Introduction
Hypertension is a complex multifactorial disorder
that is thought to result from an interaction between an
individual’s genetic background and various lifestyle and
environmental factors (1). The
genetic influence on blood pressure (BP) variability has been
estimated at 30–60% for a given individual (2), and the genetic heritability of
hypertension estimated at 30% (3). Given that hypertension is a major
risk factor for coronary artery disease, ischemic and hemorrhagic
stroke, as well as chronic kidney disease (4–6),
the personalized prevention of hypertension is an important public
health goal.
Genome-wide association studies have identified
various loci and genes associated with BP or to a predisposition to
hypertension in Caucasian populations (7–11)
or African Americans (12).
Although the genes for adducin 2 (13) and ATPase, Ca2+
transporting, plasma membrane 1 (14) have been shown to be susceptibility
loci for hypertension in Japanese individuals, the genes that
confer susceptibility to this condition in Japanese individuals
remain to be identified definitively.
We have previously identified 9 genes and
chromosomal region 3q28 as susceptibility loci for myocardial
infarction, ischemic stroke, or chronic kidney disease in Japanese
individuals by genome-wide (15–17) or candidate gene (18–20) association studies. Given that
hypertension is an important risk factor for these conditions
(4–6), we hypothesized that certain single
nucleotide polymorphisms (SNPs) at these 10 loci may contribute to
their genetic susceptibility by affecting the susceptibility to
hypertension. Therefore, the purpose of the present study was to
examine the possible association of 13 SNPs at these 10 loci with
the prevalence of essential hypertension or their association with
BP in community-dwelling Japanese individuals.
Materials and methods
Study population
Study subjects comprised 6,027 community-dwelling
individuals (2,250 subjects with essential hypertension and 3,777
controls) who were recruited to a population-based cohort study in
Inabe City (Inabe Health and Longevity Study), Mie Prefecture,
Japan. The Inabe Health and Longevity Study is a longitudinal
genetic epidemiological study of atherosclerotic, cardiovascular
and metabolic diseases (21–26). The subjects were recruited from
individuals who visited the Health Care Center of Inabe General
Hospital for an annual health checkup, and they are followed up
each year. A total of 6,027 individuals was registered between
March 2010 and September 2012, and genomic DNA was extracted from
the venous blood cells of these subjects and stored in the genomic
DNA bank of the Research Center for Genomic Medicine at Mie
University. For all the participants, medical examination data
obtained from April 2003 to March 2014 (11 years) were entered into
a database. If individuals had a medical checkup 2 or more times
per year, data from one time point for each year were entered, so
that each subject had one set of health data for each year they had
attended the clinic. All participants thus had undergone 1–11
medical examinations, and the average follow-up period was 5
years.
Subjects with hypertension either had a systolic BP
of ≥140 mmHg or a diastolic BP of ≥90 mmHg (or both) or had taken
anti-hypertensive medication. The control individuals had a
systolic BP of <140 mmHg and a diastolic BP of <90 mmHg, as
well as no history of hypertension or of taking any
anti-hypertensive medication. BP was measured at least twice with
the subjects having rested in the sitting position for >5 min;
the measurements were taken by a skilled physician or nurse
according to the guidelines of the American Heart Association
(27). The study protocol
complied with the Declaration of Helsinki and was approved by the
Committees on the Ethics of Human Research of Mie University
Graduate School of Medicine and Inabe General Hospital. Written
informed consent was obtained from all subjects prior to enrollment
in the study.
Selection and genotyping of
polymorphisms
The 13 SNPs examined in the present study (Table I) were selected from our previous
genome-wide (15–17) or candidate gene (18–20) association studies. Wild-type
(ancestral) and variant alleles of the SNPs were determined from
the dbSNP database (National Center for Biotechnology Information,
Bethesda, MD, USA) (http://www.ncbi.nlm.nih.gov/SNP).
 | Table IThe 13 SNPs examined in the present
study. |
Table I
The 13 SNPs examined in the present
study.
| Chromosomal
locus | Gene | dbSNP (NCBI) | Nucleotide
substitution | Minor
allelea |
|---|
| 1q24.1 | FAM78B | rs2116519 | C→T | C |
| 3q28 | Non-gene
region | rs9846911 | A→G | G |
| 4q25 | ALPK1 | rs2074379 | G→A
(Met732Ile) | G |
| 4q25 | ALPK1 | rs2074380 | G→A
(Gly870Ser) | A |
| 4q25 | ALPK1 | rs2074381 | A→G
(Asn916Asp) | G |
| 4q25 | ALPK1 | rs2074388 | G→A
(Gly565Asp) | G |
| 6p22.1 | BTN2A1 | rs6929846 | T→C | T |
| 6q27 | THBS2 | rs8089 | T→G | G |
| 13q12.1 | PDX1 | rs146021107 | G→- (deletion) | – |
| 13q34 | F7 | rs6046 | G→A
(Arg353Gln) | A |
| 17q25.1 | LLGL2 | rs1671021 | G→A
(Leu479Phe) | G |
| 19p13.2 | ILF3 | rs2569512 | G→A | A |
| 22q13.3 | CELSR1 | rs6007897 | C→T
(Ala2268Thr) | C |
Venous blood (5 ml) was collected into tubes
containing 50 mmol/l ethylenediaminetetraacetic acid (disodium
salt), and peripheral blood leukocytes were isolated and genomic
DNA was extracted from these cells with the use of a DNA extraction
kit (SMITEST EX-R&D; Medical and Biological Laboratories,
Nagoya, Japan). The genotypes of the 13 SNPs were determined at
G&G Science Co., Ltd. (Fukushima, Japan) by a method that
combines the polymerase chain reaction and sequence-specific
oligonucleotide probes with suspension array technology (Luminex,
Austin, TX, USA). The primers, probes and other conditions for the
genotyping of the SNPs examined in the present study are shown in
Table II. Detailed genotyping
methodology was as described previously (15,16,28).
 | Table IIPrimers, probes and other conditions
for the genotyping of the 13 SNPs examined in the present
study. |
Table II
Primers, probes and other conditions
for the genotyping of the 13 SNPs examined in the present
study.
| Gene or locus | SNP | dbSNP | Sense primer
(5′→3′) | Antisense primer
(5′→3′) | Probe 1
(5′→3′) | Probe 2
(5′→3′) | Annealing | (°C) Cycles |
|---|
| FAM78B | C→T | rs2116519 |
CCTGCACTGCTCTAGCTACTTC |
GATCCCAATTTCAACTGTGAGATC |
TCATTCCGGTCTCAGCCGCT |
CCCTCATTCCGGTTTCAGCC | 60 | 50 |
| 3q28 | A→G | rs9846911 |
AGTTGTGTGCCAGATTCTCCAG |
TCTTCACTGAGACCTTGGGAAG |
TCTCCTCTTTCAATAACAAATCTTC |
AAAGTCTCCTCTTTCAGTAACAAAT | 60 | 50 |
| ALPK1 | G→A | rs2074379 |
TCTGCTTCTTGGTCTTCTGATTC |
AGTTGGTTTCTGGAAACTCAACAA |
GAAGGATGTGTGCCTATATTCTT |
GATGTGTGCCCATATTCTTGGG | 60 | 50 |
| ALPK1 | G→A | rs2074380 |
CTCCACAGTGGATGAGGAGG |
CTTACAGAGGAATTGGGGGTC |
ACAAATGGGCACAGCTCTCATA |
TATGAGAGCCGTGCCCATTTGT | 60 | 50 |
| ALPK1 | A→G | rs2074381 |
AGGACTGCACTACCACAGAGG |
TGATTTCAGCCACCACACTGAG |
ATCAGCCTGGAAACATGCTAAAC |
AGTTTAGCATGTCTCCAGGCTG | 60 | 50 |
| ALPK1 | G→A | rs2074388 |
TGTGGAGACTGAGACTGAGCC |
TTGCTCCAAGCACTGGAAGTC |
ACTACAGCAATGATGAGGGAGC |
GCTCCCTCACCATTGCTGTAG | 60 | 50 |
| BTN2A1 | T→C | rs6929846 |
CCAAACATGGCGACCTAGGAGA |
ATCTGCCCAGGGGCACAGGC |
TTTGGGAAGGTTTGCGTCTAG |
TTTGGGAAGGTTTGTGTCTAGT | 60 | 50 |
| THBS2 | T→G | rs8089 |
AACCCAAGTGCCTTCAGAGGAT |
CTCCACATAAAGTCTCATATATCAC |
GATGTTCATCTCTGAGTTCCA |
GATGTTCATCTCTGCGTTCCA | 60 | 50 |
| PDX1 | G→- | rs146021107 |
TGGCTGTGGGTTCCCTCTGAG |
GATTTGGCACTGTGTGGCGTTC |
CGAGCAGGGGTGGCGCC |
GGCGCCACCCTGCTCGCT | 60 | 50 |
| F7 | G→A | rs6046 |
CGGCTACTCGGATGGCAGCA |
CCAAAGTGGCCCACGGTTGC |
TACCACGTGCCCCGGTAGTG |
GCCACCCACTACCAGGGCA | 60 | 50 |
| LLGL2 | G→A | rs1671021 |
GCTCCTGGCCTCACCTTGCG |
GCTGCTCTACAAACTCAGCACTG |
CTGGGCACTGAAGTTCTCGTT |
CCAACGAGAACCTCAGTGCC | 60 | 50 |
| ILF3 | G→A | rs2569512 |
ACCACCTCAACTGCAAGCTGAA |
GGAATGATCCCTCTGGGAAGGT |
GTGCAACTGCCAAAAACTGGT |
GTGCAACTGCCAAGAACTGG | 60 | 50 |
| CELSR1 | C→T | rs6007897 |
GGAGACGGAGGACTCCAGCTC |
CTTGCTGTCGACATCTTTGACAAG |
TCTTCATGGATGGCGTCGAAT |
TCTTCATGGATGGTGTCGAATC | 60 | 50 |
Statistical analysis
Quantitative data were compared between the subjects
with hypertension and the controls with the unpaired Student’s
t-test. Categorical data were compared with the χ2 test.
We examined the association of the 13 SNPs with the prevalence of
hypertension or their association with systolic, diastolic, or mean
BP based on a 5-year longitudinal cohort study. Longitudinal
changes in the prevalence of hypertension were compared between 2
groups (the dominant or recessive genetic model) with a generalized
estimating equation, as previously described (29) and with adjustment for age, gender,
body mass index (BMI) and smoking status. Longitudinal changes in
systolic, diastolic, or mean BP in all the individuals or in the
individuals not any taking anti-hypertensive medication were
compared between 2 groups (the dominant or recessive model) in a
generalized linear mixed-effect model, as previously described
(30) with adjustment for age,
gender, BMI and smoking status. The dominant or recessive model was
defined as AA vs. AB + BB or AA +
AB vs. BB (A, major allele; B, minor
allele), respectively. Age-related changes in the prevalence of
hypertension or in systolic or diastolic BP were estimated with
quadratic curves controlling for the observation year. A P-value
<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using R software
version 3-0-2 (the R Project for Statistical Computing) and JMP
Genomics version 6.0 (SAS Institute, Cary, NC, USA).
Results
Characteristics of the 6,027 study subjects (3,352
males, 2,675 females) with regard to all measurements in a 5-year
follow-up are shown in Table
III. Characteristics of the subjects with hypertension and the
controls according to cross-sectional analysis in March 2014 are
shown in Table IV. Age, the
frequency of the male gender, BMI and the prevalence of smoking
were greater in the subjects with hypertension than in the
controls.
 | Table IIICharacteristics of the study
subjects: analysis of all measurements in a 5-year follow-up. |
Table III
Characteristics of the study
subjects: analysis of all measurements in a 5-year follow-up.
| Parameter | Malea | Femalea | Alla |
|---|
| No. of
subjects | 3352 | 2675 | 6027 |
| Age (years) | 52.5±12.5
(15,959) | 52.5±11.9
(12,572) | 52.5±12.2
(28,531) |
| Height (cm) | 168.4±6.6
(15,550) | 155.2±5.9
(12,373) | 162.6±9.1
(27,923) |
| Weight (kg) | 67.0±11.0
(15,548) | 53.5±8.2
(12,373) | 61.0±12.0
(27,921) |
| Body mass index
(kg/m2) | 23.6±3.3
(15,548) | 22.2±3.2
(12,373) | 23.0±3.3
(27,921) |
| Waist circumference
(cm) | 83.2±8.7
(11,817) | 77.8±9.0
(9,541) | 80.8±9.2
(21,358) |
| Alcohol concumption
(%) | 67.4 (15,959) | 26.4 (12,572) | 49.3 (28,531) |
| Current or former
smoker (%) | 65.0 (15,959) | 8.5 (12,572) | 40.1 (28,531) |
| Systolic blood
pressure (mmHg) | 122±16
(15,541) | 119±16
(12,370) | 121±16
(27,911) |
| Diastolic blood
pressure (mmHg) | 77±12 (15,541) | 71±11 (12,370) | 75±12 (27,911) |
| Mean blood pressure
(mmHg) | 92±13 (15,541) | 87±12 (12,370) | 90±13 (27,911) |
| Ocular tension
(right, mmHg) | 14.0±3.0
(6,132) | 13.4±2.8
(4,886) | 13.7±3.0
(11,018) |
| Functional vital
capacity (l) | 3.53±0.66
(6,173) | 2.55±0.47
(4,865) | 3.10±0.76
(11,038) |
| FEV1% (%) | 82.3±7.1
(6,168) | 84.8±6.7
(4,865) | 83.4±7.0
(11,033) |
| Serum albumin
(g/l) | 44.5±2.9
(10,332) | 44.1±2.7
(8,510) | 44.3±2.8
(18,842) |
| Serum total
cholesterol (mmol/l) | 5.15±0.88
(15,121) | 5.31±0.88
(11,887) | 5.22±0.89
(27,008) |
| Serum triglyceride
(mmol/l) | 1.46±1.06
(15,639) | 1.01±0.58
(12,401) | 1.26±0.91
(28,040) |
| Serum
HDL-cholesterol (mmol/l) | 1.47±0.39
(15,627) | 1.78±0.42
(12,378) | 1.61±0.43
(28,005) |
| Serum
LDL-cholesterol (mmol/l) | 3.19±0.81
(14,997) | 3.18±0.79
(11,836) | 3.18±0.80
(26,833) |
| Fasting plasma
glucose (mmol/l) | 5.82±1.27
(15,685) | 5.39±0.93
(12,395) | 5.63±1.15
(28,080) |
| Blood hemoglobin
A1c (%) | 5.78±0.74
(10,849) | 5.64±0.54
(10,169) | 5.71±0.66
(21,018) |
| Blood urea nitrogen
(mmol/l) | 5.61±2.86
(8,889) | 5.07±2.28
(8,162) | 5.36±2.61
(17,051) |
| Serum creatinine
(μmol/l) | 88.3±116.2
(14,545) | 63.1±82.5
(11,225) | 77.3±103.6
(25,770) |
| eGFR (ml/min/1.73
m−2) | 77.2±18.0
(14,545) | 80.3±17.5
(11,225) | 78.5±17.9
(25,770) |
| Serum uric acid
(μmol/l) | 372±79
(14,368) | 273±62
(10,900) | 329±87
(25,268) |
| Serum C-reactive
protein (μg/l) | 1573±6428
(5,793) | 1207±4107
(4,938) | 1405±5486
(10,731) |
| White blood cells
(103/μl) | 5.94±1.73
(12,521) | 5.03±1.45
(9,419) | 5.55±1.68
(21,940) |
| Red blood cells
(104/μl) | 461±46
(12,651) | 415±36 (9,500) | 441±47
(22,151) |
| Hemoglobin
(g/l) | 147±13
(12,651) | 127±13 (9,501) | 139±16
(22,152) |
| Hematocrit (%) | 43.3±3.7
(12,642) | 37.5±3.4
(9,497) | 40.8±4.6
(22,139) |
| Platelets
(104/μl) | 23.1±5.5
(12,473) | 23.8±6.2
(9,398) | 23.4±5.8
(21,871) |
 | Table IVCharacteristics of subjects with
hypertension and controls: cross-sectional analysis in March
2014. |
Table IV
Characteristics of subjects with
hypertension and controls: cross-sectional analysis in March
2014.
| Parameter | Subjects with
hypertensiona | Controlsa | P-value |
|---|
| No. of
subjects | 2250 | 3777 | |
| Age (years) | 61.1±10.7
(2,250) | 50.1±12.4
(3,777) | <0.0001 |
| Gender
(male/female, %) | 62.6/37.4 | 51.5/48.5 | <0.0001 |
| Height (cm) | 161.3±9.4
(2,207) | 163.2±9.0
(3,747) | <0.0001 |
| Weight (kg) | 63.1±12.7
(2,205) | 59.7±11.6
(3,747) | <0.0001 |
| Body mass index
(kg/m2) | 24.1±3.6
(2,205) | 22.3±3.1
(3,747) | <0.0001 |
| Waist circumference
(cm) | 84.0±9.5
(1,986) | 78.5±8.5
(3,619) | <0.0001 |
| Alcohol consumption
(%) | 52.0 (2,250) | 46.0 (3,777) | <0.0001 |
| Current or former
smoker (%) | 47.7 (2,250) | 44.5 (3,777) | 0.0147 |
| Systolic blood
pressure (mmHg) | 133±15 (2,200) | 113±11 (3,745) | <0.0001 |
| Diastolic blood
pressure (mmHg) | 83±12 (2,200) | 70±10 (3,745) | <0.0001 |
| Mean blood pressure
(mmHg) | 99±12 (2,200) | 84±9 (3,745) | <0.0001 |
| Ocular tension
(right, mmHg) | 13.9±3.0 (722) | 13.3±2.9
(1,339) | <0.0001 |
| Functional vital
capacity (l) | 3.12±0.80
(768) | 3.39±0.80
(1,475) | <0.0001 |
| FEV1% (%) | 80.4±6.38
(768) | 81.7±6.6
(1,475) | <0.0001 |
| Serum albumin
(g/l) | 44.5±3.0
(1,715) | 44.7±2.4
(2,497) | 0.0302 |
| Serum total
cholesterol (mmol/l) | 5.19±0.90
(2,230) | 5.23±0.88
(3,720) | 0.0921 |
| Serum triglyceride
(mmol/l) | 1.43±0.96
(2,215) | 1.16±0.79
(3,721) | <0.0001 |
| Serum
HDL-cholesterol (mmol/l) | 1.59±0.44
(2,213) | 1.70±0.45
(3,721) | <0.0001 |
| Serum
LDL-cholesterol (mmol/l) | 3.15±0.79
(2,212) | 3.19±0.81
(3,720) | 0.0632 |
| Fasting plasma
glucose (mmol/l) | 5.90±1.36
(2,238) | 5.40±0.96
(3,718) | <0.0001 |
| Blood hemoglobin
A1c (%) | 5.84±0.78
(1,782) | 5.59±0.59
(2,681) | <0.0001 |
| Blood urea nitrogen
(mmol/l) | 5.72±2.68
(1,691) | 4.86±1.23
(2,410) | <0.0001 |
| Serum creatinine
(μmol/l) | 88.5±127.4
(2,162) | 64.8±15.1
(3,414) | <0.0001 |
| eGFR (ml/min/1.73
m−2) | 71.2±18.3
(2,162) | 80.1±14.7
(3,414) | <0.0001 |
| Serum uric acid
(μmol/l) | 349±88 (2,139) | 312±81 (3,392) | <0.0001 |
| Serum C-reactive
protein (μg/l) | 1832±9666
(775) | 826±3359
(1,338) | 0.0005 |
| White blood cells
(103/μl) | 5.51±1.74
(1,573) | 5.31±1.63
(3,034) | 0.0001 |
| Red blood cells
(104/μl) | 436±48 (1,577) | 437±43 (3,046) | 0.1928 |
| Hemoglobin
(g/l) | 139±16 (1,577) | 137±15 (3,046) | 0.0017 |
| Hematocrit (%) | 40.4±4.4
(1,576) | 40.1±4.2
(3,042) | 0.0186 |
| Platelets
(104/μl) | 21.8±5.5
(1,557) | 22.6±5.3
(3,011) | <0.0001 |
The association of the 13 SNPs with the prevalence
of hypertension was analyzed with a generalized estimating equation
and with adjustment for age, gender, BMI and smoking status
(Table V). The rs2116519 (C→T)
SNP of the family with sequence similarity 78, member B gene
(FAM78B, recessive model), rs6929846 (T→C) of the
butyrophilin, subfamily 2, member A1 gene (BTN2A1, dominant
model), rs146021107 (G→-) of the pancreatic and duodenal homeobox 1
gene (PDX1, dominant model) and rs1671021 (G→A) of the
lethal giant larvae homolog 2 gene (LLGL2, dominant model)
were significantly (P<0.05) associated with the prevalence of
hypertension.
 | Table VAssociation of polymorphisms with
hypertension analyzed for 5-year longitudinal data with a
generalized estimating equation. |
Table V
Association of polymorphisms with
hypertension analyzed for 5-year longitudinal data with a
generalized estimating equation.
| Gene or locus | SNP | Genotype |
Hypertensiona | Controlsa | P-value (dominant
model)b | P-value (recessive
model)c |
|---|
| FAM78B | rs2116519
(C→T) | TT | 1,888 (32.3) | 6,649 (30.3) | 0.3039 | 0.0266 |
| | TC | 2,959 (50.7) | 11,046 (50.3) | | |
| | CC | 991 (17.0) | 4,279 (19.5) | | |
| 3q28 | rs9846911
(A→G) | AA | 5,033 (86.2) | 19,102 (86.9) | 0.1629 | 0.1620 |
| | AG | 759 (13.0) | 2,756 (12.5) | | |
| | GG | 46 (0.8) | 116 (0.5) | | |
| ALPK1 | rs2074379
(G→A) | AA | 2,707 (46.4) | 10,004 (45.5) | 0.7330 | 0.2596 |
| | AG | 2,560 (43.9) | 9,736 (44.3) | | |
| | GG | 571 (9.8) | 2,234 (10.2) | | |
| ALPK1 | rs2074380
(G→A) | GG | 4,905 (84.0) | 18,656 (84.9) | 0.1124 | 0.1496 |
| | GA | 885 (15.2) | 3,165 (14.4) | | |
| | AA | 48 (0.8) | 153 (0.7) | | |
| ALPK1 | rs2074381
(A→G) | AA | 4,981 (85.3) | 18,815 (85.6) | 0.2390 | 0.4732 |
| | AG | 821 (14.1) | 3,038 (13.8) | | |
| | GG | 36 (0.6) | 121 (0.6) | | |
| ALPK1 | rs2074388
(G→A) | AA | 2,714 (46.5) | 10,013 (45.6) | 0.7043 | 0.2637 |
| | AG | 2,552 (43.7) | 9,721 (44.2) | | |
| | GG | 572 (9.8) | 2,240 (10.2) | | |
| BTN2A1 | rs6929846
(T→C) | CC | 4,484 (76.8) | 17,333 (78.9) | 0.0013 | 0.3602 |
| | CT | 1,275 (21.8) | 4,365 (19.9) | | |
| | TT | 79 (1.4) | 276 (1.3) | | |
| THBS2 | rs8089 (T→G) | TT | 4,895 (83.8) | 18,159 (82.6) | 0.7407 | 0.9741 |
| | TG | 902 (15.5) | 3,615 (16.5) | | |
| | GG | 41 (0.7) | 200 (0.9) | | |
| PDX1 | rs146021107
(G→-) | GG | 1,745 (29.9) | 5,983 (27.2) | 0.0031 | 0.2885 |
| | G/- | 2,839 (48.6) | 11,049 (50.3) | | |
| | -/- | 1,254 (21.5) | 4,942 (22.5) | | |
| F7 | rs6046 (G→A) | GG | 5,104 (87.4) | 19,187 (87.3) | 0.1478 | 0.8979 |
| | GA | 715 (12.2) | 2,693 (12.3) | | |
| | AA | 19 (0.3) | 94 (0.4) | | |
| LLGL2 | rs1671021
(G→A) | AA | 4,187 (71.7) | 16,353 (74.4) | 0.0372 | 0.3881 |
| | AG | 1,521 (26.1) | 5,223 (23.8) | | |
| | GG | 130 (2.2) | 398 (1.8) | | |
| ILF3 | rs2569512
(G→A) | GG | 2,563 (43.9) | 9,525 (43.3) | 0.3765 | 0.2560 |
| | GA | 2,605 (44.6) | 10,180 (46.3) | | |
| | AA | 670 (11.5) | 2,269 (10.3) | | |
| CELSR1 | rs6007897
(C→T) | TT | 5,671 (97.1) | 21,303 (96.9) | 0.6353 | not determined |
| | TC | 167 (2.9) | 671 (3.1) | | |
| | CC | 0 (0) | 0 (0) | | |
The association between the prevalence of
hypertension and age analyzed longitudinally with a generalized
estimating equation according to the SNP genotype is shown in
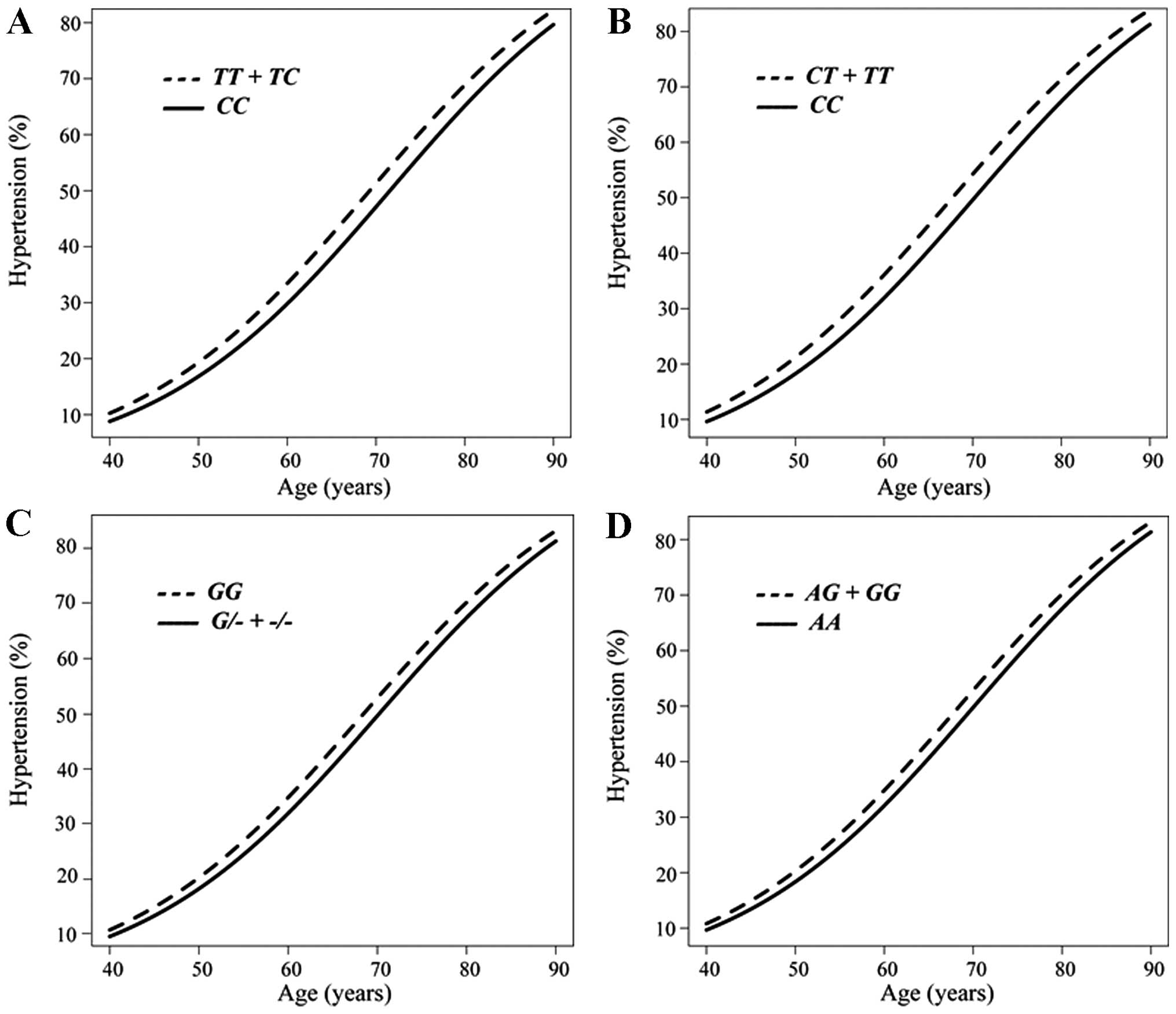
Fig. 1. The prevalence of
hypertension was greater in the combined group of subjects with the
TT or TC genotypes of rs2116519 of FAM78B than
in those with the CC genotype from 40 to 90 years of age
(Fig. 1A), in the combined group
of subjects with the CT or TT genotypes of rs6929846
of BTN2A1 than in those with the CC genotype
(Fig. 1B), in subjects with the
GG genotype of rs146021107 of PDX1 than in the
combined group of subjects with the G/- or -/- genotypes
(Fig. 1C), and in the combined
group of subjects with the AG or GG genotypes of
rs1671021 of LLGL2 than in those with the AA genotype
(Fig. 1D).
Given that 4 SNPs were significantly associated with
hypertension, the association of these SNPs with systolic,
diastolic, or mean BP in all individuals or individuals not taking
any anti-hypertensive medication were analyzed with a generalized
linear mixed-effect model, with adjustment for age, gender, BMI and
smoking status (Table VI). The
rs6929846 polymorphism of BTN2A1 was significantly
associated with systolic, diastolic and mean BP in the dominant
model among all individuals or individuals not taking any
anti-hypertensive medication, with the T allele being
associated with an increased BP. The rs146021107 SNP of PDX1
was significantly associated with systolic BP in the dominant model
among all individuals or individuals not taking any
anti-hypertensive medication, with the G allele being
associated with an increased BP. The rs2116519 polymorphism of
FAM78B was significantly associated with diastolic BP in the
recessive model among individuals not taking any anti-hypertensive
medication, with the T allele being associated with a high
BP. The rs1671021 SNP of LLGL2 was significantly associated
with diastolic and mean BP in the dominant model among individuals
not taking any anti-hypertensive medication, with the G
allele being associated with a high BP.
 | Table VIAssociation of polymorphisms with
systolic, diastolic, or mean BP in all individuals or individuals
not taking any anti-hypertensive medication analyzed for 5-year
longitudinal data with a generalized linear mixed-effect model. |
Table VI
Association of polymorphisms with
systolic, diastolic, or mean BP in all individuals or individuals
not taking any anti-hypertensive medication analyzed for 5-year
longitudinal data with a generalized linear mixed-effect model.
| Gene | SNP | BP (mmHg) | Dominant
modela
| P-value | Recessive
modela
| P-value |
|---|
| All
individuals |
| FAM78B | rs2116519
(C→T) | | TT
(8,537) | TC +
CC (19,275) | | TT +
TC (2,2542) | CC
(5,270) | |
| | Systolic | 121.0±16.7 | 120.4±16.1 | 0.3818 | 120.7±16.5 | 120.1±15.7 | 0.5823 |
| | Diastolic | 74.9±12.5 | 74.6±12.1 | 0.1260 | 74.8±12.4 | 74.1±11.8 | 0.0823 |
| | Mean | 90.2±13.1 | 89.9±12.6 | 0.1722 | 90.1±12.9 | 89.4±12.2 | 0.1814 |
| BTN2A1 | rs6929846
(T→C) | | CC
(21,817) | CT +
TT (5,995) | | CC +
CT (27,457) | TT
(355) | |
| | Systolic | 120.4±16.2 | 121.2±16.7 | 0.0061 | 120.6±16.3 | 121.4±15.4 | 0.1369 |
| | Diastolic | 74.5±12.2 | 75.2±12.4 | 0.0023 | 74.7±12.3 | 75.2±11.0 | 0.2483 |
| | Mean | 89.8±12.7 | 90.5±13.0 | 0.0019 | 90.0±12.8 | 90.6±11.7 | 0.1748 |
| PDX1 | rs146021107
(G→-) | | GG
(7,728) | G/- + -/-
(20,084) | | GG +
G/- (21,616) | -/- (6,196) | |
| | Systolic | 121.1±17.1 | 120.4±16.0 | 0.0284 | 120.8±16.4 | 120.0±16.1 | 0.3884 |
| | Diastolic | 74.5±12.8 | 74.7±12.1 | 0.2719 | 74.8±12.3 | 74.4±12.1 | 0.9222 |
| | Mean | 90.1±13.3 | 90.0±12.5 | 0.1029 | 90.1±12.8 | 89.6±12.5 | 0.6821 |
| LLGL2 | rs1671021
(G→A) | | AA
(20,540) | AG +
GG (7,272) | | AA +
AG (27,284) | GG
(528) | |
| | Systolic | 120.4±16.2 | 121.2±16.6 | 0.1943 | 120.6±16.3 | 121.6±16.1 | 0.9056 |
| | Diastolic | 74.5±12.2 | 75.2±12.4 | 0.1280 | 74.7±12.3 | 75.8±12.7 | 0.4665 |
| | Mean | 89.8±12.7 | 90.5±12.9 | 0.1315 | 90.0±12.8 | 91.1±12.8 | 0.7203 |
| Individuals not
taking any anti-hypertensive medication |
| FAM78B | rs2116519
(C→T) | | TT
(8,132) | TC +
CC (18,370) | | TT +
TC (2,1459) | CC
(5,043) | |
| | Systolic | 120.5±16.7 | 119.9±16.0 | 0.2563 | 120.2±16.4 | 119.7±15.6 | 0.5041 |
| | Diastolic | 74.6±12.5 | 74.4±12.1 | 0.2039 | 74.6±12.4 | 73.9±11.7 | 0.0495 |
| | Mean | 89.9±13.0 | 89.6±12.6 | 0.1948 | 89.8±12.9 | 89.1±12.1 | 0.1248 |
| BTN2A1 | rs6929846
(T→C) | | CC
(20,807) | CT +
TT (5,695) | | CC +
CT (26,163) | TT
(339) | |
| | Systolic | 120.0±16.1 | 120.7±16.6 | 0.0017 | 120.1±16.3 | 120.8±15.3 | 0.1734 |
| | Diastolic | 74.3±12.2 | 75.0±12.4 | 0.0008 | 74.4±12.3 | 75.0±10.8 | 0.2059 |
| | Mean | 89.5±12.7 | 90.2±13.0 | 0.0005 | 89.7±12.7 | 90.3±11.5 | 0.1678 |
| PDX1 | rs146021107
(G→-) | | GG
(7,328) | G/- + -/-
(19,174) | | GG +
G/- (20,580) | -/- (5,922) | |
| | Systolic | 120.6±17.1 | 120.0±15.9 | 0.0132 | 120.3±16.3 | 119.5±16.0 | 0.2565 |
| | Diastolic | 74.2±12.8 | 74.5±12.0 | 0.3963 | 74.5±12.3 | 74.2±12.1 | 0.8832 |
| | Mean | 89.7±13.3 | 89.7±12.5 | 0.1081 | 89.8±12.8 | 89.3±12.5 | 0.7018 |
| LLGL2 | rs1671021
(G→A) | | AA
(19,569) | AG +
GG (6,933) | | AA +
AG (26,005) | GG
(497) | |
| | Systolic | 119.9±16.2 | 120.7±16.5 | 0.0891 | 120.1±16.3 | 121.4±16.1 | 0.6847 |
| | Diastolic | 74.2±12.2 | 75.0±12.4 | 0.0468 | 74.4±12.2 | 75.7±12.7 | 0.2512 |
| | Mean | 89.5±12.7 | 90.2±12.9 | 0.0471 | 89.6±12.7 | 90.9±12.80 | 0.3889 |
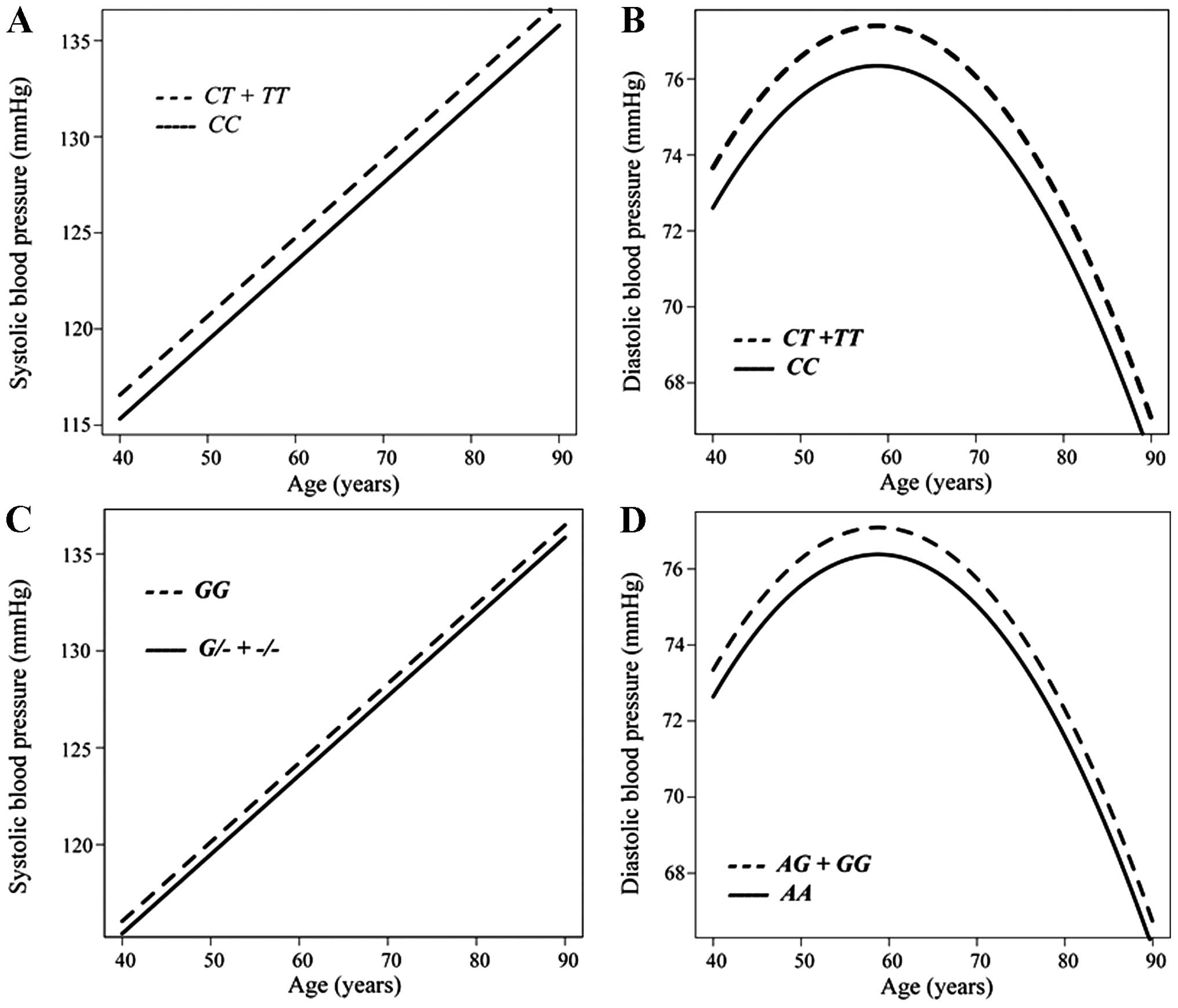
The association between systolic or diastolic BP and
age in individuals not taking any anti-hypertensive medication was
analyzed longitudinally according to genotype with a generalized
linear mixed-effect model (Fig.
2). Systolic (Fig. 2A) and
diastolic (Fig. 2B) BP were
greater in the combined group of individuals with the CT or
TT genotypes of rs6929846 of BTN2A1 than in those
with the CC genotype from 40 to 90 years of age. Systolic BP
was greater in subjects with the GG genotype of rs146021107
of PDX1 than in the combined group of individuals with the
G/- or -/- genotypes (Fig.
2C). Diastolic BP was greater in the combined group of
individuals with the AG or GG genotypes of rs1671021
of LLGL2 than in those with the AA genotype (Fig. 2D).
Discussion
Given that genetic factors, as well as interactions
between multiple genes and environmental factors are important in
the development of hypertension (1), the ability to predict the risk of
developing hypertension on the basis of genetic variants would be
beneficial for the personalized prevention of this condition. In
this study, we demonstrated that rs6929846 (T→C) of BTN2A1
was significantly associated with the prevalence of hypertension
and also with systolic, diastolic, and mean BP in
community-dwelling Japanese individuals, with the minor T
allele representing a risk factor for hypertension.
We have previously reported that rs6929846 of
BTN2A1 is significantly associated with hypertension in a
cross-sectional study of a different hospital-based population
(31). We also observed the
association of this polymorphism with hypertension in a previous
cross-sectional analysis of the Inabe Health and Longevity Study
(26). The results of the present
longitudinal population-based study are thus consistent with these
previous observations (26,31) and validate the association of
rs6929846 of BTN2A1 with hypertension.
BTN2A1 is a cell-surface transmembrane
glycoprotein and a member of the butyrophilin superfamily of
proteins. Many of these proteins regulate immune function, and
polymorphisms within the coding sequences of the corresponding
genes have been associated with the predisposition to inflammatory
diseases (32). We have
previously demonstrated that the T allele of rs6929846 of
BTN2A1 is associated with an increased risk of developing
myocardial infarction and with an increased transcriptional
activity of BTN2A1 (15).
The serum concentration of high-sensitivity C-reactive protein was
significantly greater in individuals in the combined group of
CT or TT genotypes for this SNP than in those with
the CC genotype among healthy subjects without neoplastic,
infectious, or inflammatory disease (15,33). These observations suggest that the
T allele of rs6929846 of BTN2A1 may accelerate
inflammatory processes.
Previous studies have suggested that chronic
vascular inflammation influences BP and vascular remodeling
(34–37). Systolic and diastolic BP, as well
as pulse pressure were thus found to be positively associated with
the plasma concentration of interleukin-6 in healthy men (34). The plasma concentration of
high-sensitivity C-reactive protein was also greater in individuals
with hypertension than in the controls, and it was shown to be
positively associated with systolic BP and pulse pressure (35). In addition, oxidative stress and
vascular inflammation have been shown to influence BP, suggesting
that chronic inflammation may play a key role in the pathogenesis
of hypertension (36,37). In this study, we demonstrated that
rs6929846 of BTN2A1 was significantly associated with
hypertension, with the minor T allele representing a risk
factor for this condition. The enhancement of chronic inflammation
by the T allele of rs6929846 may account for its association
with hypertension, although the molecular mechanisms underlying the
effects of this polymorphism on the development of hypertension
remain to be elucidated.
In a previous meta-analysis of cohort studies, a
reduction of 10 mmHg in systolic or 5 mmHg in diastolic BP was
estimated to result in a 22–25% decrease in the incidence of
coronary artery disease and a 36–41% decrease in that of stroke
(38). In our longitudinal
analysis, systolic, diastolic and mean BP were each increased by 1
mmHg in individuals with the TT genotype of rs6929846 of
BTN2A1 compared with those with the CC genotype. Such
a difference is small at the individual level and may not have
practical clinical implications. However, even small increments in
BP have important effects on cardiovascular morbidity and mortality
at the population level, given the high incidence of coronary
artery disease, stroke and chronic kidney disease. The reduction in
the mortality rate estimated for each 2-mmHg decrease in systolic
BP is 4% for coronary artery disease and 6% for stroke (39). Small differences in average BP at
the population level thus result in significant differences in the
population mortality rate (39).
In this study, we observed that the SNPs of
PDX1, LLGL2 and FAM78B were also associated
with the prevalence of hypertension, as well as with systolic BP
among all individuals and individuals not taking any
anti-hypertensive medication (PDX1), with diastolic and mean
BP among individuals without anti-hypertensive medication
(LLGL2), or with diastolic BP among individuals without
anti-hypertensive medication (FAM78B). FAM78B is
located at 1q24.1, which has previously been suggested to harbor
susceptibility loci for hypertension (40) and type 2 diabetes mellitus
(41), although the function of
the gene remains unclear. PDX1 is a transcriptional
activator at several genes, including those for insulin,
somatostatin, glucokinase, islet amyloid polypeptide and glucose
transporter type 2 (NCBI Gene). It contributes to the early
development of the pancreas and plays an important role in the
glucose-dependent regulation of insulin gene expression (42). A rare frameshift variant of
PDX1 was previously found to associated with type 2 diabetes
mellitus (43). We have
previously demonstrated that rs146021107 of PDX1 is
significantly associated with myocardial infarction (18,20), although, to the best of our
knowledge, the association of PDX1 polymorphisms with
hypertension has not yet been reported. LLGL2 plays a role
in asymmetric cell division, the establishment of epithelial cell
polarity and cell migration (44,45). We have previously demonstrated
that rs1671021 of LLGL2 is associated with ischemic stroke
(16), although, to the best of
our knowledge, variants of LLGL2 have not yet been
associated with hypertension.
The present study had certain limitations: i) given
that the study subjects comprised only Japanese individuals,
further studies are required on other ethnic groups. ii) It is
possible that rs6929846 of BTN2A1 is in linkage
disequilibrium with other polymorphisms in BTN2A1 or in
nearby genes that are actually responsible for the development of
hypertension. iii) The functional relevance of rs6929846 of
BTN2A1 to the pathogenesis of hypertension remains
unclear.
In conclusion, the present results suggest that
BTN2A1 is a susceptibility gene for essential hypertension
in Japanese individuals. The determination of the genotype for
rs6929846 may prove informative for the assessment of the genetic
risk for hypertension in such individuals.
Acknowledgments
This study was supported by the CREST, Japan Science
and Technology Agency (to Y.Y. and I.T.), and by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan (no. 24590746 to Y.Y.).
References
|
1
|
Lifton RP, Gharavi AG and Geller DS:
Molecular mechanisms of human hypertension. Cell. 104:545–556.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kupper N, Willemsen G, Riese H, Posthuma
D, Boomsma DI and de Geus EJ: Heritability of daytime ambulatory
blood pressure in an extended twin design. Hypertension. 45:80–85.
2005. View Article : Google Scholar
|
|
3
|
Agarwal A, Williams GH and Fisher ND:
Genetics of human hypertension. Trends Endocrinol Metab.
16:127–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kannel WB: Elevated systolic blood
pressure as a cardiovascular risk factor. Am J Cardiol. 85:251–255.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sacco RL, Benjamin EJ, Broderick JP, Dyken
M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G,
Kittner SJ, et al: American Heart Association Prevention
Conference. IV. Prevention and Rehabilitation of Stroke. Risk
factors Stroke. 28:1507–1517. 1997. View Article : Google Scholar
|
|
6
|
Yamagata K, Ishida K, Sairenchi T,
Takahashi H, Ohba S, Shiigai T, Narita M and Koyama A: Risk factors
for chronic kidney disease in a community-based population: A
10-year follow-up study. Kidney Int. 71:159–166. 2007. View Article : Google Scholar
|
|
7
|
Ehret GB, Munroe PB, Rice KM, Bochud M,
Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ,
et al: CHARGE-HF consortium: Genetic variants in novel pathways
influence blood pressure and cardiovascular disease risk. Nature.
478:103–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wain LV, Verwoert GC, O’Reilly PF, Shi G,
Johnson T, Johnson AD, Bochud M, Rice KM, Henneman P, Smith AV, et
al: Genome-wide association study identifies six new loci
influencing pulse pressure and mean arterial pressure. Nat Genet.
43:1005–1011. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newton-Cheh C, Johnson T, Gateva V, Tobin
MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S,
et al: Genome-wide association study identifies eight loci
associated with blood pressure. Nat Genet. 41:666–676. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levy D, Ehret GB, Rice K, Verwoert GC,
Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund
T, et al: Genome-wide association study of blood pressure and
hypertension. Nat Genet. 41:677–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wellcome Trust Case Control Consortium:
Genome-wide association study of 14,000 cases of seven common
diseases and 3,000 shared controls. Nature. 447:661–678. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adeyemo A, Gerry N, Chen G, Herbert A,
Doumatey A, Huang H, Zhou J, Lashley K, Chen Y, Christman M, et al:
A genome-wide association study of hypertension and blood pressure
in African Americans. PLoS Genet. 5:e10005642009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato N, Miyata T, Tabara Y, Katsuya T,
Yanai K, Hanada H, Kamide K, Nakura J, Kohara K, Takeuchi F, et al:
High-density association study and nomination of susceptibility
genes for hypertension in the Japanese National Project. Hum Mol
Genet. 17:617–627. 2008. View Article : Google Scholar
|
|
14
|
Tabara Y, Kohara K, Kita Y, Hirawa N,
Katsuya T, Ohkubo T, Hiura Y, Tajima A, Morisaki T, Miyata T, et
al: Global Blood Pressure Genetics Consortium: Common variants in
the ATP2B1 gene are associated with susceptibility to hypertension:
The Japanese Millennium Genome Project. Hypertension. 56:973–980.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada Y, Nishida T, Ichihara S, Sawabe M,
Fuku N, Nishigaki Y, Aoyagi Y, Tanaka M, Fujiwara Y, Yoshida H, et
al: Association of a polymorphism of BTN2A1 with myocardial
infarction in East Asian populations. Atherosclerosis. 215:145–152.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamada Y, Fuku N, Tanaka M, Aoyagi Y,
Sawabe M, Metoki N, Yoshida H, Satoh K, Kato K, Watanabe S, et al:
Identification of CELSR1 as a susceptibility gene for ischemic
stroke in Japanese individuals by a genome-wide association study.
Atherosclerosis. 207:144–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamada Y, Nishida T, Ichihara S, Kato K,
Fujimaki T, Oguri M, Horibe H, Yoshida T, Watanabe S, Satoh K, et
al: Identification of chromosome 3q28 and ALPK1 as susceptibility
loci for chronic kidney disease in Japanese individuals by a
genome-wide association study. J Med Genet. 50:410–418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamada Y, Matsuo H, Segawa T, Watanabe S,
Kato K, Hibino T, Yokoi K, Ichihara S, Metoki N, Yoshida H, et al:
Assessment of genetic risk for myocardial infarction. Thromb
Haemost. 96:220–227. 2006.PubMed/NCBI
|
|
19
|
Fujimaki T, Kato K, Yoshida T, Oguri M,
Watanabe S, Metoki N, Yoshida H, Satoh K, Aoyagi Y, Nishigaki Y, et
al: Association of genetic variants with myocardial infarction in
Japanese individuals with chronic kidney disease. Thromb Haemost.
101:963–968. 2009.PubMed/NCBI
|
|
20
|
Oguri M, Kato K, Yokoi K, Itoh T, Yoshida
T, Watanabe S, Metoki N, Yoshida H, Satoh K, Aoyagi Y, et al:
Association of genetic variants with myocardial infarction in
Japanese individuals with metabolic syndrome. Atherosclerosis.
206:486–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ueyama C, Horibe H, Fujimaki T, Oguri M,
Kato K and Yamada Y: Association of genetic variants of CELSR1 and
3q28 with hypertension in community-dwelling individuals. Biomed
Rep. 1:840–844. 2013.
|
|
22
|
Shimokata S, Oguri M, Fujimaki T, Horibe
H, Kato K and Yamada Y: Association between polymorphisms of the
α-kinase 1 gene and type 2 diabetes mellitus in community-dwelling
individuals. Biomed Rep. 1:940–944. 2013.
|
|
23
|
Oguri M, Fujimaki T, Horibe H, Kato K,
Ichihara S and Yamada Y: Association of a polymorphism of BTN2A1
with chronic kidney disease in community-dwelling individuals.
Biomed Rep. 1:868–872. 2013.
|
|
24
|
Fujimaki T, Horibe H, Oguri M, Kato K and
Yamada Y: Association of genetic variants of the α-kinase 1 gene
with myocardial infarction in community-dwelling individuals.
Biomed Rep. 2:127–131. 2014.PubMed/NCBI
|
|
25
|
Horibe H, Ueyama C, Fujimaki T, Oguri M,
Kato K, Ichihara S and Yamada Y: Association of a polymorphism of
BTN2A1 with dyslipidemia in community-dwelling individuals. Mol Med
Rep. 9:808–812. 2014.PubMed/NCBI
|
|
26
|
Murakata Y, Fujimaki T and Yamada Y:
Association of a butyrophilin, subfamily 2, member A1 gene
polymorphism with hypertension. Biomed Rep. 2:818–822.
2014.PubMed/NCBI
|
|
27
|
Perloff D, Grim C, Flack J, Frohlich ED,
Hill M, McDonald M and Morgenstern BZ: Human blood pressure
determination by sphygmomanometry. Circulation. 88:2460–2470. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Itoh Y, Mizuki N, Shimada T, Azuma F,
Itakura M, Kashiwase K, Kikkawa E, Kulski JK, Satake M and Inoko H:
High-throughput DNA typing of HLA-A, -B, -C, and -DRB1 loci by a
PCR-SSOP-Luminex method in the Japanese population. Immunogenetics.
57:717–729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanley JA, Negassa A, Edwardes MD and
Forrester JE: Statistical analysis of correlated data using
generalized estimating equations: An orientation. Am J Epidemiol.
157:364–375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dean CB and Nielsen JD: Generalized linear
mixed models: A review and some extensions. Lifetime Data Anal.
13:497–512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horibe H, Kato K, Oguri M, Yoshida T,
Fujimaki T, Kawamiya T, Yokoi K, Watanabe S, Satoh K, Aoyagi Y, et
al: Association of a polymorphism of BTN2A1 with hypertension in
Japanese individuals. Am J Hypertens. 24:924–929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arnett HA, Escobar SS and Viney JL:
Regulation of costimulation in the era of butyrophilins. Cytokine.
46:370–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oguri M, Kato K, Yoshida T, Fujimaki T,
Horibe H, Yokoi K, Watanabe S, Satoh K, Aoyagi Y, Tanaka M, et al:
Association of a genetic variant of BTN2A1 with metabolic syndrome
in East Asian populations. J Med Genet. 48:787–792. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chae CU, Lee RT, Rifai N and Ridker PM:
Blood pressure and inflammation in apparently healthy men.
Hypertension. 38:399–403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schillaci G, Pirro M, Gemelli F,
Pasqualini L, Vaudo G, Marchesi S, Siepi D, Bagaglia F and
Mannarino E: Increased C-reactive protein concentrations in
never-treated hypertension: The role of systolic and pulse
pressures. J Hypertens. 21:1841–1846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Savoia C and Schiffrin EL: Inflammation in
hypertension. Curr Opin Nephrol Hypertens. 15:152–158.
2006.PubMed/NCBI
|
|
37
|
Androulakis ES, Tousoulis D, Papageorgiou
N, Tsioufis C, Kallikazaros I and Stefanadis C: Essential
hypertension: Is there a role for inflammatory mechanisms? Cardiol
Rev. 17:216–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Law MR, Morris JK and Wald NJ: Use of
blood pressure lowering drugs in the prevention of cardiovascular
disease: meta-analysis of 147 randomised trials in the context of
expectations from prospective epidemiological studies. BMJ.
338:b16652009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stamler J, Rose G, Stamler R, Elliott P,
Dyer A and Marmot M: INTERSALT study findings. Public health and
medical care implications. Hypertension. 14:570–577. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ehret GB, O’Connor AA, Weder A, Cooper RS
and Chakravarti A: Follow-up of a major linkage peak on chromosome
1 reveals suggestive QTLs associated with essential hypertension:
GenNet study. Eur J Hum Genet. 17:1650–1657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shriner D, Adeyemo A and Rotimi CN: Joint
ancestry and association testing in admixed individuals. PLoS
Comput Biol. 7:e10023252011. View Article : Google Scholar
|
|
42
|
Stoffers DA, Zinkin NT, Stanojevic V,
Clarke WL and Habener JF: Pancreatic agenesis attributable to a
single nucleotide deletion in the human IPF1 gene coding sequence.
Nat Genet. 15:106–110. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Steinthorsdottir V, Thorleifsson G, Sulem
P, Helgason H, Grarup N, Sigurdsson A, Helgadottir HT,
Johannsdottir H, Magnusson OT, Gudjonsson SA, et al: Identification
of low-frequency and rare sequence variants associated with
elevated or reduced risk of type 2 diabetes. Nat Genet. 46:294–298.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Müsch A, Cohen D, Yeaman C, Nelson WJ,
Rodriguez-Boulan E and Brennwald PJ: Mammalian homolog of
Drosophila tumor suppressor lethal (2) giant larvae interacts with
basolateral exocytic machinery in Madin-Darby canine kidney cells.
Mol Biol Cell. 13:158–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yasumi M, Sakisaka T, Hoshino T, Kimura T,
Sakamoto Y, Yamanaka T, Ohno S and Takai Y: Direct binding of Lgl2
to LGN during mitosis and its requirement for normal cell division.
J Biol Chem. 280:6761–6765. 2005. View Article : Google Scholar : PubMed/NCBI
|