Introduction
Herpes simplex virus (HSV) is a common human
pathogen that infects orofacial mucosal surfaces (HSV-1) and
genital mucosal surfaces (HSV-2) (1). Severe pathological conditions may
result from infections of the cornea (keratitis) (2) or the central nervous system
(encephalitis) (3), while
infection in newborns or immunocompromised individuals may result
in severe disseminated diseases (4). Drugs used for the treatment of HSV
infections primarily belong to a group of nucleoside analogs
designed to inhibit viral DNA synthesis, such as acyclovir and its
derivatives. Nonetheless, resistant strains develop in
immunocompromised patients, particularly among HIV-infected
individuals or those who have undergone bone-marrow transplants. In
these cases, highly toxic DNA polymerase inhibitors, such as
foscarnet or cidofovir are administered (5). As a result, there is a need for the
development of novel anti-HSV agents, particularly for compounds
with a new mode of action.
Plant extracts and synthetic molecules constitute
key sources for novel anti-viral drugs (6–9).
Within these two categories, molecules with immunomodulating
capacities are particularly promising in this regard. More
specifically, previous studies have reported that the
immune-response modifiers, imiquimod and resiquimod, effectively
fight HSV infections (8,10). The non-toxic ammonium,
trichloro[1,2-ethanediolato-O,O′]-tellurate (AS101; BioMAS Ltd.,
Kfar Saba, Israel), is a low molecular-weight organic compound
(11) previously reported to be a
potent immunomodulator both in vitro and in vivo, and
has been reported to exhibit the potential for therapeutic
applications (12–14). AS101 has been shown to act
directly against several human and non-human viruses, including
murine cytomegalovirus (15), HIV
(16), West Nile virus (WNV)
(17), avian and human influenza
viruses (Mandelboim et al, personal communication) and the
human papillomavirus (HPV) (18).
The topical application of AS101 has been shown to be effective
against genital warts caused by the HPV 6 and 11 strains; it
requires a relatively short duration of treatment, triggers minimal
side-effects and is associated with a low recurrence rate (18). Phase-2 clinical trials have
recently begun (http://clinicaltrials.gov/show/NCT01555112), and a
phase-3 clinical trial is expected to commence shortly (B. Sredni,
personal communication). Since HSV infection, similar to HPV, is
manifested by dermal lesions, AS101 cream may be effective against
HSV lesions as well.
The primary objectives of this study were to
evaluate the direct anti-viral activity of AS101 against HSV-1 and
HSV-2 in a cell culture model, and to identify the stage of viral
replication that is sensitive to the drug.
Materials and methods
Cells and viruses
Monkey kidney Vero cells (Cat. no. ccl-81; American
Type Culture Collection, Manassas, VA, USA) were grown in Eagle’s
minimum essential medium-with non-essential amino acids (MEM-NAA)
containing 10% heat-inactivated fetal bovine serum (FBS). Human
kidney (HK) cells (obtained from the Department of National Health
and Welfare, Ottawa, ON, Canada) were grown in M199 medium
containing 10% FBS. All media were supplemented with streptomycin
0.8 mg/ml, penicillin 800 U/ml and mycostatin 50 U/ml. The cells
were cultured at 37°C in humidified air containing 5%
CO2. All reagents (medium and serum) were purchased from
Biological Industries Israel Beit-Haemek, Ltd. (Kibbutz
Beit-Haemek, Israel). HSV-1 (VR3 strain) was a kind gift from Dr A.
Nahmias (Emory University School of Medicine, Atlanta, GA, USA) and
HSV-2 (RL strain) was a kind gift from Dr S. Schprecher-Goldberger
(Saint-Pierre University Hospital, Laboratory of Microbiology,
Brussels, Belgium). The HSV-1 and HSV-2 clinical isolates were
isolated from patient clinical samples submitted for HSV-1 and
HSV-2 testing (the use of those samples was approved by the
Helsinki Committee of the Chaim Sheba Medical Center, Ramat-Gan,
Israel; 1007-14-SMC). The viruses were propagated to
>107 plaque-forming units (PFU) per ml on Vero cells,
as described below, aliquoted and stored at −80°C until use.
Reagents
AS101 was provided by BioMAS Ltd. It was dissolved
in PBS (pH 7.4) and maintained at 4°C.
Viral stock preparation
A confluent Vero cell monolayer was infected with
HSV-1 (VR), HSV-2 (RL), or HSV clinical isolates at a multiplicity
of infection (MOI) of 0.01. At 96 h post-infection (pi), when
significant cytopathic effects (CPE) were observed, the cell
cultures were frozen at −80°C for 24 h, thawed, and the
supernatants were then collected and centrifuged at 400 x g for 10
min. The cleared supernatants were aliquoted and stored at −80°C.
The various stocks were titrated by a standard plaque assay, as
described below.
Cytotoxicity assay
The toxicity of AS101 was tested using XTT assay,
performed according to the manufacturer’s instructions (Xenometrix
AG, Allschwil, Switzerland). In brief, the Vero and HK cells were
seeded at a density of 4×104 cells/well in 96-well
plates and incubated for 24 h at 37°C in a 5% CO2
incubator. After removing the growth medium (MEM-NAA containing 10%
heat-inactivated FBS) various concentrations of AS101 (1.5–30
µg/ml) diluted in MEM-NAA medium supplemented with 5% FBS
were placed over the cells, which were incubated for an additional
48 h. An equal volume of PBS served as the control. Before the
analysis, the culture medium was replaced with fresh MEM-NAA
medium, without serum and 50 µl XTT reaction solution was
added to each well. A mixture of culture medium and XTT reagent
solution was placed in cell-free wells and served as a background
(blank) control. The cells were then incubated for up to 2 h. The
absorbance of the reduced orange-colored tetrazolium salt was
measured by a spectrophotometer at a wavelength of 450 nm.
Absorbance at 630 nm, representing non-specific readings, and the
blank control reading, were subtracted from all readings. Cell
viability was calculated as the percentage of the mean optical
density (OD) value of the controls (PBS-treated cells).
Plaque reduction assay
The plaque reduction assay was performed as
previously described (19), with
the following adaptations: the Vero or HK cells were seeded at a
density of 106 cells/well in a 6-well plate 20 h prior
to infection. The cells were infected with either clinical isolates
or laboratory reference strains, at a concentration of 40–80
PFU/well (which enables the convenient counting of plaques),
suspended in MEM-NAA containing 2% FBS (inoculum volume 0.2
ml/well) and incubated (1 h, 37°C, 5% CO2) with constant
shaking. Following incubation, the medium was removed and the cells
were washed twice with medium and overlaid with fresh medium
containing 2% FBS, 0.4% (w/v) agar (Difco Laboratories, Jerusalem,
Israel) and 5 µg/ml AS101. AS101 was added at different time
points or at different concentrations, as indicated in the text
below. The cells were then incubated (37°C, 5% CO2) for
3–4 days until plaques appeared, and were then fixed and stained
with a fixation/staining solution containing ethanol (1.67%),
formalin (3%) and crystal violet (0.4%) diluted in saline. The
plaques were counted over a light box after removal of the agar
overlay. Differences in plaque area were determined using ImageJ
software. A plaque area of 35 representative plaques from each
treatment was taken for statistical analysis.
Virus yield titration by plaque
assay
One day prior to infection, the HK cells
(106 cells/well in a 6-well plate) were seeded in M199
medium supplemented with 10% heat-inactivated FBS. The cells were
infected with HSV-1 (VR3) or HSV-2 (RL) at an MOI of 0.1, 0.5, 1
and 2. The virus was allowed to be adsorbed for 1 h with constant
shaking, and was then removed. The cells were washed twice with
M199 medium and then overlaid with M199 medium containing 2% FBS
with or without 5 µg/ml AS101. At the indicated time points,
the supernatants were collected, centrifuged (400 x g, 10 min) and
the supernatant was diluted by 10-fold serial dilutions in culture
medium supplemented with 2% FBS. To determine the intracellular
virus titer, the infected cells were washed twice with fresh medium
and scraped into M199 medium supplemented with 2% FBS using a
rubber policeman. The suspension of the cells was then subjected to
3 cycles of freezing (liquid nitrogen) and thawing (water bath at
37°C), followed by centrifugation at (400 x g, 10 min). The
supernatant, containing the intracellular virus, was diluted by
10-fold serial dilutions and titrated on the Vero cells by plaque
assay.
Assay of virucidal activity
The direct effect of AS101 on HSV-1 and HSV-2
infectivity was assayed by mixing 0.05 ml concentrated viral stock
solutions containing 2.2×105 PFU/ml HSV-2 or
3.8×108 PFU/ml HSV-1 with 0.05 ml MEM-NAA medium
containing AS101 (10 µg/ml) or with 0.05 ml MEM-NAA medium
containing an equal volume of PBS. Both the AS101- and PBS-treated
viruses were incubated at 37°C for 2 h. The mixture was then
diluted to a concentration of 40–80 PFU/ml in MEM-NAA medium
containing 2% FBS, and placed over the Vero cells (0.2 ml diluted
virus per well on a 6-well plate) for 1 h at 37°C. The inoculum was
then removed and the Vero cell cultures were washed twice before
being overlaid with medium to allow plaque formation.
DNA extraction
DNA was extracted using the High Pure Viral Nucleic
Acid kit (Roche, Manheim, Germany) according to the manufacturer’s
instructions. DNA was recovered in 50 µl elution buffer, and
10 µl samples of 10-fold serial dilutions were
quantitatively analyzed using the TaqMan real-time PCR method, as
previously described (20).
Quantitative (real-time) PCR
A nucleotide segment of the glycoprotein B (gB)
region (nt 139 824-139 896, accession no. NC_001798) was amplified
by the use of primers and probes as described in Table I. The reaction volume of 25
µl contained 12.5 µl universal master mix (ABsolute
Blue qPCR low rox mix, Cat. no. AB-4318/B; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) 10 µl of sample DNA and
primers and probes at the concentrations described in Table I. Amplification was carried out in
a real-time PCR instrument ABI PRISM 7500 (Applied Biosystems,
Foster City, CA, USA) with the following cycling conditions:
incubation at 50°C for 2 min and denaturation at 95°C for 10 min,
followed by 60 amplification cycles at 95°C. For each positive
sample, the threshold cycle (Ct) value was recorded. The Ct is the
cycle when the fluorescence has become detectable and is in the
exponential phase of amplification. The Ct value is inversely
proportional to the log concentration of the target DNA.
 | Table IPrimers and probes used for the
TaqMan detection of the gB region. |
Table I
Primers and probes used for the
TaqMan detection of the gB region.
| Final concentration
(nM) | Sequence
(5′→3′) | Primers/probes |
|---|
| 300 |
AGATATCCTCTTTATCATCAGCACCA | HSV-2-Forward |
| 300 |
TTGTGCTGCCAAGGCGA | HSV-2-Reverse |
| 200 |
CGGCGGCGTTCGTTTGTCTG | HSV-2-Prob (5′-FAM,
3′-TAMRA) |
Preparation of HSV DNA standards
Ten-fold serial dilutions were prepared from the
commercial quantified HSV-2 DNA (Advanced Technologies, Inc.,
Columbia, MD, USA). The DNA stocks were serially diluted in RNase-
and DNase-free water. Standards were analyzed in triplicate and
used to generate a standard curve as well as a positive control for
each quantitative PCR run.
Direct and indirect immunofluorescence
assays
Confluent HK tissue cultures were infected with
HSV-2 at an MOI of 2 PFU/cell in the presence or absence of AS101.
The cells were harvested 20 h pi with a rubber policeman, and
samples of 2×106 cells/ml were applied on glass slides
using a cytocentrifuge (Shandon CytoSpin 3; Shandon, Pittsburgh,
PA, USA) at 54 x g for 4 min. The slides were fixed with acetone,
washed with PBS and subjected to immunofluorescence staining using
both direct and indirect methods. Direct immunofluorescence
staining was performed using a mixture of fluorescein-labeled
murine monoclonal antibodies against HSV-2 140 kDa RR1 protein,
HSV-2 (ICP34.5α 38 kDa) and HSV-2 gB (8H349UL; Trinity Biotech PLC,
Wicklow, Ireland), according to the manufacturer’s instructions.
Indirect immunofluorescence staining was performed using a mouse
monoclonal antibody against HSV-VP-5 (SC-13525; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), followed by the
application of FITC-labeled polyclonal rabbit anti-mouse
immunoglobulins (DakoCytomation, Glostrup, Denmark).
Software
ImageJ software was used to calculate the plaque
areas from the photographed 6-well plates.
Statistical analysis
The data presented are the means ± standard
deviation (SD) of triplicate measures of 3 or more experiments
performed independently. Error bars represent SD. Statistical
significance of the mean value was calculated using a one-sided
Student’s t-test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of AS101 on HSV-1 and HSV-2 plaque
formation
The effect of AS101 on cell viability was assessed
by XTT assay. AS101 was tolerated at all tested concentrations (up
to 30 µg/ml) by both the Vero and HK cell lines (data not
shown). The anti-viral activity of AS101 was manifested by a
reduced plaque number and a profound effect on plaque size in the
HSV-2-infected cells (Fig. 1). No
visible effect was detected on either the plaque number or the size
of the HSV-1-infected cells. This phenomenon was not restricted to
laboratory strains and was induced by fresh clinical isolates as
well. In order to verify that AS101 anti-HSV-2 activity is not
cell-type specific, the experiments were performed on Vero and HK
cell lines. The HK cells were infected with 40–80 PFU of HSV-2, and
AS101 was added post-infection at various concentrations. In
another experimental setup, AS101 (5 µg/ml) was added 16 h
prior to infection (pre-infection treatment) or it was added 16 h
prior to infection and immediately post-infection (pre- +
post-treatment). Following 4 days of incubation, the cells were
fixed and stained. AS101 inhibited HSV-2 replication in the HK
cells in a dose-dependent manner. The addition of the drug
immediately post-infection was as effective as the addition of the
drug at the time of infection and thereafter (pre- +
post-treatment), while pre-treatment of the cells only prior to
infection had a minor effect (Fig.
2). These experiments encouraged us to search in detail for the
possible anti-viral activity of AS101 against HSV-1 and HSV-2 in
our further experiments. An AS101 dose of 5 µg/ml was used
for all further experiments with the HK cells. The Vero cells were
used as an indicator cell line when titration of the virus progeny
was required.
Effect of AS101 on HSV-2 yield
production
The effect of AS101 on yield production was assessed
by infecting the HK cells with either HSV-1 or HSV-2 at different
MOI ratios. The titer of the virus progeny released into the
supernatant was determined using plaque assay on the Vero cells.
The administration of AS101 led to a ≥50% reduction in the HSV-1
yield and to an even more prominent effect (almost 2 log reduction)
on the HSV-2 yield (Table
II).
 | Table IIEffect of AS101 on HSV-1 and HSV-2
viral yield. |
Table II
Effect of AS101 on HSV-1 and HSV-2
viral yield.
| Virus | MOI | AS101 (5
µg/ml) | Log (PFU/ml) | Percentage of
inhibition |
|---|
| HSV-1 | 0.1 | − | 3.9±0.09 | 35 |
| | + | 3.7±0.08 | |
| 0.5 | − | 5.4±0.03 | 36 |
| | + | 5.1±0.04 | |
| 1 | − | 6.5±0.04 | 51 |
| | + | 6.2±0.07 | |
| 2 | − | ±0.028.5 | 47 |
| | + | ±0.068.3 | |
| HSV-2 | 0.1 | − | ±0.08 2.3 | 88 |
| | + | ±01.39 | |
| 0.5 | − | 3.3±0.04 | 76 |
| | + | ±0.072.7 | |
| 1 | − | 3.34±0.01 | 97 |
| | + | 1.84±0.2 | |
| 2 | − | 6.1±0.1 | |
| | + | 4.7±0.04 | 96 |
Effect of AS101 on the infectivity of
mature HSV-1 and HSV-2
The effect of AS101 on the infectivity of mature
cell-free viruses was examined by incubating a highly concentrated
stock of the HSV-2 RL strain or HSV-1 strain (with AS101 at a final
concentration of 5 µg/ml, as described in the Materials and
methods). No changes in viral infectivity were observed, indicating
that AS101 exhibited no virucidal activity against HSV-1 or HSV-2
(Fig. 3). All the above results
indicated that AS101 exhibited a significant anti-viral activity,
specifically against HSV-2. For this reason, we decided to focus on
the HSV-2 strain in our further explorations of the anti-viral
mechanisms of AS101.
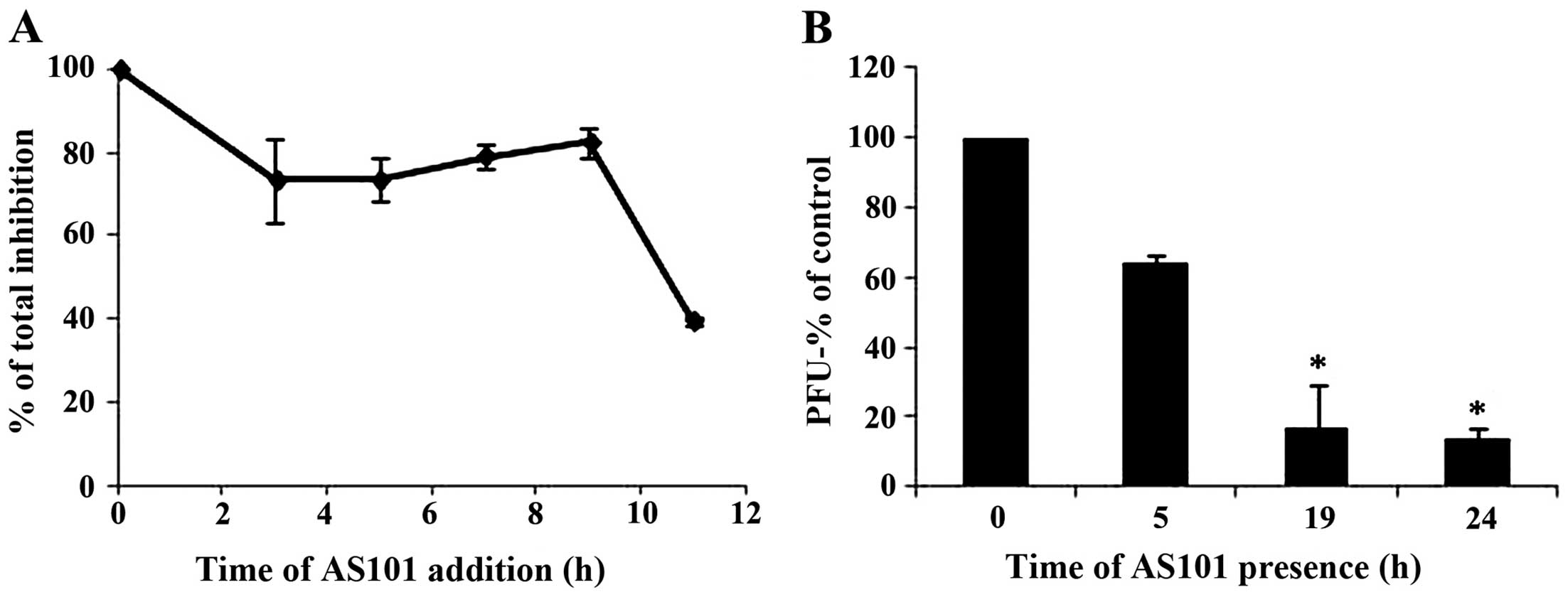
Effect of time of the addition of AS101
on HSV-2 propagation
In order to identify the stage of viral replication
that is hindered by AS101, a synchronous infection in which AS101
was added from the onset of infection until titration or at various
time points pi, was performed. In another experiment, AS101 was
provided for the first 5 h of infection or, alternatively, was
added at 5 h pi. The addition of AS101 within the first 9 h of
infection inhibited viral replication to almost the same extent
(~80%) as did treatment from the onset of infection (considered as
100% inhibition) (Fig. 4). In
addition, when AS101 was present for the first 5 h of infection and
then washed out of the system, its inhibitory effect was markedly
reduced (Fig. 4B). When only
administering AS101 at 3 h pi, approximately 20% of the anti-viral
activity observed upon its administration at the onset of infection
was lost, suggesting some effect on the early stages of
replication. This minor effect on the early stages of replication
was indistinguishable by the less sensitive plaque reduction assay
(Fig. 2B).
Effect of AS101 on HSV-2 DNA synthesis
and structural protein synthesis
The effect of AS101 on viral DNA synthesis was
evaluated in the culture supernatants and inside the cells by
quantitative PCR. No significant effect on the genome copy number
was observed in the presence of AS101 when measured from the cell
culture medium or from inside the cells (Fig. 5A). Likewise, when assessing
intracellular viral protein synthesis by the immunostaining of
viral structural proteins, staining of the viral structural
proteins was similar in the presence or absence of the compound
(Fig. 5B).
Effect of AS101 on the infectivity of
intracellular HSV-2
Since AS101 exerted an inhibitory effect on viral
infectivity, we were interested in measuring the infectivity of
intracellular viruses as well. Infectivity of both free and
cell-associated viruses from the AS101-treated and -untreated HK
cultures was determined using the plaque assay. The obtained
results revealed that the infectivity of the extracellular virus
was reduced by 2 logs by AS101, while the infectivity of the
intracellular virus was reduced by only 1 log (Fig. 6).
Discussion
HSV infections are usually effectively treated with
nucleoside analog drugs, such as acyclovir, or with DNA polymerase
inhibitors, such as forscarnet or cidofovir (3). However, the appearance of thymidime
kinase or DNA polymerase-mutated viruses, particularly among
immunocompromised patients, renders these drugs inadequate in
certain patient populations. Thus, compounds that can inhibit HSV
replication by other mechanisms are urgently required (5,21,22). The purpose of this study was to
explore the anti-viral activity of AS101 against HSV. AS101 is an
organotellurium compound that has been shown to exert anti-viral
effects against a variety of human viruses (16,17). A recent study demonstrated that
AS101 can even eradicate genital warts attributed to HPV (18).
In this study, we demonstrated that AS101 exerted a
significant anti-viral effect against HSV-2, but only a minor
effect against HSV-1. When added post-adsorption, AS101 inhibited
HSV-2-induced plaque formation, as manifested by the reduced plaque
size in the Vero cells and the reduced plaque number and viral
yield in the HK cells (Figs. 1
and 2). The incubation of mature
virions with AS101 did not alter the viral infectivity, suggesting
that AS101 does not interact with mature viruses (Fig. 3). In an attempt to identify the
stage of viral replication that is sensitive to the drug, we
performed a kinetic assay in which AS101 was added at different
time points post-infection. AS101 maintained 80% of its inhibitory
capacity when added at up to 9 h pi, a time when viral DNA
synthesis and nucleocapsid formation are already complete (23). These results are compatible with
those demonstrating that AS101 did not interfere with either viral
DNA or protein synthesis, as manifested by the equal genome copy
numbers in the presence or absence of AS101 for both free and
cell-associated viruses (Fig. 5).
On the other hand, the titer of infective viruses that burst from
infected cells was markedly lower (almost 2 log reduction) in the
presence of AS101, an effect that was most clearly demonstrated
when the cells were infected with an MOI of 1 or 2 (Table II). Of note, the effect of AS101
on HSV-2 infectivity was reduced by 1 log when we comparing the
infectivity of HSV-2 liberated from inside the cells to that of
HSV-2 found in the cell culture fluid without any intervention
(Fig. 6). Considering that AS101
is not active against mature, cell-released viruses, and that drug
uptake by the cells has not been established, these results suggest
that AS101 mainly affects the complex process of viral egress,
which takes place at the cell membrane, leading to the accumulation
of defective viral particles.
The phenomenon of the reduction of plaque size in
the presence of the drug may also result from an increase in the
number of defective viral particles. Although most of the AS101
activity is exerted during the late stages of viral replication,
kinetic assays revealed that 20–30% of the total anti-viral
activity may be attributed to the early stages of replication.
Viral entry and egress involve both cellular and viral proteins
embedded in the cell membrane and in the viral membrane envelope.
As reported in the literature, Te(IV) compounds readily interact
with thiols to form a Te-S bond (11). It has already been shown that many
of the AS101 biological activities are attributed to the ability of
its tellurium ion to interact with a cysteine residue in the active
site of proteins, thereby modifying their activity (11,13,14). HSV has at least 12
membrane-associated glycoproteins (24) that play a pivotal role in viral
penetration and egress, and are essential for viral infectivity
(25,26). For instance, glycoprotein D (gD)
is a structural component of the HSV envelope that is essential for
virus penetration, and its structure is dependent on maintenance of
three intact disulfide bonds (17). The chemical properties of AS101
suggest that it reacts with cysteine residues on both viral and
cellular glycoproteins. The differential anti-viral activity of
AS101 against HSV-1, compared to HSV-2, can also be derived from
diversity in cysteine content and function among the two HSV types
(17).
A recently published study provided evidence that
AS101 may inhibit WNV entry into Vero cells by interacting with a
putative host-cell receptor of the WNV α(V)β(3) integrin (17). Other studies have demonstrated
that α(V)β(3) integrin is a
determinant in the choice of the HSV entry pathway into cells
(27), and that HSV-1
glycoprotein H (gH) can bind to cells using the α(V)β(3) integrin (28). Therefore, the blocking of
α(V)β(3) integrin by AS101 may
partially account for its minor activity at the early stages of
viral replication. In their comprehensive analysis of HSV egress
from Vero cells, Mingo et al (29) demonstrated that HSV was released
in specific pocket-like areas on the plasma membrane that coincided
with the substrate-adherent surface and cell-cell-adherent contact
points (29). Integrins are
located at the very same points (29); an association between AS101 and
these molecules may interrupt the delicate membrane structure and
interfere with the release of the virus in a manner that leads to
the formation of defective viral particles.
HSV, similar to HPV, triggers the formation of
dermal lesions. The efficacy of an AS101 cream will be simple to
test on lesions in vaginally HSV-2-infected female mice (30). In addition, AS101 was found to be
active when added at late time points post-infection and, hence,
can be considered a therapeutic agent and not a prophylactic drug.
One major obstacle in the possible future use of AS101 as an
anti-herpetic remedy is its limited effectiveness against HSV-1.
Therefore, a compound that will synergize with AS101 in its
anti-viral activity against HSV-1 is necessary. Indeed a molecule
known as cysteamine was found to synergize the effect of AS101 as a
growth inhibitor of Jurkat cells (31). In our future research, we aim to
focus on studying the anti-viral activity of the combination of
AS101 with cysteamine, and on conducting in vivo experiments
for further evaluation of the AS101 anti-viral activity against
herpes viruses.
Acknowledgments
This study was supported by the Israeli Ministry of
Health. We wish to thank Dr S. Soupaev and Dr M. Book for their
helpful advice and Dr J. Alfandri and Dr Z. Cohen for generously
providing technical support in the laboratory. This study was part
of the Master’s thesis of Ms. Danna Sheinboim.
References
|
1
|
Wilson C: Reproductive endocrinology:
AS101 might prevent chemotherapy-induced infertility. Nat Rev
Endocrinol. 9:4412013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK,
Knickelbein JE and Hendricks RL: Herpes keratitis. Prog Retin Eye
Res. 32:88–101. 2013. View Article : Google Scholar
|
|
3
|
Rozenberg F, Deback C and Agut H: Herpes
simplex encephalitis: From virus to therapy. Infect Disord Drug
Targets. 11:235–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor TJ, Brockman MA, McNamee EE and
Knipe DM: Herpes simplex virus. Front Biosci. 7:d752–d764. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piret J and Boivin G: Resistance of herpes
simplex viruses to nucleoside analogues: Mechanisms, prevalence,
and management. Antimicrob Agents Chemother. 55:459–472. 2011.
View Article : Google Scholar :
|
|
6
|
Zhang Y, But PP, Ooi VE, Xu HX, Delaney
GD, Lee SH and Lee SF: Chemical properties, mode of action, and in
vivo anti-herpes activities of a lignin-carbohydrate complex from
Prunella vulgaris. Antiviral Res. 75:242–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yarmolinsky L, Zaccai M, Ben-Shabat S,
Mills D and Huleihel M: Antiviral activity of ethanol extracts of
Ficus binjamina and Lilium candidum in vitro. N Biotechnol.
26:307–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bernstein DI, Miller RL and Harrison CJ:
Effects of therapy with an immunomodulator (imiquimod, R-837) alone
and with acyclovir on genital HSV-2 infection in guinea-pigs when
begun after lesion development. Antiviral Res. 20:45–55. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abad MJ, Guerra JA, Bermejo P, Irurzun A
and Carrasco L: Search for antiviral activity in higher plant
extracts. Phytother Res. 14:604–607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirokawa D, Woldow A, Lee SN and Samie F:
Treatment of recalcitrant herpes simplex virus with topical
imiquimod. Cutis. 88:276–277. 2011.
|
|
11
|
Albeck A, Weitman H, Sredni B and Albeck
M: Tellurium compounds: Selective Inhibition of cysteine proteases
and model reaction with thiols. Inorg Chem. 37:1704–1712. 1998.
View Article : Google Scholar
|
|
12
|
Halperin-Sheinfeld M, Gertler A, Okun E,
Sredni B and Cohen HY: The Tellurium compound, AS101, increases
SIRT1 level and activity and prevents type 2 diabetes. Aging
(Albany NY). 4:436–447. 2012.
|
|
13
|
Sredni B, Geffen-Aricha R, Duan W, Albeck
M, Shalit F, Lander HM, Kinor N, Sagi O, Albeck A, Yosef S, et al:
Multifunctional tellurium molecule protects and restores
dopaminergic neurons in Parkinson’s disease models. FASEB J.
21:1870–1883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sredni B, Gal R, Cohen IJ, Dazard JE,
Givol D, Gafter U, Motro B, Eliyahu S, Albeck M, Lander HM, et al:
Hair growth induction by the Tellurium immunomodulator AS101:
Association with delayed terminal differentiation of follicular
keratinocytes and ras-dependent up-regulation of KGF expression.
FASEB J. 18:400–402. 2004.
|
|
15
|
Sredni B, Rosenthal-Galili Z, Michlin H,
Sobelman D, Seger Y, Blagerman S, Kalechman Y and Rager-Zisman B:
Restoration of murine cytomegalovirus (MCMV) induced
myelosuppression by AS101. Immunol Lett. 43:159–165. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vonsover A, Loya S, Sredni B, Albeck M,
Gotlieb-Stematsky T, Araf O and Hizi A: Inhibition of the reverse
transcriptase activity and replication of human immunodeficiency
virus type 1 by AS101 in vitro. AIDS Res Hum Retroviruses.
8:613–623. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Indenbaum V, Bin H, Makarovsky D, Weil M,
Shulman LM, Albeck M, Sredni B and Mendelson E: In vitro and in
vivo activity of AS101 against West Nile virus (WNV). Virus Res.
166:68–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Friedman M, Bayer I, Letko I, Duvdevani R,
Zavaro-Levy O, Ron B, Albeck M and Sredni B: Topical treatment for
human papillomavirus-associated genital warts in humans with the
novel tellurium immunomodulator AS101: Assessment of its safety and
efficacy. Br J Dermatol. 160:403–408. 2009. View Article : Google Scholar
|
|
19
|
Cantatore A, Randall SD, Traum D and Adams
SD: Effect of black tea extract on herpes simplex virus-1 infection
of cultured cells. BMC Complement Altern Med. 13:1392013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Filén F, Strand A, Allard A, Blomberg J
and Herrmann B: Duplex real-time polymerase chain reaction assay
for detection and quantification of herpes simplex virus type 1 and
herpes simplex virus type 2 in genital and cutaneous lesions. Sex
Transm Dis. 31:331–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Snoeck R: Antiviral therapy of herpes
simplex. Int J Antimicrob Agents. 16:157–159. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilson SS, Fakioglu E and Herold BC: Novel
approaches in fighting herpes simplex virus infections. Expert Rev
Anti Infect Ther. 7:559–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koyama AH and Uchida T: Quantitative
studies on the maturation process of herpes simplex virus type 1 in
Vero cells. Virus Res. 10:281–285. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Norais N, Tang D, Kaur S, Chamberlain SH,
Masiarz FR, Burke RL and Marcus F: Disulfide bonds of herpes
simplex virus type 2 glycoprotein gB. J Virol. 70:7379–7387.
1996.PubMed/NCBI
|
|
25
|
Foster TP and Kousoulas KG: Genetic
analysis of the role of herpes simplex virus type 1 glycoprotein K
in infectious virus production and egress. J Virol. 73:8457–8468.
1999.PubMed/NCBI
|
|
26
|
Foster TP, Chouljenko VN and Kousoulas KG:
Functional and physical interactions of the herpes simplex virus
type 1 UL20 membrane protein with glycoprotein K. J Virol.
82:6310–6323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gianni T and Campadelli-Fiume G:
αVβ3-integrin relocalizes nectin1 and routes herpes simplex virus
to lipid rafts. J Virol. 86:2850–2855. 2012. View Article : Google Scholar :
|
|
28
|
Parry C, Bell S, Minson T and Browne H:
Herpes simplex virus type 1 glycoprotein H binds to alphavbeta3
integrins. J Gen Virol. 86:7–10. 2005. View Article : Google Scholar
|
|
29
|
Mingo RM, Han J, Newcomb WW and Brown JC:
Replication of herpes simplex virus: Egress of progeny virus at
specialized cell membrane sites. J Virol. 86:7084–7097. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ikeda K, Yamasaki H, Minami S, Suzuki Y,
Tsujimoto K, Sekino Y, Irie H, Arakawa T and Koyama AH: Arginine
inactivates human herpesvirus 2 and inhibits genital herpesvirus
infection. Int J Mol Med. 30:1307–1312. 2012.PubMed/NCBI
|
|
31
|
Daniel-Hoffmann M, Albeck M, Sredni B and
Nitzan Y: A potential antimicrobial treatment against
ESBL-producing Klebsiella pneumoniae using the tellurium compound
AS101. Arch Microbiol. 191:631–638. 2009. View Article : Google Scholar : PubMed/NCBI
|