Introduction
In Western countries, colorectal cancer (CRC) is the
second leading cause of cancer-related mortality (1). Recent advancements in the
therapeutic strategies for this disease have successfully saved the
lives of a number of patients with early-stage disease. However,
the mortality rate of patients with advanced stages of the disease
remains high. Thus, further investigations are urgently required to
elucidate the specific molecular mechanisms responsible for the
progression of CRC.
In the majority of tumor cells, constitutive
activation of nuclear factor-κB (NF-κB) is often observed (2). The abnormal activation of NF-κB
contributes to significant cell proliferation and migration in CRC
and in other types of cancer (3),
and the inhibition of NF-κB activity has been shown to
significantly reduce cancer cell growth and enhance cell apoptosis,
indicating that NF-κB members are potential therapeutic targets in
tumors (4). Mammalian NF-κB
members mainly include p65 (RelA), p105/p50, RelB, p100/p52 and
c-Rel (5), and two distinct
pathways are involved in the regulation of NF-κB activation: the
canonical and the non-canonical pathways. The canonical pathway of
NF-κB is controlled by the IκB kinase (IKK) complex, which is
comprised of IKKα, IKKβ and IKKγ. Through the phosphorylation of
inhibitors of κB (IκBs), the IKK complex usually sequesters NF-κB
members into the cytoplasm, thereby inhibiting the nuclear
translocation of these transcription factors. The non-canonical
pathway of NF-κB is regulated by IKKα and the NF-κB-inducing kinase
(NIK). NIK, also known as mitogen-activated protein kinase kinase
kinase 14 (MAP3K14), activates NF-κB upon the stimulation of tumor
necrosis factor (TNF), leading to the activation of the canonical
pathway (6). NIK plays a key role
in receptor-initiating signaling in the non-canonical (alternative)
NF-κB pathway. It has been suggested that the activation of NF-κB
by NIK significantly promotes epithelial cell proliferation, the
inflammatory response and oncogenic signaling (7). In normal cells, the protein level of
NIK is maintained at a normal level by proteasomal degradation;
however, aberrant NIK accumulation has been observed in some cancer
cells (8). However, studies on
NIK accumulation in CRC are limited.
MicroRNAs (miRNAs or miRS) are small non-coding RNAs
that are 18–25 nt in length. Through interactions with 3′
untranslated regions (3′ UTRs), miRNAs negatively regulate the
expression of a series of target genes. Over the years, a number of
studies have demonstrated the important role of miRNAs in cancer
pathology, indicating that miRNAs can act as either oncogenes or
tumor suppressors (9–11). In many types of tumors, the
downregulation of miRNAs and the upregulation of oncogenes have
been reported, as was demonstrated for cervical cancer (12). For instance, in high-grade serous
ovarian carcinomas, miR-106 has been reported to be significantly
upregulated and to enhance cell proliferation and differentiation
(13). Furthermore, miR-519 was
found to target HuR, thereby reducing cell proliferation and cell
cycle progression in various types of tumor (14). For instance, miR-150 and miR-630
induce pancreatic cancer cell apoptosis by targeting insulin-like
growth factor 1 (IGF-1R), while miR-21 functions as an
anti-apoptotic regulator by targeting pro-apoptotic genes, such as
Fas ligand (FasL), phosphatase and tensin homolog (PTEN) and
programmed cell death protein 4 (PDCD4) (15). Taken together, these data suggest
that miR-518a-3p regulates cell proliferation and cell cycle
progression and may be associated with the progression of human
cancer.
In this study, we identified a novel miRNA,
miR-518a-3p, which regulates the protein level of NIK. Furthermore,
our results indicate that the downregulation of miR-518a-3p
abnormally activates NF-κB signaling in CRC, thereby defining the
significance of miR-518a-3p in the progression of CRC.
Materials and methods
Statement
All the subjects who participated in this study
provided written informed consent, and this study was approved by
the Ethics Committee of Jilin University (Changchun, China).
Human tissue specimens and cell
lines
CRC tissues with distant metastases (n=42) and
without distant metastases (n=40) were obtained from 82 patients
with CRC who underwent an initial surgery at the First Hospital of
Jilin University between June 2009 and January 2011. All the
patients had a histological diagnosis of CRC. Following resection,
the specimens were snap-frozen in liquid nitrogen and stored at
−80°C until RNA extraction.
The human HT-29, HCT116, SW480, SW620 and LoVo CRC
cell lines, the 293T embryonic kidney cell line, and the NCM460
normal colonic epithelial cell line were purchased from the
American Type Culture Collection (ATCC, Manassas, VA, USA). The
cells were cultured in RPMI-1640 medium containing 10% fetal bovine
serum in a humidified 37°C incubator supplemented with 5%
CO2.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the tissues and cells
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). RNA quality and concentration were determined using the
NanoDrop 2000 system (Thermo Fisher Scientific, Inc., Wilmington,
MA, USA). To quantify miR-518a-3p expression, a TaqMan MicroRNA
Assay kit (Applied Biosystems, Foster City, CA, USA) was used, and
U6 snRNA was used as a reference. To quantify the NIK mRNA level, a
SYBR Premix Ex Taq™ kit [Takara Biotechnology (Dalian) Co., Ltd.,
Liaoning, Japan] was used, and β-actin expression was used as an
endogenous control. Quantitative PCR was performed using the
Applied Biosystems 7900 Fast Real-Time PCR system (Applied
Biosystems). The data were analyzed using the 2−ΔΔct
method.
Western blot analysis
Western blot analysis was performed as previously
described (16). Briefly, total
cellular protein was isolated, and the protein concentration was
determined using the Bradford DC protein assay (Bio-Rad, Hercules,
CA, USA). Forty micrograms of protein were separated by SDS-PAGE
and transferred onto polyvinylidene fluoride (PVDF) membranes. The
membranes were then incubated with the following primary
antibodies: NIK (#4994, 1:1,000), X-linked inhibitor of apoptosis
(XIAP; #14334, 1:1,000), FLICE-like inhibitory protein (FLIP;
#8510, 1:1,000), B-cell lymphoma-extra large (Bcl-xL; #2764,
1:1,000) (all from Cell Signaling Technology, Inc., Boston, MA,
USA) and GAPDH (1:2,000; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), and the proteins were visualized by the ECL
procedure (Amersham Biosciences Corp., Piscataway, NJ, USA).
Oligonucleotide transfection
The hsa-miR-518a-3p mimic, negative control (NC)
oligonucleotides, has-miR-518a-3p inhibitor and the scramble
oligonucleotides were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The cells were plated in a 6-well plate the day
prior to transfection. Using Lipofectamine 2000 (Invitrogen Life
Technologies), the LoVo cells were transfected with the
hsa-miR-518a-3p mimic or NC (50 nmol/l), and the LoVo cells were
infected with the has-miR-518a-3p inhibitor or scramble
oligonucleotides (100 nmol/l). Twenty-four hours later, the cells
were collected, and in vitro assays were performed.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
To examine the effect of miR-518a-3p on cell
viability, 5,000 cells/well in 100 μl of medium were seeded
in 96-well plates and transfected with miR-518a-3p mimics (50 nM)
or negative control (50 nM), as described above. At 24 h after
transfection, 20 μl of MTT reagent (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) was added to the wells
and then incubated with the cells for 4 h. After removing the
medium, the blue formazan was dissolved in 200 μl of
dimethyl sulfoxide (DMSO) (Beijing Solarbio, Scienc e &
Technology Co., Ltd.), and the absorbance was measured at 550 nm.
Wells containing only LoVo cells served as the blanks.
Immunofluorescence
The LoVo cells were cultured on 6-well chamber
slides and fixed with 4% paraformaldehyde for 10 min at −20°C. The
slides were washed in PBS 3 times, and incubated with a polyclonal
antibody against NIK (1:50 diluted in PBS with 1% BSA, 50
μl/slide) for 2 h at room temperature. After washing with
PBS 3 times (5 min/time), the slides were incubated with
TRITC-conjugated anti-mouse IgG (1:100 diluted in PBS with 1% BSA,
50 μl/slide) for 1 h at room temperature. After washing the
slides in PBS 3 times, the slides were incubated with Hoechst 33258
(10 μg/ml) for 5 min. The slides were then washed again and
examined under a fluorescence microscope (Axio Observer; Zeiss,
Göttingen, Germany).
Hoechst 33258 staining
The LoVo cells were cultured in 6-well plates. After
48 h of transfection with miR-518a-3p mimics, inhibitors or
negative control, the cells were washed with PBS and then stained
with Hoechst 33258 (10 μg/ml) (Beijing Solarbio Science
& Technology Co., Ltd.) for 5 min before being washed 3 times
with PBS.
Quantification of apoptotic cells
To quantify the apoptotic cells, flow cytometry was
performed using an Annexin V-fluorescein-5-isothiocyanate Apoptosis
Detection kit (BioVision, Inc., Milpitas, CA, USA). Forty-eight
hours after transfection with miR-518a-3p mimics (50 nM) or
negative control (50 nM), the LoVo cells were placed in a 5-ml
tube. The cells were then washed with cold PBS and resuspended at a
final concentration of 1×106 cells/ml. FITC-Annexin V (5
μl) and propidium iodide were gently mixed and incubated
with the cells for 15 min at room temperature. Within 1 h after
incubation, the samples were analyzed by flow cytometry.
Vector construction and dual-luciferase
reporter assays
Before the luciferase assays were performed, the
potential miR-518a-3p binding site in the NIK 3′ UTR was predicted
using TargetScan (www.targetscan.org) and miRanda (www.microRNA.org). The 3′ UTR of the NIK mRNA and a
mutant NIK mRNA were then synthesized and cloned into the
XbaI site of a pGL3 basic vector (Promega Corp., Madison,
WI, USA) downstream of the luciferase stop codon, and these
plasmids were designated as pGL3-wt-NIK and pGL3-mt-NIK,
respectively. Subsequently, the 293T cells (1×105
cells/well) were cultured in 24-well plates and co-transfected with
the pGL3-control (0.4 mg), pGL3-wt-NIK (0.4 mg) or the pGL3-mt-NIK
(0.4 mg) plasmid, as well as the pRL-TK luciferase reporters (25
ng/well) and pcDNA-miR-518a-3p (20 nmol/l) or pcDNA-miR-NC (20
nmol/l) using Lipofectamine 2000 (Invitrogen Life Technologies).
Forty-eight hours later, the cells were harvested, and the
luciferase activity was measured using a Dual-Luciferase Reporter
Assay kit (Promega Corp.).
Inhibition of NIK by RNA
interference
NIK-specific shRNA (shNIK) and negative control were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China)
Cells (1×105/well) in a 6-well plate were transfected
with 50 nM shNIK or negative control for 48 h using HiPerFect
Transfection Reagent (Qiagen GmbH, Düsseldorf, Germany) as
described above.
Statistical analysis
The data are expressed as the means ± SD, and
statistical significance was analyzed using the Student’s t-test
(two-tailed). All statistical analyses were performed using SPSS
13.0 software or the GraphPad Prism 5.0 software package. The
Kaplan-Meier method and the log-rank test were performed to analyze
the prognostic significance. A value of P<0.05 was considered to
indicate a statistically significant difference in all tests.
Results
miR-518a-3p is significantly
downregulated in CRC cell lines and CRC tissues with
metastases
First, we analyzed the expression of miR-518a-3p in
5 CRC cell lines and in the normal colonic cell line, NCM460.
miR-518a-3p was significantly downregulated in the CRC cell lines
compared with the NCM460 cells (all P<0.05) (Fig. 1A). Among the 5 CRC cell lines, the
LoVo cells showed the lowest miR-518a-3p expression level, and the
HCT116 cells exhibited the highest miR-518a-3p level (Fig. 1A). To examine the expression of
miR-518a-3p in CRC tissues, the miR-518a-3p level was measured in
CRC tissues with metastases (n=42) and normal tissues without
metastases (n=40). Of note, miR-518a-3p expression was markedly
lower in the CRC tissues than in the normal tissues (P<0.001;
Fig. 1B).
NIK is a direct target of
miR-518a-3p
To determine the functional significance of the
downregulation of miR-518a-3p, we attempted to identify the target
genes of miR-518a-3p using computational algorithms. A
computational search predicted one binding site for miR-518a-3p in
the NIK 3′ UTR (Fig. 2A). To
experimentally identify the target genes of miR-518a-3p, we
performed reporter-based screens as described below. Luciferase-3'
UTR reporter assays demonstrated a marked negative effect against
upstream gene expression by the NIK 3′ UTR sequence. As shown in
Fig. 2B, treatment with a
miR-518a-3p inhibitor increased NIK 3′ UTR reporter activity,
suggesting the involvement of endogenous miR-518a-3p in NIK
down-regulation. To identify the regulatory sequence in the 3' UTR
of NIK, we constructed additional reporters with mutated sequences
in the seed region (Fig. 2C). The
reporter containing the mutated miR-518a-3p seed sequence prevented
the effects of anti-miR-518a-3p treatment (Fig. 2D), and miR-518a-3p inhibition
inversely rescued the NIK level, which revealed that the cellular
miR-518a-3p level negatively affected that of the NIK protein
through its 3' UTR sequence. These lines of evidence collectively
demonstrate that miR-518a-3p recognizes and regulates NIK mRNA
through specific binding to its 3′ UTR.
miR-518a-3p negatively regulates NF-κB
signaling through NIK expression
The transient introduction of the miR-518a-3p
precursor into the LoVo cells resulted in the downregulation of NIK
at the protein level and was associated with the downregulation of
the phosphorylated IκBα level and NF-κB activity (Fig. 3A and B). By contrast, the
inhibition of miR-518a-3p resulted in the accumulation of NIK
protein expression in the LoVo cells (Fig. 3C and D). The manipulation of the
miR-518a-3p level clearly indicated that the miR-518a-3p level
negatively correlated with cellular NF-κB activity. These results
collectively demonstrate that miR-518a-3p inhibits the basal and
receptor-initiated activities of the non-canonical NF-κB pathway
and that miR-518a-3p plays a critical role in the negative
regulation of the NF-κB pathway by manipulating the expression of
NIK.
miR-518a-3p suppresses LoVo cell growth
by inhibiting NF-κB
Although it has been documented that the abnormal
accumulation of NIK in cells acts as a constitutive activator of
the NF-κB pathway (17), the
mechanisms underlying the overproduction of NIK remain to be
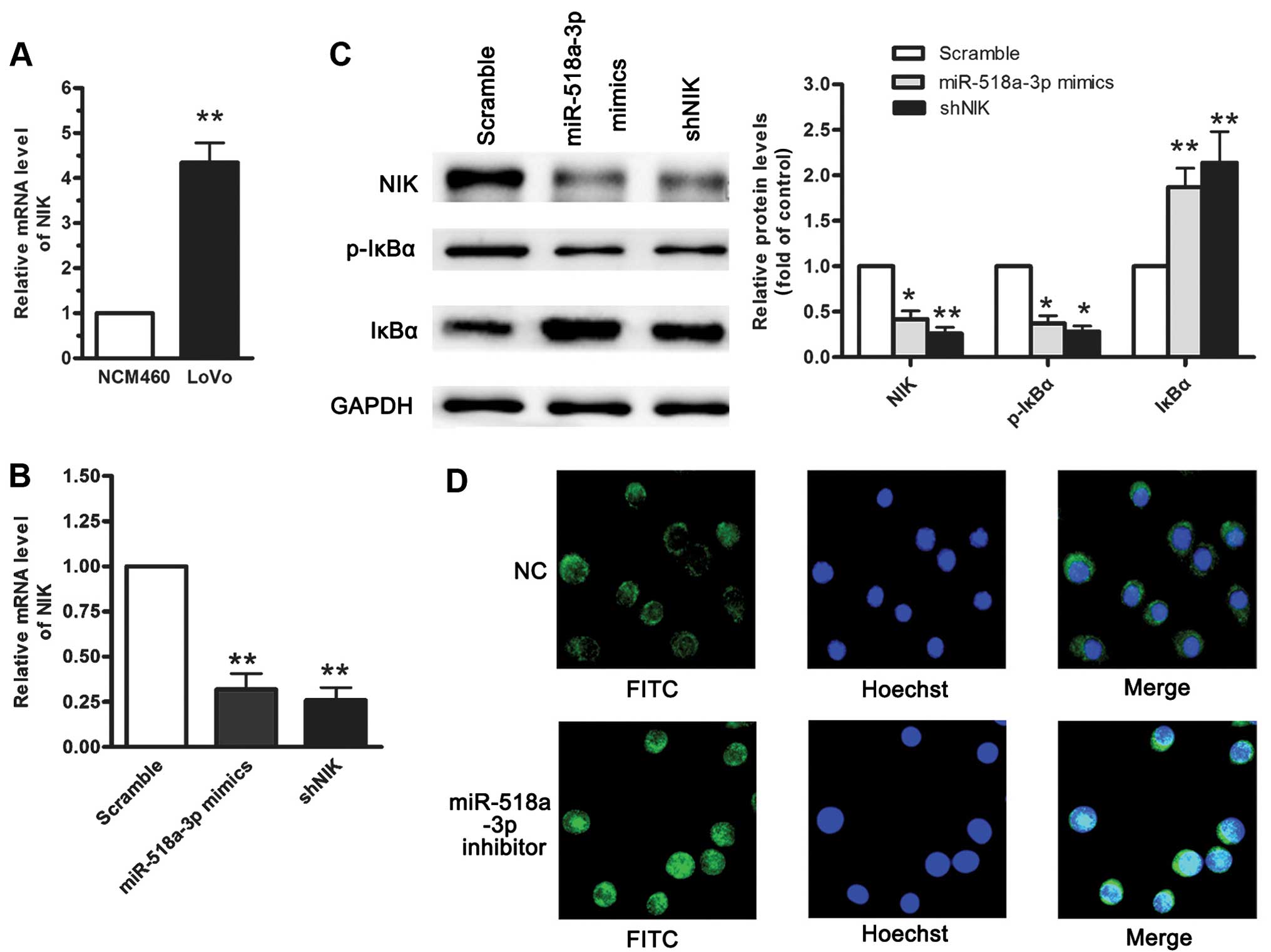
elucidated. RT-qPCR revealed that the abnormal accumulation of NIK
in the LoVo cells acts as a constitutive activator of the NF-κB
pathway when compared with the NCM460 cells (Fig. 4A). To investigate the functional
roles of NIK and miR-518a-3p, we established NCM460 cells stably
expressing miR-518a-3p or NIK-specific shRNA (shNIK) using
retroviral vectors. RT-qPCR and western blot analysis revealed that
the enforced expression of miR-518a-3p or transfection with shNIK
reduced the mRNA and protein levels of NIK, as well as the levels
of p-IκBα, but not those of IκBα (Fig. 4B and C). Furthermore, the enhanced
translocation of nuclear RelA increased the activity of the
canonical and non-canonical NF-κB pathways (Fig. 4D). The re-expression of NIK led to
the activation of NF-κB that was inhibited by miR-518a-3p,
suggesting a reciprocal association between the level of
miR-518a-3p and that of NIK.
miR-518a-3p promotes apoptosis by
inhibiting NF-κB
We hypothesized that the miR-518a-3p-mediated NF-κB
modulation may affect cellular apoptosis as numerous studies have
demonstrated that NF-κB activation is a strong anti-apoptotic
factor in CRC cells (6,7). We found that the suppression of NIK
by miR-518a-3p or shNIK resulted in the downregulation of a subset
of genes involved in resistance to apoptosis, including Bcl-xL,
XIAP and FLIP (Fig. 5A), which
suggests that miR-518a-3p plays a role in promoting apoptosis
through the inhibition of NF-κB activity. To assess the biological
function of miR-518a-3p in apoptotic signals, we transfected
miR-518a-3p mimics into the LoVo cells, and found that
trans-fection with miR-518a-3p mimics promoted the apoptosis of the
LoVo cells (Fig. 5B). In
addition, miR-518a-3p overexpression led to the activation of
caspase-3 (Fig. 5C).
Collectively, these findings indicate that miR-518a-3p mediates
apoptosis through the suppression of NIK in colonic cell lines. To
demonstrate the role of miR-518a-3p in cancer cell survival, we
examined whether the transfection of miR-518a-3p mimics resulted in
a killing effect against the cancer cells, and the number of
apoptotic cells was determined by Hoechst staining. The results
revealed that the expression of miR-518a-3p facilitated tumor cell
death (Fig. 5D). Since the
suppression of NIK by shRNA also had a strong killing effect, NIK
and NF-κB may thus be crucial players in the survival of CRC cells.
Taken together, these lines of experimental evidence definitively
support two notions: i) miR-518a-3p functions as a tumor suppressor
in CRC cells; and ii) NIK-regulated NF-κB is important for CRC cell
survival.
Discussion
In various types of tumor, including Hodgkin's
lymphoma, breast cancer, prostate cancer and CRC, the constitutive
activation of NF-κB significantly promotes abnormal cancer cell
proliferation and inhibits cell death (18-20). Furthermore, NF-κB plays key roles
in different cellular functions, such as inflammation, innate
immunity and lymphocytic development (21). A deeper understanding of NF-κB
signaling may thus lead to progress being made in the determination
of the molecular pathology of various types of cancer, such as
CRC.
Increasing evidence has suggested the important role
of miRNAs in cancer progression (22,23). In CRC, some miRNAs have been
suggested to widely participate in the pathology of tumorigenesis
and metastasis. For instance, miR-21, miR-31 and miR-192 have been
shown to increase the resistance of CRC cells to 5-fluorouracil
(5-FU) (24–26), and a polymorphism in pre-miR-27a
has been shown to significantly correlate with the risk of
developing CRC (27).
Furthermore, miR-182 is increased in colorectal carcinoma,
suggesting that it is a potential prognostic factor for CRC
(28–30). However, the role of miR-518a-3p in
CRC remaines to be elucidated. In this study, we mainly focused on
the functional significance of miR-518a-3p in abnormal CRC cell
proliferation. Our results revealed a profound downregulation of
miR-518a-3p in all CRC cases, suggesting that the loss of
miR-518a-3p is a prerequisite for the development of CRC. These
results indicate that the downregulation of miR-518a-3p may be a
common occurrence in CRC and that miR-518a-3p may be used as a
biomarker to predict clinical outcome and metastasis in patients
with CRC.
Using miRBase, we first identified NIK as a possible
target gene of miR-518a-3p. In the present study, we identified NIK
as a target of miR-518a-3p. First, luciferase-3' UTR reporter
assays revealed that the NIK 3' UTR sequence plays a role in its
negative regulation. By combining a specific inhibitor and
mutations in the miR-518a-3p-binding site, we demonstrated that
miR-518a-3p recognizes and negatively regulates the 3' UTR of NIK
(Fig. 2A). Second, by introducing
an miR-518a-3p precursor or inhibitor, we demonstrated that the
amount of miR-518a-3p inversely correlates with the levels of
expression and downstream signaling of NIK. Collectively, we
provide definitive evidence demonstrating that miR-518a-3p
negatively regulates NIK expression and activity. It is well known
that the NIK level directly activates NF-κB signaling in various
cell types (7), and we
experimentally demonstrated that the negative role of miR-518a-3p
in cytokine-induced NIK accumulation is widely important in the
non-canonical regulation of NF-κB in CRC cell types.
Furthermore, the overexpression of miR-518a-3p and
inhibition by shNIK significantly inhibited CRC cell proliferation
and enhanced CRC cell apoptosis. Moreover, the restoration of
miR-518a-3p suppressed NF-κB activity in the CRC cells and led to
the impairment of the proliferative capacity and enhanced the
apoptosis of the cells. Our results indicate that the suppression
of NF-κB enhances CRC cell death, which is in line with previous
observations (18). Since it is
highly possible that miR-518a-3p and relevant factors are vital for
NF-κB signaling, their aberrant expression would be of great
importance to the abnormal signaling and clinical outcomes of
CRC.
In the present study, we found that the decreased
expression of miR-518a-3p and the elevated expression of NIK lead
to the activation of NF-κB in CRC cells. We also demonstrated that
the restoration of miR-518a-3p partially impaired the NF-κB-induced
aberrant CRC cell proliferation. Furthermore, given that NF-κB is a
pivotal transcriptional regulator in normal and oncogenic
functions, the understanding of the role of epigenetic regulators
and miR-518a-3p in NF-κB signaling may enhance our understanding of
the molecular mechanisms of CRC cell function. These findings
suggest that an aberrant gene expression pattern correlates with
the malignant phenotype, which provides important clues as to the
clinical manifestations and may help identify therapeutic targets
in CRC cells.
In conclusion, we demonstrate that the
downregulation of miR-518a-3p is responsible for oncogenic NF-κB
activation and for the malignant phenotypes of CRC. Moreover, our
results provide evidence indicating that miR-518a-3p is an
important tumor suppressor.
Acknowledgments
This study was funded by the Science and Technology
Program. We appreciate the work from all researchers in this
article. We also acknowledge additional support from the Division
of Clinical Epidemiology, First Hospital of Jilin University Dr
Jiang's laboratory.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hironaka N, Mochida K, Mori N, Maeda M,
Yamamoto N and Yamaoka S: Tax-independent constitutive IkappaB
kinase activation in adult T-cell leukemia cells. Neoplasia.
6:266–278. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prasad S, Ravindran J and Aggarwal BB:
NF-kappaB and cancer: How intimate is this relationship. Mol Cell
Biochem. 336:25–37. 2010. View Article : Google Scholar
|
|
4
|
Claudius AK, Kankipati CS, Kilari RS,
Hassan S, Guest K, Russell ST, Perry CJ, Stark LA and Nicholl ID:
Identification of aspirin analogues that repress NF-κB signalling
and demonstrate anti-proliferative activity towards colorectal
cancer in vitro and in vivo. Oncol Rep. 32:1670–1680.
2014.PubMed/NCBI
|
|
5
|
Yang H, Qi H, Ren J, Cui J, Li Z, Waldum
HL and Cui G: Involvement of NF-κB/IL-6 pathway in the processing
of colorectal carcinogenesis in colitis mice. Int J Inflam.
2014:1309812014. View Article : Google Scholar
|
|
6
|
Thu YM, Su Y, Yang J, Splittgerber R, Na
S, Boyd A, Mosse C, Simons C and Richmond A: NF-κB inducing kinase
(NIK) modulates melanoma tumorigenesis by regulating expression of
pro-survival factors through the β-catenin pathway. Oncogene.
31:2580–2592. 2012. View Article : Google Scholar :
|
|
7
|
Thu YM and Richmond A: NF-κB inducing
kinase: A key regulator in the immune system and in cancer.
Cytokine Growth Factor Rev. 21:213–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Annunziata CM, Davis RE, Demchenko Y,
Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W,
et al: Frequent engagement of the classical and alternative
NF-kappaB pathways by diverse genetic abnormalities in multiple
myeloma. Cancer Cell. 12:115–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao JL and Starczynowski DT: Role of
microRNA-146a in normal and malignant hematopoietic stem cell
function. Front Genet. 5:2192014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bandres E, Bitarte N, Arias F, Agorreta J,
Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ,
et al: microRNA-451 regulates macrophage migration inhibitory
factor production and proliferation of gastrointestinal cancer
cells. Clin Cancer Res. 15:2281–2290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Guo X, Zhang H, Xiang Y, Chen J,
Yin Y, Cai X, Wang K, Wang G, Ba Y, et al: Role of miR-143
targeting KRAS in colorectal tumorigenesis. Oncogene. 28:1385–1392.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Wu H, Li P, Zhao Y, Liu M and
Tang H: NF-κB-modulated miR-130a targets TNF-α in cervical cancer
cells. J Transl Med. 12:1552014. View Article : Google Scholar
|
|
13
|
Liu Z, Gersbach E, Zhang X, Xu X, Dong R,
Lee P, Liu J, Kong B, Shao C and Wei JJ: miR-106a represses the Rb
tumor suppressor p130 to regulate cellular proliferation and
differentiation in high-grade serous ovarian carcinoma. Mol Cancer
Res. 11:1314–1325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abdelmohsen K, Srikantan S, Kuwano Y and
Gorospe M: miR-519 reduces cell proliferation by lowering
RNA-binding protein HuR levels. Proc Natl Acad Sci USA.
105:20297–20302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farhana L, Dawson MI, Murshed F, Das JK,
Rishi AK and Fontana JA: Upregulation of miR-150* and
miR-630 induces apoptosis in pancreatic cancer cells by targeting
IGF-1R. PLoS One. 8:e610152013. View Article : Google Scholar
|
|
16
|
Guo J, Li M, Meng X, Sui J, Dou L, Tang W,
Huang X, Man Y, Wang S and Li J: MiR-291b-3p induces apoptosis in
liver cell line NCTC1469 by reducing the level of RNA-binding
protein HuR. Cell Physiol Biochem. 33:810–822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Odqvist L, Sánchez-Beato M, Montes-Moreno
S, et al: NIK controls classical and alternative NF-κB activation
and is necessary for the survival of human T-cell lymphoma cells.
Clin Cancer Res. 19:2319–2330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Wang X, Liu M, Qi X and Li J: NF-κB
signaling inhibition and anticancer activities of LLDT-246 on human
colorectal cancer HCT-116 cells in vitro. Biomed Pharmacother.
68:527–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hajder J, Marisavljević D, Stanisavljević
N, Mihaljević B, Kovcin V, Marković O and Zivković R: BCL10
aberations and NF-kappa B activation involving p65 are absent or
rare in primary gastric MALT lymphoma. Vojnosanit Pregl.
71:1040–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Celegato M, Borghese C, Casagrande N,
Carbone A, Colombatti A and Aldinucci D: Bortezomib down-modulates
the survival factor interferon regulatory factor 4 in Hodgkin
lymphoma cell lines and decreases the protective activity of
Hodgkin lymphoma-associated fibroblasts. Leuk Lymphoma. 55:149–159.
2014. View Article : Google Scholar
|
|
21
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kupcinskas J, Bruzaite I, Juzenas S,
Gyvyte U, Jonaitis L, Kiudelis G, Skieceviciene J, Leja M, Pauzas
H, Tamelis A, et al: Lack of association between miR-27a, miR-146a,
miR-196a-2, miR-492 and miR-608 gene polymorphisms and colorectal
cancer. Sci Rep. 4:59932014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodríguez-Montes JA and Menéndez Sánchez
P: Role of micro-RNA in colorectal cancer screening. Cir Esp.
92:654–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valeri N, Gasparini P, Braconi C, Paone A,
Lovat F, Fabbri M, Sumani KM, Alder H, Amadori D, Patel T, et al:
MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating
human DNA MutS homolog 2 (hMSH2). Proc Natl Acad Sci USA.
107:21098–21103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang CJ, Stratmann J, Zhou ZG and Sun XF:
Suppression of microRNA-31 increases sensitivity to 5-FU at an
early stage, and affects cell migration and invasion in HCT-116
colon cancer cells. BMC Cancer. 10:6162010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boni V, Bitarte N, Cristobal I, Zarate R,
Rodriguez J, Maiello E, Garcia-Foncillas J and Bandres E:
miR-192/miR-215 influence 5-fluorouracil resistance through cell
cycle-mediated mechanisms complementary to its post-transcriptional
thymidilate synthase regulation. Mol Cancer Ther. 9:2265–2275.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Sun X, Wang Y, Liu X, Xuan Y and
Hu S: Association between miR-27a genetic variants and
susceptibility to colorectal cancer. Diagn Pathol. 9:1462014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Yang MH, Wang XY, Lin J and Ding
YQ: Increased expression of miRNA-182 in colorectal carcinoma: An
independent and tissue-specific prognostic factor. Int J Clin Exp
Pathol. 7:3498–3503. 2014.PubMed/NCBI
|
|
29
|
Huh JH, Kim TH, Kim K, Song JA, Jung YJ,
Jeong JY, Lee MJ, Kim YK, Lee DH and An HJ: Dysregulation of
miR-106a and miR-591 confers paclitaxel resistance to ovarian
cancer. Br J Cancer. 109:452–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plummer SM, Holloway KA, Manson MM, Munks
RJ, Kaptein A, Farrow S and Howells L: Inhibition of
cyclo-oxygenase 2 expression in colon cells by the chemopreventive
agent curcumin involves inhibition of NF-kappaB activation via the
NIK/IKK signalling complex. Oncogene. 18:6013–6020. 1999.
View Article : Google Scholar : PubMed/NCBI
|