction in a
dose-dependent manner (Fig. 5).
These data suggested that CWE inhibited the TNF-α-induced nuclear
translocation of NF-κB.
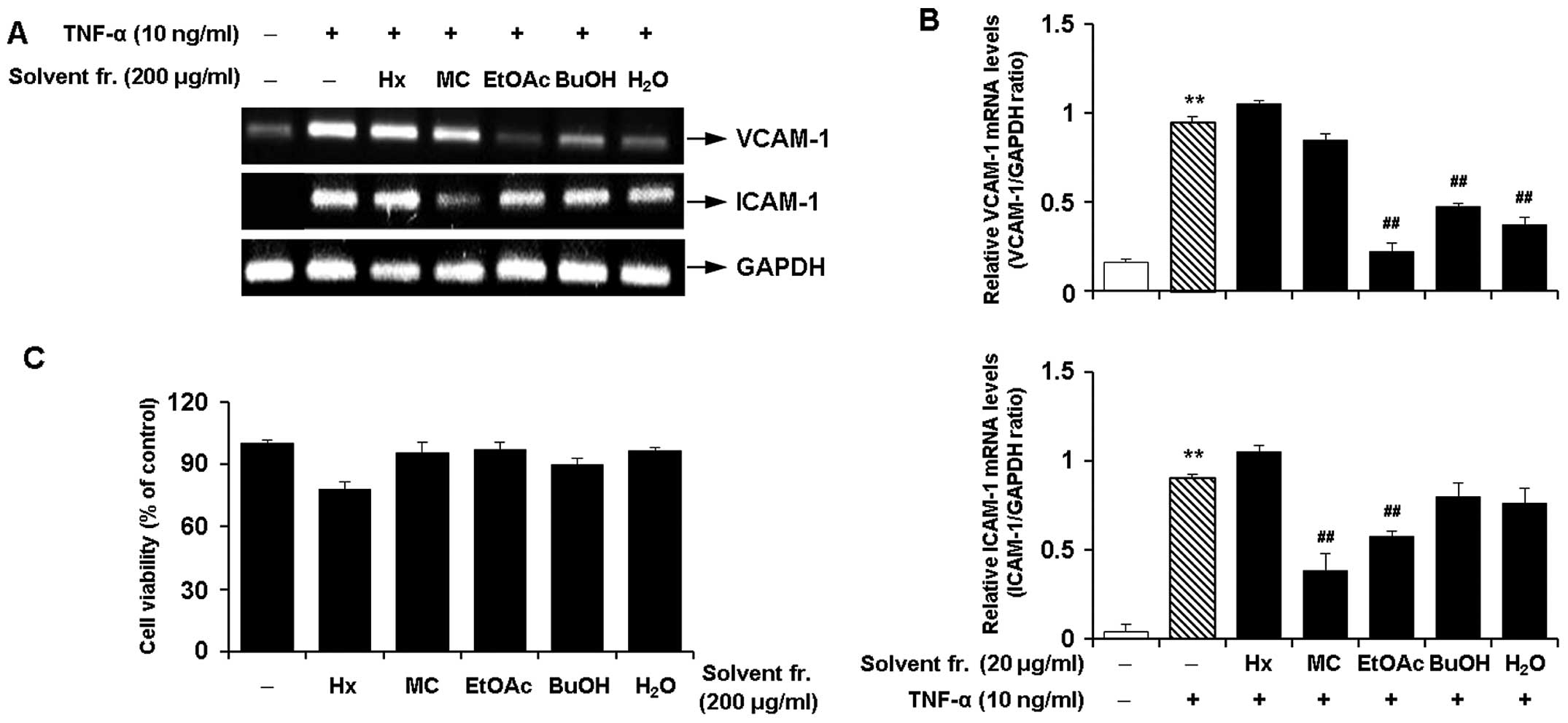
Effects of solvent fractions of CWE on
the TNF-α-induced expression of adhesion molecules in HASMCs
We performed solvent fractionation of CWE and
evaluated the effects of the fractions on the expression of
adhesion molecules to select the most promising fraction (Fig. 6). RT-PCR revealed that the
fraction with ethyl acetate (EtOAc) inhibited the mRNA expression
of VCAM-1 and ICAM-1 by approximately 80 and 40% in the
TNF-α-stimulated HASMCs, respectively (Fig. 7A and B). The EtOAc fraction was
found to be more active with lower cytotoxicity (Fig. 7C) than the other fractions.
Effect of the EtOAc fraction of CWE on
the TNF-α-induced expression of adhesion molecules in HASMCs
As described above, the EtOAc fraction had the
maximum inhibitory effect on the expression of VCAM-1 and ICAM-1
and did not elicit cytotoxicity. Hence, we investigated the
dose-response effects of this fraction on the expression of VCAM-1
and ICAM-1 in the TNF-α-stimulated HASMCs. The EtOAc fraction
markedly inhibited the TNF-α-induced mRNA expression of VCAM-1 and
ICAM-1 in a dose-dependent manner (Fig. 8).
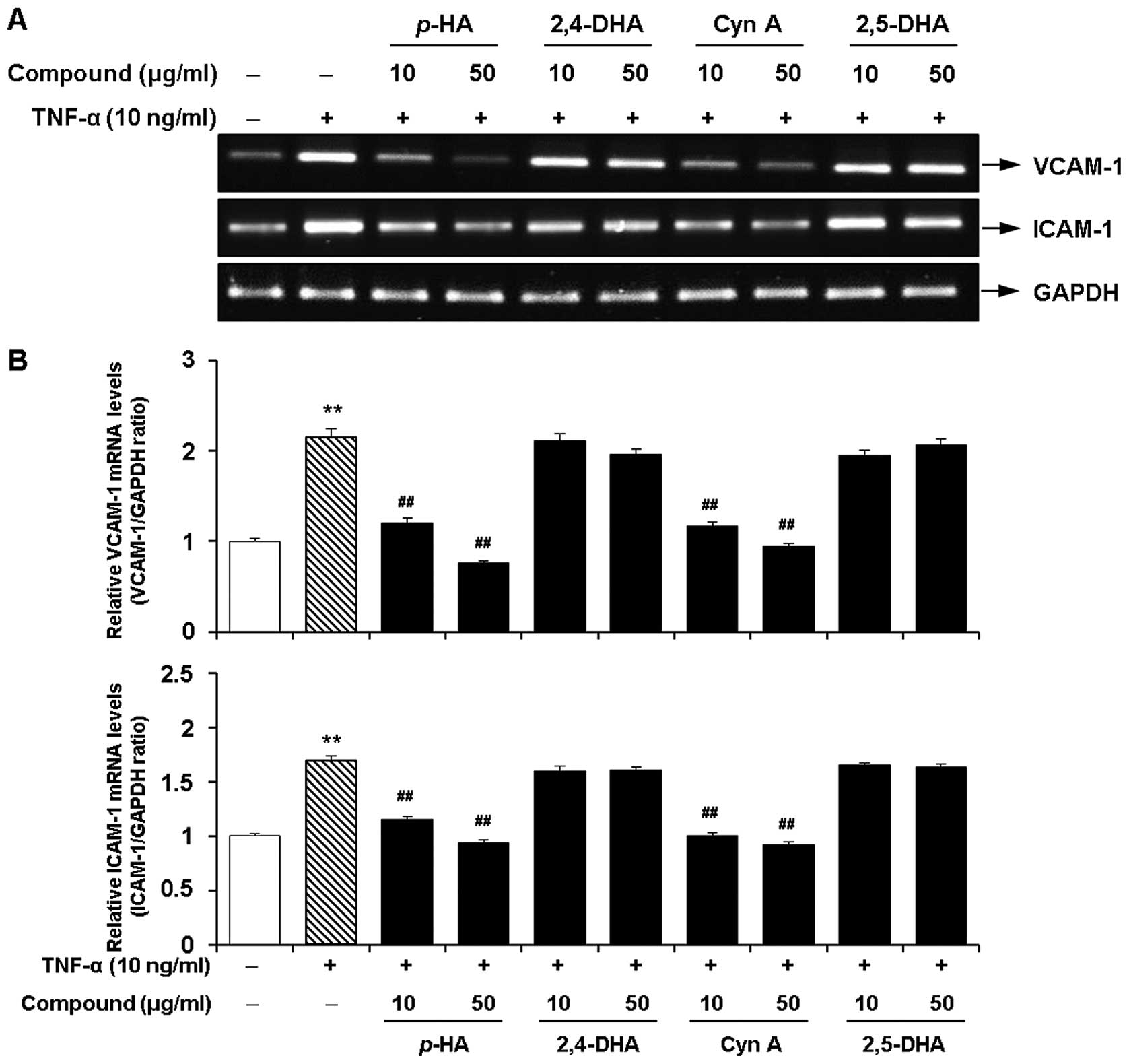
Inhibitory effects of the major
components of the EtOAc fraction of CWE on the TNF-α-induced
expression of adhesion molecules in HASMCs
Next, we investigated whether 4 major acetophenones
from the EtOAc fraction of CWE (p-HA, 2,4-DHA, Cyn A and
2,5-DHA) inhibit the TNF-α-induced expression of VCAM-1 and ICAM-1
in the HASMCs. Among these components, p-HA and Cyn A
significantly inhibited the mRNA expression of VCAM-1 and ICAM-1 at
10 and 50 μg/ml (Fig. 9).
However, treatment with 2,4-DHA and 2,5-DHA had little or no effect
on the expression of VCAM-1 and ICAM-1. These 4 components did not
affect cell viability at the concentrations tested (data not
shown).
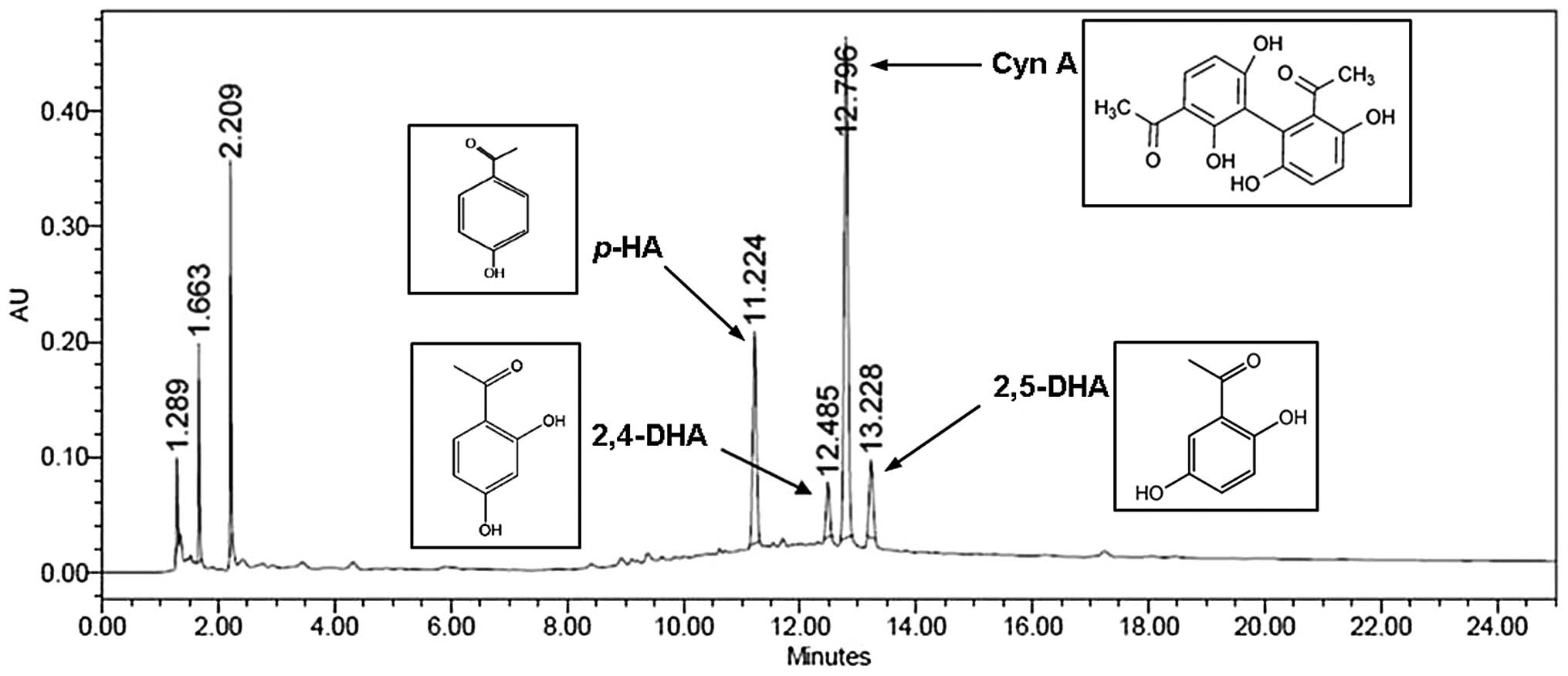
Quantitative analysis of CWE
We applied HPLC for the simultaneous quantification
of p-HA, 2,4-DHA, Cyn A and 2,5-DHA in CWE. The levels of
p-HA, 2,4-DHA, Cyn A and 2,5-DHA identified at the retention
times of 11.22, 12.49, 12.80 and 13.23 min were 3.8, 4.0, 21.0 and
1.0 mg/g, respectively (Fig.
10).
Discussion
The arterial media comprises mainly of vascular
smooth muscle cells (VSMCs). VSMCs contribute to the response to
environ mental stresses and repair of the walls of blood vessels
from vascular injury (21–23).
In the vascular inflammatory reaction, the interactions of VSMCs
with monocytes via CAMs are crucial events (24–26). Sutides have demonstrated that
interactions between transmigrated monocytes and VSMCs induce
monocyte pro-coagulant activity, pro-inflammatory responses and
vascular dysfunction (27,28).
The strong expression of CAMs, such as VCAM-1 and ICAM-1 in VSMCs
in atherosclerotic lesions can facilitate the accumulation of
transmigrated leukocytes within the vascular walls (29). Therefore, the inhibition of these
mediators may be a promising strategy for the prevention and
treatment of vascular inflammatory diseases (29,30).
The present study demonstrated the anti-inflammatory
effects of CWE in TNF-α-stimulated human aortic SMCs. During the
extraction or preparation of natural products, organic solvents,
such as ethanol, methanol, acetone, ethyl acetate, dichloromethane
or hexane are frequently used. Among these, ethanol is the most
common completely biodegradable, edible and food-grade solvent
(31). We selected the ethanol
solvent and prepared various root extracts of C. wilfordii
at an ethanol concentration range of 10 to 100%. We found that the
90% ethanol extract provided the optimal condition for the root of
C. wilfordii to elicit the inhibition of VCAM-1 expression
in the TNF-α-stimulated HASMCs. CWE inhibited the TNF-α-induced
expression of VCAM-1 and ICAM-1 in the HASMCs in a dose-dependent
manner.
Several studies have demonstrated that, in addition
to endothelial cells, VSMCs also express ICAM-1 and VCAM-1 in
atherosclerosis and vascular diseases (29,30). The expression of these molecules
in VSMCs may facilitate the accumulation of transmigrated
leukocytes within the vascular walls. It is well known that
interactions between leukocytes and VSMCs can occur via CAMs, which
can be antagonized by the inhibition of ICAM-1 and/or VCAM-1
(4). To confirm this hypothesis,
we examined the effects of CWE on the monocyte THP-1 adherence to
TNF-α-stimulated SMCs; we observed a marked reduction in monocyte
adhesion in the CWE-treated groups in a dose-dependent manner.
We performed an animal experiment to confirm the
suppressive effects of CWE on the expression of CAMs in the
thoracic aortas of hypercholesterolemic mice. The administration of
an ATH diet for 12 weeks resulted in the significantly increased
expression of ICAM-1 and VCAM-1 in the aortic tissues. Several
lines of evidence have suggested that exposure to a
high-cholesterol diet potentiates systemic vascular inflammation,
which leads to hypercholesterolemia and atherosclerosis. For
example, Zhang et al (9)
and Shi et al (32)
demonstrated that the consumption of high-fat meals increases the
plasma levels of TNF-α, IL-6, ICAM-1 and VCAM-1 and leads to
vascular dysfunction. Our results clearly demonstrated that the
administration of CWE downregulated the expression of ICAM-1 and
VCAM-1 in ATH diet-fed mice.
NF-κB is an ubiquitous transcription factor crucial
for the expression of inflammatory mediators (including CAMs) in
VSMCs (33). It has been well
established that NF-κB activation is associated with the nuclear
translocation of the p65 component of the complex (34,35). We found that CWE inhibited the
TNF-α-induced translocation of p65 to the nucleus. This finding
suggests that the inhibitory effects of CWE on the expression of
CAMs may be associated with the suppression of expression of NF-κB
in VSMCs.
Several studies have demonstrated that active
components from natural products can be converted into therapeutic
agents (36–38). In a similar approach, we attempted
to identify pharmacologically active components from CWE. CWE was
fractionated with various solvents, and the EtOAc fraction showed
maximal efficacy for the inhibition of the expression of VCAM-1 and
ICAM-1 in the TNF-α-stimulated HASMCs. Subsequently, we performed
further sub-fractionation and purification of the chemical
components in the EtOAc fraction and identified 4 acetophenones:
p-HA, 2,4-DHA, Cyn A and 2,5-DHA (Fig. 6).
Acetophenones are the major endogenous volatile
organic compounds in plants. There is emerging evidence that
acetophenones exert beneficial effects on vascular diseases. Ha
et al (39) reported that
acetophenones isolated from Paeonia suffruticosa Andr.
stimulated the phosphorylation of endothelial nitric oxide synthase
in human umbilical vein endothelial cells, which plays a role in
vascular protection. Senejoux et al (40) also demonstrated that the naturally
occurring acetophenone, apocynin, induced relaxation in aortic
rings in vitro and reduced vascular pressure in
spontaneously hypertensive rats. They demonstrated that apocynin
exerted a vasorelaxant effect through the inhibition of the calcium
ion-related contraction in VSMCs and the regulation of the
production of endothelium-derived nitric oxide.
In the present study, we also investigated the
anti-inflammatory effects of 4 acetophenones from CWE. We found
that 2 components, p-hydroxyacetophenone and Cyn A, exerted
suppressive effects on the expression of VCAM-1 and ICAM-1 in
TNF-α-stimulated VSMCs. Therefore, we suggest that the
anti-inflammatory properties of CWE, such as the inhibition of the
expression of VCAM-1 and ICAM-1, and the reduction in monocyte
adhesion to VSMCs, were mainly exerted by 2 types of acetophenones,
p-hydroxyacetophenone and Cyn A. To clarify our hypothesis,
we examined the amounts of the 2 acetophenones in the CWE we used.
We found that CWE contained approximately 3.8 mg/g of
p-hydroxyacetophenone and 21.0 mg/g of Cyn A,
respectively.
We investigated the mechanisms through which CWE
exerts beneficial effects on the prevention of vascular
inflammation. We identified the 2 bioactive components of CWE,
p-hydroxyacetophenone and Cyn A. These results suggest that
the root of C. wilfordii and/or its active components may
have potential application in the prevention of atherosclerosis and
vascular inflammatory diseases.
Acknowledgments
This study was supported by the Bio-industry
Technology Development Program, Ministry of Agriculture, Food and
Rural Affairs (SRAA), Republic of Korea.
Abbreviations:
|
CWE
|
Cynanchum wilfordii ethanol
extract
|
|
TNF-α
|
tumor necrosis factor-α
|
|
HASMCs
|
human aortic smooth muscle cells
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
VCAM-1
|
vascular cell adhesion molecule-1
|
|
NF-κB
|
nuclear factor-κB
|
|
2,4-DHA
|
2,4-dihydroxyacetophenone
|
|
2,5-DHA
|
2,5-dihydroxyacetophenone
|
|
p-HA
|
p-hydroxyacetophenone
|
|
Cyn A
|
cynandione A
|
References
|
1
|
Willerson JT and Ridker PM: Inflammation
as a cardiovascular risk factor. Circulation. 109:2–10. 2004.
View Article : Google Scholar
|
|
2
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Packard RR and Libby P: Inflammation in
atherosclerosis: from vascular biology to biomarker discovery and
risk prediction. Clin Chem. 54:24–38. 2008. View Article : Google Scholar
|
|
4
|
Braun M, Pietsch P, Schrör K, Baumann G
and Felix SB: Cellular adhesion molecules on vascular smooth muscle
cells. Cardiovasc Res. 41:395–401. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Granger DN and Senchenkova E: Inflammation
and the Microcirculation. Morgan & Claypool Life Sciences; San
Rafael, CA: 2010
|
|
6
|
Meager A: Cytokine regulation of cellular
adhesion molecule expression in inflammation. Cytokine Growth
Factor Rev. 10:27–39. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradley JR: TNF-mediated inflammatory
disease. J Pathol. 214:149–160. 2008. View Article : Google Scholar
|
|
8
|
Popa C, Netea MG, van Riel PL, van der
Meer JW and Stalenhoef AF: The role of TNF-alpha in chronic
inflammatory conditions, intermediary metabolism, and
cardiovascular risk. J Lipid Res. 48:751–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Park Y, Wu J, Chen Xp, Lee S,
Yang J, Dellsperger KC and Zhang C: Role of TNF-alpha in vascular
dysfunction. Clin Sci (Lond). 116:219–230. 2009. View Article : Google Scholar
|
|
10
|
Hwang BY, Kim SE, Kim YH, Kim HS, Hong YS,
Ro JS, Lee KS and Lee JJ: Pregnane glycoside multidrug-resistance
modulators from Cynanchum wilfordii. J Nat Prod. 62:640–643. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HS: Effects of Cynanchum wilfordii
extract on serum lipid components and enzyme activities in
hyperlipidemic and streptozotocin-induced diabetic rats. Korean J
Hum Ecol. 7:1–11. 2004.
|
|
12
|
Shan L, Zhang WD, Zhang C, Liu RH, Su J
and Zhou Y: Antitumor activity of crude extract and fractions from
root tuber of Cynanchum auriculatum Royle ex Wight. Phytother Res.
19:259–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shan L, Liu RH, Shen YH, Zhang WD, Zhang
C, Wu DZ, Min L, Su J and Xu XK: Gastroprotective effect of a
traditional Chinese herbal drug ‘Baishouwu’ on experimental gastric
lesions in rats. J Ethnopharmacol. 107:389–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niu JZ, Ye BK and Wang DF: Observation of
the protection effect of Baishouwu to the liver of the high serum
cholesterol mouse. Ji Sheng Chong Yu Yi Xue Kun Chong Xue Bao.
3:266–268. 1998.
|
|
15
|
Choi DH, Lee YJ, Oh HC, Cui YL, Kim JS,
Kang DG and Lee HS: Improved endothelial dysfunction by Cynanchum
wilfordii in apolipoprotein E(-/-) mice fed a high
fat/cholesterol diet. J Med Food. 15:169–179. 2012. View Article : Google Scholar :
|
|
16
|
Yoon MY, Choi NH, Min BS, Choi GJ, Choi
YH, Jang KS, Han SS, Cha B and Kim JC: Potent in vivo antifungal
activity against powdery mildews of pregnane glycosides from the
roots of Cynanchum wilfordii. J Agric Food Chem. 59:12210–12216.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SB, Lee SM, Park JH, Lee TH, Baek NI,
Park HJ, Lee H and Kim J: Cynandione A from Cynanchum wilfordii
attenuates the production of inflammatory mediators in LPS-induced
BV-2 microglial cells via NF-κB inactivation. Biol Pharm Bull.
37:1390–1396. 2014. View Article : Google Scholar
|
|
18
|
Koo HJ, Sung YY and Kim HK: Inhibitory
effects of Akebia quinata ethanol extract on TNF-α-mediated
vascular inflammation in human aortic smooth muscle cells. Mol Med
Rep. 7:379–383. 2013.
|
|
19
|
Collins T, Read MA, Neish AS, Whitley MZ,
Thanos D and Maniatis T: Transcriptional regulation of muscle cell
adhesion molecules: NF-κB and cytokine inducible enhancers. FASEB
J. 9:899–909. 1995.PubMed/NCBI
|
|
20
|
Chen C, Chou C, Sun Y and Huang W: Tumor
necrosis factor α-induced activation of downstream NF-κB site of
the promoter mediates epithelial ICAM-1 expression and monocyte
adhesion: involvement of PKCα, tyrosin kinase, and IKK2, but not
MAPKs, pathway. Cell Signal. 13:543–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fotis L, Agrogiannis G, Vlachos IS,
Pantopoulou A, Margoni A, Kostaki M, Verikokos C, Tzivras D,
Mikhailidis DP and Perrea D: Intercellular adhesion molecule
(ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 at the early
stages of atherosclerosis in a rat model. In Vivo. 26:243–250.
2012.PubMed/NCBI
|
|
22
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moiseeva EP: Adhesion receptors of
vascular smooth muscle cells and their functions. Cardiovasc Res.
52:372–386. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Glass CK and Witztum JL: Atherosclerosis.
the road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross R: Atherosclerosis is an inflammatory
disease. Am Heart J. 138:419–420. 1999. View Article : Google Scholar
|
|
26
|
Springer TA: Traffic signals for
lymphocyte recirculation and leukocyte emigration: the multistep
paradigm. Cell. 76:301–314. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Hojo Y, Ikeda U, Takahashi M and
Shimada KJ: Interaction between monocytes and vascular smooth
muscle cells enhances matrix metalloproteinase-1 production.
Cardiovasc Pharmacol. 36:152–161. 2000. View Article : Google Scholar
|
|
28
|
Cai Q, Lanting L and Natarajan R:
Interaction of monocytes with vascular smooth muscle cells
regulates monocyte survival and differentiation through distinct
pathways. Arterioscler Thromb Vasc Biol. 24:2263–2270. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Libby P and Li H: Vascular cell adhesion
molecule-1 and smooth muscle cell activation during atherogenesis.
J Clin Invest. 92:538–539. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kasper HU, Schmidt A and Roessner A:
Expression of the adhesion molecules ICAM, VCAM, and ELAM in the
arteriosclerotic plaque. Gen Diagn Pathol. 141:289–294.
1996.PubMed/NCBI
|
|
31
|
Chemat F, Vian MA and Cravotto G: Green
extraction of natural products: concept and principles. Int J Mol
Sci. 13:8615–8627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi Q, Vandeberg JF, Jett C, Rice K,
Leland MM, Talley L, Kushwaha RS, Rainwater DL, Vandeberg JL and
Wang XL: Arterial endothelial dysfunction in baboons fed a
high-cholesterol, high-fat diet. Am J Clin Nutr. 82:751–759.
2005.
|
|
33
|
Li P, Sanz I, O’Keefe RJ and Schwarz EM:
NF-kappa B regulates VCAM-1 expression on fibroblast-like
synoviocytes. J Immunol. 164:5990–5997. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Waddick KG and Uckun FM: Innovative
treatment programs against cancer. II. Nuclear factor-kappa B
(NF-kB) as a molecular target. Biochem Pharmacol. 57:9–17. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baker DD, Chu M, Oza U and Rajgarhia V:
The value of natural products to future pharmaceutical discovery.
Nat Prod Rep. 24:1225–1244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Butler MS: Natural products to drugs:
natural product derived compounds in clinical trials. Nat Prod Rep.
22:162–195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katiyar C, Gupta A, Kanjilal S and Katiyar
S: Drug discovery from plant sources: An integrated approach. Ayu.
33:10–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ha do T, Trung TN, Hien TT, Dao TT, Yim N,
Ngoc TM, Oh WK and Bae K: Selected compounds derived from Moutan
Cortex stimulated glucose uptake and glycogen synthesis via AMPK
activation in human HepG2 cells. J Ethnopharmacol. 131:417–424.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Senejoux F, Girard-Thernier C, Berthelot
A, Bévalot F and Demougeot C: New insights into the mechanisms of
the vasorelaxant effects of apocynin in rat thoracic aorta. Fundam
Clin Pharmacol. 27:262–270. 2013. View Article : Google Scholar
|