Introduction
The control of host-microbe homeostasis in the
mucosal epithelium is essential for preventing
microorganism-triggered inflammatory diseases. A number of common
pathogenic bacteria are able to invade epithelial cells. Intestinal
pathogens, such as Salmonella, Shigella,
Yersinia and Escherichia coli are able to invade
intestinal epithelial cells (1).
Respiratory pathogens, such as Pseudomonas aeruginosa
(2), Haemophilus
influenzae (3),
Streptococcus pneumonia (4) and Klebsiella pneumoniae
(5) can enter airway epithelial
cells. Uropathogens, such as Escherichia coli and
Klebsiella pneumoniae (6)
and Stenotrophomonas maltophilia (7) invade the host urothelium. Studies
have demonstrated that some host cells have the ability to remove
intracellular bacteria (8,9).
We discovered that Klebsiella pneumoniae is capable of
invading human T24 cells and that these cells, in turn, are capable
of eliminating the infection.
Innate immunity plays an important role in the
removal of invasive intracellular bacteria from epithelial cells.
As regards invertebrates, the Drosophila melanogaster
intestinal immune response relies mainly on 2 types of
complementary and synergistic molecular effectors that restrict the
proliferation of microorganisms: antimicrobial peptides and
reactive oxygen species (ROS) (10). Oxidative defense mechanisms
involving ROS play important roles in innate immunity (11). ROS mainly derive from
mitochondrial metabolism or from regulated nicotinamide adenine
dinucleotide phosphate-oxidase (NADPH) oxidase (NOX) activity
(12). The NOX family includes
NOX1-5, dual oxidase (DUOX)1 and DUOX2 (13), all of which function as components
of the mucosal immune response (14). In fact, the T helper (Th)1 and Th2
cytokines promote host defense and pro-inflammatory responses by
regulating the expression of DUOX1 and DUOX2 in human airways
(11). Moreover, as previously
demonstrated, DUOX2/dual oxidase maturation factor 2 (DUOXA2)
co-transfected human thyroid cells treated with phorbol
12-myristate 13-acetate (PMA) exhibit increased
H2O2 generation, which is associated with
PMA-mediated DUOX2 phosphorylation (15).
ROS generation is necessary to initiate an innate
immune response in normal human nasal epithelial cells (16), and this response mediates
epithelial cell H2O2 production to counteract
the replication of the respiratory syncytial virus (17) and influenza A virus (18) and prevent gastric Helicobacter
felis colonization and the inflammatory response (10). DUOX2 activity is a possible source
of ROS in all of these immune responses. Additionally, in the
Listeria monocytogenes infection model, the simultaneous
overexpression of nucleotide-binding oligomerization domain
containing 2 (NOD2) and DUOX2 was found to result in cooperative
protection against bacterial cytoinvasion (19). Therefore, as the main NOX, DUOX2
plays a major role in host defense in epithelial cells, including
airway and intestinal epithelial cells, and acts as a host defense
mechanism in phagocytes through the generation of ROS.
Klebsiella pneumonae is a common uropathogen
found in diabetic patients and hospital settings and comprises up
to 5% of urinary tract infections (20,21). While the antimicrobial substances
in urine (such as urea) and the mucin of urinary tract mucosal
surfaces provide a physiochemical barrier, intrinsic antibacterial
activity may play an important role in maintaining bladder
sterility.
Although the activation of DUOX1 has been shown to
protect mouse urothelial cells from pathogens through the
production of H2O2 (22), to the best of our knowledge, DUOX2
expression in bladder cancer epithelial cells has not been reported
to date. Furthermore, whether Klebsiella pneumonae invades
human T24 cells and whether DUOX2 plays a role in eliminating
Klebsiella pneumonae through ROS signaling in T24 cells
remains unknown. Thus, the aim of this study was to investigate
DUOX2 expression in T24 cells and to determine whether DUOX2
contributes to immune host defense and the elimination of the
Klebsiella pneumonae K5 strain in T24 cells through ROS
production.
Materials and methods
Cell culture and treatment
T24 human bladder carcinoma cells (maintained in our
laboratory) were cultured in RPMI-1640 medium (HyClone
Laboratories, Inc., Logan, UT, USA) containing 10% newborn calf
serum (Lanzhou Minhai Bioengineering Co., Ltd., Gansu, China) and
grown at 37°C in a humidified atmosphere with 5% CO2.
The experiments were performed using cells in the exponential
growth phase.
The pro-inflammatory cytokines, interferon-γ
(IFN-γ), tumor necrosis factor (TNF-)α, interleukin (IL)-1β and
IL-17 (0–20 ng/ml); the NOX activator, PMA (0–1 nM); the NOD2
cognate ligand, muramyl dipeptide (MDP; 0–1 µg/ml);
N-acetylmuramyl-D-alanyl-D-isoglutamine (MDP-DD; an MDP negative
control; 0–1 µg/ml); the NOX inhibitor, diphenyleneiodonium
(DPI ; 0–50 nM/ml) and the antioxidant, catalase (CAT; 0–1,000
U/ml), were purchased from Sigma (St. Louis, MO, USA). The
cytokines were dissolved in phosphate-buffered saline (PBS) with 1%
bovine serum albumin (BSA). DPI and PMA were dissolved in dimethyl
sulfoxide (DMSO). MDP and MDP-DD were dissolved in distilled water.
CAT was dissolved in RPMI-1640 medium. Untreated cells were used as
controls.
Knockdown of DUOX2 using small
interfering RNA (siRNA)
The T24 cells were transfected with 20 nM/1 siRNA
targeting DUOX2 (DUOX2 siRNA; sense, 5′-GGCAGGAGACUAU GUUCUATT-3′
and antisense, 5′-UAGAACAUAGUCUCCU GCCTT-3′) according to the
manufacturer’s instructions. In brief, the T24 cells were seeded in
6- or 24-well culture plates 24 h prior to transfection.
Lipofectamine 2000 (5 µl; Invitrogen Life
Technologies, Carlsbad, CA, USA) and DUOX2 siRNA (10
µl; Shanghai GenePharma Co., Ltd., Shanghai, China)
were added to serum-free cell culture medium prior to use and were
then mixed. The mixture was incubated for 20 min before being added
to the T24 cells (~80% confluent) which were grown for an
additional 48 h prior to use.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions, and the RNA concentration was measured at an
absorbance of 260 nm using a spectrophotometer (ULTROSPEC 2100;
Biochrom Ltd., Cambridge, UK). The quality of the isolated RNA was
confirmed on a 1% agarose gel. qPCR was performed using SYBR Premix
Ex Taq (Takara Bio, Inc., Shiga, Japan) and a Bio-Rad C1000
real-time system (Bio-Rad, Hercules, CA, USA) according to the
manufacturer’s instructions. The primers for DUOX2 were sense,
5′-AACCTAAGCAGCTCA CAACT-3′ and antisense,
5′-CAAGAGCAATGATGGTGAT-3′), as described in a previous study
(7). The primers for GAPDH were
sense, 5′-GGTCTCCTCTGACTTCAACA-3′ and anti-sense, 5′-AGCC
AAATTCGTTGTCATAC-3′. For each PCR reaction, cDNA was synthesized
from 3 mg of RNA. The thermal cycling conditions involved an
initial step consisting of denaturation at 95°C for 30 sec, and
then 40 cycles of the following 3 steps were performed:
denaturation at 94°C for 45 sec, annealing at 55°C for 45 sec and
elongation at 72°C for 1 min. The results of RT-qPCR were analyzed
using the 2−ΔΔCt method.
Western blot analysis
The T24 cells were treated with siRNA, the cytokines
(IFN-γ, TNF-α, IL-1β and IL-17), PMA, MDP or MDP-DD for 24 h and
lysed in 2X lysis buffer (250 mM Tris-Cl, pH 6.5, 2% SDS, 4%
β-mercaptoethanol, 0.02% bromophenol blue and 10% glycerol). The
cell lysate (30 mg protein) was electrophoresed in sample buffer
containing 10% SDS, transferred onto PVDF membranes that were
blocked for 3 h in Tris-buffered saline (TBS; 50 mM Tris-Cl, pH
7.5, and 150 mM NaCl) containing 0.1% Tween-20 and 3% BSA, and
subsequently incubated overnight at 4°C with a primary antibody
against DUOX2 (ab170308; Abcam Plc, Cambridge, UK) at a ratio of
1:500. The membranes were then washed 3 times with
Tween-10/Tris-buffered saline (TTBS; 0.5% Tween-20 in TBS) and
incubated with an appropriate horseradish peroxidase-conjugated
secondary antibody (5127s; Cell Signaling Technology, Beverly, MA,
USA). Protein bands were visualized using ECL reagent (Thermo
Fisher Scientific, Waltham, MA, USA).
Removal of intracellular bacteria
The K5 bacterial strain was first grown in
Luria-Bertani (LB) medium for 4–5 h at 37°C while being shaken, and
then further subcultured for 16–18 h. A total of 100 ml of bacteria
grown to the exponential growth phase (OD650 nm, 0.4–0.6) was
centrifuged at 10,000 x g at room temperature for 2 min and
resuspended in PBS. The T24 cells were incubated with the bacteria
and resuspended in RPMI-1640 medium at 37°C for 2 h. The
extracellular bacteria were then removed by washing 3 times with
PBS supplemented with 100 mg/ml gentamicin to kill the
extracellular bacteria. The cell monolayer was then washed twice
with PBS and solubilized in 200 µl 0.5% Triton X-100 in PBS
at 37°C for 15 min for cell lysis. The solute was serially diluted
in LB and plated on LB agar plates followed by incubation at 37°C,
and on the following day the colonies were counted.
Measurement of ROS levels
The T24 cells were incubated with 10 µM
2′,7′-dichlorofuorescin diacetate (DCFDA; Molecular Probes, Eugene,
USA) for 30 min and washed with 1 ml of PBS solution at least 5
times to remove the extracellular ROS. The levels of DCFDA
fluorescence were measured at an excitation wavelength of 488 nm
and an emission wavelength of 525 nm using a microplate reader
(Thermo Fisher Scientific). The values were averaged in order to
obtain the mean relative fluorescence intensity.
Statistical analysis
Statistical analysis was performed using the t-test
with SPSS version 19.0 software (IBM Corp., Armonk, NY, USA). A
p-value <0.05 was considered to indicate a statistically
significant difference.
Results
DUOX2 expression induced by infection
with Klebsiella pneumoniae strain K5 suppresses the persistence of
K5 in T24 cells
To observe the intracellular destiny of the K5
bacterial strain in the T24 cells, the intracellular bacterial
colonization level in the lysates was counted at different time
points following the bacterial infection of the T24 cells by
RT-qPCR. At the same time, we examined whether DUOX2 activity
affected the persistence of the K5 strain in the T24 cells. The
number of viable intracellular bacteria peaked at 12 h, with a 52%
increase being observed at 12 h compared to the 2-h time point
(p<0.01); however, this number significantly decreased at 24, 48
and 72 h post-infection of the T24 cells with the K5 bacterial
strain when compared with the number observed at 12 h (Fig. 1A; all p<0.01). This suggests
that the intracellular bacterial growth was effectively restricted
in the T24 cells. The expression levels of DUOX2 following
infection of the T24 cells with the K5 bacterial strain were
significantly elevated at 12, 24, 48 and 72 h, reaching peak levels
at 48 h (Fig. 1B and C).
 | Figure 1Infection with the Klebsiella
pneumoniae K5 bacterial strain induces dual oxidase 2 (DUOX2)
expression in T24 cells. Bacteria and T24 cells were incubated with
the K5 bacteria for 2 h, and the extracellular bacteria were
removed with gentamicin (100 mg/ml). The number of viable
intracellular bacterial was calculated using the plate count
method, and DUOX2 expression was measured by western blot analysis
at 2, 12, 24, 48 and 72 h post-infection. (A) Number of viable
intracellular bacteria. (B) Relative DUOX2 protein band intensity.
(C) DUOX2 protein expression measured by western blot analysis.
Data are representative results of at least 3 independent
experiments, each performed in triplicate.
*,**p<0.01, compared to the number of viable bacteria
at 2 and 12 h, respectively. CFU, colony forming units. |
Treatment with IFN-γ, TNF-α, IL-1β, PMA
or MDP induces DUOX2 expression in T24 cells infected with the K5
bacterial strain
We verified that the T24 cells expressed DUOX2
(Fig. 1 B and C). The T24 cells
were then treated with the cytokines, TNF-α, INF-γ, IL-1β, IL-17,
or with PMA or MDP, and all treatments decreased the number of
viable intracellular bacteria when compared with the control group
(p<0.05; Fig. 2B). The DUOX2
expression levels in the T24 cells were significantly increased by
all treatments, with INF-γ (4.17-fold) and MDP (3.88-fold) having
the greatest effect on DUOX2 expression when compared with the
control group (p<0.05; Fig.
2A, C and D).
 | Figure 2Chemical factors induce dual oxidase
2 (DUOX2) expression in T24 cells infected with the Klebsiella
pneumoniae K5 bacterial strain 24 h postinfection. T24 cells
were incubated with the K5 bacterial strain for 2 h, followed by
treatment with various cytokines, phorbol 12-myristate 13-acetate
(PMA), muramyl dipeptide (MDP) and
N-acetylmuramyl-D-alanyl-D-isoglutamine (MDP-DD) for 2 h. The
extracellular bacteria were removed with gentamicin (100 mg/ml).
The number of viable intracellular bacteria was calculated using
the plate count method, and DUOX2 expression was measured by qPCR
and, western blot analysis. (A) DUOX2 was upregulated following
treatment with PMA, MDP and cytokines. Treatment with MDP-DD had no
effect. (B) The number of viable intracellular bacteria was
decreased following treatment with the reagentst. (C) Relative
DUOX2 protein band intensity. (D) DUOX2 protein expressino was
upregulated following treatment. Data are representative of at
least 3 independent experiments, performed in triplicate. CFU,
colony forming units; CTRL, control; IFN γ, interferon-γ;
TNF-α tumor necrosis factor; IL, interleukin. *p<0.05, compared
to the control group. |
DUOX2 knockdown leads to an increase in
the number of viable K5 bacteria
The T24 cells were transfected with DUOX2 siRNA
(siDUOX2) before being infected with the K5 bacterial strain. The
extracellular bacteria were removed, and this was followed by
treatment with IFN-γ, TNF-α, IL-1β, IL-17, PMA, MDP or MDP-DD
Transfection of the cells with siDUOX2 reduced DUOX2 expression
which was induced by IFN-γ, TNF-α, IL-1β, IL-17, PMA and MDP
(Fig. 3A).
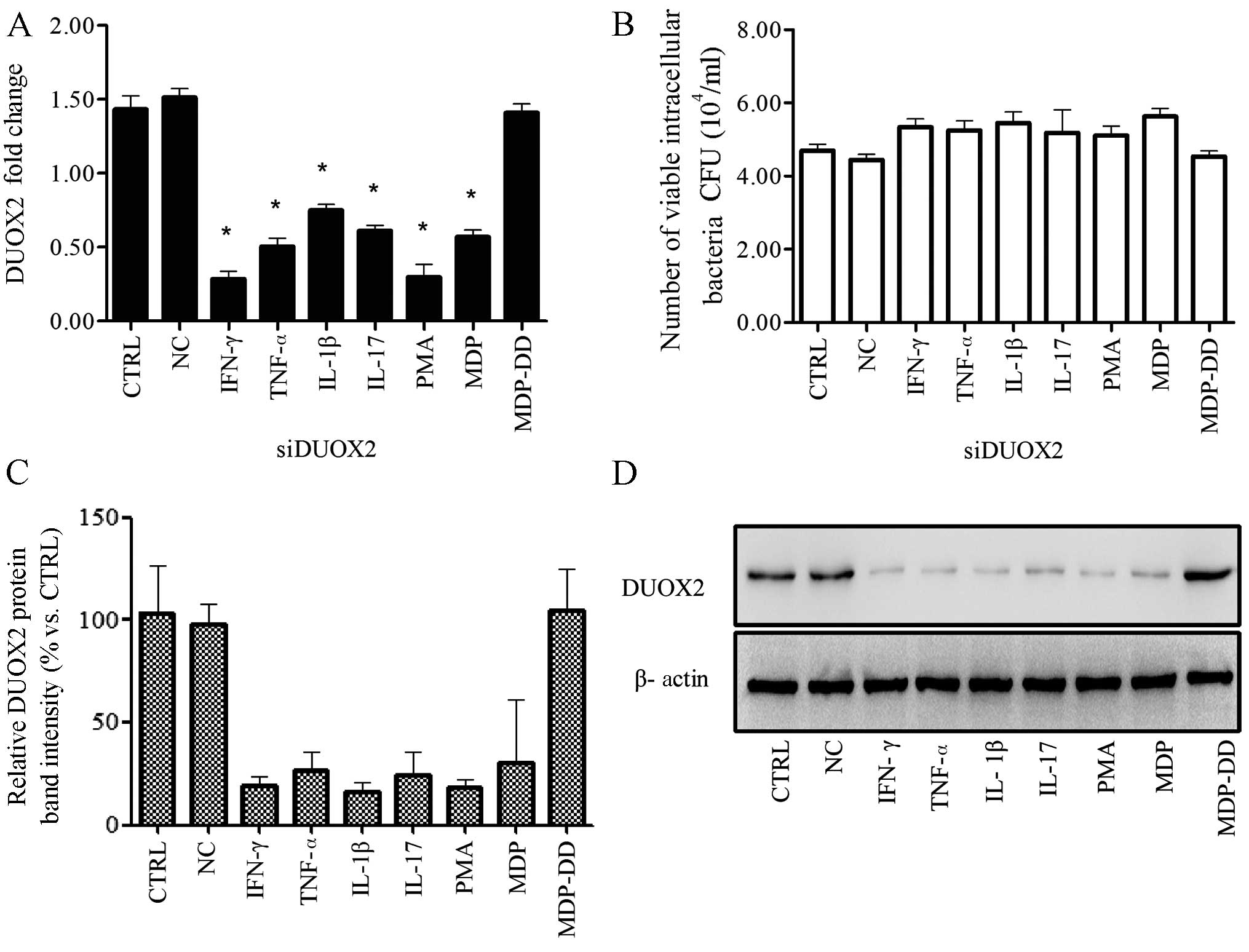 | Figure 3Knockdown of dual oxidase 2 (DUOX2)
leads to increased cell colonization 24 h post-infection with the
Klebsiella pneumoniae K5 bacterial strain. One day prior to
the knockdown of DUOX2 by siRNA, the T24 cells were incubated with
the K5 bacteria for 2 h, and this was followed by treatment with
various cytokines, phorbol 12-myristate 13-acetate (PMA), muramyl
dipeptide (MDP) or N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP-DD)
for 2 h. The extracellular bacteria were removed with gentamicin
(100 mg/ml). The number of viable intracellular bacteria was
calculated using the plate count method, and DUOX2 expression was
measured by qPCR and western blot analysis. (A) DUOX2 was
downregulated following transfection with siRNA and treatment with
cytokines, PMA, or MDP. (B) The number of viable intracellular
bacteria increased following transfection with siRNA and treatment
with the chemical reagents. (C) Relative DUOX2 intensity following
transfection with siRNA, and treatment with the cytokines, PMA, or
MDP. (D) DUOX2 protein expression was downregulated following
transfection with siRNA and treatment with the cytokines, PMA, or
MDP. Data are representative of at least 3 independent experiments,
performed in triplicate. CFU, colony forming units; CTRL, control;
IFN γ, interferon-γ; TNF-α tumor necrosis factor; IL, interleukin.
*p<0.05, compared to the control group. |
The knockdown of DUOX2 using siRNA affected the
antibacterial activity, and the number of viable intracellular
bacteria increased by varying degrees (p>0.05) compared with the
control group (Fig. 3B). The
protein expression of DUOX2 was also suppressed to varying degrees
following transfection of the cells with siDUOX2 (p<0.01;
Fig. 3C and D).
Treatment with CAT or DPI suppresses the
clearance of bacteria mediated by cytokine-, PMA- or MDP-induced
DUOX2 expression in T24 cells infected with the K5 bacterial
strain
To determine whether the NOX inhibitor, DPI, or the
H2O2 inhibitor, CAT affects the expression of
DUOX2 in the T24 cells infected with the K5 bacterial strain, the
T24 cells were incubated with the K5 bacterial strain for 2 h, the
extracellular bacteria were removed by treatment with gentamicin
(100 mg/ml) for 1 h, and the cells were then treated with CAT or
DPI for 1 h After 1 h, the inhibitor was replaced with IFN-γ,
TNF-α, IL-1β, IL-17, PMA, MDP or MDP-DD for 24 h. Both CAT and DPI
suppressed the induction of DUOX2 expression by all cytokines, PMA,
and MDP (p<0.01; Fig. 4 A and
C) compared with the control group. This led to no significant
changes being observed in the viable intracellular bacterial
numbers compared with the control group (P>0.05; Fig. 4B and D).
 | Figure 4Treatment with catalase (CAT;
H2O2 inhibitor) or diphenyleneiodonium (DPI;
NOX inhibitor) decreases dual oxidase 2 (DUOX2) expression and
suppresses the clearance of bacteria mediated by cytokine-, phorbol
12-myristate 13-acetate (PMA)- or muramyl dipeptide (MDP)-induced
DUOX2 expression in T24 cells infected with the Klebsiella
pneumoniae K5 bacterial strain. T24 cells were incubated with
the K5 bacteria for 2 h and then treated with the inhibitors (CAT
and DPI) for 2 h, and the extracellular bacteria were removed with
gentamicin (100 mg/ml). The number of viable intracellular bacteria
was calculated using the plate count method, and dual oxidase 2
(DUOX2) expression was measured by qPCR. The cells were treated
with (A and C) CAT (0–1,000 U/ml) or (C and D) DPI (0–50 nM/ml).
DUOX2 expression was downregulated and the number of viable
intracellular bacteria was increased following treatment with the
inhibitors. Data are representative result of at least 3
independent experiments, performed in triplicate. CFU, colony
forming units; CTRL, control; IFN γ, interferon-γ; TNF-α tumor
necrosis factor; IL, interleukin; MDP-DD,
N-acetylmuramyl-D-alanyl-D-isoglutamine. *p<0.05,
compared to the control group. |
Treatment with cytokines, PMA or MDP
increases intracellular ROS levels and this increase is reversed by
CAT and DPI
To identify the mechanisms responsible for the
generation of intracellular ROS induced by infection with the K5
bacterial strain, the T24 cells were incubated with the K5
bacterial strain for 2 h, treated with gentamicin for 2 h to remove
extracellular bacteria, and then treated with the inhibitors (CAT
or DPI) for 2 h. The T24 cells were then incubated with 10
µM DCFDA for 30 min, and the DCFDA fluorescence levels were
then measured at 488/525 nm. All reagents (cytokines and PMA and
MDP) were found to increase ROS generation (p<0.05; Fig. 5A compared to the control group;
however, no changes in ROS levels were observed in the
MDP-DD-treated cells. Treatment with siDUOX2, CAT or DPI inhibited
the generation of intracellular ROS compared to the control group
(p<0.05; Fig. 5A-C).
Discussion
It is known that DUOX1 is expressed in uroepithelial
cells (22); however, to the best
of our knowledge, whether DUOX2 plays a role in the removal of the
K5 bacterial strain has not been reported to date. Moreover,
whether DUOX2 inhibits bacterial cytoinvasion through the ROS
signaling-mediated innate immune response in uroepithelial cells
also remains unknown. The present study demonstrated that bladder
epithelial cells have an innate antimicrobial mechanism that relies
on ROS, and that DUOX2 plays an important role in the removal of
the K5 bacterial strain through a ROS-mediated innate immune
response which promotes the elimination of intracellular bacteria.
The number of viable bacteria peaked at 12 h post-infection and
significantly diminished thereafter, as DUOX2 expression increased
(Fig. 1), indicating that
infection with the K5 bacterial strain infection induced DUOX2
expression, which in turn led to the removal of the bacteria.
The role of DUOX2 in the removal of bacteria was
confirmed by treatment with multiple cytokines (IFN-γ, TNF-α, IL-1β
and IL-17), PMA and MDP. We observed that each treatment induced
DUOX2 expression and also reduced the number of viable
intracellular bacteria (Fig. 2).
This observation was corroborated by the knockdown of DUOX2
expression with siRNA (Fig. 3).
Following the knockdown of DUOX2 expression, treatment with the
cytokines, PMA or MDP did not decrease the number of viable
intracellular bacteria, which remained relatively unaltered
compared with the control group (Fig.
3B). These findings are in line with the following findings
from other studies: the knockdown of endogenous DUOX2 expression by
RNA interference (RNAi) was shown to increase bacterial
cytoinvasion and to abrogate the cytoprotective effects exerted by
NOD2 in Caco-2 cells (23);
Drosophila dual oxidase (dDuox) was shown to be essential
for the maintenance of the intestinal redox system (24); and activated DUOX was shown to
protect zebrafish larvae from infection with Salmonella
enterica serovar Typhimurium (25).
In this study, we demonstrated that treatment with
CAT (H2O2 inhibitor and DPI (NOX inhibitor)
decreased the DUOX2 expresion levels induced by treatment with the
cytokines, PMA, or MDP. As a result, following treatment with CAT
and DPI, the numbers of viable bacterial numbers were relatively
unaltered when compared to the control groups, even those which had
been treated with the cytokines, PMA, or MDP (Fig. 4B and D). Additionally, treatment
with all of the cytokines, PMA, or MDP increased the levels of ROS,
while treatment with siDUOX2, CAT and DPI decreased the ROS levels,
suggesting that DUOX2 promotes the elimination of bacterial from
T24 cells through the ROS signaling pathway.
IFN-γ, TNF-α and IL-1β are well-known classic
pro-inflammatory and anti-pathogenic cytokines. IFN-γ increases
DUOX2 expression levels in the respiratory epithelium (26). TNF-α, in synergy with IFN-β,
induces DUOX2 NOX expression (17). Signal transduction and
inflammatory responses induced by IL-1β are related to the activity
of NOX-1 in Caco-2 cells (27,28). IL-17 is essential for host defense
against a number of microbes, particularly extracellular bacteria
and fungi (29), and it induces
NOX production in human cartilage, chondrocytes and osteoarthritic
human and mouse cartilage (30,31). As an immunoadjuvant and
peptidoglycan (a structural subunit of the bacterial cell wall),
MDP enhances the ability of phagocytes to engulf bacteria,
increases natural killer (NK) cell lethality and promotes T cell
activation in order to strengthen host resistance to pathogen
infection. MDP is a well-known ligand for the intracellular NOD2
receptor (32).
NOX appears to be a particularly important enzyme
for ROS generation in non-phagocytic cells, and it is a prominent
generator of ROS in the airway epithelium (33,34). Mounting evidence indicates that
intracellular ROS facilitates cellular damage or stress and
contributes to innate immune activation (35). ROS enhance host immunity through
the prevention of pathogen-induced pro-inflammatory cytokines
(36–38).
Nuclear factor (NF)-κB is a critical signaling
molecule in H2O2-induced inflammation and
responses evoked by a variety of stimuli, including growth factors,
lymphokines, ultraviolet irradiation, pharmacological agents and
oxidative stress (39). A growing
body of evidence indicates that endogenous ROS are characteristic
of typical second messengers responsible for the activation of
NF-κB in a variety of cell types, including neutrophils,
lymphocytes, endothelial cells and epithelial cells (19). In particular, the NOX family
member, DUOX2, is involved in NOD2-dependent ROS production
(19). DUOX2 functions as a
molecular switch that enhances the protective properties of NOD2
through both direct (generation of bactericidal ROS) and indirect
mechanisms (by amplifying NF-κB signaling) (16). PMA, a protein kinase C activator,
activates NAPDH protein molecules, induces DUOX2 phosphorylation to
generate high levels of H2O2 (15,30) and promotes antibacterial effects
exerted by the NOX family. The antibacterial role of PMA may be
evidenced through the NF-κB signaling pathway. MDP is a
peptidoglycan moiety derived from commensal and pathogenic bacteria
and a ligand of its intracellular sensor NOD2. The ex vivo
stimulation of human carotid plaques with MDP has been shown to
lead to the enhanced activation of the inflammatory signaling
pathways, p38 and MAPK, and the NF-κB-mediated release of
pro-inflammatory cytokines (40).
IFN-β and IL-1β can cooperate synergistically to upregulate DUOX2
expression, and the contribution of NF-κB signaling to DUOX2
expression cannot be excluded, since IL-1β signaling also activates
NF-κB (22). TNF-α, IFN-γ, IL-1β
and IL-17 are important cytokines that regulate host immune
defense, and thus we hypothesized that they may also function
through NF-κB signaling to increase DUOX2 expression in infected
T24 cells.
Mechanistically, NOX-mediated ROS generation
constitutes a well-known host defense mechanism in phagocytes, and
DUOX1 and DUOX2 provide extracellular H2O2 to
lactoperoxidase (LPO) to produce antimicrobial hypothiocyanite ions
(11). DUOX-generated
H2O2, in conjunction with LPO and thiocyanate
(SCN−), kills bacteria in the respiratory tract (41,42). The presence of
H2O2 in the airway allows LPO to oxidize the
airway surface liquid (ASL) component, SCN (27), thereby generating antimicrobial
hypothiocyanite (OSCN) in order to support oxidant-mediated
bacterial killing in the airways (42). In a previous study, Pseudomonas
aeruginosa flagellin infected human airway epithelial cells to
facilitate host invasion through the inactivation of the
DUOX/LPO/SCN−protective system (42). OSCN− is abundantly
found in saliva and other mucosal secretions, acting as an
effective microbicidal or microbiostatic agent, and the
DUOX/LPO/SCN− antimicrobial system is fully assembled
only in the final stages of saliva formation, with
H2O2 provided by DUOX2 (43). H2O2
generated by DUOX in the airway epithelium supports the production
of bactericidal OSCN(-) in the presence of the airway surface
liquid components, LPO, and SCN(-), and OSCN(-) eliminates
Staphylococcus aureus and Pseudomonas aeruginosa on
airway mucosal surfaces (11).DUOX1 also plays a role in the
protection of urothelial cells, and alterations in ROS production
in the urothelium may contribute to the development of bladder
diseases (22).
In conclusion, the findings of the present study
demonstrate that innate immunity may protect T24 bladder cancer
cells from invasion by the K5 bacterial strain through the
upregulation of DUOX2 expression, mediated through ROS signaling.
Our findings provide insight into the mechanisms through which
uroepithelial cells are protected from cytoinvasion by the K5
bacterial strain, and may thus lead to the development of novel
therapeutic strategies for urinary tract infections.
References
|
1
|
Meca G, Sospedra I, Valero MA, Mañes J,
Font G and Ruiz MJ: Antibacterial activity of the enniatin B,
produced by Fusarium tricinctum in liquid culture, and cytotoxic
effects on Caco-2 cells. Toxicol Mech Methods. 21:503–512. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rhim AD, Stoykova L, Glick MC and Scanlin
TF: Terminal glycosylation in cystic fibrosis (CF): Aa review
emphasizing the airway epithelial cell. Glycoconj J. 18:649–659.
2001. View Article : Google Scholar
|
|
3
|
Swords WE, Chance DL, Cohn LA, Shao J,
Apicella MA and Smith AL: Acylation of the lipooligosaccharide of
Haemophilus influenzae and colonization: an htrB mutation
diminishes the colonization of human airway epithelial cells.
Infect Immun. 70:4661–4668. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loose M, Hudel M, Zimmer KP, Garcia E,
Hammerschmidt S, Lucas R, Chakraborty T and Pillich H: Pneumococcal
hydrogen peroxide-induced stress signaling regulates inflammatory
genes. J Infect Dis. 211:306–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cortés G, Alvarez D, Saus C and Albertí S:
Role of lung epithelial cells in defense against Klebsiella
pneumoniae pneumonia. Infect Immun. 70:1075–1080. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosen DA, Pinkner JS, Walker JN, Elam JS,
Jones JM and Hultgren SJ: Molecular variations in Klebsiella
pneumoniae and Escherichia coli FimH affect function and
pathogenesis in the urinary tract. Infect Immun. 76:3346–3356.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zgair AK and Al-Adressi AM:
Stenotrophomonas maltophilia fimbrin stimulates mouse bladder
innate immune response. Eur J Clin Microbiol Infect Dis.
32:139–146. 2013. View Article : Google Scholar
|
|
8
|
Michelet C, Avril JL, Cartier F and Berche
P: Inhibition of intracellular growth of Listeria monocytogenes by
antibiotics. Antimicrob Agents Chemother. 38:438–446. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakagawa I, Amano A, Mizushima N, Yamamoto
A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, et
al: Autophagy defends cells against invading group A Streptococcus.
Science. 306:1037–1040. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grasberger H, El-Zaatari M, Dang DT and
Merchant JL: Dual oxidases control release of hydrogen peroxide by
the gastric epithelium to prevent Helicobacter felis infection and
inflammation in mice. Gastroenterology. 145:1045–1054. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rada B and Leto TL: Oxidative innate
immune defenses by Nox/Duox family NADPH oxidases. Contrib
Microbiol. 15:164–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Enyedi B and Niethammer P:
H2O2: a chemoattractant? Methods Enzymol.
528:237–255. 2013. View Article : Google Scholar
|
|
13
|
Geiszt M and Leto TL: The Nox family of
NAD(P)H oxidases: host defense and beyond. J Biol Chem.
279:51715–51718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lees MS, H Nagaraj S, Piedrafita DM, Kotze
AC and Ingham AB: Molecular cloning and characterisation of ovine
dual oxidase 2. Gene. 500:40–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rigutto S, Hoste C, Grasberger H,
Milenkovic M, Communi D, Dumont JE, Corvilain B, Miot F and De
Deken X: Activation of dual oxidases Duox1 and Duox2: differential
regulation mediated by camp-dependent protein kinase and protein
kinase C-dependent phosphorylation. J Biol Chem. 284:6725–6734.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HJ, Kim CH, Ryu JH, Kim MJ, Park CY,
Lee JM, Holtzman MJ and Yoon JH: Reactive oxygen species induce
antiviral innate immune response through IFN-λ regulation in human
nasal epithelial cells. Am J Respir Cell Mol Biol. 49:855–865.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fink K, Martin L, Mukawera E, Chartier S,
De Deken X, Brochiero E, Miot F and Grandvaux N: IFNβ/TNFα
synergism induces a non-canonical STAT2/IRF9-dependent pathway
triggering a novel DUOX2 NADPH oxidase-mediated airway antiviral
response. Cell Res. 23:673–690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strengert M, Jennings R, Davanture S,
Hayes P, Gabriel G and Knaus UG: Mucosal reactive oxygen species
are required for antiviral response: Rrole of Duox in influenza a
virus infection. Antioxid Redox Signal. 20:2695–2709. 2014.
View Article : Google Scholar
|
|
19
|
Lipinski S, Till A, Sina C, Arlt A,
Grasberger H, Schreiber S and Rosenstiel P: DUOX2-derived reactive
oxygen species are effectors of NOD2-mediated antibacterial
responses. J Cell Sci. 122:3522–3530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ronald A and Ludwig E: Urinary tract
infections in adults with diabetes. Int J Antimicrob Agents.
17:287–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hansen DS, Gottschau A and Kolmos HJ:
Epidemiology of Klebsiella bacteraemia: Aa case control study using
Escherichia coli bacteraemia as control. J Hosp Infect. 38:119–132.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donkó A, Ruisanchez E, Orient A, Enyedi B,
Kapui R, Péterfi Z, de Deken X, Benyó Z and Geiszt M: Urothelial
cells produce hydrogen peroxide through the activation of Duox1.
Free Radic Biol Med. 49:2040–2048. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El Hassani RA, Benfares N, Caillou B,
Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D,
Ohayon R, et al: Dual oxidase2 is expressed all along the digestive
tract. Am J Physiol Gastrointest Liver Physiol. 288:G933–G942.
2005. View Article : Google Scholar
|
|
24
|
Ha EM, Oh CT, Bae YS and Lee WJ: A direct
role for dual oxidase in Drosophila gut immunity. Science.
310:847–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flores MV, Crawford KC, Pullin LM, Hall
CJ, Crosier KE and Crosier PS: Dual oxidase in the intestinal
epithelium of zebrafish larvae has anti-bacterial properties.
Biochem Biophys Res Commun. 400:164–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harper RW, Xu C, Eiserich JP, Chen Y, Kao
CY, Thai P, Setiadi H and Wu R: Differential regulation of dual
NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2
cytokines in respiratory tract epithelium. FEBS Lett.
579:4911–4917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geiszt M, Lekstrom K, Brenner S, Hewitt
SM, Dana R, Malech HL and Leto TL: NAD(P)H oxidase 1, a product of
differentiated colon epithelial cells, can partially replace
glycoprotein 91phox in the regulated production of superoxide by
phagocytes. J Immunol. 171:299–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geiszt M, Lekstrom K, Witta J and Leto TL:
Proteins homologous to p47phox and p67phox support superoxide
production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol
Chem. 278:20006–20012. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O’Quinn DB, Palmer MT, Lee YK and Weaver
CT: Emergence of the Th17 pathway and its role in host defense. Adv
Immunol. 99:115–163. 2008. View Article : Google Scholar
|
|
30
|
Miljkovic D and Trajkovic V: Inducible
nitric oxide synthase activation by interleukin-17. Cytokine Growth
Factor Rev. 15:21–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trajkovic V, Stosic-Grujicic S, Samardzic
T, Markovic M, Miljkovic D, Ramic Z and Mostarica Stojkovic M:
Interleukin-17 stimulates inducible nitric oxide synthase
activation in rodent astrocytes. J Neuroimmunol. 119:183–191. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Willems MM, Zom GG, Meeuwenoord N,
Ossendorp FA, Overkleeft HS, van der Marel GA, Codée JD and
Filippov DV: Design, automated synthesis and immunological
evaluation of NOD2-ligand-antigen conjugates. Beilstein J Org Chem.
10:1445–1453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HJ, Kim CH, Ryu JH, Joo JH, Lee SN,
Kim MJ, Lee JG, Bae YS and Yoon JH: Crosstalk between
platelet-derived growth factor-induced Nox4 activation and MUC8
gene overexpression in human airway epithelial cells. Free Radic
Biol Med. 50:1039–1052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van der Vliet A: NADPH oxidases in lung
biology and pathology: hHost defense enzymes, and more. Free Radic
Biol Med. 44:938–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arnoult D, Carneiro L, Tattoli I and
Girardin SE: The role of mitochondria in cellular defense against
microbial infection. Semin Immunol. 21:223–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao XP, Standiford TJ, Rahman A, Newstead
M, Holland SM, Dinauer MC, Liu QH and Malik AB: Role of NADPH
oxidase in the mechanism of lung neutrophil sequestration and
microvessel injury induced by Gram-negative sepsis: Studies in
p47phox−/− and gp91phox−/− mice. J Immunol.
168:3974–3982. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koarai A, Sugiura H, Yanagisawa S,
Ichikawa T, Minakata Y, Matsunaga K, Hirano T, Akamatsu K and
Ichinose M: Oxidative stress enhances toll-like receptor 3 response
to double-stranded RNA in airway epithelial cells. Am J Respir Cell
Mol Biol. 42:651–660. 2010. View Article : Google Scholar :
|
|
38
|
Zmijewski JW, Lorne E, Zhao X, Tsuruta Y,
Sha Y, Liu G and Abraham E: Antiinflammatory effects of hydrogen
peroxide in neutrophil activation and acute lung injury. Am J
Respir Crit Care Med. 179:694–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hou Y, An J, Hu XR, Sun BB, Lin J, Xu D,
Wang T and Wen FQ: Ghrelin inhibits interleukin-8 production
induced by hydrogen peroxide in A549 cells via NF-kappaB pathway.
Int Immunopharmacol. 9:120–126. 2009. View Article : Google Scholar
|
|
40
|
Kim JY, Omori E, Matsumoto K, Núñez G and
Ninomiya-Tsuji J: TAK1 is a central mediator of NOD2 signaling in
epidermal cells. J Biol Chem. 283:137–144. 2008. View Article : Google Scholar
|
|
41
|
Forteza R, Salathe M, Miot F, Forteza R
and Conner GE: Regulated hydrogen peroxide production by Duox in
human airway epithelial cells. Am J Respir Cell Mol Biol.
32:462–469. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gattas MV, Forteza R, Fragoso MA, Fregien
N, Salas P, Salathe M and Conner GE: Oxidative epithelial host
defense is regulated by infectious and inflammatory stimuli. Free
Radic Biol Med. 47:1450–1458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Geiszt M, Witta J, Baffi J, Lekstrom K and
Leto TL: Dual oxidases represent novel hydrogen peroxide sources
supporting mucosal surface host defense. FASEB J. 17:1502–1504.
2003.PubMed/NCBI
|



















