Introduction
Noise-induced hearing loss (NIHL) is a major health
issue in a number of industrialized countries, despite reduced
occupational noise exposure, strict standards for hearing
protection and extensive public health awareness campaigns
(1). It has been demonstrated
that the loss of hair cells in the auditory end organ accounts for
the majority of ear pathological conditions (2). Cochlear hair cells include the inner
hair cells and three rows of outer hair cells. Inner hair cells are
the primary sensory cells that convert the mechanical acoustic
input into receptor potential and the release of neurotransmitters,
triggering action potentials in the auditory centers of the brain.
The major function of outer hair cells is to enhance the
performance of the cochlea, particularly at low sound-intensity
levels (3).
Histone deacetylases (HDACs) are a class of enzymes
that remove acetyl groups from the lysine residues of target
proteins, promoting chromatin condensation and reduced
transcription (4). Four classes
of mammalian HDACs have been identified and classified based on
sequence homology to the original yeast enzymes and domain
organization (5,6). HDAC1 is a member of class I HDACs,
which are expressed in the majority of tissues and cell types and
function as nuclear proteins and transcriptional repressors. HDAC4
belongs to class II HDACs, which are expressed in the heart,
skeletal muscle and brain and function as a shuttle between the
cytoplasm and the nucleus (7).
The N-terminal tails of the core H3 histones contain specific amino
acid sequences for acetylation, methylation, phosphorylation,
ubiquitination and sumoylation (8), and H3K9 is a common modification
site of acetylation (9). Previous
studies have demonstrated that the treatment of mice with kanamycin
decreases the acetylation of histone H3 in the nuclei of outer hair
cells (3,10).
Suberoylanilide hydroxamic acid (SAHA) is a commonly
used HDAC inhibitor that effectively blocks all class I and class
II HDACs. Studies have indicated that SAHA inhibits the growth of
ovarian cancer cells, while inducing the expression of imprinted
tumor suppressor genes (11–13). A previous study by our research
group also demonstrated that the kanamycin-induced hypoacetylation
of histones is associated with aminoglycoside-induced hair cell
loss, and that the restoration of histone acetylation with HDAC
inhibitors prevents damage to inner ear hair cells in vitro
(3). However, whether there is an
association between HDACs and NIHL, and the role of the HDAC
inhibitor, SAHA, in preventing NIHL remains to be elucidated.
In the present study, hair cell death was assessed
following the exposure of adult CBA/J mice to broadband noise (BBN)
to induce hearing loss. Following an injection of SAHA, the protein
levels of HDAC1 and HDAC4 in the cochlear tissue and the levels of
acetyl-histone H3 (Lys9) (H3-AcK9) in the outer hair cells were
measured, the number of hair cells was quantified and their
morphology was assessed, and comparisons were made between the mice
injected with SAHA and the untreated mice in the control group.
Materials and methods
Antibodies and reagents
Unless otherwise stated, all materials were
purchased from Gibco (Grand Island, NY, USA). Polyclonal rabbit
antibodies against HDAC1 (Cat. no. 7872), HDAC4 (Cat. no. 11418)
and H3-AcK9 (Cat. no. 33361), as well as the corresponding
secondary antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). GAPDH antibodies (Cat. no. 367714) were
obtained from Upstate Biotechnology, Inc. (Lake Placid, NY, USA).
Alexa Fluor 594 secondary antibody (Cat. no. A24923),
FITC-phalloidin (Cat. no. F432) and Hoechst 33342 (Cat. no. H3570)
were purchased from Molecular Probes (Eugene, OR, USA). SAHA was
purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). ECL Plus
Western Blotting Detection reagents were purchased from GE
Healthcare UK Ltd. (Little Chalfont, Buckinghamshire, UK).
Animals
Sexually mature (12 weeks old) male CBA/J mice were
purchased from the Model Animal Research Center of Nanjing
University (Nanjing, China). The animals were maintained on a 12-h
light/12-h dark schedule and had free access to water and a
standard mouse diet (Purina 5025) for 1 week before the
commencement of the experiments. The animals were then randomly
assigned to 3 experimental groups following weight stratification:
i) the control group (G1), animals were placed in the noise
exposure monitoring device, but were not exposed to noise; ii) the
DMSO group (G2), animals were exposed to noise and received an
intraperitoneal injection of DMSO (10%) 3 days before they were
exposed to noise; and iii) the SAHA group (G3), animals were
exposed to noise and received an intraperitoneal injection of SAHA
(25 mg/kg) 3 days before they were exposed to noise. Each group
comprised 20 mice. All procedures were carried out in accordance
with the Guide for the Care and Use of Laboratory Animals and were
approved by the Medical Research and Ethics Committee (the Fourth
Military Medical University).
Exposure to noise and auditory brainstem
response (ABR)
The mice were kept in separate stainless-steel wire
cages (9×9×9 cm) and the animals in the G2 and G3 groups were
exposed to BBN with a frequency spectrum from 2 to 20 kHz at a
sound pressure level (SPL) of 110 dB for 2 h to induce a permanent
threshold shift (PTS), while the mice in the G1 group were not
exposed to noise in the same chamber. The experimental period
lasted for 2 weeks.
Noise was created using a sound exposure chamber.
Audio CD sound files were created and equalized with audio editing
software (Adobe Systems Audition 3; Adobe Systems, Inc., San Jose,
CA, USA). In order to ensure uniformity throughout the sound field,
a sound level meter (model 1200; Quest Technologies Software, Aliso
Viejo, CA, USA) was used to calibrate the sound levels at multiple
locations within the sound chamber prior to and following the
exposure of the mice to noise.
At the end of the experimental period, the mice were
anesthetized with an intraperitoneal injection of xylazine (20
mg/kg) and ketamine (100 mg/kg), and were then placed in a sound
isolation and electrically shielded booth (Acoustic Systems,
Indianapolis, IN, USA). ABRs were measured at 4, 8, 12, 24, 32 and
48 kHz using TDT system 3 hardware and SigGen/BioSig software (both
from Tucker-Davis Technologies, Alachua, FL, USA). Thresholds were
estimated between the lowest stimulus level at which a response was
observed and the level without a response. All ABR measurements
were conducted by the same experimenter blinded to the treatment
conditions.
Western blot analysis
Cochlear tissue was homogenized and lysed with RIPA
lysis buffer (100 mM NaCl, 50 mM Tris-HCl pH 7.5, 1% Triton X-100,
1 mM EDTA, 10 mM β-glycerophosphate and 2 mM sodium vanadate and
protease inhibitor). Protein concentrations were determined using
the Micro BCA protein assay (Pierce, Rockford, IL, USA). A 40-mg
sample of protein in each lane was separated by 10% SDS-PAGE and
electroblotted onto polyvinylidenedifluoride (PVDF) membranes in a
semi-dry trans-blot apparatus. Subsequently, the PVDF membranes
were blocked by incubating them in 5% non-fat milk in TBST buffer
at room temperature for 1 h. The PVDF membranes were then incubated
with anti-HDAC1, anti-HDAC4, anti-H3-AcK9, or anti-GAPDH antibodies
for 1 h at room temperature. Following 3 washes in TBST buffer,
HRP-conjugated secondary antibodies were introduced and ECL was
used for detection.
Immunofluorescence staining and
quantification of cell number
The mice were perfused through the heart with PBS
and 4% paraformaldehyde (PFA) for 30 min under physiological
pressure. The cochlear tissue was isolated and fixed with 4%
paraformaldehyde for 12 h at 4°C and was then rinsed in PBS and
decalcification buffer in a 4% solution of sodium EDTA (pH 7.4).
The EDTA solution was changed daily for 3 days at 4°C. Following
decalcification, the cochleae were embedded in OCT-freezing medium
and cut into 10-µm-thick serial sections. The corresponding
sections were placed into 3% hydrogen peroxide for 2.5 h to quench
endogenous peroxidase and were then incubated in a solution for
blocking non-specific antibody binding overnight at 4°C.
Subsequently, the sections were processed with a primary antibody
(1:100) for 4 days at 4°C, washed in PBS and then incubated
overnight at 4°C with Alexa Fluor 594-conjugated secondary
antibodies. Nuclear material was detected by incubating the
sections with Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) for 30
min at room temperature. A sample size of 5 mice was used in each
experimental group. Images were captured using a fluorescence
microscope (BX50; Olympus, Essex, UK) using a DP Controller and DP
Manager (Olympus, Essex, UK) software and a confocal microscope
(FV-500; Olympus, Essex, UK). The number of cells expressing one or
both markers of interest in each field was counted manually by 2
independent blinded observers using Adobe Photoshop. The percentage
of hair cells was calculated as the average of 6 high-power fields
of view normalized to the total number of cells per view.
Morphometric analysis
Hair cell morphometric changes were observed by
phalloidin staining and scanning electron microscopy (SEM). The
phalloidin-stained stereociliary bundles and circumferential
F-actin rings on the cuticular plate of outer and inner hair cells
allowed the determination of cells that were present or missing.
The cochleae from the noise-exposed and unexposed groups were fixed
and stained simultaneously with identical solutions and processed
in parallel. FITC-conjugated phalloidin was used for labeling the
hair cell structure to identify the comparable parts of hair cells
for the capture of confocal images. Each condition was replicated
in 4 different animals. SEM was performed after the cells were
fixed in ice-cold 2.5% glutaraldehyde and 1% osmium tetroxide 2 h
at 4°C. Subsequently, the cells were dehydrated in an acetone
gradient, then soaked and embedded in epoxy resin 618. Semi-thin
sections (1.0-µm-thick) were prepared after trimming and
were located by light microscopy after staining. Ultrathin sections
(70-nm-thick) were prepared and photographed using a scanning
electron microscope (Olympus, Tokyo, Japan) following staining with
uranyl acetate and uranium-lead acid.
Data analysis
Each experiment was repeated at least 3 times. The
results are presented as the means ± standard (SD). Statistical
analyses were performed using a paired or unpaired Student’s t-test
for direct 2-group comparisons and the Tukey-Kramer post-hoc test
after a one-way analysis of variance (ANOVA) F-test for
multiple-group comparisons. A P<0.05 was considered to indicate
a statistically significant difference.
Results
Permanent NIHL in adult CBA/J mice
The exposure of 12-week-old male CBA/J mice to BBN
at 110 dB SPL for 2 h resulted in PTS at 4, 8, 12, 24, 32 and 48
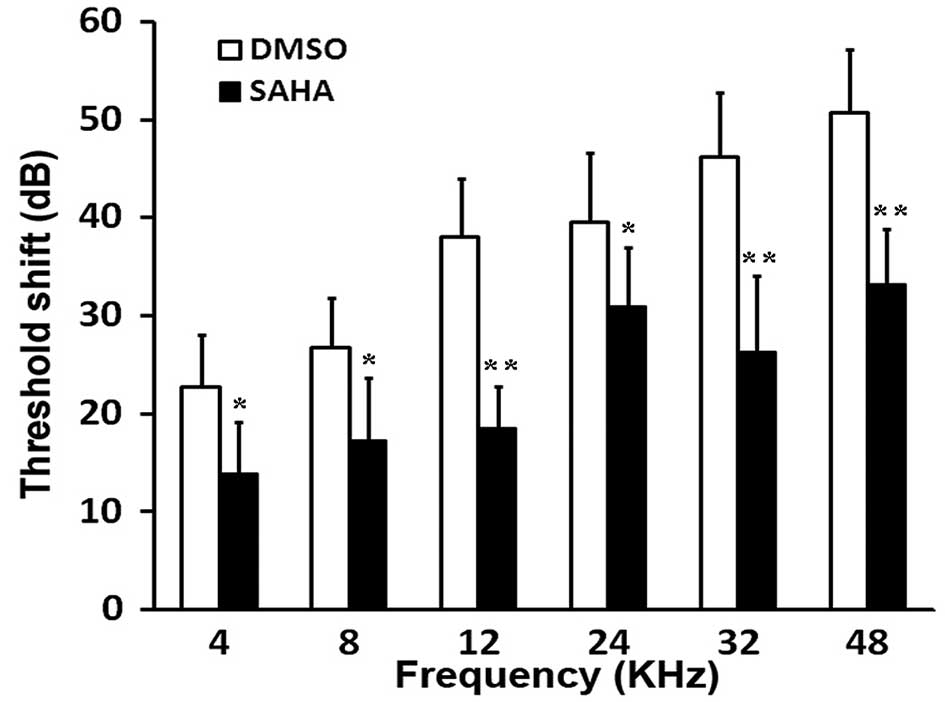
kHz, 2 weeks following exposure to noise (Fig. 1). Compared with the DMSO-injected
group (G2), pre-treatment with the HDAC inhibitor, SAHA,
significantly reduced PTS (P<0.05 and P<0.01; Fig. 1).
Traumatic noise exposure increases the
espression of HDAC1 and HDAC4, but decreases H3-AcK9 levels
Western blot analysis revealed that the expression
of HDAC1 and HDAC4 increased 1 h following exposure to noise and
subsequently reached a plateau above the control levels (Fig. 2A). The quantification of the ratio
of the protein band densities of HDAC1 revealed an approximately
2-fold increase in protein expression compared with the control
group, and an approximately 3-fold increase was observed in the
HDAC4 protein level (Fig. 2A). In
contrast to the increase in HDAC1 and HDAC4 expression, the H3-AcK9
levels decreased 1 h following exposure to noise and showed lower
band densities (Fig. 2B). The
quantification of the band densities of H3-AcK9 to total H3
confirmed the significance of this decrease (Fig. 2B).
 | Figure 2HDACs are transiently upregulated by
traumatic noise. Western blot analysis detected HDAC-1 and HDAC-4
protein expression in the cochlear tissue. (A) One hour following
the exposure of the mice to noise, there was an approximately
2-fold increase in the protein expression of HDAC1 and an
approximately 3-fold increase in the HDAC4 protein levels compared
with the control group. (B) Western blot analysis detected the
H3-AcK9 protein levels in the cochlear tissue 1 h following
exposure to noise and showed a significant decrease compared to
pre-exposure. Con, control group; I, 0 h after exposure to noise; 1
h, 1 h after exposure to noise; *P<0.05 and
**P<0.01. (C) Immunofluorescence staining of the
entire cochlea for HDAC4 (red) indicated a transient increase
(white arrows) in the number of outer hair cells expressing HDAC4 1
h after exposure to noise. (D) Immunofluorescence staining of the
entire cochlea for H3-AcK9 (red) indicated a transient decrease
(white arrows) in the number of outer hair cells expressing H3-AcK9
1 h after exposure to noise. Nuclei are stained blue with Hoechst
33342 and Hensen’s cells are identified by their position on the
surface preparation and in comparison with remaining hair cells.
Blue color represents Hoechst 33342 staining of the nuclei. The
figure shows representative images from the basal segment (n=3 at
each time point). Scale bar, 20 µm. HDAC, histone
deacetylase; H3-AcK9, acetyl-histone H3 (Lys9); OHC1, OHC2, OHC3,
outer hair cells of the first, second and third row, respectively;
IHC, inner hair cells. |
To address the issue of whether the expression of
HDAC1, HDAC4 and H3-AcK9 is altered in the entire cochlea, an
immunofluoresence assay was carried out. No apoptotic cells were
observed in the normal controls. However, 1 h following exposure to
noise, the outer hair cells in the basal segment showed typical
cell death characteristics with the majority of cells exhibiting
signs of apoptosis characterized by chromatin condensation (white
arrows in Fig. 2C and D). This
corresponded with an increase in HDAC4 levels and a decrease in
H3-AcK9 levels.
Accoustic trauma increases hair cell
loss, which is blocked following treatment with the HDAC inhibitor,
SAHA
Seven days following the exposure of the mice to
noise, the ratio of cochlear hair cell loss was calculated.
Corresponding with the ABR functional measurements, large numbers
of hair cells were lost in the basal and the middle regions of the
epithelium. Outer hair cell loss began at a 5% distance from the
apex and increased to 60% cell loss the end of the epithelium.
Inner hair cell loss also began at a 5% distance from the apex and
reached an average of 30% loss of hair cells at the end of the
basal region (Fig. 3A).
Further analysis of hair cell loss following
pre-treatment with the HDAC inhibitor, SAHA, revealed an
interesting pattern. Compared with G2 (group injected with DMSO),
the number of hair cells lost in the G3 (group injected with SAHA)
was significantly decreased, particularly in the basal regions of
the epithelium. Outer hair cell loss began at a 35% distance from
the apex and increased to 40% cell loss the end of the epithelium,
while inner hair cell loss began at an 80% distance from the apex
and reached an average of 30% loss of hair cells at the end of the
basal region (Fig. 3B),
suggesting that SAHA partially inhibited the loss of hair cells
induced by noise exposure, particularly outer hair cell loss.
The HDAC inhibitor, SAHA, protects outer
hair cells against morphological changes induced by noise
exposure
In order to investigate the morphological changes
occurring in the ourter hair cells induced by noise exposure, and
whether the HDAC inhibitor, SAHA, protects the cells against
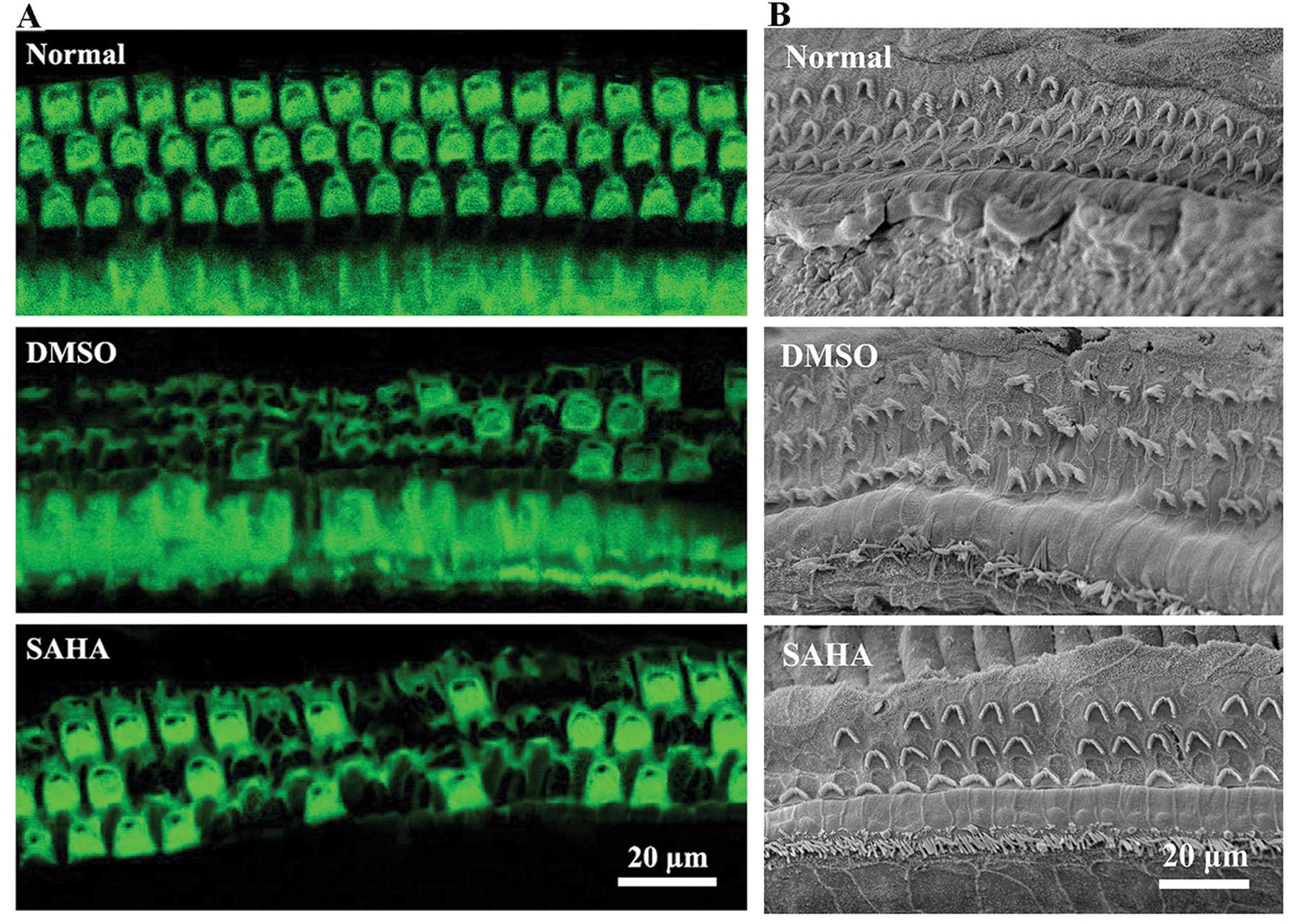
noise-induced damage, phalloidin staining (Fig. 4A) and SEM analysis (Fig. 4B) were carried out.
The results revealed that SAHA protected the outer
hair cells against noise-induced damage. As shown in Fig. 4, the normal outer hair cells in
the control group exhibited a normal appearance. In G2 (DMSO
treatment), 2 weeks following exposure to noise, cell morphology
was significantly altered with outer hair cell loss and cilia
damage. In G3 (SAHA treatment), in comparison to incubation with
DMSO alone, essentially doubled the number of surviving outer hair
cells and attenuated cilia damage. Thus, these results indicate
that the HDAC inhibitor, SAHA protects outer hair cells against
noise-induced morphological changes and prevents hair cell
loss.
Discussion
NIHL is a type of permanent hearing impairment that
results from prolonged exposure to high levels of noise (14). Hearing loss caused by recreational
and occupational noise exposure is the second most common cause of
sensorineural hearing loss following presbycusis (15). Histone acetylation and
deacetylation, as important epigenetic modifications, are
responsible for maintaining chromatin stability (16). An imbalance in histone acetylation
has been implicated in a wide range of transcriptional
dysfunctions, gene silencing and the development of
neurodegenerative disorders (17–19). In the present study, we
hypothesized that histone acetylation is involved in the death of
inner ear sensory cells due to NIHL, and that the HDAC inhibitor,
SAHA, may be used as a potential therapeutic agent to prevent
hearing loss.
HDACs are a class of enzymes that remove acetyl
groups from the lysine residues of target proteins, thus promoting
chromatin condensation and reduced transcription (4). The overexpression or sustained HDAC
activity has been reported in leukemia, lymphomas and other types
of cancers, as well as in neurodegenerative disorders (20–22). Acetylated histones, such H3 and
H4, serve as in vitro and in vivo markers for
successful HDAC inhibition (23).
In this study, in order to determine the histone
acetylation levels following exposure to BBN, an animal model of
NIHL was established, and the protein levels of HDAC1, HDAC4 and
H3-AcK9 were measured by western blot analysis. The results
revealed that, 2 weeks following the exposure of CBA/J mice to BBN,
PTS at 4, 8, 12, 24, 32 and 48 kHz was evident in the ABR test, and
a large number of hair cells was lost in the basal and middle
regions of the epithelium, which presented as permanent NIHL in the
adult CBA/J mice. The HDAC1 and HDAC4 levels began to increase 1 h
following exposure to noise and subsequently reached a plateau
above the control levels, while the H3-AcK9 levels decreased at the
same time point in the entire cochlea. This timing and location
suggest that a change in histone acetylation may be one of the
triggers to cell loss in the early stages of noise exposure.
HDAC inhibitors are a class of compounds that
interfere with the function of histone deacetylase (24). Previous studies have investigated
HDAC inhibitors as possible treatment strategies for cancer,
neurodegenerative disorders and inflammatory diseases (25–28). It has been shown that the
inhibition of HDAC1 and HDAC3 relieves Huntington’s disease
(HD)-like phenotypes in model systems and may be a target for human
HD (29). In addition, as
previously demonstrated, a novel class of pimelic
o-aminobenzamide HDAC inhibitors may be used as therapeutic
agents in neurodegenerative diseases, such as Friedreich’s ataxia
(30). Small-molecule inhibitors
of class I, II and IV HDACs have been shown to exhibit anticancer
activity with good safety profiles, particularly in patients with
hematologic malignancies (31).
Although the mechanisms of action of HDAC inhibitors remain
unclear, they are emerging therapeutic agents that have been
clinically validated in cancer patients with hematologic
malignancies, including cutaneous T-cell lymphoma (32).
In the present study, pre-treatment with the HDAC
inhibitor, SAHA, significantly reduced the threshold shifts 2 weeks
following exposure to BBN, which suggests that SAHA may exert a
protective effect against NIHL. To clarify the site and mechanism
of action of SAHA, we investigated hair cell loss following
pre-treatment with SAHA. The results revealed that following
pre-treatment with SAHA, outer hair cell loss began at a 35%
distance from the apex and increased to 40% cell loss at the end of
the epithelium, while inner hair cell loss began at an 80% distance
from the apex and reached an average of 30% loss of hair cells at
the end of the basal region, which was significantly decreased
compared with the control group. These data suggest that SAHA
partically inhibits noise-induced hair cell loss, particularly
outer hair cell loss. Further analysis of the morphological changes
in noise-damaged outer hair cells by phalloidin staining and SEM
revealed that SAHA protected the outer hair cells against
noise-induced loss and morphological changes. This study lends
strong support to the hypothesis that traumatic noise leads to the
dysfunction of HDAC, causing cell loss and morphological changes in
cochlear hair cells, particularly outer hair cells. Morevoer, the
HDAC inhibitor, SAHA, significantly decreases NIHL, and may thus be
a potential therapeutic strategy for the prevention of hearing
loss. Several HDAC inhibitors have already been approved for
clinical use (33,34). Such inhibitors may benefit the
large population of patients exposed to noise for long periods of
time and are at risk of developing irreversible hearing and balance
deficits.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81271069).
References
|
1
|
Sliwinska-Kowalska M and Davis A:
Noise-induced hearing loss. Noise Health. 14:274–280. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodyear RJ, Jones SM, Sharifi L, Forge A
and Richardson GP: Hair bundle defects and loss of function in the
vestibular end organs of mice lacking the receptor-like inositol
lipid phosphatase PTPRQ. J Neurosci. 32:2762–2772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen FQ, Schacht J and Sha SH:
Aminoglycoside-induced histone deacetylation and hair cell death in
the mouse cochlea. J Neurochem. 108:1226–1236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shakespear MR, Halili MA, Irvine KM,
Fairlie DP and Sweet MJ: Histone deacetylases as regulators of
inflammation and immunity. Trends Immunol. 32:335–343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao L, Cueto MA, Asselbergs F and Atadja
P: Cloning and functional characterization of HDAC11, a novel
member of the human histone deacetylase family. J Biol Chem.
277:25748–25755. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morrison BE, Majdzadeh N, Zhang X, Lyles
A, Bassel-Duby R, Olson EN and D’Mello SR: Neuroprotection by
histone deacetylase-related protein. Mol Cell Biol. 26:3550–3564.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Ruijter A, Van Gennip A, Caron H, Kemp
S and van Kuilenburg A: Histone deacetylases (HDACs):
characterization of the classical HDAC family. Biochem J.
370:737–749. 2003. View Article : Google Scholar
|
|
8
|
Sadri-Vakili G and Cha JH: Mechanisms of
disease: histone modifications in Huntington’s disease. Nat Clin
Pract Neuro. 2:330–338. 2006. View Article : Google Scholar
|
|
9
|
Strasák L, Bártová E, Harnicarová A,
Galiová G, Krejcí J and Kozubek S: H3K9 acetylation and radial
chromatin positioning. J Cell Physiol. 220:91–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang H, Sha SH and Schacht J: Kanamycin
alters cytoplasmic and nuclear phosphoinositide signaling in the
organ of Corti in vivo. J Neurochem. 99:269–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiebert SW and Stengel KR: Class I HDACs
affect DNA replication, repair and chromatin structure:
implications for cancer therapy. Antioxid Redox Signal. Jun
26–2014.Epub ahead of print.
|
|
12
|
Feng W, Zhang B, Cai D and Zou X:
Therapeutic potential of histone deacetylase inhibitors in
pancreatic cancer. Cancer Lett. 347:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rikiishi H: Autophagic and apoptotic
effects of HDAC inhibitors on cancer cells. J Biomed Biotechnol.
2011:Article ID 830260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rabinowitz PM: Noise-induced hearing loss.
Am Fam Physician. 61:2759–2760. 2000.
|
|
15
|
Nair C: Noise-induced hearing loss.
InnovAiT. 7:204–208. 2014.
|
|
16
|
Kingston RE and Narlikar GJ: ATP-dependent
remodeling and acetylation as regulators of chromatin fluidity.
Genes Dev. 13:2339–2352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stilling RM and Fischer A: The role of
histone acetylation in age-associated memory impairment and
Alzheimer’s disease. Neurobiol Learn Mem. 96:19–26. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song C, Kanthasamy A, Anantharam V, Sun F
and Kanthasamy AG: Environmental neurotoxic pesticide increases
histone acetylation to promote apoptosis in dopaminergic neuronal
cells: relevance to epigenetic mechanisms of neurodegeneration. Mol
Pharmacol. 77:621–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gräff J and Tsai LH: Histone acetylation:
molecular mnemonics on the chromatin. Nat Rev Neurosci. 14:97–111.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bassett SA and Barnett MP: The role of
dietary histone deacetylases (HDACs) inhibitors in health and
disease. Nutrients. 6:4273–4301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zwergel C, Valente S, Jacob C and Mai A:
Emerging approaches for histone deacetylase inhibitor drug
discovery. Expert Opin Drug Discov. 10:599–613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konsoula Z and Barile FA: Epigenetic
histone acetylation and deacetylation mechanisms in experimental
models of neurodegenerative disorders. J Pharmacol Toxicol Methods.
66:215–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vieyra D, Loewith R, Scott M, Bonnefin P,
Boisvert FM, Cheema P, Pastyryeva S, Meijer M, Johnston RN and
Bazett-Jones DP: Human ING1 proteins differentially regulate
histone acetylation. J Biol Chem. 277:29832–29839. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marks PA, Miller T and Richon VM: Histone
deacetylases. Curr Opin Pharmacol. 3:344–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chuang DM, Leng Y, Marinova Z, Kim HJ and
Chiu CT: Multiple roles of HDAC inhibition in neurodegenerative
conditions. Trends Neurosci. 32:591–601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Halili MA, Andrews MR, Sweet MJ and
Fairlie DP: Histone deacetylase inhibitors in inflammatory disease.
Curr Top Med Chem. 9:309–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang L: Targeting histone deacetylases
for the treatment of cancer and inflammatory diseases. J Cell
Physiol. 209:611–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dietz KC and Casaccia P: HDAC inhibitors
and neurodegeneration: at the edge between protection and damage.
Pharmacol Res. 62:11–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia H, Pallos J, Jacques V, Lau A, Tang B,
Cooper A, Syed A, Purcell J, Chen Y, Sharma S, et al: Histone
deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate
polyglutamine-elicited phenotypes in model systems of Huntington’s
disease. Neurobiol Dis. 46:351–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soragni E, Xu C, Cooper A, Plasterer HL,
Rusche JR and Gottesfeld JM: Evaluation of histone deacetylase
inhibitors as therapeutics for neurodegenerative diseases. Methods
Mol Biol. 793:495–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roger T, Lugrin J, Le Roy D, Goy G,
Mombelli M, Koessler T, Ding XC, Chanson AL, Reymond MK and
Miconnet I: Histone deacetylase inhibitors impair innate immune
responses to Toll-like receptor agonists and to infection. Blood.
117:1205–1217. 2011. View Article : Google Scholar
|
|
32
|
Kim HJ and Bae SC: Histone deacetylase
inhibitors: molecular mechanisms of action and clinical trials as
anti-cancer drugs. Am J Transl Res. 3:166–179. 2011.PubMed/NCBI
|
|
33
|
Piekarz R and Bates S: A review of
depsipeptide and other histone deacetylase inhibitors in clinical
trials. Curr Pharm Des. 10:2289–2298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prince HM, Bishton MJ and Harrison SJ:
Clinical studies of histone deacetylase inhibitors. Clin Cancer
Res. 15:3958–3969. 2009. View Article : Google Scholar : PubMed/NCBI
|