Introduction
Curcumin, a hydrophobic polyphenol derived from the
rhizome of the herb, Curcuma longa (turmeric), has a wide
spectrum of biological and pharmacological activities. Chemically,
curcumin is a bis-α,β-unsaturated β-diketone (commonly known as
diferuloylmethane), which exhibits keto-enol tautomerism having a
predominant keto form in acidic and neutral solutions and a stable
enol form in alkaline media. Turmeric has traditionally been used
in the treatment of a number of ailments, particularly as an
anti-inflammatory agent, and curcumin has been identified as the
active component (1). Curcumin
has been shown to possess anti-inflammatory (2), antibacterial (3,4),
antifungal and anti-yeast (5),
anti-hypercholesterolemic (6),
anticancer (7–10), antimutagenic (11), antiparasitic (12), anti-tumor-promoting (13), anti-proliferative (14), as well as MDR modulator effects
(15), among others. In previous
studies of ours, we reported that curcumin specifically inhibited
the activity of DNA polymerase λ (Pol λ), a DNA
repair/recombination Pol, with a half-maximal inhibitory
concentration (IC50) of 7.0 μM (16,17). This compound also suppressed the
lipopolysaccharide (LPS)-induced production of tumor necrosis
factor-α (TNF-α) in RAW264.7 murine macrophages and attenuated
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear
inflammation in mice (16,18).
We have also previousy suggested that the anti-inflammatory effects
of curcumin may due to the inhibition of Pol λ activity (19).
Several investigators, ourselves included, have
investigated plasma curcumin levels in clinical trials and have
reported that plasma levels remained low despite the intake of gram
doses of curcumin (20–23). Furthermore, a curcumin intake
>8 g was not shown to increase blood curcumin levels in healthy
volunteers (24). Thus, the low
bioavailability of curcumin is a major issue facing investigators
seeking to verify the therapeutic efficacy of this promising agent
in clinical trials. Therefore, we focused on improving the
bioavailability of curcumin through the use of an innovative drug
delivery system, whereby curcumin is incorporated into a lipid
emulsion of various nano-order diameters (i.e., lipid
nanoemulsion).
Emulsion-based delivery systems are being
increasingly used in the pharmaceutical industry to incorporate
lipophilic bioactive components (25). Nanoemulsions, or oil-in-water
(O/W) emulsions, can be prepared by solubilizing lipophilic
bioactive components within the oil phase, and then homogenizing
this phase with an aqueous phase containing a water-soluble
emulsifier. The size of the droplets produced depends on the
composition of the system and the homogenization method employed.
O/W conventional emulsions and nanoemulsions are both
thermodynamically unstable systems that consist of
emulsifier-coated lipid droplets dispersed within an aqueous medium
(26). Lipid emulsions have also
been used as a promising drug delivery system to target tissues
(27,28). Moreover, a number of studies have
demonstrated the validity of lipid emulsions as a parenteral drug
delivery device (29–31). They have certain advantages, such
as good biocompatibility, biodegradability, physical stability and
ease of large-scale production. In addition, they can incorporate
hydrophobic and amphipathic drugs due to their structural
characteristics. Since curcumin is hydrophobic in nature, it can be
incorporated into a lipid emulsion. Thus, lipid emulsions represent
a promising means with which to deliver curcumin.
In the present study, we prepared curcumin lipid
nanoemulsions of various particle sizes and assessed the effects of
particle size the on physiological activities, such as the
anti-inflammatory and anti-allergoc activities, of curcumin in
vitro and in vivo.
Materials and methods
Materials
Curcumin and LPS were purchased from Sigma-Aldrich,
Inc. (St. Louis, MO, USA). Murine macrophages (RAW264.7) and rat
basophilic leukemia (RBL-2H3) cells were obtained from the American
Type Culture Collection (ATCC, Manassas, VA, USA). All other
reagents were of analytical grade and were obtained from Nacalai
Tesque, Inc. (Kyoto, Japan).
Preparation of curcumin lipid
nanoemulsions
The curcumin lipid nanoemulsions (O/W emulsions)
were prepared using a slightly modified thin-film hydration method
at room temperature (24°C) as previously described (32,33). In brief, the emulsions consisted
of soybean oil, hydrogenated L-α-phosphatidylcholine (HEPC) from
egg yolk, water, curcumin and an appropriate co-surfactant, such as
polyoxyethylene [20] sorbitan monooleate (Tween-80). The soybean
oil and HEPC were dissolved in 4 ml of chloroform. Curcumin was
dissolved in 4 ml of chloroform. Tween-80 was dissolved in 2 ml of
chloroform. A mixture of these 3 solutions was dried by rotary
evaporation for 20 min, and then subjected to vacuum desiccation
for 3 h to generate a dry thin film. The film was hydrated with 30
ml of distilled water warmed at 50°C in a bath-type sonicator
(Branson-Yamato 2510, Bransonic; Emerson-Japan, Kanagawa, Japan).
In order to obtain the desired emulsion particle size, the hydrated
curcumin emulsion was sonicated for 60 min maximum with a bath-type
sonicator thermostated to 25–55°C. The sonication was performed as
follows: 3 min of sonication and subsequent 2 min of cooling, which
was repeated for 60 min.
Measurement of particle size
The particle size of the lipid nanoemulsions was
measured by a dynamic light scattering method using a Zetasizer
3000HSA (Malvern Instruments Ltd., Worcestershire, UK) at room
temperature. Particle size data were expressed as the means of the
Z-average of 3 independent batches of the nanoemulsions.
Cell culture
RAW264.7 murine macrophages were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1.0 g/l
glucose and sodium pyruvate plus 10% fetal bovine serum (FBS), 5 mM
L-glutamine, 50 U/ml penicillin and 50 U/ml streptomycin. Rat
basophilic leukemia RBL-2H3 cells were cultured in Eagle’s minimum
essential medium (EMEM) supplemented with 10% FBS, 5 mM
L-glutamine, 50 U/ml penicillin and 50 U/ml streptomycin. All cells
in standard medium were cultivated in a humidified incubator of 95%
air and 5% CO2 at 37°C.
Animals
Male 8-week-old C57BL/6j mice (body weight range,
22–27 g) and male 6-week-old ICR mice (body weight range, 25–27 g)
were obtained from Japan SLC, Inc. (Hamamatsu, Japan). Mice that
had been bred in-house with free access to a standard diet (MF;
Oriental Yeast Co., Ltd., Osaka, Japan) and water were used for all
the experiments. All mice were maintained under a 12 h light/dark
cycle and housed at a room temperature of 25°C. All animal studies
were approved by the Kobe Gakuin University Animal Committee (Kobe,
Japan) according to the university guidelines for the ‘Care and Use
of Laboratory Animals’.
Measurement of TNF-α secretion in
RAW264.7 cells
The RAW264.7 cells were seeded at 4×104
cells/well in a 96-well plate and incubated for 24 h. The cells
were then treated with a final concentration of 6.5 μg/ml of
the test compound for 30 min prior to the addition of 50 ng/ml LPS,
a major component of the outer membrane of Gram-negative bacteria.
Following LPS stimulation for 24 h, the cell culture medium was
collected and the amount of secreted TNF-α was quantified using a
commercially available enzyme-linked immunosorbent assay (ELISA)
development system (eBioscience Co., Ltd., Kobe, Japan) in
accordance with the manufacturer’s instructions.
Measurement of serum levels of TNF-α in
mice
The C57BL/6j mice were given only water for 24 h
prior to the oral administration of the test samples. The mice were
orally administered 8 mg/kg body weight of the test compound or
200 μl of distilled water as a vehicle control. After 2 h,
the mice were intraperitoneally injected with 250 μg/kg body
weight of LPS dissolved in phosphate-buffered saline (PBS) or 200
μl of PBS as a vehicle control. After 1 h, the mice were
sacrificed, and whole blood was collected. The serum was separated
by centrifugation at 5,000 × g for 10 min at 4°C. The serum TNF-α
levels were measured using the above-mentioned ELISA kit.
Measurement of β-hexosaminidase release
in RBL-2H3 cells
It has been previously reported that the release of
β-hexosaminidase positively correlates with the release of
histamine, which is a major component of mast cell granules
(34); therefore, in this study,
mast cell degranulation was estimated using a β-hexosaminidase
release assay as previously described by Sato et al
(35), with minor modifications.
Briefly, the RBL-2H3 cells were seeded at 2×105
cells/well in a 24-well plate and incubated for 24 h. The cells
were washed with Tyrode’s buffer (8 mg/ml NaCl, 1 mg/ml glucose, 1
mg/ml NaHCO3, 0.2 mg/ml KCl and 0.05 mg/ml
NaH2PO4) containing 1 mM CaCl2 and
0.5 mM MgCl2. Individual test compounds were added at a
final concentration of 6.5 μg/ml in Tyrode’s buffer. The
cells were then stimulated with 5 μM of A23187 (a calcium
ionophore) and incubated for 90 min. Following centrifugation for 3
min at 10°C, 100 μl of the cell supernatant were mixed with
substrate solution (2 mM
p-nitrophenyl-N-acetyl-β-D-glucosaminide in 0.1 M
sodium citrate buffer, pH 4.5). The mixture was incubated for 90
min at 37°C and the reaction was then terminated by the addition of
stop buffer composed of 0.2 M glycine buffer at pH 11.0. The
absorbance at 405 nm was measured using a microplate reader
(Vmax-K; Molecular Devices, LLC, Sunnyvale, CA, USA). The effects
of the test compounds on the release of β-hexosaminidase are
expressed as a percentage, calculated using the following formula:
percentage activity = [(β-hexosaminidase release with test
compound)/β-hexosaminidase release without test compound] ×100.
Measurement of anti-anaphylactic activity
in mice
The passive cutaneous anaphylaxis (PCA) reaction was
conducted as described in a previous study (35). The ICR mice were fasted 24 h prior
to the experiment. The mice were sensitized by an intradermal
injection of 0.1 μg of anti-dinitrophenyl (DNP)
immunoglobulin E (IgE) in the ear under chloral hydrate anesthesia,
and 3 h later, a test compound (100 mg/kg) or saline were orally
administered. The mice were then challenged by an intravenous
administration of 0.2 ml (1 mg/ml) of DNP-labeled human serum
albumin containing 2% Evans blue dye. The animals in the control
group were administered saline. Thirty minutes after the challenge,
the mice were subsequently sacrificed by diethyl ether anesthesia
and the ears were removed and weighed. These resulting solutions
were incubated in 200 μl of 1 N KOH overnight at 64°C for
the measurement of the amount of Evans blue dye present in the
exudates. The dissolved tissue solution was added to 400 μl
of a mixture of acetone and 0.6 N phosphoric acid (5/13, v/v), and
the optical density of the resulting solution was measured at 620
nm. The amount of dye in the exudates was calculated from an Evans
blue standard curve and the results are expressed as the percentage
of the mean exudate dye amount from the mice treated with the test
compound compared to the controls.
Measurement of curcumin concentration in
serum following the oral administration of the test compounds in
mice
The C57BL/6j mice were given only water for 24 h
prior to the oral administration of the test compounds. The mice
were orally administered 8 mg/kg body weight of the test compound
or 20 0 μl of distilled water as a vehicle control. Blood
was collected every 1 h from the tail vein until 5 h after the oral
administration. Serum was separated by centrifugation at 5,000 × g
for 10 min at 4°C. Subsequently, one volume of acetonitrile was
added to each volume of serum for deproteinization, and the mixture
was centrifuged at 15,000 × g for 15 min. Following centrifugation,
the acetonitrile phase was collected and passed through a 0.22
μm Millipore membrane filter (Millipore, Bedford, MA, USA)
prior to analysis by high-performance liquid chromatography
(HPLC).
The HPLC system comprised of a chromatographic pump
(LC-20AD), a fluorescence detector (RF-10AXL) and a degasser
(DGU-20A3) (all from Shimadzu Co., Ltd., Kyoto, Japan). A mobile
phase consisting of acetonitrile and 20 mM formic acid (45:55, v/v)
was used at a flow rate of 2 ml/min. A reversed-phase Mayysil
RP-18GP column (4.6×150 mm, particle size 5 μm; Kanto
Chemical Co., Inc., Tokyo, Japan) was used for the HPLC separation.
The mobile phase was degassed using a bath-type sonicator
(Branson-Yamato 2510, Bransonic; Emerson-Japan) before use. The
HPLC r unning time was 10 min and fluorescence was detected at Ex
400 nm/Em 527 nm. The amount of curcumin in serum was calculated
from the standard curve of fluorescence absorption by curcumin and
the results were expressed as a percentage of the mean curcumin
amount from the mice treated with the test compound compared with
the controls.
Statistical analysis
All data are expressed as the means ± standard
deviation (SD) of at least 6 independent determinations for each
experiment. The statistical significance between each experimental
group was analyzed using the Steel-Dwass test, and a probability
value of 0.01 and 0.05 was used as the criterion of
significance.
Results
Formulation of curcumin lipid
nanoemulsions
The 5 formulations of curcumin lipid nanoemulsion
are listed in Table I. A curcumin
loading dose of 15 mg was used. In this experiment, soybean oil was
used as the oil component of the curcumin lipid nanoemulsions,
since curcumin is a lipophilic molecule that exhibits limited
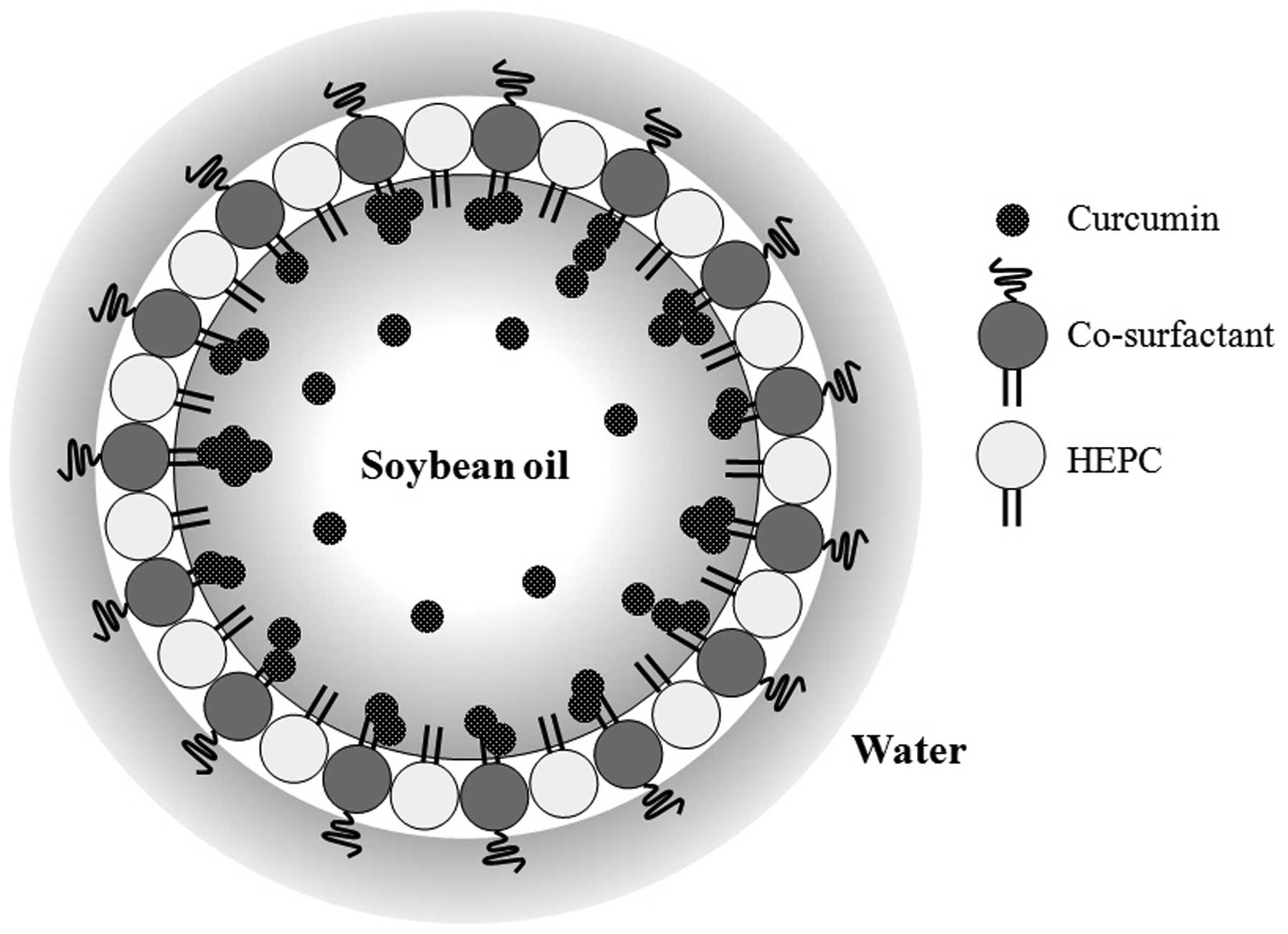
solubility in water. A schematic illustrates the possible structure
of the curcumin lipid nanoemulsion (Fig. 1). HEPC and a co-surfactant, such
as Tween-80, surround the soybean oil in which curcumin is
dissolved.
 | Table IFormulation of the curcumin lipid
nanoemulsions. |
Table I
Formulation of the curcumin lipid
nanoemulsions.
| Components and
conditions | Formulation
|
|---|
| 50-nm Blank
emulsion | 50-nm Curcumin
emulsion | 100 nm-Curcumin
emulsion | 200 nm-Curcumin
emulsion | Curcumin
suspension |
|---|
| Soybean oil
(ml) | 1 | 1 | 1 | 1 | – |
| Curcumin (mg) | – | 15 | 15 | 15 | 15 |
| HEPC (mg) | 250 | 250 | 250 | 250 | – |
| Tween-80 (mg) | 350 | 350 | 350 | 360 | – |
| Distilled water
(ml) | 30 | 30 | 30 | 30 | 30 |
| Total sonication
time (min) | 60 | 60 | 5 | 3 | 60 |
| Temperature of
water bath (°C) | 33 | 33 | 25 | 30 | 33 |
The preparation conditions were optimized according
to the sonication time and the temperature of the water bath.
Curcumin lipid nanoemulsions with particle sizes of 50, 100 and 200
nm were successfully prepared. The particle size distribution of
these nanoemulsions appeared as a single peak except for the 200-nm
sample (Fig. 2A). We also
prepared a 50-nm blank emulsion without curcumin and a water
suspended curcumin sample by sonication for 60 min at 33°C (i.e.,
curcumin suspension) as controls. The particle diameter of the
curcumin suspension could not be measured, as the particle size of
the curcumin in this suspension was too large. The nanoemulsions
were homogeneous, optically opaque systems during storage at 4°C
for over one month, whereas the curcumin suspension consisted of a
yellow turbid layer on the top, and was unstable in dispersion
(Fig. 2B).
Inhibitory effect of curcumin lipid
nanoemulsions on LPS-induced TNF-α secretion in vitro
LPS-stimulated basophils produce and release
cytokines, eventually recruiting inflammatory cells with
neutrophils and eosinophils (36,37). The inflammatory cytokine, TNF-α,
activates the nuclear factor-κB (NF-κB) signaling pathway by
binding to the TNF-α receptor, thereby initiating an inflammatory
response implicated in various inflammatory diseases (38). The inhibitory effects of the
various particle sizes of the curcumin lipid nanoemulsions against
LPS-induced TNF-α secretion in RAW264.7 cells were examined and the
anti-inflammatory effects of the formulations were determined. In
the RAW264.7 cells, none of the prepared curcumin lipid
nanoemulsions tested showed cytotoxicity at 10 μg/ml (data
not shown); these nanoemulsions and the curcumin suspension had no
effect on the proliferation of peritoneal macrophages. The RAW264.7
cells treated with 6.5 μg/ml of blank emulsion produced 693
pg/ml of TNF- α following stimulation with 50 ng/ml of LPS, and the
TNF- secretion in the controls was set at 100%. As shown in
Fig. 3A, we found that 6.5
μg/ml of the 100-nm curcumin lipid nanoemulsion
significantly suppressed the LPS -induced production of TNF-α, and
the suppressive effects of the formulations were ranked as follows:
100-nm curcumin emulsion > 200-nm curcumin emulsion > 50-nm
curcumin emulsion ≈ curcumin suspension. This result suggested that
the particle size of the curcumin lipid nanoemulsions is important
for the suppression of TNF-α production.
Inhibitory effects of curcumin lipid
nanoemulsions on LPS-induced serum TNF-α production in vivo
To evaluate the anti-inflammatory effects of
curcumin lipid nanoemulsions in vivo, we investigated their
inhibitory activity against LPS-induced serum TNF-α production in
mice. As shown in Fig. 3B, the
administration of LPS (250 μg/kg) significantly increased
the production of TNF-α; however, the blank emulsion at a dose of 8
mg/kg had no effect (100% TNF-α production). The 100-nm curcumin
lipid nanoemulsion (8 mg/kg) had the most prominent suppressive
effect on the production of TNF-α among the formulations tested.
The 50- and 200-nm curcumin lipid nanoemulsions slightly suppressed
TNF-α production, whereas the curcumin suspension showed minimal
effects. These in vivo results support the results of the
in vitro experiment with cultured RAW264.7 cells.
Inhibitory effects of curcumin lipid
nanoemulsions on the release of β-hexosaminidase in vitro
The release of β-hexosaminidase is commonly used as
an indicator of mast cell degranulation (39). The release of histamine and other
chemical mediators from mast cells is important in initiating the
immediate type anaphylactic reaction (40). Thus, the effect of the curcumin
lipid nanoemulsions on the release of β-hexosaminidase was
investigated using RBL-2H3 cells treated with the calcium
ionophore, A23187. The results confirmed that the nanoemulsions and
the curcumin suspension did not affect RBL-2H3 cell growth and did
not inhibit β-hexosaminidase enzyme activity (data not shown). The
degree of degranulation was calculated from β-hexosaminidase
activity in the supernatant and cell lysate. As a result, the 50-,
100- and 200-nm curcumin nanoemulsions inhibited the release of
β-hexosaminidase by 35, 61 and 40%, respectively, at a dose of 10
μg/ml compared to the controls (curcumin suspension)
(Fig. 4A). These results indicate
the importance of particle size in the activity of curcumin lipid
nanoemulsions.
Anti-allergic effect of curcumin lipid
nanoemulsions in vivo
The IgE-mediated PCA reaction is frequently used as
a tool for the in vivo investigation of the mechanisms of
immediate hypersensitivity reactions (35). In this study, we examined the
in vivo bioactivity of curcumin lipid nanoemulsions against
allergic reactions. The curcumin nanoemulsions (50, 100 and 200 nm)
inhibited the PCA reaction in mice by 31, 85 and 69%, respectively,
at a dose of 8 mg/kg (Fig. 4B).
By contrast, under the same conditions, the 50-nm curcumin emulsion
and the curcumin suspension had no effect on the PCA reaction.
These in vivo results support the observed in vitro
inhibition of β-hexosaminidase release activity. The anti-allergic
activity was ranked in the following order: 100-nm curcumin
emulsion > 200-nm curcumin emulsion > 50-nm curcumin emulsion
≈ curcumin suspension.
Serum absorption of curcumin lipid
nanoemulsions in mice
From the above results, the 100-nm curcumin lipid
nanoemulsion showed the strongest anti-inflammatory and
anti-allergic activities in vitro and in vivo among
the emulsions tested. In order to investigate in vivo serum
absorption of the curcumin lipid nanoemulsions, we examined serum
curcumin concentrations in mice over time. Fig. 5 shows the blood curcumin
concentrations following treatment with the 50-, 100- and 200-nm
curcumin emulsions. Two hours after the oral administration of the
test compounds at a dose of 8 mg/kg, the highest curcumin blood
concentration was observed with the 100-nm lipid nanoemulsion,
followed by the 200- and 50-nm curcumin lipid nanoemulsions. These
results suggest that the absorption efficiency differs according to
the lipid nanoemulsion size, with the 100-nm particle size showing
efficient serum curcumin absorption in mice.
Discussion
Curcumin is a lipophilic molecule that exists in the
enol-tautomer form. It exhibits limited solubility in water, slight
solubility in methanol, and good solubility in dimethyl sulfoxide
(DMSO) and chloroform (41). To
overcome its limited water-solubility, a number of new approaches
have been investigated to deliver curcumin effectively using a
lipid-based nanoparticle carrier method, such as liposome
encapsulation (42,43). The formulation of the curcumin
lipid nanoemulsion in this study was modified from that for
sulfoquinovosyl acylglycerol-containing emulsions previously
demonstrated (44). The main
components of those emulsions were soybean oil, distilled water,
sulfoquinovosyl acylglycerol, and HEPC from egg yolk. HCO-60 was
used as a co-surfactant to effectively reduce the particle size, as
well as load the nanoemulsion with the target compound (i.e.,
sulfoquinovosyl acylglycerol). Tween-80 was also effectively used
as a co-surfactant to reduce the particle size of nanoemulsions. In
a previous study of ours, we demonstrated that Tween-80 produced
much smaller particles in curcumin lipid emulsions compared to
HCO-60 (45). Thus, Tween-80 was
used as the co-surfactant in this study. Notably, Tween-80 has been
commonly used as a stabilizer in commercially available lipid
emulsion preparations for some time (46,47). Thus, it is an attractive
co-surfactant for curcumin nanoemulsions as it produces a curcumin
emulsion with a particle size that is sufficiently small for
long-term circulation in the blood and is capable of extravasating
through blood capillaries in tissues under inflammatory and/or
allergic conditions.
A good emulsion was obtained in the preliminary
experiments with HEPC. Hence, HEPC was selected as the emulsifier
for the curcumin lipid nanoemulsions (Table I). In order to evaluate the
effects of co-surfactant and the sonication time on the mean
particle diameter of lipid nanoemulsions, a series of samples was
prepared with Tween-80 as the co-surfactant and soybean oil as the
oil component by increasing the sonication time, with 15 mg of
initial curcumin loading (Table
I). The nanoemulsions were prepared at an HEPC:co-surfactant
weight ratio of 1:1.4. Thus, the outer monolayer of the oil core in
the lipid nanoemulsions was composed of an HEPC: Tween-80 molar
ratio of 5.7:4.3, assuming that all surfactant molecules were
arranged on the interface of the oil core and water.
Soybean oil was assessed for use as the oil
component. It has been widely used as a model system for lipid
emulsion studies, and is commonly used for commercially available
fat emulsions (48,49). In general, the formula of
intralipid was composed of 10–30% w/w soybean oil. The viscosity
range of soybean oil is between 69 and 10,000 mPa·s at 24°C
(50). In this study, the
curcumin nanoemulsion with soybean oil showed smaller particle
diameters of 50 to 200 nm (Fig.
2). This difference in the particle diameter may be attributed
to the lower viscosity of the soybean oil, which readily allows the
breakup of oil droplets during ultrasonication, generating
smaller-sized nanoemulsions (32).
The relatively small size of the droplets in
nanoemulsions (<200 nm) means that they often have different
physicochemical and biological properties than conventional
emulsions (>200 nm). Furthermore, nanoemulsions have been
reported to have better stability against particle aggregation and
gravitational separation due to their small droplet size (26,51). The bioavailability of encapsulated
lipophilic components within the gastrointestinal tract may be
increased by using nanoemulsions due to their relatively small
droplet size (52). Thus, one of
the objectives of the present study was to determine the influence
of droplet size on curcumin bioactivities, such as
anti-inflammatory activity (Fig.
3) and anti-allergic activity (Fig. 4). The results of this study
demonstrated that the 100-nm curcumin lipid nanoemulsion exhibited
the highest bioactivity when orally administered to mice. These
phenomena may be due to the fact that curcumin reaches a maximum
blood concentration with the 100-nm lipid nanoemulsion (Fig. 5). We are currently attempting to
clarify the molecular mechanisms regulating the efficient serum
absorption of curcumin, delivered in a 100-nm lipid nanoemulsion.
Future studies on curcumin lipid nanoemulsions are warranted in
pre-clinical in vivo models of inflammation, allergies and
other diseases that may benefit from the effects of curcumin.
In conclusion, in this study, we focused on the
in vitro and in vivo bioactivities of curcumin, such
as the anti-inflammatory and anti-allergy effects. We investigated
the effects of particle diameter (50, 100 and 200 nm) on the
bioactivities of curcumin lipid nanoemulsions, and found that the
100-nm emulsion had the best bioactivity both in vitro and
in vivo. The oral administration of the 100-nm nanoemulsion
resulted in the highest serum curcumin absorption in mice. Curcumin
lipid nanoemulsions provide an opportunity to expand the clinical
repertoire of this efficacious agent by enabling its aqueous
dispersion. The 100-nm curcumin nanoemulsion represents a promising
tool when testing the potential bioactive effects of curcumin in
clinical trials.
Acknowledgments
This study was supported in part by the Ministry of
Education, Culture, Sports, Science and Technology (MEXT,
Japan)-Supported Program for the Strategic Research Foundation at
Private Universities, 2012–2016. Y.M. acknowledges Grantsin-Aid for
Scientific Research (C) (no. 24580205) from MEXT, and the 25th
(2014) Cosmetology Research Foundation (Japan).
Abbreviations:
|
Pol
|
DNA polymerase
|
|
TNF-α
|
tumor necrosis factor-α
|
|
LPS
|
lipopolysaccharide
|
|
TPA
|
12-O-tetradecanoylphorbol-13-acetate
|
|
O/W
|
oil-in-water
|
|
HEPC
|
hydrogenated
L-α-phosphatidylcholine
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
PCA
|
passive cutaneous anaphylaxis
|
|
DNP
|
anti-dinitrophenyl
|
|
IgE
|
immunoglobulin E
|
|
HPLC
|
high-performance liquid
chromatography
|
|
SD
|
standard deviation
|
References
|
1
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ammon HP, Safayhi H, Mack T and Sabieraj
J: Mechanism of antiinflammatory actions of curcumine and boswellic
acids. J Ethnopharmacol. 38:113–119. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banerjee A and Nigam SS: Antimicrobial
efficacy of the essential oil of Curcuma longa. Indian J Med Res.
68:864–866. 1978.PubMed/NCBI
|
|
4
|
Bhavani Shankar TN and Sreenivasa Murthy
V: Effect of turmeric (Curcuma longa) fractions on the growth of
some intestinal and pathogenic bacteria in vitro. Indian J Exp
Biol. 17:1363–1366. 1979.PubMed/NCBI
|
|
5
|
Sawada T, Yamahara J, Shimazu S and Ohta
T: Evaluation of crude drugs by bioassay. III. Comparison with
local variation of contents and the fungistatic action of essential
oil from the root of Curcuma longa. Shoyakugaku Zasshi. 25:11–16.
1971.
|
|
6
|
Rao DS, Sekhara NC, Satyanarayana MN and
Srinivasan M: Effect of curcumin on serum and liver cholesterol
levels in the rat. J Nutr. 100:1307–1315. 1970.PubMed/NCBI
|
|
7
|
Limtrakul P, Anuchapreeda S, Lipigorngoson
S and Dunn FW: Inhibition of carcinogen induced c-Ha-ras and c-fos
proto-oncogenes expression by dietary curcumin. BMC Cancer. 1:1–7.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anuchapreeda S, Limtrakul P,
Thanarattanakorn P, Sittipreechacharn S and Chanarat P: Inhibitory
effect of curcumin on WT1 gene expression in patient leukemic
cells. Arch Pharm Res. 29:80–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anuchapreeda S, Thanarattanakorn P,
Sittipreechacharn S, Chanarat P and Limtrakul P: Curcumin inhibits
WT1 gene expression in human leukemic K562 cells. Acta Pharmacol
Sin. 27:360–366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anuchapreeda S, Tima S, Duangrat C and
Limtrakul P: Effect of pure curcumin, demethoxycurcumin, and
bisdemethoxycurcumin on WT1 gene expression in leukemic cell lines.
Cancer Chemother Pharmacol. 62:585–594. 2008. View Article : Google Scholar
|
|
11
|
Polasa K, Raghuram TC, Krishna TP and
Krishnaswamy K: Effect of turmeric on urinary mutagens in smokers.
Mutagenesis. 7:107–109. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roy RG, Madesayaa NM, Ghosh RB,
Gopalakrishnan DV, Murthy NN, Dorairaj TJ and Sitaraman NL: Study
on inhalation therapy by an indigenous compound on P. vivax and P.
falciparum infections - a preliminary communication. Indian J Med
Res. 64:1451–1455. 1976.PubMed/NCBI
|
|
13
|
Azuine MA and Bhide SV: Chemopreventive
effect of turmeric against stomach and skin tumors induced by
chemical carcinogens in Swiss mice. Nutr Cancer. 17:77–83. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dorai T, Cao YC, Dorai B, Buttyan R and
Katz AE: Therapeutic potential of curcumin in human prostate
cancer. III. Curcumin inhibits proliferation, induces apoptosis,
and inhibits angiogenesis of LNCaP prostate cancer cells in vivo.
Prostate. 47:293–303. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anuchapreeda S, Muangmoonchai R and
Limtrakul P: Effect of curcuminoids on MDR1 gene promoter activity
in human cervical carcinoma cells. Chiang Mai Med Bull. 41:189–202.
2002.
|
|
16
|
Mizushina Y, Hirota M, Murakami C, Ishidoh
T, Kamisuki S, Shimazaki N, Takemura M, Perpelescu M, Suzuki M,
Yoshida H, et al: Some anti-chronic inflammatory compounds are DNA
polymerase λ-specific inhibitors. Biochem Pharmacol. 66:1935–1944.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeuchi T, Ishidoh T, Iijima H, Kuriyama
I, Shimazaki N, Koiwai O, Kuramochi K, Kobayashi S, Sugawara F,
Sakaguchi K, et al: Structural relationship of curcumin derivatives
binding to the BRCT domain of human DNA polymerase λ. Genes Cells.
11:223–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishida M, Nishiumi S, Mizushina Y,
Fujishima Y, Yamamoto K, Masuda A, Mizuno S, Fujita T, Morita Y,
Kutsumi H, et al: Monoacetylcurcumin strongly regulates
inflammatory responses through inhibition of NF-κB activation. Int
J Mol Med. 25:761–767. 2010.PubMed/NCBI
|
|
19
|
Mizushina Y, Nishida M, Azuma T and
Yoshida M: Inhibition of DNA polymerase λ, a DNA repair enzyme, and
anti-inflammation: chemical knocked out analysis for DNA polymerase
λ using curcumin derivatives. DNA Repair and Human Health. Chapter
31. InTech. Vengrova S: pp. 777–792. 2011
|
|
20
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
21
|
Garcea G, Berry DP, Jones DJ, Singh R,
Dennison AR, Farmer PB, Sharma RA, Steward WP and Gescher AJ:
Consumption of the putative chemopreventive agent curcumin by
cancer patients: Assessment of curcumin levels in the colorectum
and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers
Prev. 14:120–125. 2005.PubMed/NCBI
|
|
22
|
Kanai M, Yoshimura K, Asada M, Imaizumi A,
Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y,
et al: A phase I/II study of gemcitabine-based chemotherapy plus
curcumin for patients with gemcitabine-resistant pancreatic cancer.
Cancer Chemother Pharmacol. 68:157–164. 2011. View Article : Google Scholar
|
|
23
|
Sharma RA, Euden SA, Platton SL, Cooke DN,
Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer
SM, et al: Phase I clinical trial of oral curcumin: Biomarkers of
systemic activity and compliance. Clin Cancer Res. 10:6847–6854.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vareed SK, Kakarala M, Ruffin MT, Crowell
JA, Normolle DP, Djuric Z and Brenner DE: Pharmacokinetics of
curcumin conjugate metabolites in healthy human subjects. Cancer
Epidemiol Biomarkers Prev. 17:1411–1417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porter CJ, Trevaskis NL and Charman WN:
Lipids and lipid-based formulations: Optimizing the oral delivery
of lipophilic drugs. Nat Rev Drug Discov. 6:231–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Solans C, Izquierdo P, Nolla J, Azemar N
and Garcia-Celma MJ: Nanoemulsions. Curr Opin Colloid Interface
Sci. 10:102–110. 2005. View Article : Google Scholar
|
|
27
|
Wheeler JJ, Wong KF, Ansell SM, Masin D
and Bally MB: Polyethylene glycol modified phospholipids stabilize
emulsions prepared from triacylglycerol. J Pharm Sci. 83:1558–1564.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu F and Liu D: Long-circulating
emulsions (oil-in-water) as carriers for lipophilic drugs. Pharm
Res. 12:1060–1064. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurihara A, Shibayama Y, Mizota A, Yasuno
A, Ikeda M, Sasagawa K, Kobayashi T and Hisaoka M: Lipid emulsions
of palmitoylrhizoxin: Effects of composition on lipolysis and
biodistribution. Biopharm Drug Dispos. 17:331–342. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Mesfin GM, Rodríguez CA, Slatter
JG, Schuette MR, Cory AL and Higgins MJ: Venous irritation,
pharmacokinetics, and tissue distribution of tirilazad in rats
following intravenous administration of a novel supersaturated
submicron lipid emulsion. Pharm Res. 16:930–938. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maranhão RC, Garicochea B, Silva EL,
Dorlhiac-Llacer P, Cadena SM, Coelho IJ, Meneghetti JC, Pileggi FJ
and Chamone DA: Plasma kinetics and biodistribution of a lipid
emulsion resembling low-density lipoprotein in patients with acute
leukemia. Cancer Res. 54:4660–4666. 1994.PubMed/NCBI
|
|
32
|
Miyamoto M, Hirano K, Ichikawa H, Fukumori
Y, Akine Y and Tokuuye K: Preparation of gadolinium-containing
emulsions stabilized with phosphatidylcholine-surfactant mixtures
for neutron-capture therapy. Chem Pharm Bull (Tokyo). 47:203–208.
1999. View Article : Google Scholar
|
|
33
|
Miyamoto M, Hirano K, Ichikawa H, Fukumori
Y, Akine Y and Tokuuye K: Biodistribution of gadolinium
incorporated in lipid emulsions intraperitoneally administered for
neutron-capture therapy with tumor-bearing hamsters. Biol Pharm
Bull. 22:1331–1340. 1999. View Article : Google Scholar
|
|
34
|
Razin E, Mencia-Huerta JM, Stevens RL,
Lewis RA, Liu FT, Corey E and Austen KF: IgE-mediated release of
leukotriene C4, chondroitin sulfate E proteoglycan,
β-hexosaminidase, and histamine from cultured bone marrow-derived
mouse mast cells. J Exp Med. 157:189–201. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato H, Kobayashi Y, Hattori A, Suzuki T,
Shigekawa M and Jippo T: Inhibitory effects of water-soluble
low-molecular-weight β-(1,3–1,6) D-glucan isolated from
Aureobasidium pullulans 1A1 strain black yeast on mast cell
degranulation and passive cutaneous anaphylaxis. Biosci Biotechnol
Biochem. 76:84–88. 2012. View Article : Google Scholar
|
|
36
|
Soler-Rodriguez AM, Zhang H, Lichenstein
HS, Qureshi N, Niesel DW, Crowe SE, Peterson JW and Klimpel GR:
Neutrophil activation by bacterial lipoprotein versus
lipopolysaccharide: differential requirements for serum and CD14. J
Immunol. 164:2674–2683. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzuki T, Hashimoto S, Toyoda N, Nagai S,
Yamazaki N, Dong HY, Sakai J, Yamashita T, Nukiwa T and Matsushima
K: Comprehensive gene expression profile of LPS-stimulated human
monocytes by SAGE. Blood. 96:2584–2591. 2000.PubMed/NCBI
|
|
38
|
Aggarwal BB: Signalling pathways of the
TNF superfamily: A double-edged sword. Nat Rev Immunol. 3:745–756.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Supajatura V, Ushio H, Nakao A, Akira S,
Okumura K, Chisei R and Ogawa H: Differential responses of mast
cell Toll-like receptors 2 and 4 in allergy and innate immunity. J
Clin Invest. 109:1351–1359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Metcalfe DD, Peavy RD and Gilfillan AM:
Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin
Immunol. 124:639–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Payton F, Sandusky P and Alworth WL: NMR
study of the solution structure of curcumin. J Nat Prod.
70:143–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li L, Braiteh FS and Kurzrock R:
Liposome-encapsulated curcumin: In vitro and in vivo effects on
proliferation, apoptosis, signaling, and angiogenesis. Cancer.
104:1322–1331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maiti K, Mukherjee K, Gantait A, Saha BP
and Mukherjee PK: Curcumin-phospholipid complex: Preparation,
therapeutic evaluation and pharmacokinetic study in rats. Int J
Pharm. 330:155–163. 2007. View Article : Google Scholar
|
|
44
|
Mizushina Y, Maeda N, Kawasaki M, Ichikawa
H, Murakami C, Takemura M, Xu X, Sugawara F, Fukumori Y, Yoshida H,
et al: Inhibitory action of emulsified sulfoquinovosyl acylglycerol
on mammalian DNA polymerases. Lipids. 38:1065–1074. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Anuchapreeda S, Fukumori Y, Okonogi S and
Ichikawa H: Preparation of lipid nanoemulsions incorporating
curcumin for cancer therapy. J Nanotechnol Article.
2012:2703832012.
|
|
46
|
Hung CF, Hwang TL, Chang CC and Fang JY:
Physicochemical characterization and gene transfection efficiency
of lipid emulsions with various co-emulsifiers. Int J Pharm.
289:197–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chinsriwongkul A, Opanasopit P,
Ngawhirunpat T, Chareansriwilaiwat N, Sila-On W and Ruktanonchai U:
Physicochemical properties of lipid emulsions formulated with
high-load all-trans-retinoic acid. PDA J Pharm Sci Technol.
61:461–471. 2007.
|
|
48
|
Texier I, Goutayer M, Da Silva A, Guyon L
and Djaker N: Cyanine-loaded lipid nanoparticles for improved in
vivo fluorescence imaging. J Biomed Opt. 14:0540052009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goutayer M, Dufort S, Josserand V, Royere
A, Heinrich E, Vinet F, Bibette J, Coll JL and Texier I: Tumor
targeting of functionalized lipid nanoparticles: assessment by in
vivo fluorescence imaging. Eur J Pharm Biopharm. 75:137–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hurstand WJ and Martin RA Jr: The analysis
of phospholipids in soy lecithin by HPLC. J Am Oil Chem Soc.
61:1462–1463. 1984. View Article : Google Scholar
|
|
51
|
Sonneville-Aubrun O, Simonnet JT and
L’Alloret F: Nanoemulsions: A new vehicle for skincare products.
Adv Colloid Interface Sci. 108–109:145–149. 2004. View Article : Google Scholar
|
|
52
|
Patel D and Sawant KK: Oral
bioavailability enhancement of acyclovir by self-microemulsifying
drug delivery systems (SMEDDS). Drug Dev Ind Pharm. 33:1318–1326.
2007. View Article : Google Scholar : PubMed/NCBI
|