Introduction
Osteoarthritis (OA) is a prevalent degenerative
disease of the joints affecting a large number of individuals
worldwide. It is estimated that 2/3 individuals older than 60 years
of age suffer from OA and the number of individuals suffering from
this disease is increasing (1).
Among the clinical manifestations of the disease, those including
joint pain are the most common. These manifestations appear with
functional limitation at different levels, reducing the quality of
life of affected patients. The pathological changes associated with
OA are subchondral bone remodeling, synovial inflammation and the
progressive degeneration of articular cartilage (2).
The cause of the cartilage damage involves the
destruction of the shift which maintains the balance between
chondrocyte anabolic and catabolic capacities. This balance, by
means of producing various types of proteins associated with the
matrix, enzymes, as well as cytokines, maintains the extracellular
matrix (2). OA is triggered by
the imbalance between chondrocyte catabolic and anabolic
activities. For most individuals, in spite the fact that the
etiology of OA has not yet been thoroughly elucidated, it is
acceptable to say that the production of excess inflammatory
cytokines is key to the development of OA (3). It has been demonstrated that
interleukin-1β (IL-1β), one of the cytokines with pro-inflammatory
effects, is of particular importance to the development of the
disease (4). By increasing the
production of matrix metalloproteinases (MMPs), IL-1β facilitates
the degradation of the cartilage matrix (4). At the same time, it may induce the
expression of inducible nitric oxide (NO) synthase (iNOS) and
cyclooxygenase-2 (COX-2) [which contributes to the production of
higher levels of NO and prostaglandin E2
(PGE2)] (5). It has
been found that NO is capable of upregulating the production of
inflammatory cytokines and MMPs, as well as inducing chondrocyte
apoptosis. PGE plays a role in both joint pain and bone resorption.
PGE2 is also capable of mediating cartilage matrix
degradation by promoting the activity of MMPs and other
inflammatory cytokines (6).
To date, there is no available totally effective
therapy for OA as a multifactorial degenerative joint disorder.
Current treatment strategies for individuals suffering from this
disease aim at controlling joint swelling and pain, and delaying
the progression of the disease, as well as improving the quality of
life of patients (5). Primarily,
balneotherapy has been adopted as a treatment strategy. This
ancient treatment method is applied by immersion in water with
various chemical, thermal and mechanical properties (7). A previous study demonstrated that
for rats with chronic experimental arthritis, therapy using a
sulfur bath produced an anti-inflammatory result (8). This method has been adopted for the
treatment of a number of patients with OA and rheumatic diseases;
as previously demonstrated, patients spent 2–3 weeks being
subjected to sulphurous mud-bath therapy at a spa in Italy
(9). In addition, it has been
discovered by some recently conducted research that hydrogen
sulfide (H2S) is possibly a very important contributor
to the positive effects of sulfur baths (10).
It is now considered that H2S, carbon
monoxide (CO) and NO belong to the same group of endogenously
produced gas molecules. Nevertheless, studies on the biological
function of H2S are limited and fewer than those on CO
and NO. Emerging evidence indicates that H2S is crucial
for a number of pathological and physiological processes, such as
for example, in metabolic disorders including diabetes (11). Relevant studies have indicated
that 30–100 μM concentrations of physiological
H2S exert antinociceptive effects (12). In spite of the fact that relevant
data have provided information regarding the mechanisms of action
and precise sites of action of the inflammatory mediator,
H2S, these mechanisms and sites of action have not yet
been fully elucidated. It seems that the upregulation of, for
example, the production of CO, is capable of mediating some of the
anti-inflammatory and antinociceptive effects of H2S.
This mediating process, by means of inflammatory stimuli, leads to
the inhibition of the nuclear factor-κB (NF-κB) pathway and the
downregulation of iNOS expression (13).
In terms of the potential role of H2S in
OA, to the best of our knowledge, there is no relevant information
currently available. According to our previous studies, the
concentrations of H2S contained in the synovial fluid
aspirated from the knee joints of patients with OA are higher than
those in the synovial fluid from individuals without OA
(unpublished data). Analogously, other studies have demonstrated
that human cartilage cells have the capacity to synthesize
H2S. This is observed as part of an acute response to
the so-called pro-inflammatory mediators, including IL-1β, tumor
necrosis factor-α (TNF-α) and lipopolysaccharide (LPS). It has been
suggested that cytokine-induced H2S synthesis may be
used to prevent oxidative injury to joint cells (14,15). According to previous findings,
both the decrease in leukocyte velocity and synovial leukocyte
adherence (independent of the dose used) may be caused by
H2S (16), indicating
the anti-inflammatory effects of H2S in OA. However, the
anti-inflammatory mechanisms of action and effects of
H2S on chondrocyte inflammatory responses induced by
IL-1β have not yet been fully clarified. In the process, NF-κB as
an inducible transcription factor is important for the control of
the transcription of inflammatory response genes (17,18). It is known that mitogen-activated
protein kinases (MAPKs) function as upstream activators of NF-κB.
It is evident that as regards NF-κB activity, extracellular
signal-regulated kinase (ERK) is an important temporal regulator.
When ERK1/2 is depleted using inhibitors, NF-κB activation is
reduced, inhibiting NF-κB-dependent gene transcription (19). Therefore, based on these findings,
the present study was conducted to determine the following: i) the
effects of H2S on the IL-1β-induced expression of
chondrocyte catabolic factors and ii) whether H2S
protects chondrocytes from damage through the ERK-NF-κB channel in
OA. To better conduct the evaluation of the chondroprotective
effects of H2S and to offer an advanced basis for the
clinical treatment of OA in the future, further knowledge of the
underlying mechanisms may be required.
Materials and methods
Reagents
Sodium hydrosulfide (NaHS), recombinant human IL-1β,
collagenase type II, PD98058 (an inhibitor of ERK), Toluidine blue,
Safranin O, and hematoxylin and eosin were purchased from
Sigma-Aldrich (St. Louis, MO,USA). Dulbecco’s Modified Eagle’s
Medium (DMEM), penicillin and streptomycin, fetal bovine serum
(FBS), and 0.25% trypsin were obtained from Gibco-BRL (Grand
Island, NY, USA). The cell counting kit-8 (CCK-8) was purchased
from Dojindo Laboratories (Kumamoto, Japan). The Griess reagent
assay kit was obtained from the Beyotime Institute of Biotechnology
(Haimen, China). The enzyme-linked immunosorbent assay (ELISA) kit
was provided by Boster Bio-Engineering Limited Company (Wuhan,
China). TRIzol Reagent and the One-Step qPCR kit were purchased
from Tiangen Biotech Co. Ltd. (Beijing, China). Antibodies against
ERK1/2/phospho-ERK1/2 (Thr202/Tyr204), IκBα/phospho-IκBα (Ser32/36)
and NF-κB p65/phospho-NF-κB p65 (Ser536) and β-actin were purchased
from Cell Signaling Technology, Inc. (Beverly, MA, USA). The NF-κB
(p65) transcription factor assay kit was purchased from Active
Motif (Carlsbad, CA, USA). All other chemicals were purchased from
Sigma-Aldrich unless otherwise stated.
Chondrocyte isolation and culture
The purpose and nature of the study were clearly
explained to the patients and written informed consent was obtained
from all patients, and the study was approved by the Institutional
Ethics Review Committee of the Affiliated Hospital of Qingdao
University, Qingdao, China. Our obtained human cartilage samples
were selected from OA sufferers who had undergone total knee
arthroplasty. OA was diagnosed according to the Diagnostic and
Therapeutic Criteria Committee of the American Rheumatism
Association. Patients with the same grade of OA were included in
our study. Patients who had received an intra-articular injection
of steroids were excluded from our study.
After the cartilage was subjected to 30 min of
digestion with 0.25% trypsin, it was then digested with 2 mg/ml
collagenase II in DMEM for 6 h. The cells were suspended in DMEM
with 15% FBS and antibiotics and then seeded in tissue culture
flasks. Confluent cells were split at 1:3. An inverted phase
contrast microscope (Olympus CK40, Germany) was used to observe the
morphology of the chondrocytes cultured in vitro. The
chondrocytic phenotype was confirmed by microscopic evaluation, by
staining for glycosaminoglycan production (hematoxylin and eosin,
Toluidine blue and Safranin O staining). Cells between passages 3
and 4 were used in the experiments.
H2S donor
NaHS is universally adopted and in this regard, it
functions as an H2S donor. NaHS is used to obtain
H2S, as well as to define the H2S
concentration in a solution. Compared with the defining method of
measuring bubbling H2S gas, this method is not only more
reproductive, but also more accurate. Immediately prior to use from
a solution of 200 mM NaHS stock, the solution containing NaHS was
prepared. NaHS is dissociated to HS− and Na+
(in solution) at first. It is then dissociated to HS−
and binds to H+; H2S is then formed. Under
certain conditions, 18.5% of H2S/HS− exists
(in a non-dissociated form). Without knowing the active form of
H2S, as regards the total number of the forms of
H2S, we used the terminology ‘H2S’.
Therefore, a solution of H2S with a concentration at the
level of approximately 1/3 of the NaHS original concentration was
provided, as previously described (20).
Cell viability assay
We detected cell viability using the CCK-8 assay and
cultured the chondrocytes in the exponential phase at
1x104 cells/ml concentration in 96-well plates. Each
group contained 4 duplicate wells. As soon as the cells reached
70–80% confluence, the cells were treated using conditioned medium,
which contained various concentrations of H2S.
Subsequently, 10 μl of CCK-8 solution were
added to each well and the cells were placed in an incubator for
incubation. The incubation period lasted for 4 h. We performed
measurements for absorbance at 450 nm, with the help of a
microplate reader (Molecular Devices, Sunnyvale, CA, USA), and
calculated the percentage cell viability in 4 wells, calculating
the optical density (OD) value in specific groups.
The calculation was realized based on a relevant
formula: cell viability percentage = (OD treatment group - OD blank
group)/(OD control group - OD blank group) x100. The experiment was
carried out in triplicate.
Measurement of MMP-13, PGE2
and NO production in culture supernatants
We cultured the human OA chondrocytes
(1×106 cells/ml) in 6-well plates in complete DMEM with
10% FBS. The human OA chondrocytes (>85% confluent) were
serum-starved before being treated with various doses of NaHS
(0.06, 0.15, 0.3, 0.6 and 1.5 mM) for 30 min followed by
stimulation with or without IL-1β (10 ng/ml) for 24 h. Twenty-four
hours after the addition of IL-1β, we collected the cell
conditioned medium and then stored it at −80°C until analysis. The
concentration of PGE2 and MMP-13 was quantified using
MMP-13- and PGE2-specific ELISA kits in accordance with
the instructions of the manufacturer (Boster Bio-Engineering
Limited Company). The plates were read at 450 nm. In order to
measure NO production, we measured the nitrite concentration in the
culture supernatants using Griess reagent. The absorbance was read
at 540 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for MMP-13, iNOS and COX-2
Based on the instructions of Tiangen Biotech Co.,
Ltd. (Beijing, China) we used TRIzol reagent to extract the total
RNA (1 μg), which was reverse transcribed into cDNA. We used
the One-Step qPCR kit from the same company during amplification.
We also used SYBR-Green detection to determine the mRNA expression
of COX-2, iNOS and MMP-13 following the normalization of the
values. During the performing of PCR, the 7300 sequence detection
system was used. The primers used for PCR were as follows: MMP-13
forward, 5'-CGCCAGAAGAATCTGT CTAA-3' and reverse,
5'-CCAAATTATGGAGGAGATGC-3'; iNOS forward,
5'-CCTTACGAGGCGAAGAAGGACAG-3' and reverse,
5'-CAGTTTGAGAGAGGAGGCTCCG-3'; COX-2 forward,
5'-TTCAAATGAGATTGTGGGAAAA TTGCT-3' and reverse,
5'-AGATCATCTCTGCCTGAGTAT CTT-3'; and GAPDH forward
5'-GGTATCGTCGAAGGA CTCATGAC-3' and reverse, 5'-ATGCCAGTGAGCTTCCCG
TTCAGC-3'. The thermal cycling conditions for quantitative PCR were
95°C for 2 min followed by 45 cycles (at different temperatures and
for different periods of time). We used the temperature of 68°C for
data collection. Glyceraldehyde 3- phosphate dehydrogenase (GAPDH)
was used as an internal control. We quantified the quantitative PCR
data using the ΔCT method with the formula: n=2−(ΔCT targeted
gene - ΔCT GAPDH).
Western blot analysis
Western blot analysis was performed to determine the
effects of NaHS on IL-1β-dependent ERK1/2-phosphorylation,
IκBα-phosphorylation, IκBα degradation, NF-κB p65 translocation and
NF-κB p65 activity. Whole cell lysates, the chondrocyte monolayer
nuclear and cytoplasmic extracts were washed with Hanks’ solution 3
times and whole cell proteins were extracted using lysis buffer on
ice for half an hour. Centrifugation (700 × g) was performed to
remove the cell debris. The supernatants were stored at −80°C until
use. We measured the total protein concentration in the
cytoplasmic, nuclear and whole cell extracts using the BCA protein
assay kit (Uptima; Interchim, Montlucon, France). During the
process, BSA was used as a standard. When the adjustments for equal
amounts of total protein were made, we separated the proteins under
reducing conditions. The separated proteins were then transferred
onto PVDF membranes. The membranes were then blocked for 30 min at
room temperature in fresh blocking buffer [0.1% Tween-20 in
Tris-buffered saline (TBS-T) containing 5% fat-free milk] and then
incubated with either anti-ERK1/2 (1:1,000 dilution), anti-p-ERK1/2
(1:1,000 dilution), anti-IκBα (1:1,000 dilution),anti-p-IκBα
(1:1,000 dilution), anti-NF-κB p65 (1:1,000 dilution), anti-p-NF-κB
p65 (1:1,000 dilution), or anti-β-actin antibodies (1:5,000
dilution) in freshly prepared TBS-T with 3% skimmed milk overnight
with gentle agitation at 4°C. Following 3 washes with TBS-T, the
membranes were incubated with HRP-conjugated goat anti-rabbit
secondary antibody (1:3,000 dilution; Kangchen Biotech, Shanghai,
China) in TBS-T with 3% skimmed milk for 1.5 h at room temperature.
The membranes were rinsed 3 times with TBS-T, developed in ECL
solution and visualized using X-ray film. All the experiments were
repeated for no less than 3 times. ImageJ 1.47 software was used to
analyze and scan the films for quantification.
NF-κB activity assay
Based on relevant instructions provided by the
manufacturer (Active Motif), the TransAM™ NF-κB p65 transcription
factor assay kit was used to analyze the DNA-binding activity of
NF-κB. In brief, the kit with an ELISA format as its basis is used
to perform the anlaysis in a 96-well plate. During this process, in
the wells, the oligonucleotide which contained the NF-κB
consensus-binding sequence (5′-GGGACTTTCC-3′) was immobilized. We
incubated the nuclear extracts in the wells and then used a primary
antibody to perform the detection of bound NF-κB p65. We then
utilized an HRP-conjugated secondary antibody for the detection of
the bound primary antibody. The HRP-conjugated secondary antibody
also laid the foundation for colorimetric quantification. Using a
microplate reader (Molecular Devices), we took measurements for the
enzymatic product at 450 nm. For the purpose of monitoring the
specificity of the assay, we added (mutated) competitive control
into the wells with the wild-type (mutated) NF-κB consensus
oligonucleotide before adding the nuclear extracts.
Statistical analysis
All experiments reported in this study were
performed independently at least 3 times and the data are expressed
as the means ± SD. Statistical significance was assessed by one-way
analysis of variance (ANOVA) using SPSS 13.0 software. Differences
were considered statistically significant at P<0.05.
Results
Chondrocyte morphology and cytochemical
characteristics
The chondrocytes were allowed to adhere to the
bottom of dishes for 4 to 12 h. In all monolayer cultures, the
chondrocytes were typically flattened and elongated in shape.
Images which were obtained following staining with hematoxylin and
eosin revealed that the nuclei were stained an amethyst color and
that the cytoplasm was stained pink (Fig. 1A). We used both Safranin O and
Toluidine blue staining for the purpose of identifying the
chondrocytes. It was found that the nuclei were stained a crimson
color (Fig. 1B), or dark blue
(Fig. 1C). Under the influence of
chondroitin and glycosaminoglycan staining, the cellular cytoplasm
was stained light blue or light red. Therefore, as shown by these
results, the OA chondrocytic phenotype was evident.
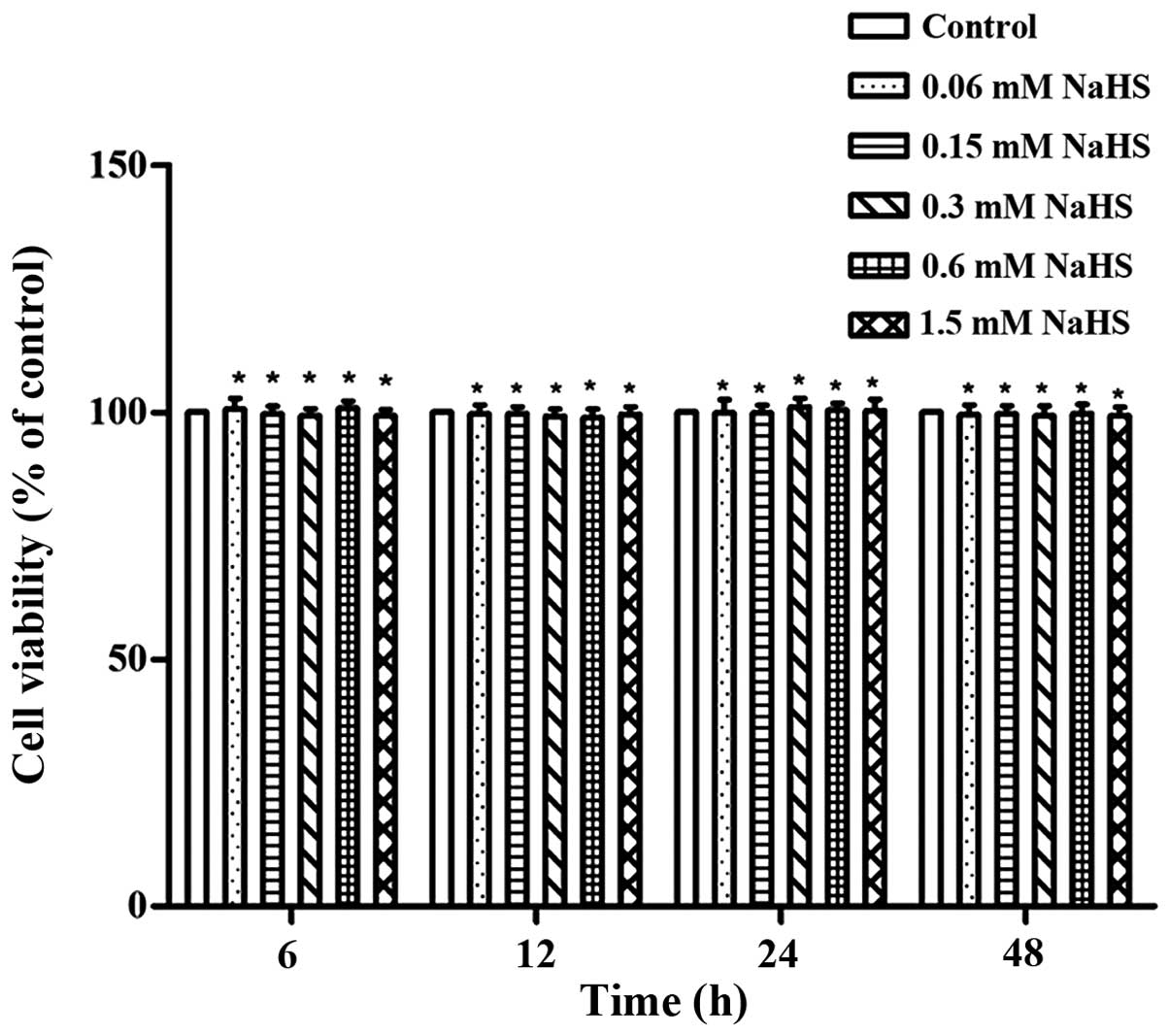
Effects of H2S on articular
chondrocyte viability
From the various H2S concentrations we
used in this study, no major cytotoxic effects on the OA
chondrocytes were observed. This was demonstrated by the assessment
of cell viability using CCK-8 assay (P>0.05; Fig. 2).
H2S suppresses the
IL-1β-induced secretion of NO, PGE2 and MMP-13 by OA
chondrocytes
As shown in Fig.
3, we determined the effects of H2S on the
generation of MMP-13, PGE2 and NO induced by IL-1β.
Various concentrations of NaHS (0, 0.06, 0.15, 0.3, 0.6 or 1.5 mM)
were used to treat the human OA chondrocytes for 30 min prior to
stimulation for 24 h with IL-1β (10 ng/ml). It was observed that
stimulation with IL-1β, compared with the unstimulated controls,
markedly promoted the production of PGE2, NO, and MMP-13
(P<0.05). However, treament of the OA chondrocytes with NaHS
(0.06, 0.15, 0.3 or 0.6 mM) prior to stimulation with IL-1β led to
a significant decrease in the production of MMP-13, PGE2
and NO (P<0.05), with the most prominent effects being observed
with the dose of 0.3 mM NaHS. CCK-8 assay revealed that cell
viability was not affected by H2S; the inhibitory
effects of H2S did not actually result in reduced cell
viability, even though the IL-1β-induced production of NO,
PGE2 and MMP-13 in the chondrocytes was not inhibited by
treatment with NaHS at the dose of 1.5 mM (P>0.05).
The induction of the expression of COX-2,
MMP-13 and iNOS by IL-1β in OA chondrocytes is attenuated by
H2S
The expression and production of COX-2, MMP-13 and
iNOS is crucial to the degradation of cartilage and human OA
chondrocytes (stimulated with IL-1β). We used RT-qPCR to determine
the effects of H2S on the gene expression levels of
COX-2, MMP-13 and iNOS induced by IL-1β. The results revealed that
the mRNA expression of COX-2, MMP-13 and iNOS was markedly
inhibited by NaHS (0.06, 0.15, 0.3 and 0.6 mM) (P<0.05; Fig. 4). The maximum response was
observed at the dose of 0.3 mM NaHS, in spite the fact that no
obvious inhibitory effect on the mRNA expression levels was
observed at the dose of 1.5 mM NaHS (P>0.05).
Effect of IL-1β on NF-κB signaling and
ERK1/2 activation
The activation the MAPK and NF-κB signaling pathways
induced by IL-1β is of great importance to the regulation cytokine
expression. We investigated the effects of IL-1β on the
phosphorylation of ERK1/2, IκBα and NF-κB p65, as well as on the
NF-κB binding activity in the OA chondrocytes at different time
points. In the control (unstimulated) OA chondrocytes, no
detectable phosphorylation of ERK1/2, IκBα and NF-κB p65 was
observed. The phosphorylation of NF-κB p65, ERK1/2 and IκBα in the
OA chondrocytes was markedly induced by stimulation with IL-1β
within a very short period of time (following treatment for 0.5, 1
and 2 h). The phosphorylation levels reached peak levels at 2 h and
then decreased to normal levels. However, stimulation with IL-1β
for longer periods of time (6 or 24 h) did not increase the
phosphorylation levels of of ERK1/2, IκBα and NF-κB p65 (Fig. 5A–C). Moreover, as regards the
NF-κB binding activity, which was in accordance with the changes
occurring during ERK1/2 activation, even though an increase was
observed within a short period of time, the activity was reduced as
time progressed (Fig. 5D). Based
on the time course, the 2 h time period was selected for use in the
subsequent experiments.
Effect of exposure time of OA
chondrocytes to H2S on ERK1/2 and NF-κB signaling
Without the involvement of IL-1β, we performed
120-min time-course experiments using 0.3 mM NaHS, and then
conducted another 2-h stimulation process with IL-1β (10 ng/ml).
The cells were lysed, and the phosphorylation levels of NF-κB p65,
IκBα and ERK1/2 were determined by western blot analysis. The NF-κB
binding activity was determined with the use of an NF-κB p65
transcription assay kit. The brief exposure of the OA chondrocytes
to NaHS led to the inhibition of the activation of NF-κB and ERK1/2
signaling induced by IL-1β (Fig.
6). The most prominent inhibitory effect of H2S was
observed in the cells pre-treated with H2S for half an
hour. Thus, pre-treatment for 30 min was used in the subsequent
experiments.
Effect of PD98059 (as an ERK pathway
inhibitor) on the suppressive effects of H2S on the
IL-1β-induced activation of NF-κB signaling
PD98059, as one of the effective inhibitors of ERK
was used by us in an effort to determine the involvement of ERK in
the suppressive effects of H2S on the IL-1β-induced
activation of NF-κB signaling. The cells were pre-incubated with
PD98059 (50 μM) for 1 h. The cells were then treated for
half an hour with NaHS (0.1 mM). Subsequently, the cells were
stimulated for 2 h with IL-1β (10 ng/ml). Following the collection
of the cell conditioned medium, the NF-κB p65 transcription assay
kit was used to perform NF-κB activity assay. In addition, the
levels of PGE2 and MMP-13 generated in the culture
medium were quantified with the use of specific ELISA kits. We also
used Griess reagent to measure the NO production. The inhibitory
effects of H2S on the IL-1β-induced activation of NF-κB
signaling were suppressed by PD98059 (Fig. 7A). The inhibitory effects of
H2S on the secretion of PGE2, MMP-13 and NO
(induced by IL-1β) were also abolished by PD98059 (Fig. 7B–D).
Discussion
Although the molecular biology of cartilage damage
in OA has been extensivey investigated (21–23), no single causative factor for
joint damage in OA has been identified to date. Generally speaking,
when the development of OA begins, synovium-produced inflammatory
mediators and cytokines are released. After being activated,
chondrocytes produce PGE2, MMPs, TNF-α and NO. Those
molecules facilitate catabolic activity and cause cartilage
structural changes (24). IL-1β
is not only important in the pathogenesis of OA, but also acts as a
powerful mediator of catabolic processes in articular chondrocytes
(25). This study used IL-1β to
mimic the pathophysiology of OA. As we had hypothesized, in
response to stimulation with IL-1β, an increase in the production
of NO, MMP-13 and PGE2 and in the expression of COX-2
and iNOS was observed. Our data demonstrate the effects of IL-1β on
the progression of OA.
A recent study demonstrated that pro-inflammatory
cytokines induce CSE (one of the H2S synthesizing
enzymes) expression and its activation through
p38-ERK-NF-κB-dependent pathways in chondrocytes, thus increasing
H2S synthesis (14).
Nevertheless, to the best of our knowledge, no investigation on the
effects of H2S on human OA chondrocytes has been
conducted to date. Therefore, this study has a huge significance in
this regard. We demonstrated that exogenous H2S at the
doses used in this study attenuated the IL-1β-induced inflammatory
responses in vitro. Our results demonstrate that
H2S exerts an anti-inflammatory effect by inhibiting
iNOS/NO and COX-2/PGE2 expression (or production), as
well as MMP-13 production by suppressing ERK1/2 and NF-κB
signaling.
Although H2S tissue production and the
levels of H2S in the blood have been the subject of much
controversy, certain studies have identified generous basal plasma
H2S levels in the 50–150 μM range according to
the spectrophotometric approach based on the indicator dye,
methylene blue (26). The
H2S concentration range in this study was decided
according to the H2S physiological level in the human
body. A previous study also revealed the cytoprotective effects of
H2S at micromolar concentrations (27). Certain cellular effects are linked
with the modulation of kinase pathways or intracellular caspases.
It was proven in this study that NaHS at the dose of 0.3 mM had the
most prominent cytoprotective effects. Previous data indicated that
exposure to H2S (millimolar) at higher concentrations
may be cytotoxic to cells. This is caused by many factors, such as
the generation of free radicals (28). According to our data, at the
concentration of 1.5 mM NaHS, H2S loses its
cytoprotective effects. It should be noted that we did not observe
any cytotoxic effects of H2S. The reason for this
difference in results between our study and other studies may be
due to the different inflammatory models used, or different
H2S donor doses used.
Among the pro-inflammatory factors, NO is a major
toxic substance with a rapid, as well as spontaneous reaction
[reacts with a superoxide anion (O2−)]. Through this
reaction, it forms both peroxynitrous acid (ONOOH; the conjugate
acid of peroxynitrite) and a peroxynitrite anion. Currently 3
isoformsof NOS have been identified. Among these isoforms, iNOS is
capable of inducing high levels of NO production, apart from its
being synthesized for response to inflammatory stimuli (29). In our study, we demonstrated that
H2S is capable of preventing the production of NO
induced by IL-1β, as well as the expression of iNOS at the mRNA
level in OA chondrocytes. Our results revealed that the inhibitory
effects of H2S on the IL-1β-induced iNOS protein
expression were mediated through the inhibition of NO production.
Our results were concurrent with those of Hu et al (30). According to their findings,
H2S is capable of inhibiting inflammation in microglia
stimulated with LPS, due to the fact that exogenous H2S
and endogenous H2S markedly suppress NO production. The
suppression of iNOS following stimulation with LPS is involved in
the mechanisms behind the anti-inflammatory effects of
H2S. H2S-NO interactions seem to be more and
more possible. Another study revealed that H2S, by
certain means, promotes both iNOS expression and NO production
(31). H2S
biosynthesis in LPS-induced endotoxemia has been shown to be
reduced by nitro-flurbiprofen-provided NO. The reason for this may
lie in transduction inhibition through the NF-κB pathway (31). It is possible that NO is capable
of controlling endogenous H2S biosynthesis and
regulating CSE expression in a positive manner. It seems that
gaseous mediator interactions are crucial; thus further studies are
warranted to investigate these interactions.
Although joint pain is one of the symptoms of OA,
information regarding the mechanisms responsible for OA-induced
pain is limited. It has been demonstrated that knee nociceptors may
be sensitized by peripheral pro-inflammatory mediators, such as
PGE2 and COX-2 (32).
IL-1β stimulates the production of COX-2 as a major inducer of pain
inflammation and a mediator in osteoarthritic joints, rather than
that of COX-1. In addition, PGE2 exerts catabolic
effects on chondrocytes (33,34). In our study, in spite of the fact
that only traces of COX-2 and PGE2 expression were
observed in the unstimulated human chondrocytes, the expression of
PGE2 and COX-2 was enhanced following stimulation with
IL-1β. We demonstrated that the addition of H2S
suppressed PGE2 production and elevated COX-2 gene
expression in the IL-1β-stimulated cells. Therefore, it is possible
that H2S exerts an anti-nociceptive effect, similar to
that of NSAIDs. This effect, by means of suppressing COX-2 and
PGE2 expression, seems to ameliorate OA symptoms. In the
study by Distrutti et al, H2S was shown to be a
promising analgesic. The systemic administration of different
H2S donors led to the opening of K+(ATP)
channels, inhibiting visceral nociception (35). Nevertheless, it was demonstrated
by Kawabata et al that either endogenous or exogenous
H2S has a peripheral activity that may promote pain. It
seems that the mechanisms of H2S-induced hyperalgesia
rely on the adjustment of T-type Ca2+ channel activity,
but are not dependent on K+(ATP) channels (36). Apart from H2S, CO and
NO also show nociceptive-related ambiguous activity (23).
MMPs are synthesized in articular joints by synovial
cells and chondrocytes. MMP-13 is considered a main cause for the
degradation of collagens and aggrecan in cartilage. It is
considered that OA chondrocytes express MMP-13 (37). Thus, MMP-13 has become a
therapeutic target with the most potential. In the present study,
we wished to determine whether H2S exerts
chondroprotective effects through the suppression of MMP-13 in
chondrocytes. Our results revealed that H2S inhibited
MMP-13 expression induced by IL-1β. This inhibition occurred at the
protein and mRNA level and was independent of the dose used in
human OA chondrocytes. The results obtained in the study by Vacek
et al were also similar. Their study demonstrated that
H2S inhibited collagen helix destruction and MMP
activation by suppressing oxidative stress (38).
The underlying cellular targets of the protective
effects of H2S on IL-1β-induced OA remain to be
elucidated. In this study, we analyzed the potential molecular
mechanisms responsible for the inhibitory effects of H2S
on inflammatory mediators in response to IL-1β in chondrocytes.
Among the downstream effects of IL-1β stimulation, many of these
effects are regulated by the activation of NF-κB signaling
(39,40). NF-κB is retained in the cytoplasm
with IκBα in an inactive state. IL-1β has the capacity to activate
NF-κB, resulting in the change of the position of NF-κB p65 so as
to regulate gene expression (17). Our results revealed that IL-1β
induced IκBα and NF-κB p65 phosphorylation, which was reduced by an
exogenous H2S donor (NaHS) at the concentration of 0.3
mM in short-term treatment (15–30 min). However, the
phosphorylation of IκBα and NF-κB p65 was not further inhibited by
long-term H2S treatment (60–120 min) following
stimulation with IL-1β. This reveals that the effects of
H2S may be produced rapidly, a fact having been
confirmed in a previous study, which demonstrated that the
inhibitory effects of H2S are not determined by the
release rate (41). Our results
are consistent with the findings of a previous study on the
protective role of GYY4137 (13).
On the whole, we found new evidence to support the hypothesis that
the NO-, PGE2- and MMP-13-mediated inflammation and
cytotoxicity are regulated by the activation of NF-κB and that
H2S protests against inflammation induced by IL-1β by
inhibiting NF-κB signaling.
It has also been suggested by previous findings that
the regulatory role of H2S on MAPK phosphorylation may
exist and that the regulation contributes to different types of
pathological and physiological roles in all types of tissues and
cells (42,43). The fact that the IL-1β-induced
phosphorylation of ERK1/2 was decreased in fibroblast-like
synoviocytes (FLS) of OA was also previously demonstrated (42,44). Since it is known that ERK1/2 is an
upstream activator of NF-κB signaling, we conducted investigations
on the relevant functions of ERK1/2. Therefore, we used the PD98059
inhitibor to block the activation of ERK1/2. The inhibition of
ERK1/2 by pre-treatment with PD98059 abolished the inhibitory
effects of H2S on cytokines following stimulation with
IL-1β, while attenuating its inhibitory effects on the activation
of NF-κB signaling induced by IL-1β. These findings, in a
straightforward manner, indicate that the cytoprotective effects of
H2S in OA may involve the ERK-NF-κB signaling pathway.
Nevertheless, attention should be paid to the fact that
pre-treatment with PD98059 did not completely abolish the
cytoprotective effects of H2S. This means that
H2S may regulate the activation of NF-κB signaling
through mediators apart from ERK1/2. According to previous
research, the inhibitory effects of H2S on inflammation
are exerted by suppressing the channels for p38 and NF-κB signaling
and by upregulating HO-1 expression (45). It is possible that the different
stimuli and different types of cells used may attribute to the
different results obtained by different studies. Nevertheless, the
regulatory mechanisms of H2S in chondrocyte inflammation
remain to be fully elucidated. Further studies are warranted to
determine the precise channels of signaling involved in this
process.
In conclusion, this study proves that a
cytoprotective effect against OA induced by IL-1β is conferred by
H2S, and that the process is realized by suppressing the
channel for ERK-NF-κB signaling in chondrocytes, which is activated
by IL-1β. This study has provided new insight into the
cytoprotective effects H2S in OA. The data from our
study also suggest that H2S may prove to be a potential
therapeutic agent for the treatment of OA.
Acknowledgments
The completion of this study was possible due to the
assistance and support of the Natural Science Foundation of China
(grant NSFC 81141078 to K.S.).
References
|
1
|
Arden NK and Leyland KM: Osteoarthritis
year 2013 in review: clinical. Osteoarthritis Cartilage.
21:1409–1413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sulzbacher I: Osteoarthritis: histology
and pathogenesis. Wien Med Wochenschr. 163:212–219. 2013.
View Article : Google Scholar
|
|
3
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar
|
|
4
|
Wojdasiewicz P, Poniatowski LA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balaganur V, Pathak NN, Lingaraju MC, et
al: Chondroprotective and anti-inflammatory effects of
S-methylisothiourea, an inducible nitric oxide synthase inhibitor
in cartilage and synovial explants model of osteoarthritis. J Pharm
Pharmacol. 66:1021–1031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldring MB, Otero M, Plumb DA, et al:
Roles of inflammatory and anabolic cytokines in cartilage
metabolism: signals and multiple effectors converge upon MMP-13
regulation in osteoarthritis. Eur Cell Mater. 21:202–220.
2011.PubMed/NCBI
|
|
7
|
Verhagen AP, Bierma-Zeinstra SM, Boers M,
et al: Balneotherapy for osteoarthritis. Cochrane Database Syst
Rev. 4:CD0068642007.PubMed/NCBI
|
|
8
|
Ekundi-Valentim E, Santos KT, Camargo EA,
Denadai-Souza A, Teixeira SA, Zanoni CI, Grant AD, Wallace J,
Muscará MN and Costa SK: Differing effects of exogenous and
endogenous hydrogen sulphide in carrageenan-induced knee joint
synovitis in the rat. Br J Pharmacol. 159:1463–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Costantino M, Filippelli A, Quenau P,
Nicolas JP and Coiro V: Sulphur mineral water and SPA therapy in
osteoarthritis. Therapie. 67:43–48. 2012.In French. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kloesch B, Liszt M and Broell J:
H2S transiently blocks IL-6 expression in rheumatoid
arthritic fibroblast-like synoviocytes and deactivates p44/42
mitogen-activated protein kinase. Cell Biol Int. 34:477–484. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang R: Hydrogen sulfide: the third
gasotransmitter in biology and medicine. Antioxid Redox Signal.
12:1061–1064. 2010. View Article : Google Scholar
|
|
12
|
Lowicka E and Beltowski J: Hydrogen
sulfide (H2S) - the third gas of interest for
pharmacologists. Pharmacol Rep. 59:4–24. 2007.
|
|
13
|
Li L, Fox B, Keeble J, et al: The complex
effects of the slow-releasing hydrogen sulfide donor GYY4137 in a
model of acute joint inflammation and in human cartilage cells. J
Cell Mol Med. 17:365–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fox B, Schantz JT, Haigh R, et al:
Inducible hydrogen sulfide synthesis in chondrocytes and
mesenchymal progenitor cells: is H S a novel cytoprotective
mediator in the inflamed joint? J Cell Mol Med. 16:896–910. 2012.
View Article : Google Scholar
|
|
15
|
Whiteman M, Haigh R, Tarr JM, Gooding KM,
Shore AC and Winyard PG: Detection of hydrogen sulfide in plasma
and knee-joint synovial fluid from rheumatoid arthritis patients:
relation to clinical and laboratory measures of inflammation. Ann
NY Acad Sci. 1203:146–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andruski B, McCafferty DM, Ignacy T,
Millen B and McDougall JJ: Leukocyte trafficking and pain
behavioral responses to a hydrogen sulfide donor in acute
monoarthritis. Am J Physiol Regul Integr Comp Physiol.
295:R814–R820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kloesch B, Liszt M, Krehan D, Broell J,
Kiener H and Steiner G: High concentrations of hydrogen sulphide
elevate the expression of a series of pro-inflammatory genes in
fibroblast-like synoviocytes derived from rheumatoid and
osteoarthritis patients. Immunol Lett. 141:197–203. 2012.
View Article : Google Scholar
|
|
19
|
Jiang B, Xu S, Hou X, Pimentel DR, Brecher
P and Cohen RA: Temporal control of NF-kappaB activation by ERK
differentially regulates interleukin-1beta-induced gene expression.
J Biol Chem. 279:1323–1329. 2004. View Article : Google Scholar
|
|
20
|
Zhi L, Ang AD, Zhang H, Moore PK and
Bhatia M: Hydrogen sulfide induces the synthesis of proinflammatory
cytokines in human monocyte cell line U937 via the ERK-NF-kappaB
pathway. J Leukoc Biol. 81:1322–1332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stolz M, Gottardi R, Raiteri R, et al:
Early detection of aging cartilage and osteoarthritis in mice and
patient samples using atomic force microscopy. Nat Nanotechnol.
4:186–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gossan N, Zeef L, Hensman J, et al: The
circadian clock in murine chondrocytes regulates genes controlling
key aspects of cartilage homeostasis. Arthritis Rheum.
65:2334–2345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsubota M and Kawabata A: Role of hydrogen
sulfide, a gasotransmitter, in colonic pain and inflammation.
Yakugaku Zasshi. 134:1245–1252. 2014.In Japanese. View Article : Google Scholar
|
|
24
|
Houard X, Goldring MB and Berenbaum F:
Homeostatic mechanisms in articular cartilage and role of
inflammation in osteoarthritis. Curr Rheumatol Rep. 15:3752013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Li F, Fan C, Wang C and Ruan H:
Effects and relationship of ERK1 and ERK2 in interleukin-1β-induced
alterations in MMP3, MMP13, type II collagen and aggrecan
expression in human chondrocytes. Int J Mol Med. 27:583–589.
2011.PubMed/NCBI
|
|
26
|
Szabo C: Hydrogen sulphide and its
therapeutic potential. Nat Rev Drug Discov. 6:917–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kimura H: The physiological role of
hydrogen sulfide and beyond. Nitric Oxide. 41:4–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ang SF, Moochhala SM, MacAry PA and Bhatia
M: Hydrogen sulfide and neurogenic inflammation in polymicrobial
sepsis: involvement of substance P and ERK-NF-κB signaling. PLoS
One. 6:e245352011. View Article : Google Scholar
|
|
29
|
Bentz M, Zaouter C, Shi Q, et al:
Inhibition of inducible nitric oxide synthase prevents lipid
peroxidation in osteoarthritic chondrocytes. J Cell Biochem.
113:2256–2267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu LF, Wong PT, Moore PK and Bian JS:
Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation
by inhibition of p38 mitogen-activated protein kinase in microglia.
J Neurochem. 100:1121–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeong SO, Pae HO, Oh GS, et al: Hydrogen
sulfide potentiates interleukin-1beta-induced nitric oxide
production via enhancement of extracellular signal-regulated kinase
activation in rat vascular smooth muscle cells. Biochem Biophys Res
Commun. 345:938–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang RX, Ren K and Dubner R:
Osteoarthritis pain mechanisms: basic studies in animal models.
Osteoarthritis Cartilage. 21:1308–1315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Boer TN, Huisman AM, Polak AA, et al:
The chondroprotective effect of selective COX-2 inhibition in
osteoarthritis: ex vivo evaluation of human cartilage tissue after
in vivo treatment. Osteoarthritis Cartilage. 17:482–488. 2009.
View Article : Google Scholar
|
|
34
|
Li X, Ellman M, Muddasani P, et al:
Prostaglandin E2 and its cognate EP receptors control human adult
articular cartilage homeostasis and are linked to the
pathophysiology of osteoarthritis. Arthritis Rheum. 60:513–523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Distrutti E, Sediari L, Mencarelli A, et
al: Evidence that hydrogen sulfide exerts antinociceptive effects
in the gastrointestinal tract by activating KATP channels. J
Pharmacol Exp Ther. 316:325–335. 2006. View Article : Google Scholar
|
|
36
|
Kawabata A, Ishiki T, Nagasawa K, et al:
Hydrogen sulfide as a novel nociceptive messenger. Pain. 132:74–81.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rosa SC, Rufino AT, Judas FM, Tenreiro CM,
Lopes MC and Mendes AF: Role of glucose as a modulator of anabolic
and catabolic gene expression in normal and osteoarthritic human
chondrocytes. J Cell Biochem. 112:2813–2824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vacek TP, Qipshidze N and Tyagi SC:
Hydrogen sulfide and sodium nitroprusside compete to
activate/deactivate MMPs in bone tissue homogenates. Vasc Health
Risk Manag. 9:117–123. 2013.PubMed/NCBI
|
|
39
|
Shakibaei M, Csaki C, Nebrich S and
Mobasheri A: Resveratrol suppresses interleukin-1beta-induced
inflammatory signaling and apoptosis in human articular
chondrocytes: potential for use as a novel nutraceutical for the
treatment of osteoarthritis. Biochem Pharmacol. 76:1426–1439. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ding QH, Cheng Y, Chen WP, Zhong HM and
Wang XH: Celastrol, an inhibitor of heat shock protein 90β potently
suppresses the expression of matrix metalloproteinases, inducible
nitric oxide synthase and cyclooxygenase-2 in primary human
osteoarthritic chondrocytes. Eur J Pharmacol. 708:1–7. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perry MM, Hui CK, Whiteman M, et al:
Hydrogen sulfide inhibits proliferation and release of IL-8 from
human airway smooth muscle cells. Am J Respir Cell Mol Biol.
45:746–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kloesch B, Liszt M, Steiner G and Broll J:
Inhibitors of p38 and ERK1/2 MAPkinase and hydrogen sulphide block
constitutive and IL-1β-induced IL-6 and IL-8 expression in the
human chondrocyte cell line C-28/I2. Rheumatol Int. 32:729–736.
2012. View Article : Google Scholar
|
|
43
|
Zhang H, Moochhala SM and Bhatia M:
Endogenous hydrogen sulfide regulates inflammatory response by
activating the ERK pathway in polymicrobial sepsis. J Immunol.
181:4320–4331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sieghart D, Kiener HP, Kloesch B and
Steiner G: A9.3 Hydrogen sulfide and MEK1 inhibition reduce
IL-1beta-induced activation of fibroblast-like synoviocytes. Ann
Rheum Dis. 73:A932014. View Article : Google Scholar
|
|
45
|
Oh GS, Pae HO, Lee BS, et al: Hydrogen
sulfide inhibits nitric oxide production and nuclear factor-kappaB
via heme oxygenase-1 expression in RAW264.7 macrophages stimulated
with lipopolysaccharide. Free Radic Biol Med. 41:106–119. 2006.
View Article : Google Scholar : PubMed/NCBI
|