Introduction
Pulmonary hypertension (PH) is a serious
complication of chronic obstructive pulmonary disease (COPD) and
develops in 30–70% of COPD patients, increasing morbidity and
mortality (1,2). The pathophysiology underlying PH
development in COPD is poorly understood and is possibly
multifactorial. A consistent finding in COPD patients is the close
association between the severity of hypoxemia and pulmonary artery
pressure (PAP), supporting a major role for alveolar hypoxia
(1–3). Alveolar hypoxia causes constriction
of pulmonary arteries, and sustained alveolar hypoxia induces
pulmonary vascular remodeling. Pathological studies of lung
specimens from COPD patients have shown extensive pulmonary
vascular remodeling with prominent intimal thickening, medial
hypertrophy and muscularization of small arterioles (4–6).
Hypoxia-inducible factor (HIF)-1 is a heterodimeric
transcription factor composed of α and β subunits, and regulates
the expression of hundreds of genes in response to hypoxia,
including numerous genes associated with hypoxic PH (HPH) (7). HIF-1β is ubiquitously expressed,
whereas HIF-1α expression is O2-regulated (8,9).
Under normoxic conditions, HIF-1α is hydroxylated on two proline
residues by prolyl hydroxylase domain proteins, which use
O2 as a substrate, marking the protein for
ubiquitination and proteasomal degradation (10,11). Under hypoxic conditions, HIF-1α
accumulates and dimerizes with HIF-1β, allowing for the
transcription of target genes. The heterodimeric complex regulates
the expression of genes involved in the pathology of numerous
diseases, including HPH. Our previous study (12) indicated that HIF-1α upregulates
the expression of its downstream target genes, including vascular
endothelial growth factor (VEGF), by transcriptional
activation during hypoxia. Its involvement in the remodeling of
rats with HPH suggests that HIF-1α acts as a ‘molecular switch’ in
hypoxic pulmonary vascular remodeling (12–14).
The ubiquitin system is a key intracellular
signaling pathway for post-translational modification of proteins
(15). Small ubiquitin-related
modifier-1 (SUMO-1) is an 11.5-kDa ubiquitin-related protein with
18% similarity with ubiquitin (16–18). SUMO-1 expression has been
described in the nuclear pore complex and within nuclei in a wide
range of tissues and cell types (16,19,20). Previous studies have demonstrated
that the SUMO-1 gene is highly conserved between humans and
mice (20,21), with mRNA and protein expressed in
the brain and heart (22–24). SUMO-1 has been suggested to
primarily prevent proteasome-mediated degradation of proteins, in
contrast to ubiquitin (15,25). Previous data have shown that
SUMO-1 stabilizes nuclear proteins by a process known as
sumoylation (26). In addition,
several studies have demonstrated that SUMO-1 interacts with
various transcription factors and further modulates their
activities (16,17,20,25). For instance, SUMO-1 modifies
HIF-1α and further regulates its transcription activity in
vitro (27). Of note, it was
recently shown that increased levels of SUMO-1 participate in the
modulation of HIF-1α function through sumoylation in the adult
mouse brain and heart (28).
However, the interaction between SUMO-1 and HIF-1α has not been
studied in the context of HPH, either in vivo or in
vitro. We hypothesized that another post-translational
modification other than ubiquitination may serve as a regulatory
factor to protect HIF-1α from degradation.
In the present study, SUMO-1 expression was
assessed under hypoxic conditions and the interaction between
SUMO-1 and HIF-1α was investigated. SUMO-1 expression was
markedly upregulated at gene and protein levels under hypoxia in a
rat HPH model and in vitro (in pulmonary artery smooth
muscle cells, PASMCs). In addition, results suggested that SUMO-1
interacted with HIF-1α, which resulted in modulation of HIF-1α
function by the sumoylation pathway.
Materials and methods
Animals
Forty adult male Wistar rats (150–250 g, 8 weeks
old) were randomly divided into 5 groups (n=8 in each group). The
animals were kept in a specific pathogen-free class experimental
animal room, at 15–28°C and 45–55% relative humidity. The animals
were used for experiments after 7 days of adaptive feeding.
All the animals, procedures and experiments were
approved by the Animal Care and Use Committee of the Zhongnan
University (Changsha, China).
Rat model of chronic hypoxia-induced
PH
The protocol for the exposure of the animals to
hypoxia and normoxia was identical to that reported previously by
our laboratory (13). Wistar rats
were placed in custom-made plexiglas chambers continuously supplied
with a mixture of air and N2 (10% O2) for 3,
7, 14 and 21 days (H3, H7, H14 and H21, respectively). Normoxic
animals were kept under normal room air conditions. Oxygen levels
were measured with a gas analyzer (MB80 oxygen transmitter; Zhuhai
S.E.Z. Hangto Science & Tech. Co., Ltd., Zhuhai, China), and
the animals were allowed free access to food and water. Rats were
removed from chambers for <5 min twice weekly to replenish food
and water, and to clean cages. At the end of the exposure period,
animals were anesthetized with sodium pentobarbital (50 mg/kg)
administered intraperitoneally. PH was evaluated in rats by
measuring right ventricular (RV) systolic pressure (RVSP) and RV
hypertrophy. RV pressure was measured using a SPR-671 Mikro-Tip
pressure catheter (Model SPR-671, size 1.4F; Millar Instruments,
Inc., Houston, TX, USA) via the cannulation of the right jugular
vein. Heart and lungs were removed following exsanguination. RV was
separated from the left ventricle and septum (LV + S). The mass
ratio of RV/(LV + S) was determined as the right ventricular
hypertrophy index (RVHI), as previously described (14).
Isolation of intralobar pulmonary
arteries
Intralobar pulmonary arteries were isolated from
normoxic and hypoxic male Wistar rats (29). Following exsanguination, the lungs
were removed and transferred into petri dishes filled with
HEPES-buffered salt solution (HBSS) containing 130 mM NaCl, 5 mM
KCl, 1.2 mM MgCl2, 1.5 mM CaCl2, 10 mM HEPES
and 10 mM glucose (pH 7.4). Third- and fourth-generation
intrapulmonary arteries (200–800 µm) were isolated.
PASMC isolation and culture
PASMCs were enzymatically isolated and transiently
cultured (30). Briefly, lungs
were extracted from untreated animals following sacrifice, and
intrapulmonary arteries were dissected in HBSS containing 130 mM
NaCl, 5 mM KCl, 1.2 mM MgCl2, 1.5 mM CaCl2,
10 mM HEPES and 10 mM glucose (pH 7.2). Pulmonary arteries were
carefully cleaned from the connective tissue, and the endothelial
layer was removed by gently rubbing the luminal surface with a
cotton swab. Subsequently, the clean arteries were sequentially
incubated in ice-cold HBSS (30 min) and reduced Ca2+ (20
µM) HBSS (20 min, room temperature), following which they
were digested in reduced Ca2+ HBSS containing
collagenase (Type I, 1,750 U/ml), papain (6.4 U/ml), bovine serum
albumin (2 mg/ml) and dithiothreitol (1 mM) at 37°C for 20 min.
Following washing with Ca2+-free HBSS, single smooth
muscle cells (identified as expressing α-actin at ≥95%) were gently
dispersed from tissues by trituration in Ca2+-free HBSS,
and cultured (16–24 h, 37°C, 5% CO2) on 25-mm glass
coverslips in Ham’s F-12 medium supplemented with 0.5% fetal calf
serum, 100 U/ml streptomycin and 0.1 mg/ml penicillin in a modular
incubator chamber (Billups-Rothenberg, Del Mar, CA, USA) under 1%
O2-5% CO2 for 2, 6, 12 and 24 h for hypoxia,
and 21% O2-5% CO2 for 2, 6, 12 and 24 h for
normoxic analysis, respectively, prior to reverse
transcription-polymerase chain reaction (RT-PCR) and western blot
analysis.
Semi-quantitative RT-PCR
Pulmonary arteries and PASMCs of normoxic and
hypoxic conditions were mechanically homogenized. Subsequently,
total RNA was extracted using an RNeasy Fibrous Mini kit (Qiagen,
Valencia, CA, USA) according to the manufacturer’s instructions.
Genomic DNA contamination was removed with TURBO DNA-free DNase
(Ambion, Austin, TX, USA). Total RNA concentrations were measured
by spectrophotometry (DU-70; Beckman Coulter Inc., Brea, CA, USA)
at 260 nm, and 1 µg of RNA was used for reverse
transcription with random hexamer primers and SuperScript III RNase
H-reverse transcriptase (Invitrogen, Carlsbad, CA, USA), according
to the manufacturer’s instructions. The resulting first-strand cDNA
samples were used as templates for PCR amplification. Sense and
antisense primers specific for SUMO-1, HIF-1α,
VEGF and β-actin (Table I)
were used in PCR reactions containing PCR SuperMix (Gibco,
Invitrogen). PCR conditions were: Denaturation at 94°C for 30 sec,
annealing at 60°C for 45 sec and extension at 72°C for 90 sec for a
total of 35 cycles; and final extension at 72°C for 10 min. PCR
products were analyzed by 1.5% agarose gel electrophoresis and
visualized by staining with ethidium bromide. Parallel reactions
were run for each RNA sample in the absence of SuperScript II to
access the degree of genomic DNA contamination.
 | Table IPrimer sequences for RT-PCR. |
Table I
Primer sequences for RT-PCR.
| Gene | Accession
number | Source | Primer pair
sequence, sense/antisense | Product size,
bp | Location in
sequence |
|---|
| HIF-1α | NM024359.1 | Rat |
5′-GCCCCTACTATGTCGCTTTC-3′ | 433 | 2247–2679 |
| | |
5′-GGCCCAAACTAAACTATCTGA-3′ | | |
| SUMO-1 | NM001009672.1 | Rat |
5′-ATTGCCCTTCTTCCTTTA-3′ | 218 | 560–777 |
| | |
5′-TTCCACAGTTCGGTTCTC-3′ | | |
| VEGF | NM053653.1 | Rat |
5′-CATCCACCATGCACTTGCTGT-3′ | 490 | 80–569 |
| | |
5′-GGCTGCTCCAAACTCCTTCCA-3′ | | |
| β-actin | NM031144 | Rat |
5′-CCTAAGGCCAACCGTGAA-3′ | 635 | 223–205 |
| | |
5′-CTAGGAGCCAGGGCAGTAATC-3′ | | |
Western blot analysis and
co-immunoprecipitation
Pulmonary arteries from rats or PASMC primary
cultures were washed in phosphate-buffered saline (PBS). Pulmonary
arteries were snap-frozen in liquid nitrogen, crushed, homogenized
using a mortar and pestle and resuspended in ice-cold cell lysis
buffer (Beyotime Institute of Biotechnology, Shanghai, China)
containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% deoxycholic
acid, 0.1% SDS, 0.5% Nonidet P-40 (NP-40) and protease inhibitor
cocktail. The homogenates were centrifuged at 1,000 x g for 5 min
(4°C) and the supernatants were collected. Protein concentration
was estimated in supernatants by the bicinchoninic acid assay and
10 µg of protein was resolved on 8% SDS-PAGE gel and
electrotransferred onto nitrocellulose membranes (Shanghai Jining
Co., Ltd., Shanghai, China). Subsequent to blocking in 5% (w/v)
skimmed dry milk in PBS containing 0.05% Tween-20 (PBST) for 1 h at
room temperature, membranes were incubated at 4°C overnight with
specific primary antibodies raised in rabbits against SUMO-1 (Cat.
no. SC-9060), HIF-1α (Cat. no. sc-10790), VEGF (Cat. no. sc-507)
and β-actin (Cat. no. sc-130619) (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Subsequently, membranes were washed with PBST and
incubated with peroxidase-conjugated goat-anti-rabbit secondary
antibodies (Cat. no. BA1003; Wuhan Boster Bio., Co., Ltd., Wuhan,
China) at room temperature for 1 h. Following washing, signals were
detected with enhanced chemiluminescence, and membranes were imaged
on a Gel Logic 200 image system (Tanon Gis-2010; Tanon Science and
Technology Co., Ltd., Shanghai, China).
Co-immunoprecipitation experiments were performed
using a commercial kit (Wuhan Boster Bio., Co., Ltd.). Total
proteins were extracted with ice-cold lysis buffer containing 25 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% NP-40, 1% SDS, 200 µM
sodium deoxycholate, 1 mM dithiothreitol, 5 mM EDTA, 0.5 mM
phenylmethanesulfonylfluoride, 10 mM N-ethylmaleimide (NEM), 10 mM
iodoacetamide and a cocktail of protease inhibitors. Anti-SUMO-1
antibodies (Santa Cruz Biotechnology, Inc.) were added to 500
µg of protein extracts and incubated for 4 h at room
temperature. Immune complexes were obtained by addition of 50
µl of pansorbin cells. The resulting immobilized immune
complexes were washed in radioimmunoprecipitation assay buffer,
supplemented with 10 mM NEM and 10 mM iodoacetamide. The bound
proteins were eluted by boiling in 30 µl of SDS
sample-reducing loading buffer for 5 min. Immunoprecipitation
complexes were analyzed by western blot analysis using the
anti-HIF-1α antibody, as described above.
Immunohistochemistry
Immunohistochemical analysis (Wuhan Boster Bio.,
Co., Ltd.) was performed as previously described (14). In brief, paraffin-embedded lung
sections were mounted on poly-L-lysine slides. Slides (6 µm)
were dewaxed and sections rehydrated by immersion in ethanol
gradient and distilled water. After antigen retrieval, endogenous
peroxidase activity was blocked with 3% H2O2
in methanol for 30 min. Sections were pre-incubated in PBS
supplemented with 1% bovine serum albumin and 10% normal horse
serum for 1 h. Endogenous biotin was blocked using an
avidin/biotin-blocking kit and slides were incubated overnight with
primary antibodies raised in rabbits against SUMO-1 or HIF-1α.
Negative controls were incubated with PBS only. Subsequent to
washing, sections were sequentially incubated with biotinylated
goat-anti-rabbit secondary antibodies and streptavidin-horseradish
peroxidase (HRP). Subsequently, sections were visualized by a color
reaction with diaminobenzidine as the substrate. Brown and yellow
cells indicated positive staining. Finally, the sections were
counterstained with hematoxylin and mounted, and protein expression
levels were quantified by a pathology image analysis system
(PIPS-2020; Chongqing Thme Co, Ltd., Chongqing, China). Eight rats
per group were studied.
In situ hybridization
In situ hybridization was performed using a
commercial kit (Wuhan Boster Bio., Co., Ltd.). Sections (4
µm) were mounted on charged slides, dewaxed, dehydrated and
washed. Slides were placed in Declere (Wuhan Boster Bio., Co.,
Ltd.) for 15 min, washed and treated with proteinase K (25
µg/ml) for 1 min. Subsequently, endogenous peroxidase was
blocked using 3% H2O2. To block biotin,
sections were incubated with avidin for 15 min, and avidin was
blocked by incubation with biotin for 15 min. Sections were covered
with 10 µl of the cagA probe (4 µg/µl). For
DNA denaturation, slides were incubated at 95°C for 5 min, 4°C for
10 min, and hybridized overnight at 37°C in a humid chamber.
Subsequent to washing with HWb (Research Genetics Inc., Huntsville,
AL, USA) at 60°C for 10 min, slides were sequentially incubated in
protein-blocking buffer for 30 min and streptavidin-HRP for 30 min.
Following the reaction with diaminobenzidine, positive staining
appeared brown and yellow. Finally, the sections were
counterstained with hematoxylin and mounted. Expression levels of
mRNA were quantified by a pathology image analysis system (JD801;
Beijing Time Technologies Co., Ltd., Beijing, China).
Lentiviral vector construction and
transfection
According to the nucleotide sequence of the rat
SUMO-1 gene (NM024359.1, NM001009672.1 and NM053653.1), 4
small interfering RNAs (SUMO-1-RNAi-LV2 at 5×108
TU/ml, SUMO-1-RNAi-LV3 at 5×108 TU/ml,
SUMO-1-RNAi-LV4 at 2×108 TU/ml and pGC
FU-RNAi-NC-LV at 1x109 TU/ml as the negative control)
and 2 SUMO-1 overexpression fragments (SUMO-1-LV at
2×108 TU/ml and pGC FU-GFP-LV at 2×109 TU/ml
as the negative control) were constructed and synthesized by
Shanghai GeneChem Co. (Shanghai, China). The sequences were
transfected into PASMCs in 6-well cell culture plates, including
blank control, no-load lentivirus and small
interference/overexpression groups, and cultured for 4-6 h in a
modular incubator chamber. Lentiviral transfection rates were
observed by fluorescence microscopy (Olympus, Tokyo, Japan). Cells
were grown to 90% confluency and the media replaced for
synchronization, followed by further culture for 12 h under
normoxia (21% O2-5% CO2) and hypoxia (1%
O2-5% CO2), respectively. Finally, mRNA and
protein levels of SUMO-1, HIF-1α, VEGF were tested by RT-PCR and
western blot analysis.
Statistical analysis
The statistical package SPSS 19.0 (SPSS Inc.,
Chicago, IL, USA) was used for all the analyses. Data are expressed
as mean ± standard deviation. The group t-test was used to compare
data between two groups. Analysis of variance was used to determine
statistically significant differences among multiple groups, with
Newman-Keuls test comparing differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Verification of the hypoxia-induced PHP
model
Rats exhibited profound pulmonary arterial
hypertension and right ventricular hypertrophy when examined 21
days after hypoxia induction. Mean PAP (mPAP) was measured as an
indicator of PAP in conscious rats (mPAP in normoxic animals was
15.9±1.3 mmHg). As expected, hypoxic animals developed PH after 7
days of hypoxia (P<0.05). PH peaked at 14 days, and remained at
high levels (Table II). In
addition, pulmonary arterioles in normoxic animals were thin,
whereas after 7 days of hypoxia, they developed increased medial
thickness characteristic of PH (Table II). Quantification of the
structural changes in several lung sections from animals exposed to
different hypoxia time periods (3, 7, 14 or 21 days) revealed
significantly increased medial thickness of pulmonary arterioles in
comparison with normoxic controls. Right ventricular hypertrophy
resulting from right ventricle pressure overload is a hallmark of
PH. After 14 days of hypoxia, the RVHI was significantly increased
in comparison with controls (P<0.05), and further increased at
21 days of hypoxia. These results indicate that right ventricular
hypertrophy had developed after 14 days of hypoxia.
 | Table IIHistological and non-histological
parameters of rats exposed to normoxia or hypoxia. |
Table II
Histological and non-histological
parameters of rats exposed to normoxia or hypoxia.
| Group | mPAP, mmHg | RVHI, % | WA, % | WT, % | LA, % |
|---|
| Control | 15.9±1.3 | 23.2±2.1 | 35.0±2.2 | 15.7±1.6 | 65.0±2.2 |
| H3 | 17.1±1.4 | 23.6±2.1 | 36.6±2.0 | 16.5±1.7 | 63.4±2.0 |
| H7 |
21.3±1.6a,b | 24.7±1.7 | 41.4±2.8a,b | 19.0±1.8a,b | 58.6±2.8a,b |
| H14 | 26.5±1.7a–c | 27.0±1.8a,c | 52.0±4.0a–c | 23.1±1.9a–c | 48.1±4.0a–c |
| H21 | 28.0±2.0a–c | 29.0±1.5a–d | 58.5±4.7a–d | 26.1±1.6a–d | 41.5±4.7a–d |
| F | 91.188 | 13.866 | 76.366 | 52.715 | 76.351 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
SUMO-1, HIF-1α and VEGF levels in rat
pulmonary arteries following exposure to hypoxia
The mRNA levels of SUMO-1, HIF-1α and
VEGF in pulmonary arteries from control and hypoxia-treated
rats were assessed using in situ hybridization and RT-PCR
(Fig. 1). SUMO-1 mRNA
levels were extremely low in the control group (Fig. 1A), increased by day 3 of hypoxia
(Fig. 1B) and peaked at 14 days
(Fig. 1D). Although SUMO-1
mRNA levels were reduced in the H21 group, they were higher
compared with the values obtained from the control group (Fig. 1E). The same trend was obtained for
pulmonary artery SUMO-1 mRNA (Fig. 1L and M) as assessed by RT-PCR and
in situ hybridization (Fig.
1K). The SUMO-1 protein was hardly detected in the pulmonary
arteries from control animals (Fig.
1F). However, the H3 group (Fig.
1G) showed overtly increased protein levels, which peaked in
the H14 group (Fig. 1I) and
remained high at 21 days (H21 group; Fig. 1J). SUMO-1 expression in lung
arteries assessed by immunoblotting (Fig. 1O and P) was consistent with
immunohistochemistry data (Fig.
1N).
 | Figure 1Hypoxia induces ubiquitin-related
modifier-1 (SUMO-1) expression in rat lung arteries. Time
course of SUMO-1 mRNA expression by in situ
hybridization in rat lung arteries exposed to (A) normoxia or
hypoxia for (B) 3, (C) 7, (D) 14 and (E) 21 days (magnification,
x400). Immunohistochemistry staining of SUMO-1 protein in rat lung
arteries exposed to (F) normoxia or hypoxia for (G) 3, (H) 7, (I)
14 and (J) 21 days (magnification, x400). Detection of
SUMO-1 mRNA and protein by (M) RT-PCR and (P) western blot
analysis; β-actin was used as reference. Densitometric analyses:
(K) (N), (L) and (O) represent (A–E), (F–J), (M) and (P),
respectively. Data are expressed as mean ± standard deviation
(n=5). P<0.05 vs. acontrol group; bgroup
H3; cgroup H7. RT-PCR, reverse transcription-polymerase
chain reaction. |
Subsequently, in situ hybridization and
immunohistochemistry were used to evaluate the expression of HIF-1α
mRNA and protein, respectively (Fig.
2). Detectable amounts of HIF-1α mRNA were found in the
pulmonary arteries from the controls (Fig. 2A), but no clear change was evident
at days 3 and 7 of hypoxia (P>0.05). However, the H14 and H21
groups showed higher mRNA levels compared with the control, H3 and
H7 groups. The trend for HIF-1α gene expression in lung
arteries (Fig. 2L and M) observed
by RT-PCR was consistent with in situ hybridization data
(Fig. 2K). Immunohistochemistry
and immunoblotting analyses revealed no clear HIF-1α protein signal
in the controls, while a statistical significance was observed at
day 3 of hypoxia in the intima and media of pulmonary arterioles,
compared with the controls (P<0.05). A peak was obtained by day
7 (H7 group) and by day 14, protein levels began to drop, a trend
that continued to day 21 (Fig. 2N and
O).
 | Figure 2Expression of HIF-1α in rat lung
arteries following exposure to normoxia and hypoxia. Time course
analysis of HIF-1α (A to E) mRNA and (F–J) protein
expression in rat lung arteries. N, H3, H7, H14 and H21 represent
rat lung exposure to normal room air (normoxia) as control or 10%
O2 (hypoxia) for 3, 7, 14 and 21 days, respectively
(magnification, x400). Analysis of HIF-1α mRNA and protein by (M)
RT-PCR and (P) western blot analysis; β-actin was used as a
reference. Densitometric analyses in (K), (N), (L), and (O)
represent (A–E), (F–J), (M) and (P), respectively. Data are
expressed as mean ± standard deviation (n=4). P<0.05 vs.
acontrol group; bgroup H3; cgroup
H7; dgroup H14. Hypoxia-inducible factor-1α, HIF-1α;
RT-PCR, reverse transcription-polymerase chain reaction. |
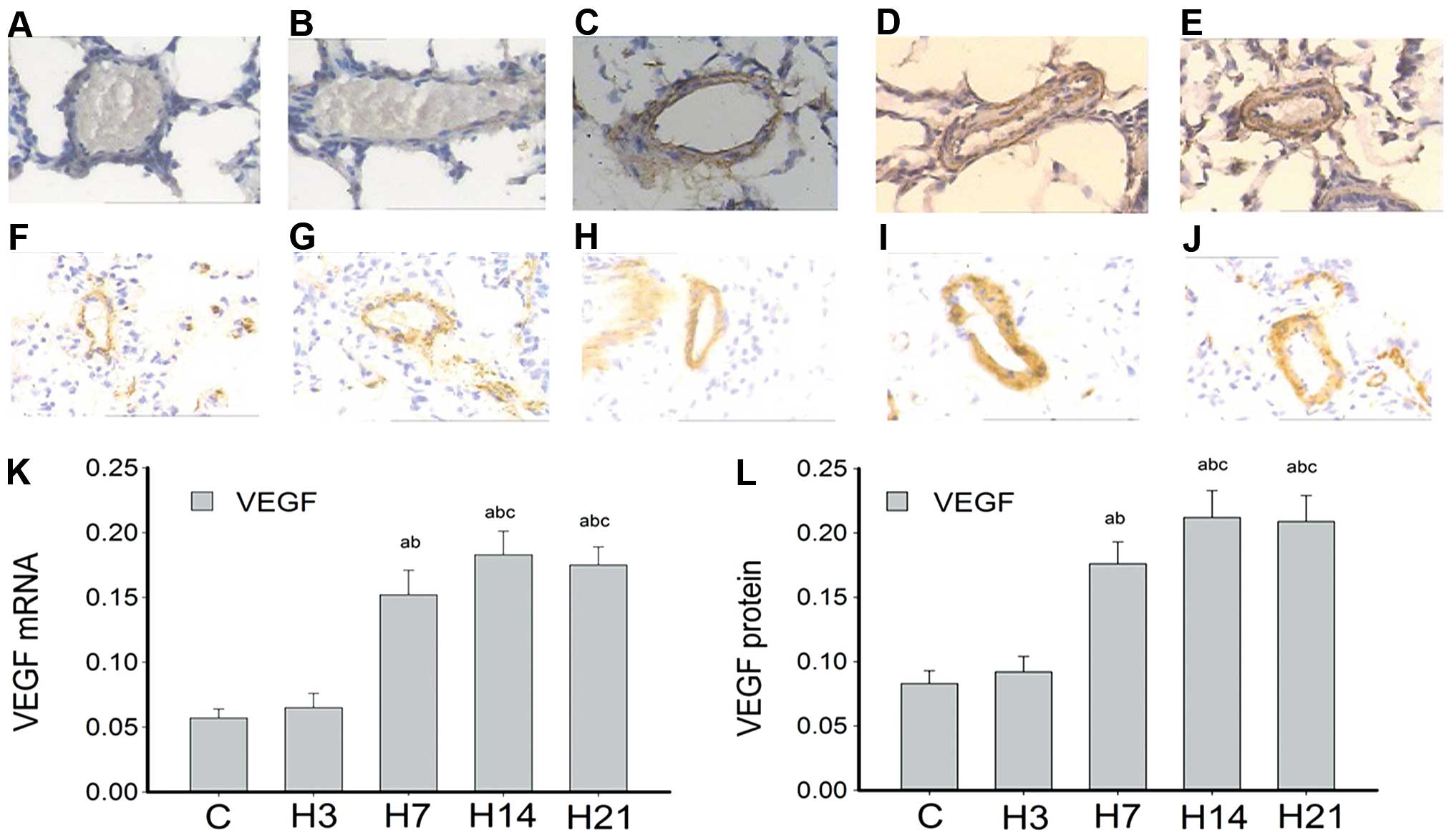
Fig. 3A–E and F–J
present the time course analysis of VEGF mRNA and protein
expression, respectively, in rat lung arteries. VEGF mRNA
was hardly detected in the control and H3 groups. However, samples
collected after 7 days of hypoxia showed that VEGF mRNA
levels were markedly higher compared with that of the controls,
with a statistical significance at P<0.05. Notably, mRNA
expression peaked at day 14 and remained high until day 21
(Fig. 3K). VEGF mRNA was
also mainly expressed at the intima and media. Accordingly, the
levels of VEGF protein were extremely low in the control and H3
groups, significantly raised in the H7 group, peaked at day 14 and
remained high at day 21 of hypoxia (Fig. 3L).
SUMO-1, HIF-1α and VEGF expression levels
in rat PASMCs following hypoxia
The results presented suggest that hypoxia induces
SUMO-1, HIF-1α and VEGF gene expression in
animals. However, it is not clear whether this is true at the cell
level, in vitro. Therefore, RT-PCR and western blot analysis
were used to assess the expression levels of SUMO-1, HIF-1α and
VEGF in PAMSCs, at mRNA and protein levels (Fig. 4). SUMO-1 mRNA levels began
to increase in PAMSCs after hypoxia for 2 h (H2h), compared with
normoxia (control group), peaked after 12 h (H12h), and remained
higher than the control values over time, until 24 h (H24h)
(Fig. 4C). HIF-1α mRNA
levels immediately increased in PAMSCs after 2-h hypoxia compared
with the normoxia group; however, no significant difference was
found between all the hypoxia groups (H2h, H6h, H12h and H24h) and
controls (P>0.05) as shown in Fig.
4D. As for VEGF, the mRNA expression levels gradually
increased with hypoxia time, reaching a peak after 12 h, and still
exhibiting high levels at 24 h (Fig.
4E).
 | Figure 4Ubiquitin-related modifier-1
(SUMO-1), HIF-1α and vascular endothelial growth factor (VEGF) are
increased by hypoxia in pulmonary arterial smooth muscle cells
(PASMCs) isolated from rat lungs. Time-course analysis of SUMO-1,
HIF-1α and VEGF expression by (A) RT-PCR and (B) western blot
analysis in rat PASMCs cultured in complete medium equilibrated
with either 21% O2 (normoxia) or 1% O2
(hypoxia) for 2, 6, 12 and 24 h (H2h, H6h, H12h and H24h,
respectively). β-actin was used as a reference. Densitometric
analyses in (C and F), (D and G) and (E and H) represent the levels
of SUMO-1, HIF-1α and VEGF mRNA and protein, respectively. Data are
expressed as mean ± standard deviation from four independent
experiments. P<0.05 vs. acontrol group;
bgroup H2h; cgroup H6h. RT-PCR, reverse
transcription-polymerase chain reaction. |
Western blot data showed that SUMO-1 protein levels
began to increase in PAMSCs after 6 h of hypoxia, compared with the
normoxia group, peaked after 12 h but tended to decrease at 24 h
(Fig. 4B and F). However, HIF-1α
protein levels steadily increased and maintained higher levels from
6 to 24-h hypoxia, peaking after 12 h (Fig. 4G), consistent with VEGF protein
levels (Fig. 4H). Therefore, we
may conclude that hypoxia overtly enhances mRNA and protein
expression of SUMO-1, HIF-1α and VEGF in rat PASMCs in a
time-dependent manner.
Effect of rat SUMO-1 gene regulation in
PASMCs
As shown previously, hypoxia promotes the expression
of SUMO-1, HIF-1 and VEGF in PASMCs. To
confirm the role of SUMO-1 in sumoylational modification of HIF-1α,
gene silencing (SUMO-1-RNAi-LV) and overexpression
(SUMO-1-LV) were carried out in rat PASMCs.
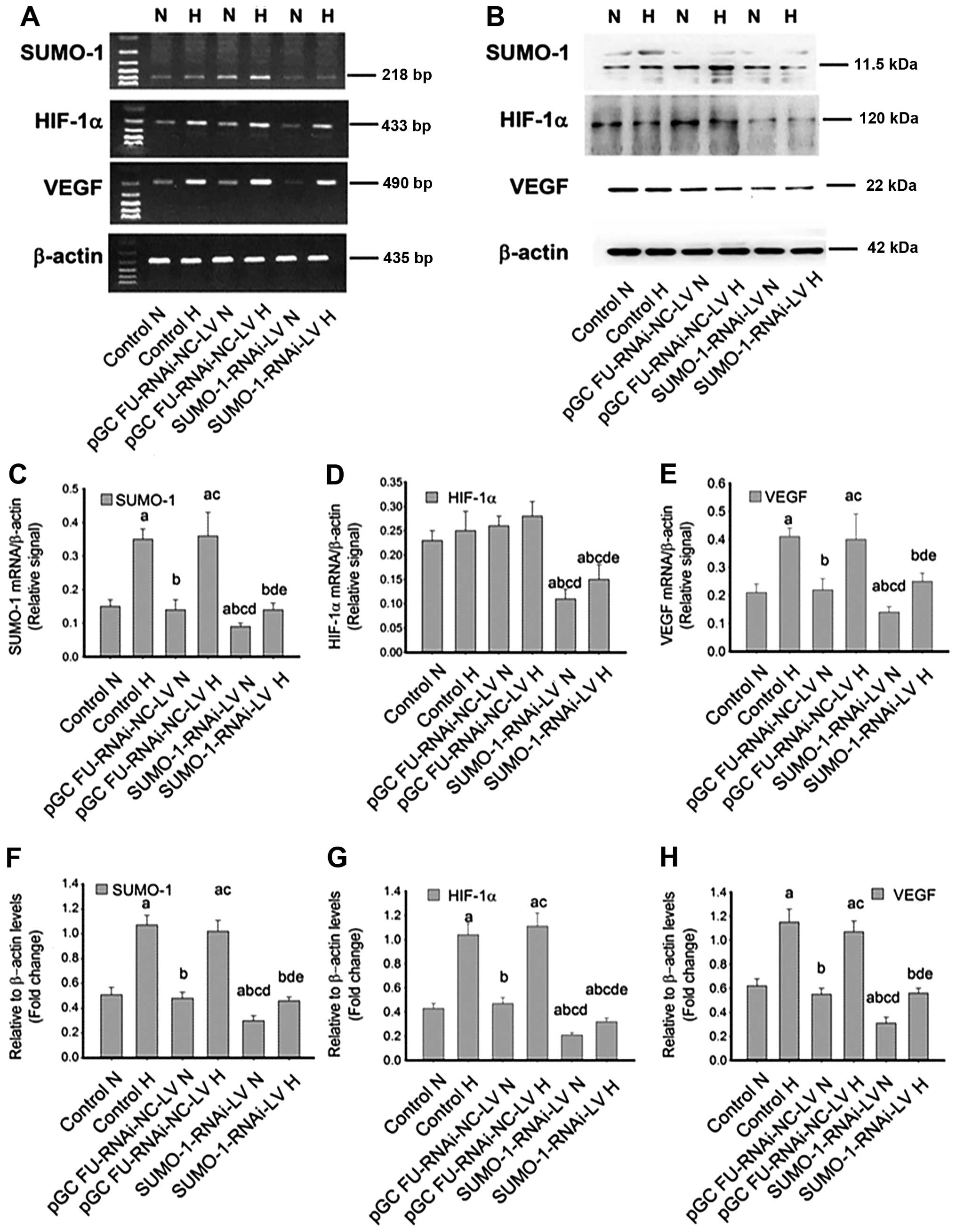
Following SUMO-1 gene silencing, RT-PCR and
immunoblotting revealed a more pronounced decrease in the
expression of HIF-1α and VEGF, compared with that of the control
groups and cells transfected with pGC FU-RNAi-NC-LV (Fig. 5A and B). Compared with the
corresponding normoxia groups (control, pGC FU-RNAi-NC-LV and
SUMO-1-RNAi-NC-LV groups), SUMO-1 and VEGF
mRNA expression were increased in all hypoxia groups (Fig. 5C and E). Whether under normoxia or
hypoxia, SUMO-1, HIF-1α and VEGF in the SUMO-1-RNAi-NC-LV
group were clearly reduced, with a statistically significant
difference (P<0.05) (Fig.
5C–H).
Subsequent to SUMO-1 gene overexpression,
RT-PCR and immunoblotting data demonstrated that the expression of
HIF-1α and VEGF (mRNA and protein) was increased compared with that
of the control and pGC FU-RNAi-NC-LV groups (Fig. 6A and B). Compared with the
corresponding normoxia groups (control, pGC FU-RNAi-NC-LV and
SUMO-1-LV groups), SUMO-1α and VEGF expression
was increased in all the hypoxia groups (Fig. 6C and E). In addition, whether
under normoxia or hypoxia, SUMO-1, HIF-1α and VEGF levels in the
SUMO-1-LV group were all clearly enhanced, with a
statistically significant difference (P<0.05), as shown in
Fig. 6C–H. Therefore,
SUMO-1 gene silencing and overexpression assays confirmed
that SUMO-1 may participate in the modulation of HIF-1α through
sumoylation and upregulate HIF-1α expression, as well as
HIF-1α downstream target genes, including VEGF, in
rat PASMCs.
There was no difference between pGC FU-RNAi-NC-LV or
pGC FU-GFP-LV and controls under normoxia and hypoxia (all
P>0.05) (Figs. 5D, E, G and H,
and 6D, E, G and H), suggesting
that the negative controls of SUMO-1 knockdown and
overexpression did not affect hypoxia-induced HIF signaling.
HIF-1α sumoylational modification in rat
lung arteries and PASMCs
To further confirm the findings, HIF-1α
sumoylational modification of rat lung arteries was compared after
exposure to hypoxia for 0, 3, 7, 14 and 21 days, and PASMCs after
2, 6, 12 and 24 h of exposure to hypoxia (Fig. 7). The data clearly demonstrated
that HIF-1α in the control group did not bear any sumoylational
modification. However, hypoxia-treated cells showed significant
sumoylational modification from day 3, which peaked by 14 days and
remained high until 21 days (Fig.
7A). Similarly, HIF-1α in the control cells exhibited no
sumoylational modification, and hypoxia groups showed overt
sumoylational modifications, more evident at 12 and 24 h (Fig. 7B).
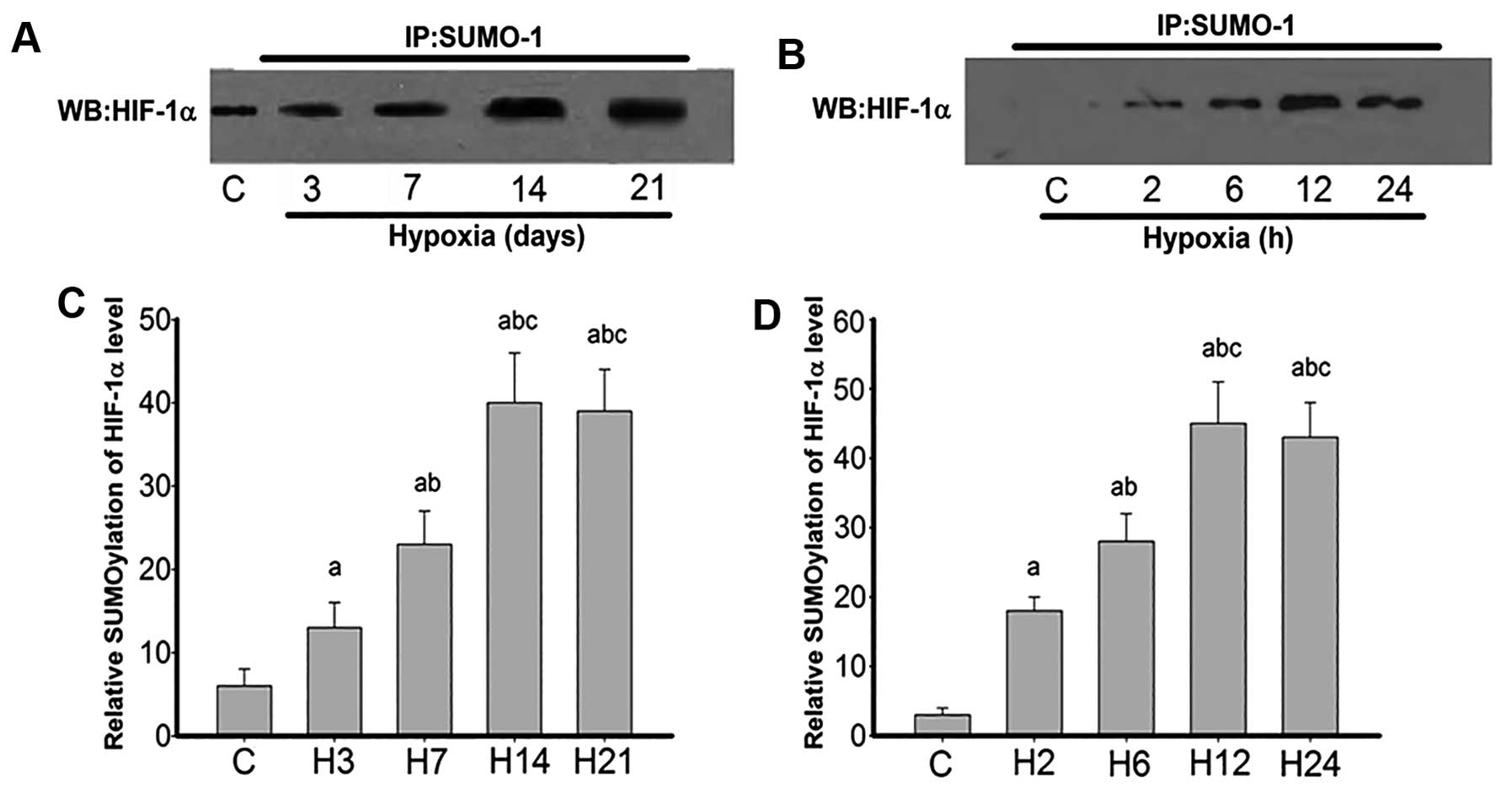 | Figure 7Co-immunoprecipitation of
ubiquitin-related modifier-1 (SUMO-1) with HIF-1α in rat lung
arteries and pulmonary arterial smooth muscle cells (PASMCs)
exposed to hypoxia. Immunoprecipitation (IP) was performed with the
anti-SUMO-1 antibody. For western blot analysis (WB), the primary
antibody against HIF-1α was used. (A) SUMO-1 and HIF-1α
co-immunoprecipitation at lung arteries exposed to hypoxia 3, 7, 14
and 21 days (H3, H7, H14 and H21, respectively). (C) Quantification
of (A). Data are expressed as mean ± standard deviation (SD) (n=4).
P<0.05 vs. acontrol group; bgroup H3;
cgroup H7. (B) SUMO-1 and HIF-1α co-immunoprecipitation
in PASMCs exposed to hypoxia for 0, 2, 6, 12 and 24 h. (D)
Quantification of (B). Data are expressed as mean ± SD from four
independent experiments. P<0.05 vs. acontrol group;
bgroup H2; cgroup H6. Hypoxia-inducible
factor-1α, HIF-1α. |
Discussion
In the present study, the results suggest that
SUMO-1 gene expression is enhanced by hypoxic stimulation in
rat PASMCs and the rat HPH model. Furthermore, there may be an
association between SUMO-1 and HIF-1α in response to hypoxic
stimulation in vivo and in vitro, suggesting that
sumoylation of HIF-1α may be important in the stabilization and
action of the HIF-1α protein.
HIF-1 is a heterodimer of two basic
helix-loop-helix/PAS proteins, HIF-1α and HIF-1β (8), and its activation plays an important
role in tissue preservation as a response to regional hypoxia
(31). HIF-1α is rapidly degraded
under normoxic conditions by the ubiquitin-proteasome system;
HIF-1β is constitutively expressed in the nucleus and its level is
not significantly affected by oxygen levels (32,33). It is known that hypoxia stabilizes
HIF-1α, and nucleus-bound translocation of the stabilized HIF-1α
allows for the formation of the HIF-1αβ heterodimer that becomes
transcriptionally active (33).
HIF-1α, as a transcription factor induced by hypoxia, regulates the
expression of >100 genes involved in cellular adaptation and
survival (34,35). It has been shown that HIF-1α can
be regulated by various post-translational modifications, including
hydroxylation, acetylation and phosphorylation. Therefore, HIF-1α
plays a central role in the cellular response to hypoxia, and its
expression levels are tightly controlled through synthesis and
degradation (34,35).
The present study indicates an important role for
sumoylation of HIF-1α in hypoxic PH. The covalent conjugation of
SUMOs to proteins has received increasing attention since its
discovery (36,37), due to the involvement of target
proteins in gene expression, chromatin structure, signal
transduction or maintenance of the genome (38). SUMO modification has emerged as an
important regulatory mechanism for protein function and
localization. Sumoylation is a dynamic process, catalyzed by
SUMO-specific E1, E2 and E3s (39). In mammals, SUMO is first activated
by an E1 heterodimer comprised of SAE1 and SAE2, transferred to an
E2-conjugating Ubc9 enzyme and conjugated by an E3 ligase to target
proteins. Three mammalian E3 enzymes have been described:
Ran-binding protein2, protein inhibitor of activated STAT and
polycomb protein 2 (40). For
instance, transient global cerebral ischemia induces significant
increases in the sumoylation of proteins, including transcription
factors (41). In particular,
hypoxia increases SUMO-1 mRNA and protein levels in the brain, and
the induced SUMO-1 is co-expressed with HIF-1α in neurons (39).
However, controversy exists regarding the cellular
mechanism of HIF-1α modulation through sumoylation. It is generally
believed that sumoylation of transcription factors blocks their
activation (26,42). Thus, conjugation of SUMO to HIF-1α
decreases its activity (43), and
hypoxia-induced sumoylation of HIF-1α leads to its ubiquitination
and degradation (44). By
contrast, Bae et al (45)
reported that HIF-1α upregulation through SUMO-1 modification at
Lys(391)/Lys(477) residues increases its stability and enhances
transcriptional activity, and sumoylation of HIF-1α blocks its
degradation (41). However,
sumoylation of HIF-1α in the pathogenesis of hypoxia-induced PH
remains poorly understood.
The present results suggest that hypoxia induces
SUMO-1 expression and promotes HIF-1α and VEGF mRNA and
protein expression in PASMCs. Increased SUMO-1 mRNA and protein
levels following hypoxia stimulation and involvement in HIF-1α
post-translational modifications is a well-described process
(39). Therefore, an increase in
SUMO-1 may contribute to HIF-1α stabilization under hypoxic
conditions, which is important for HIF-1α-dependent gene activation
(such as activation of VEGF, erythropoietin, glucose
transporters and endothelial cell proliferation) involved in
vascular biology, cellular metabolism and tissue tolerance to
hypoxia (46). VEGF has been
shown in vivo and in vitro to be the principal
mediator of hypoxia-induced angiogenesis (46–49). In the present study, sumoylation
does not appear to be the only mechanism responsible for increased
HIF-1α expression. In addition to increased protein levels due to a
decreased degradation (8,9), HIF-1α mRNA expression is also
increased. Previous studies suggest that hypoxia per se
increases HIF-1α mRNA expression through a number of
mechanisms, including the mTOR pathway (50), changes in redox status within the
cells (51) and
lipopolysaccharide exposure (52). Further studies are required to
examine the exact mechanisms involved in HIF-1α mRNA
expression, particularly in HPH.
In order to further verify the association between
sumoylation modification and HIF-1α, gene silencing and
overexpression were used to modulate SUMO-1 in rat PASMCs. The
present data suggested that HIF-1α and VEGF expression, at mRNA and
protein levels, was decreased following SUMO-1 gene
knockdown, and increased upon overexpression. These findings also
suggested that SUMO-1 plays a critical role in HIF-1α
sumoylational modification. In addition, the co-immunoprecipitation
experiment suggests an association between SUMO-1 and HIF-1α, but
did not allow the assessment of the exact nature of this
association. Further studies are required regarding this point.
Notably, Shao et al (39) reported that RWD-containing
sumoylation enhancer (RSUME) can be induced by hypoxia, enhancing
HIF-1α sumoylation and promoting its stabilization and
transcriptional activity during hypoxia. Disruption of the RWD
domain of RSUME demonstrated that RWD is critical for RSUME action.
Therefore, we can speculate that RSUME plays a central role in the
regulation of sumoylation, having indirectly demonstrated that
sumoylation of HIF-1α stabilizes HIF-1α and enhances its
transcriptional activity.
In conclusion, to the best of our knowledge, this
is the first study investigating the effect of hypoxia on
SUMO-1 gene expression in PASMCs and rat HPH models. There
was an association between the hypoxia-induced increase in
SUMO-1 expression and HIF-1α induction in response to
hypoxia, but our results did not allow us to determine the extent
and the exact nature of this association. Further studies are
required to explore this association.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 30971329 and
81270118) and the Medical Scientific Research Projects of Hunan
Province (nos. B2008-025 and B2011-101).
References
|
1
|
Chatila WM, Thomashow BM, Minai OA, Criner
GJ and Make BJ: Comorbidities in chronic obstructive pulmonary
disease. Proc Am Thorac Soc. 5:549–555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaouat A, Naeije R and Weitzenblum E:
Pulmonary hypertension in COPD. Eur Respir J. 32:1371–1385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scharf SM, Iqbal M, Keller C, et al:
Hemodynamic characterization of patients with severe emphysema. Am
J Respir Crit Care Med. 166:314–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kosanovic D, Dahal BK, Peters DM, Seimetz
M, Wygrecka M, Hoffmann K, Antel J, Reiss I, Ghofrani HA, Weissmann
N, Grimminger F, Seeger W and Schermuly RT: Histological
characterization of mast cell chymase in patients with pulmonary
hypertension andchronic obstructive pulmonary disease. Pulm Circ.
4:128–136. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ulasli SS, Eyuboglu FO, Verdi H and Atac
FB: Associations between endothelial nitric oxide synthase A/B,
angiotensin converting enzyme I/D and serotonin transporter L/S
gene polymorphisms with pulmonary hypertension in COPD patients.
Mol Biol Rep. 40:5625–5633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wright JL, Petty T and Thurlbeck WM:
Analysis of the structure of the muscular pulmonary arteries in
patients with pulmonary hypertension and COPD: National Institutes
of Health nocturnal oxygen therapy trial. Lung. 170:109–124. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimoda LA and Semenza GL: HIF and the
lung: Role of hypoxia-inducible factors in pulmonary development
and disease. Am J Respir Crit Care Med. 183:152–156. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar
|
|
9
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaakkola P, Mole DR, Tian YM, et al:
Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation
complex by O2-regulated prolyl hydroxylation. Science.
292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivan M, Kondo K, Yang H, et al: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li QF and Dai AG: Hypoxia -inducible
factor-1 alpha regulates the role of vascular endothelial growth
factor on pulmonary arteries of rats with hypoxia-induced pulmonary
hypertension. Chin Med J (Engl). 117:1023–1028. 2004.
|
|
13
|
Li QF and Dai AG: Hypoxia inducible
factor-1 alpha correlates the expression of heme oxygenase 1 gene
in pulmonary arteries of rat with hypoxia-induced pulmonary
hypertension. Acta Biochim Biophys Sin (Shanghai). 36:133–140.
2004. View Article : Google Scholar
|
|
14
|
Jiang Y, Dai A, Li Q and Hu R: Hypoxia
induces transforming growth factor-beta1 gene expression in the
pulmonary artery of rats via hypoxia-inducible factor-1alpha. Acta
Biochim Biophys Sin (Shanghai). 39:73–80. 2007. View Article : Google Scholar
|
|
15
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Melchior F: SUMO - nonclassical ubiquitin.
Annu Rev Cell Dev Biol. 16:591–626. 2000. View Article : Google Scholar
|
|
17
|
Müller S, Hoege C, Pyrowolakis G and
Jentsch S: SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell
Biol. 2:202–210. 2001. View Article : Google Scholar
|
|
18
|
Hochstrasser M: SP-RING for SUMO: New
functions bloom for a ubiquitin-like protein. Cell. 107:5–8. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saitoh H, Pu RT and Dasso M: SUMO-1:
Wrestling with a new ubiquitin-related modifier. Trends Biochem
Sci. 22:374–376. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su HL and Li SS: Molecular features of
human ubiquitin-like SUMO genes and their encoded proteins. Gene.
296:65–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Howe K, Williamson J, Boddy N, Sheer D,
Freemont P and Solomon E: The ubiquitin-homology gene PIC1:
Characterization of mouse (Pic1) and human (UBL1) genes and
pseudogenes. Genomics. 47:92–100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamitani T, Kito K, Nguyen HP,
Fukuda-Kamitani T and Yeh ET: Characterization of a second member
of the sentrin family of ubiquitin-like proteins. J Biol Chem.
273:11349–11353. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okura T, Gong L, Kamitani T, et al:
Protection against Fas/APO-1- and tumor necrosis factor-mediated
cell death by a novel protein, sentrin. J Immunol. 157:4277–4281.
1996.PubMed/NCBI
|
|
24
|
Shao R, Zhang FP, Rung E, Palvimo JJ,
Huhtaniemi I and Billig H: Inhibition of small ubiquitin-related
modifier-1 expression by luteinizing hormone receptor stimulation
is linked to induction of progesterone receptor during ovulation in
mouse granulosa cells. Endocrinology. 145:384–392. 2004. View Article : Google Scholar
|
|
25
|
Hochstrasser M: Ubiquitin-dependent
protein degradation. Annu Rev Genet. 30:405–439. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Desterro JM, Rodriguez MS and Hay RT:
SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation.
Mol Cell. 2:233–239. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tojo M, Matsuzaki K, Minami T, et al: The
aryl hydrocarbon receptor nuclear transporter is modulated by the
SUMO-1 conjugation system. J Biol Chem. 277:46576–46585. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan JY, Tsai CY, Wu CH, et al:
Sumoylation of hypoxia-inducible factor-1alpha ameliorates failure
of brain stem cardiovascular regulation in experimental brain
death. PLoS One. 6:e173752011. View Article : Google Scholar
|
|
29
|
Li X, Lu W, Fu X, et al: BMP4 increases
canonical transient receptor potential protein expression by
activating p38 MAPK and ERK1/2 signaling pathways in pulmonary
arterial smooth muscle cells. Am J Respir Cell Mol Biol.
49:212–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J: Extraction and identification of
primary rat pulmonary artery smooth muscle cells and effects of
hypoxia on the proliferation. Chin J Respir Crit Care Med.
11:147–152. 2012.
|
|
31
|
Rosenberger C, Heyman SN, Rosen S, et al:
Up-regulation of HIF in experimental acute renal failure: Evidence
for a protective transcriptional response to hypoxia. Kidney Int.
67:531–542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wood SM, Gleadle JM, Pugh CW, Hankinson O
and Ratcliffe PJ: The role of the aryl hydrocarbon receptor nuclear
translocator (ARNT) in hypoxic induction of gene expression.
Studies in ARNT-deficient cells. J Biol Chem. 271:15117–15123.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ema M, Hirota K, Mimura J, et al:
Molecular mechanisms of transcription activation by HLF and
HIF1alpha in response to hypoxia: Their stabilization and redox
signal-induced interaction with CBP/p300. EMBO J. 18:1905–1914.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martinez-Sanchez G and Giuliani A:
Cellular redox status regulates hypoxia inducible factor-1
activity. Role in tumour development. J Exp Clin Cancer Res.
26:39–50. 2007.PubMed/NCBI
|
|
35
|
Yee Koh M, Spivak-Kroizman TR and Powis G:
HIF-1 regulation: Not so easy come, easy go. Trends Biochem Sci.
33:526–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahajan R, Delphin C, Guan T, Gerace L and
Melchior F: A small ubiquitin-related polypeptide involved in
targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell.
88:97–107. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matunis MJ, Coutavas E and Blobel G: A
novel ubiquitin-like modification modulates the partitioning of the
Ran-GTPase-activating protein RanGAP1 between the cytosol and the
nuclear pore complex. J Cell Biol. 135:1457–1470. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hay RT: SUMO: A history of modification.
Mol Cell. 18:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shao R, Zhang FP, Tian F, et al: Increase
of SUMO-1 expression in response to hypoxia: Direct interaction
with HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett.
569:293–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mossessova E and Lima CD: Ulp1-SUMO
crystal structure and genetic analysis reveal conserved
interactions and a regulatory element essential for cell growth in
yeast. Mol Cell. 5:865–876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang W, Sheng H, Homi HM, Warner DS and
Paschen W: Cerebral ischemia/stroke and small ubiquitin-like
modifier (SUMO) conjugation - a new target for therapeutic
intervention? J Neurochem. 106:989–999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng J, Bawa T, Lee P, Gong L and Yeh ET:
Role of desumoylation in the development of prostate cancer.
Neoplasia. 8:667–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Berta MA, Mazure N, Hattab M, Pouyssegur J
and Brahimi-Horn MC: SUMOylation of hypoxia-inducible factor-1alpha
reduces its transcriptional activity. Biochem Biophys Res Commun.
360:646–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng J, Kang X, Zhang S and Yeh ET:
SUMO-specific protease 1 is essential for stabilization of
HIF1alpha during hypoxia. Cell. 131:584–595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bae SH, Jeong JW, Park JA, et al:
Sumoylation increases HIF-1alpha stability and its transcriptional
activity. Biochem Biophys Res Commun. 324:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Semenza GL: Expression of
hypoxia-inducible factor 1: Mechanisms and consequences. Biochem
Pharmacol. 59:47–53. 2000. View Article : Google Scholar
|
|
47
|
Semenza GL: HIF-1: Mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol (1985). 88:1474–1480. 2000.
|
|
48
|
Semenza GL, Agani F, Feldser D, et al:
Hypoxia, HIF-1, and the pathophysiology of common human diseases.
Adv Exp Med Biol. 475:123–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ravi R, Mookerjee B, Bhujwalla ZM, et al:
Regulation of tumor angiogenesis by p53-induced degradation of
hypoxia-inducible factor 1alpha. Genes Dev. 14:34–44.
2000.PubMed/NCBI
|
|
50
|
Hudson CC, Liu M, Chiang GG, et al:
Regulation of hypoxia-inducible factor 1alpha expression and
function by the mammalian target of rapamycin. Mol Cell Biol.
22:7004–7014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin WS, Kong ZL, Shen ZF, Jin YZ, Zhang WK
and Chen GF: Regulation of hypoxia inducible factor-1alpha
expression by the alteration of redox status in HepG2 cells. J Exp
Clin Cancer Res. 30:612011. View Article : Google Scholar
|
|
52
|
Blouin CC, Page EL, Soucy GM and Richard
DE: Hypoxic gene activation by lipopolysaccharide in macrophages:
implication of hypoxia-inducible factor 1alpha. Blood.
103:1124–1130. 2004. View Article : Google Scholar
|