Introduction
Bone marrow-derived mesenchymal stem cells (BM-MSCs)
are multipotent adult cells that possess the potential to
differentiate into a variety of cell types, including adipocytes,
osteocytes, chondrocytes and neurons. The multipotential
differentiation capacity makes this type of cell important
candidates for tissue regenerative medicine (1). Over the past decade, various studies
have indicated that the osteogenic differentiation of BM-MSCs is a
complex process, regulated by a number of osteogenic transcription
factors, such as peroxisome proliferator-activated receptor-γ,
muscle segment homeobox 2 and runt-related transcription factor 2
(Runx2) (2,3). However, the molecular mechanism that
controls the osteogenic differentiation of BM-MSCs is not well
understood.
Annexin A1 (ANX A1), a member of the Annexin
superfamily, plays an essential role in cell differentiation,
proliferation and apoptosis. ANX A1 acts as a substrate for the
epidermal growth factor (EGF) receptor tyrosine kinase and through
its implication in the mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase (ERK) glucocorticoid
signaling pathway, performs important signaling functions in cell
proliferation and differentiation (4). It has been reported that the
phosphorylation of ANX A1 by EGF has an antiproliferative effect on
macrophages due to the constitutive activation of the MAPK/ERK
signaling pathway (5). The
molecular mechanism behind the involvement of ANX A1 in cell
differentiation is complex. ANX A1 is frequently dysregulated in
various types of cancer and its expression is associated with tumor
differentiation. The increased expression of ANX A1 has been
reported in pancreatic cancer, colon adenocarcinoma and
hepatocellular carcinoma (6–8).
However, certain studies have demonstrated that the expression of
ANX A1 is downregulated in thyroid, breast, and head and neck
cancer (9–11). Furthermore, an increase of ANX A1
expression with differentiation has been reported to occur in
normal epithelial cells (12). A
recent study by Bizzarro et al (13) demonstrated that ANX A1 is
upregulated during myoblast cell differentiation and a functional
knockdown of the ANX A1 protein inhibits myogenic differentiation.
Furthermore, an anomalous development of the craniofacial bone has
been observed in ANX A1 null mice (14). This finding indicated that
attenuation of ANX A1 may cause bone dysostosis. However, the
functional role of ANX A1 in bone development remains unclear, and
knowledge has been confined to a relatively small number of
published findings.
ERK is the most extensively investigated MAPK family
member, which is predominantly known for its role in the regulation
of BM-MSC proliferation and osteogenic differentiation. The
inhibition of extracellular matrix-induced ERK1/2 activation by
PD98059 [an MAPK kinase (MEK) inhibitor] suppresses osteogenic
differentiation of human MSCs (15). Additionally, ANX A1 specifically
regulates the activity of the ERK cascade (16). Studies indicate that ANX A1
specifically regulates cell differentiation and proliferation
through the ERK/MAPK signaling pathway (17). Thus, it may be hypothesized that
aberrant activation of the ERK signaling pathway by the regulated
expression of ANX A1 may influence BM-MSC osteogenic
differentiation.
The role of ANX A1 in BM-MSC osteogenic
differentiation and proliferation remains unclear. Therefore, in
the present study, the inhibition of BM-MSC osteogenic
differentiation as a result of ANX A1 downregulation was
investigated.
Materials and methods
Reagents and antibodies
The following antibodies were used for western blot
analysis: Rabbit anti-rat ANX A1 (ab65844) was obtained from Abcam
(Cambridge, USA); and phospho-p44/42 MAPK (Thr202/Tyr204) rabbit
mAb (catalogue no. 4370); p44/42 MAPK rabbit mAb (catalogue no.
4695); phospho-p38 MAPK (Thr180/Tyr182) rabbit mAb (catalogue no.
4511); p38 MAPK rabbit mAb (catalogue no. 8690); and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) rabbit mAb (catalogue no. 5174)
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA). All the primary antibodies were used at a dilution of
1:1,000. The secondary anti-rabbit IRDye 800CW antibody was
obtained from LI-COR Biosciences (Lincoln, NE, USA).
Isolation and culture of BM-MSCs
The management of experimental animals used in the
present study were according to the Regulations for the
Administration of Affairs Concerning Experimental Animals (approved
by the State Council of China and promulgated by decree no. 2 of
the State Science and Technology Commission on November 14,
1988).
The BM-MSCs were isolated by adherence to plastic
surfaces, as previously described (18). Briefly, thigh bones were isolated
from 6 Sprague Dawley rats (body weight, 150–200 g; maintained
under SPF raising conditions before surgery) and bone marrow
containing mononuclear cells was flushed out with
phosphate-buffered saline (PBS) using a syringe. The cell
suspension was filtered through a 100-μm strainer, and the
cells were counted and centrifuged (500 x g for 10 min). The pellet
was resuspended in Dulbecco’s modified Eagle’s medium (DMEM; Gibco
Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; GE Healthcare Life Sciences, Logan, UT, USA),
100 U/ml penicillin and 100 μg/ml streptomycin (Gibco Life
Technologies). The harvested cells were seeded at a density of
1×106/well in 6-well plates with the medium changed
every 3 days. Cells were passaged upon reaching 70–80%
confluence.
ANX A1 knockdown via short hairpin RNA
(shRNA)
Fourth-passage BM-MSCs were used for transfection.
The shRNA (fluorescent dye-labeled shRNA; Shanghai GenePharma Co.,
Ltd., Shanghai, China) was designed to target the common sequence
of rat ANX A1. The sense sequence of shRNA-ANXA1 was 5′-GCCT CACA
ACCA TTGT GAAG T-3′, and a scrambled shRNA served as a negative
control. The uniqueness of the designed shRNA was confirmed using
the National Center for Biotechnology Information Basic Local
Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
The BM-MSCs were plated at a density
5×103/well in 96-well culture plates prior to
transfection. After the cells had adhered for 24 h, the culture
medium was changed to antibiotic-free serum media. Transducing
lentiviral particles (Shanghai GenePharma Co., Ltd.) expressing ANX
A1 shRNA or scrambled shRNA were added at the quantity that was
calculated to reach an optimal multiplicity of infection of 80
units. After a 48-h incubation, the viral medium was removed and
the cells were collected for further experiments. BM-MSCs
expressing green fluorescent protein (GFP) reporter were analyzed
under an Olympus CKX41SF fluorescence microscope (Olympus, Tokyo,
Japan) 48 h post-transfection. The transfection efficiency was
measured by flow cytometry (BD Accuri™ C5 flow cytometer; BD
Biosciences, San Jose, CA, USA) 5 days post-transfection.
ANX A1 knockdown cells were designated as ANX
A1-knockdown (ANX A1-KD) BM-MSCs and the cells that were infected
with scrambled shRNA were termed negative control BM-MSCs.
Osteogenic differentiation
To evaluate their osteogenic capacity, the cells
(1×105 cells/well) were seeded in 6-well plates and
cultured in high-glucose DMEM supplemented with 10% FBS, 100 U/ml
penicillin, 100 mg/ml streptomycin, 0.05 mM ascorbate, 1 μM
dexamethasone and 10 mM β-glycerophosphate for 18 days.
Osteogenesis was confirmed by Alizarin red S (Solarbio, Beijing,
China) staining and alkaline phosphatase (ALP) activity. The images
were captured using an Olympus CKX41SF microscope (Olympus).
For Alizarin red S staining, the cells were seeded
in 6-well plates and grown in osteogenic-inducing media until the
indicated time point (10 and 18 days), when the cells were fixed in
a solution of 4% formaldehyde. The cells were stained in 1 ml
Alizarin red S solution for 20 min at room temperature and washed
with ultra pure water. Alizarin red S-stained areas were evaluated
via phase contrast microscopy (Olympus CKX41SF; Olympus). After
staining, the cultures were incubated with 10% cetylpyridinium
chloride at room temperature for 1 h to release the calcium-bound
Alizarin. The absorbance of the released Alizarin red S was
measured at a wavelength of 570 nm using a Multiskan MK3
spectrophotometer (Thermo Fisher Scientific, Inc., Rockford, IL,
USA) (19).
BM-MSCs cultured in osteogenic-inducing media were
collected from each well and assayed for ALP activity. The activity
of ALP was measured by an Alkaline Phosphatase Activity
Colorimetric assay kit (BioVision, Inc., Milpitas, CA, USA)
according to the manufacturer’s instructions; the optical density
(OD) was measured at 490 nm.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
proliferation assay
BM-MSC proliferation was measured using an MTT assay
(Solarbio). Briefly, ~5×103 cells were plated in 96-well
plates for each appropriate time point (day 0, 1, 3, 5 and 7). The
MTT solution was then added and maintained for 4 h at 37°C.
Dimethyl sulfoxide (150 μl) was added to each well for 10
min to solubilize the crystals. The OD value was measured at a
wavelength of 490 nm using a spectrophotometer (Multiskan MK3;
Thermo Fisher Scientific, Inc.).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA extraction was performed using
the RNAprep Pure Cell kit (Tiangen Biotech Co., Ltd., Beijing,
China) according to the manufacturer’s instructions. To generate
single stranded cDNA, RNA was reverse transcribed using a Revert
Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific
Inc.). PCR was performed with the ABI7500 system (Applied
Biosystems Life Technologies, Foster City, CA, USA) using Fast
Start Universal SYBR-Green Master (Roche Diagnostics, Indianapolis,
IN, USA). The primer sequences used for RT-qPCR were as follows:
Forward, 5′-ACCA GAAG AAGT ACGG AA-3′ and reverse, 5′-AACA ACGG
CTAA GAGA TG-3′ for ANX A1; forward, 5′-GACA ACTT TGGC ATCG TGGA-3′
and reverse, 5′-ATGC AGGG ATGA TGTT CTGG-3′ for GAPDH; forward,
5′-CTGG GCTT AGAT GGAC-3′ and reverse, 5′-CTAT TATG GGCT GGGT-3′
for Runx2; forward, 5′-AACC AAGC GTGG AAAC-3′ and reverse, 5′-TGGA
ACTC GCCT GACT-3′ for osteopontin (OPN); forward, 5′-GGCA GTAA GGTG
GTGA A-3′ and reverse, 5′-CCTG GAAG CCAA TGTG-3′ for osteocalcin
(OC).
The cycling conditions were as follows:
Pre-incubation, 95°C for 5 min; PCR, 95°C for 30 sec and 58°C for
30 sec over 42 cycles; and final elongation, 72°C for 60 sec.
Expression levels of the relative genes were calculated using the
2−ΔΔCt method and GAPDH mRNA served as an internal
control.
Western blot analysis
Proteins were extracted from the BM-MSCs using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Beijing, China) and the total protein concentration
was measured using a Bradford assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer’s instructions.
Proteins (40–60 μg/lane) were separated on a 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and
electrophoretically transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA) for 1 h at 100 mA.
Membranes were incubated in 3% bovine serum albumin/PBS-Tween for 1
h at room temperature. Primary antibodies were used at a dilution
of 1:1,000 and applied by overnight (at least 12 h) incubation at
4°C. Subsequently, the membrane was incubated with a
fluorescent-conjugated secondary antibody at a dilution of 1:10,000
for 1 h. Blots were analyzed using the Odyssey imaging system
(LI-COR Biosciences).
Statistical analysis
All data were expressed as the mean ± standard
deviation. The statistical significance between groups was
determined by Student’s t-test or one-way analysis of variance.
Statistical analyses were performed using SPSS version 13.0 (SPSS,
Inc., Chicago, IL, USA) and P<0.05 was considered to indicate a
statistically significant difference.
Results
Successful knockdown of ANX A1 in rat
BM-MSCs
To investigate the effect of ANX A1 on proliferation
and differentiation of BM-MSCs, a stable knockdown cell line was
generated using lentiviral particles. The microphotograph in
Fig. 1A depicts GFP-expressing
cells 2 days after infection. Flow cytometric analysis confirmed
that BM-MSCs achieved transfection efficiencies of ≤80% for the ANX
A1-KD group and ≤75% for the negative control group (Fig. 1A).
Downregulation of ANX A1 at the transcription and
translation levels was confirmed by SYBR-Green RT-qPCR and western
blot analysis. On average, treatment with shRNA targeted to ANX A1
resulted in a 68.1% reduction in ANX A1 mRNA levels (P<0.01;
n=5) and a 76.2% decrease in ANX A1 protein levels (P<0.01, n=5)
in ANX A1-KD BM-MSCs when compared with scrambled shRNA (Fig. 1B). No significant difference was
observed in the reduction of protein levels between the
utransfected BM-MSCs and the negative control BM-MSCs
(P<0.05).
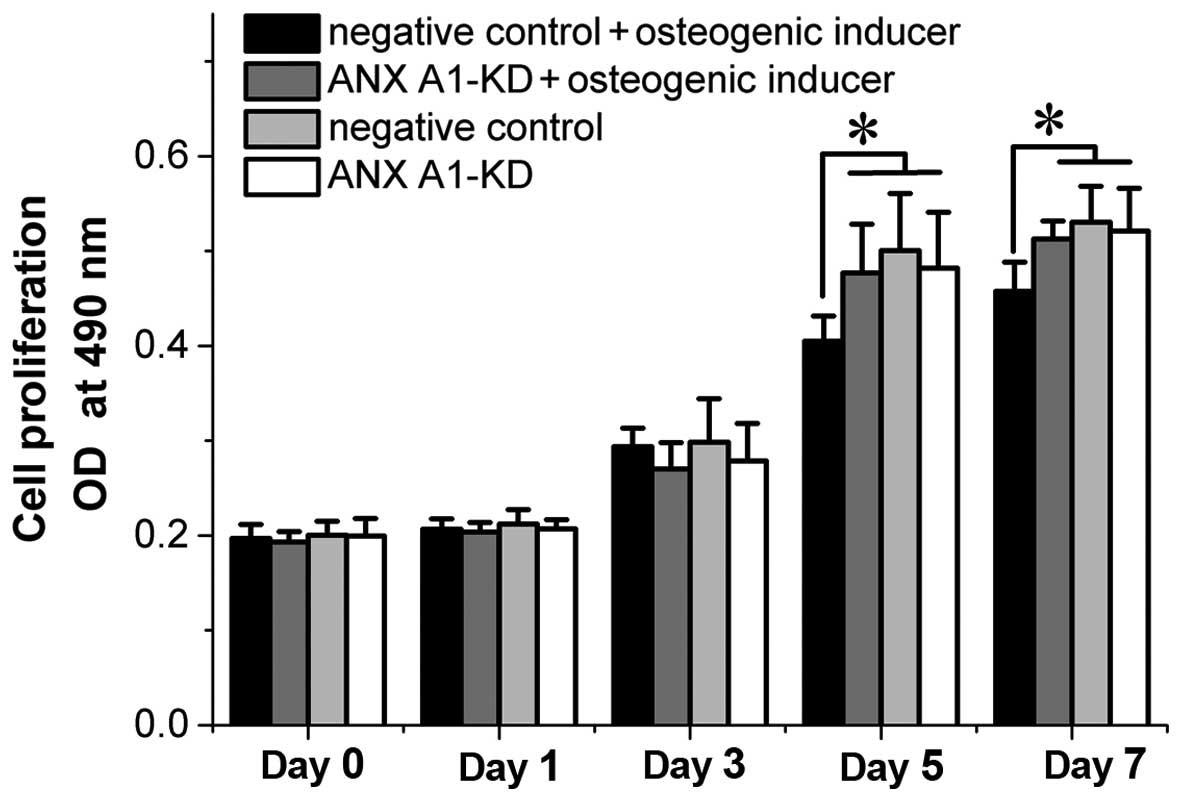
Effect of ANX A1 on BM-MSC
proliferation
Expression levels of ANX A1 have been found to
affect cell proliferation in vitro (20,21). To determine whether the knockdown
of ANX A1 affected BM-MSC proliferation, the proliferation rate of
BM-MSCs was analyzed by MTT assay. Although ANX A1-KD cells
cultured in normal conditions exhibited a marginal decrease in the
proliferation rate, no significant difference was identified
between the ANX A1-KD and negative control cells. However, when
cells were cultured in the osteogenic-inducing media, the negative
control cells exhibited an inhibited proliferation rate at day 5
and 7 when compared with the ANX A1-KD cells (P<0.05; Fig. 2).
ANX A1 expression is upregulated during
the later stages of osteogenic differentiation
The expression of ANX A1 in the negative control
BM-MSCs during differentiation was analyzed to determine how
endogenous ANX A1 is regulated throughout BM-MSC osteogenic
differentiation. For osteogenic differentiation, the cells were
cultured with osteogenic-inducing media. BM-MSC differentiation
into osteoblasts was induced and these cells were used to analyze
the expression of ANX A1 during differentiation. Following
osteogenic-inducing media treatment, a marginal decrease in the
expression of ANX A1 was apparent at day 1 and 3, and subsequently
an ongoing increase was observed from day 5 to 18 (P<0.01;
Fig. 3A). Therefore, it was
hypothesized that the expression of ANX A1 may be involved in the
later stages of osteogenic differentiation.
 | Figure 3Knockdown of ANX A1 by shRNA resulted
in reduced differentiation of BM-MSCs. (A) Analysis of ANX A1
protein expression during osteogenesis. GAPDH served as an internal
control and the quantitative analysis of ANXA1 expression was
performed by densitometry. The amount of ANXA1 was normalized to
the amount of GADPH. Quantitative analysis of the ANX A1 protein
expression is presented in the bar chart. *P<0.05 and
**P<0.01 vs. day 0 (n=5 per group). (B) The shape of
the negative control BM-MSCs became angular during osteogenesis
compared with the ANX A1-KD BM-MSCs (magnification, ×200). (C)
Analysis of Alizarin red S staining and quantification of matrix
mineralization (magnification, ×100). Knockdown of ANX A1
significantly inhibited calcified matrix synthesis. (D–F) Silencing
of ANX A1 by shRNA significantly suppressed Runx2, OPN and OC gene
expression. **P<0.01 vs. the negative control, as
determined by one-way analysis of variance, n=5 per group. KD,
knockdown; ANX A1, Annexin A1; shRNA, short hairpin RNA; BM-MSC,
bone marrow-derived mesenchymal stem cells; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; Runx2, runt-related transcription factor
2; OPN, osteopontin; OC, osteocalcin. |
Downregulation of ANX A1 inhibits BM-MSC
osteogenic differentiation
To analyze whether ANX A1 downregulation leads to
inhibition of BM-MSC differentiation into osteoblasts, BM-MSCs were
infected with the shRNA-ANX A1 plasmid. In addition, BM-MSCs were
infected with scrambled shRNA that served as a negative control.
The ANX A1-KD BM-MSCs and the negative control BM-MSCs were induced
to differentiate into mature osteoblasts, and the Alizarin red S
staining was performed to investigate the maturity of the
osteoblasts.
Following 10 days of exposure to osteogenic-inducing
media, the negative control cells became angular in shape, with
increased cell extensions observed during osteogenesis, whereas the
ANX A1-KD BM-MSCs were spindle-shaped (Fig. 3B). The ALP activity of ANX A1-KD
BM-MSCs cultured in osteogenic medium was significantly lower than
that in the negative control BM-MSCs (day 10, 0.224±0.074 vs.
0.646±0.126 nmol/min/mg and day 18, 0.319±0.039 vs. 0.691±0.096
nmol/min/mg; P<0.01, n=5).
Alizarin red S staining was performed after 10 and
18 days of culturing in the osteogenic-inducing media. The negative
control BM-MSCs showed matrix mineralization with more intense
Alizarin red S staining when compared with the ANX A1-KD BM-MSCs
(Fig. 3C). Quantitative analysis
of mineralization by measuring the absorbance of Alizarin red S at
570 nm revealed a significant difference between the two groups on
days 10 and 18 (P<0.01; Fig.
3C), indicating that the knockdown of ANX A1 inhibited BM-MSC
osteogenic differentiation.
To further investigate the influence of ANX A1
knockdown on the BM-MSCs osteogenic differentiation, the expression
of Runx2 mRNA and its downstream target genes, OPN and OC was
evaluated. RT-qPCR analysis revealed that silencing ANX A1 using
shRNA significantly suppressed the Runx2, OPN and OC gene
expression after 10 and 18 days of culturing in osteogenic-inducing
media (P<0.01; Fig. 3D–F).
Knockdown of ANX A1 decreases ERK1/2
phosphorylation during osteogenic differentiation
It has been demonstrated that the ERK-MAPK signaling
pathway is pivotal during MSC differentiation (22). Therefore, the phosphorylation
status of ERK1/2 in BM-MSCs, which had been stimulated with
osteogenic-inducing media from day 1 to 18, was investigated.
Phosphorylation of ERK1/2 was initially elevated in the negative
control BM-MSCs following exposure to osteogenic-inducing media for
5 days, and remained highly phosphorylated at day 7, 10 and 18
(Fig. 4A). However, the
phosphorylation of ERK1/2 in the ANX A1-KD BM-MSCs was
significantly inhibited when compared with the negative control
BM-MSCs (P<0.01).
In an attempt to elucidate whether the knockdown of
ANX A1-mediated inhibition of BM-MSC osteogenic differentiation
involves the activation of p38 MAPK, the status of p38
phosphorylation during the course of differentiation of BM-MSCs
into osteoblasts was analyzed. The results presented in Fig. 4B demonstrate that the
phosphorylation of p38 MAPK was significantly elevated and remained
highly phosphorylated from day 1 to 18; however, the absence of ANX
A1 did not alter the p38 MAPK phosphorylation.
Taken together, these results support the hypothesis
that the silencing of ANX A1 with shRNA significantly reduces the
differentiation of BM-MSCs by inhibiting the activation of ERK1/2,
but not that of p38 MAPK.
Involvement of ERK1/2 activation in
osteogenic differentiation
Due to previous reports describing that ANX A1
modulates the ERK signaling pathway at a proximal site (16) and knockdown of ANX A1 inhibits
ERK1/2 activation (23), an
inhibitor of MEK/ERK, PD98059, was used in the current study to
mimic the inhibitory effect caused by knockdown of ANX A1 on ERK1/2
activation. In the present study, negative control cells or ANX
A1-KD cells were treated with osteogenic-inducing media
supplemented with or without 20 μM PD98059. Treatment of
negative control cells with PD98059 resulted in a significant
inhibition of ERK1/2 activity (P<0.01). In addition, a further
decrease of ERK1/2 activity was observed in ANX A1-KD cells treated
with PD98059 (P<0.01; Fig.
5A). A significant decrease of calcified matrix synthesis and
ALP activity was observed in the cell culture with PD98059, as well
as in the ANX A1-KD cell culture with osteogenic-inducing media
alone (P<0.01; Fig. 5B–D).
Furthermore, it was observed that ANX A1-KD cells in combination
with PD98059 treatment further inhibited the ALP activity and
calcified matrix synthesis when compared with the ANX A1-KD cells
without PD98059 treatment (P<0.01; Fig. 5B and C).
 | Figure 5Effect of PD98059 on the ability of
BM-MSCs to undergo osteogenic differentiation. (A) Treatment of
BM-MSCs with PD98059 produced a significant inhibition of ERK1/2
phosphorylation. (B and C) Results of ALP activity and the
spectroscopic calcium assay at day 18. PD98059 treatment resulted
in a significant decrease of calcified matrix synthesis and ALP
activity (**P<0.01). (D) Osteogenic differentiation
demonstrated by staining with Alizarin red S 18 days after
induction reveals that PD98059 treatment inhibits mineralization
(magnification, ×100). (E–G) Quantitative reverse transcription
polymerase chain reaction analysis of Runx2, OPN and OC gene
expression. Treatment of cells with PD98059 produced a significant
inhibition of the expression levels of those genes
(**P<0.01). Statistical significance was determined
by one-way analysis of variance. *P<0.05 and
**P<0.01, n=5 per group. p, phosphorylated; KD,
knockdown; ANX A1, Annexin A1; BM-MSC, bone marrow-derived
mesenchymal stem cell; ERK1/2, extracellular signal-regulated
kinase 1/2; ALP, alkaline phosphatase; Runx2, runt-related
transcription factor 2; OPN, osteopontin; OC, osteocalcin. |
The effect of PD98059 on the osteogenic gene
expression was also assessed. Consistent with the data obtained
from ERK1/2 activity, ANX A1-KD cells together with PD98059
treatment resulted in a reduced decrease of Runx2, OPN and OC mRNA
expression when compared with ANX A1-KD cells alone (P<0.01;
Fig. 5E–G). In addition,
treatment of the negative control cells with 20 μM PD98059
produced a significant suppression of Runx2, OPN and OC mRNA
expression when compared with the untreated negative control cells
(P<0.01), however, no significant difference was noted when
compared with the group of ANX A1-KD cells without PD98059
treatment (P<0.05; Fig.
5E–G).
Taken together, these data demonstrate that the
activation of ERK1/2 is necessary for osteogenic differentiation
and that treatment with PD98059 may enhance the inhibitory effect
of ANX A1 knockdown on the osteogenic differentiation of
BM-MSCs.
Discussion
ANX A1, the first characterized member of the
Annexin super-family, is found in numerous cells and tissues,
including the lungs, bone marrow and intestine (24). Annexins are associated with the
cell membrane or cytoskeleton in a calcium-dependent manner.
Accumulating evidence has demonstrated that the expression level of
ANX A1 is involved in cell proliferation and differentiation
(25). The present study was
undertaken to investigate the effects of ANX A1 on BM-MSC
proliferation and osteogenic differentiation. The results
demonstrated that the knockdown of ANX A1 with shRNA in BM-MSCs
resulted in reduced osteogenic differentiation. It was also found
that the knockdown of ANX A1 caused a reduction in ERK1/2
phosphorylation and osteogenic gene expression during osteogenic
differentiation. Furthermore, downregulation of ANX A1 was observed
to decrease the rate of proliferation reduction following BM-MSC
incubation in osteogenic-inducing media.
Although the expression of ANX A1 has been
associated with various types of cellular differentiation (25), to the best of our knowledge,
little previous evidence exists implicating ANX A1 in the
differentiation of BM-MSCs into osteoblasts. In the present study,
it was found that the expression of ANX A1 marginally decreased on
day 1 and 3 following exposure to an osteogenic medium, however, it
was increased markedly on day 5 and remained elevated up to day 18.
To confirm the role of ANX A1 in osteogenic differentiation,
BM-MSCs were transfected with shRNA-ANXA1. The results demonstrate
that knockdown of ANX A1 significantly inhibited the expression of
the osteogenic genes (Runx2, OPN and OC) and resulted in reduced
differentiation of BM-MSCs into osteocytes, implying that the
upregulation of ANX A1 in the later stages of differentiation may
be required for further osteogenic differentiation.
The differentiation of BM-MSCs into osteoblasts is a
complex process involving the interplay of numerous effectors that
regulate, positively and negatively, a network of signaling
pathways. The ERK, p38 and c-Jun N-terminal kinase (JNK) MAPKs are
cell signaling pathways that are pivotal in cell differentiation.
The role of p38 MAPK in osteogenic differentiation remains unclear,
and previous studies gave controversial results. Certain studies
claim that activation of ERK1/2, but not JNKs or the p38 MAPKs,
promotes osteogenesis (26),
whereas others state that p38 MAPKs promote osteogenic
differentiation (27). However,
studies have confirmed that the ERK signaling pathway affects
osteogenesis via different mechanisms during the differentiation
process (28). ANX A1
specifically modulates the ERK signaling cascade at an upstream
site. Increasing the expression of ANX A1 leads to constitutive
activation of ERK1/2 kinase (16). A recent study found that ERK
activation was significantly decreased in ANX A1 KD-DU145 cells
(23). However, it has been
reported that p38 MAPK function, upstream of dexamethasone-induced
ANX A1 synthesis, and the p38 MAPK inhibitor, SB203580 prevent
dexamethasone-induced ANX A1 expression (29). In the present study, a sustained
phosphorylation of ERK1/2 was observed from day 5 to 18 during
negative control BM-MSC differentiation into osteoblasts. This
phosphorylation was correlated with the expression of ANX A1, which
was upregulated in the later stages of differentiation.
Furthermore, knockdown of ANX A1 significantly inhibited the
phosphorylation of ERK1/2 during BM-MSC osteogenic differentiation.
Although activation of the p38 MAPK signaling cascade has been
associated with BM-MSC osteogenic differentiation (27), the present study demonstrates that
knockdown of ANX A1 exerts no apparent effect on altering p38 MAPK
phosphorylation. Therefore, it was hypothesized in the current
study that ANX A1 regulation of ERK1/2 phosphorylation may be
involved in osteogenic differentiation.
In order to further confirm the involvement of the
ERK1/2 signaling pathway in BM-MSC osteogenic differentiation, the
effect of PD98059 on the ability of negative control BM-MSCs to
undergo osteogenic differentiation was analyzed in the present
study. It was found that blocking ERK1/2 phosphorylation resulted
in decreased mineralization, ALP activity and expression of
osteogenic genes (Runx2, OPN and OC) when BM-MSCs were incubated in
osteogenic-inducing media. This result is consistent with earlier
observations made by Jaiswal et al (30). Although knockdown of ANX A1
resulted in a decrease of ERK1/2 phosphorylation, the inhibition
was not complete. Therefore, ANX A1-KD cells were treated with
PD98059, to establish whether it would potentiate the inhibitory
effects of ANX A1 knockdown on osteogenic differentiation. As
expected, a more marked reduction in mineralization and osteogenic
gene expression was observed in the ANX A1-KD cells treated with
PD98059, when compared with the group of ANX A1-KD cells not
treated with PD98059. These results further support the hypothesis
that osteogenic differentiation of BM-MSCs requires activation of
ERK1/2 and that the inhibitory effect of ANX A1 knockdown on BM-MSC
osteogenic differentiation involves ERK1/2 inhibition.
ANX A1 regulates ERK signaling pathway activation
and inhibits cyclin D1 expression, therefore reducing cell
proliferation (20). Evidence
reveals that ANX A1 functions as an inhibitor of signal
transduction pathways that leads to cell proliferation in JACRO
cells (21) and lymphocytes
(31). However, the effects of
over-expression of ANX A1 in rapidly proliferating hepatocytes
(7), as well as human foreskin
fibroblasts (32) indicates that
the underlying mechanism is more complex. Although downregulation
of ANX A1 has been traditionally associated with increased cellular
proliferation, the data from the present study indicates that the
proliferation rate of ANX A1-KD BM-MSCs was not significantly
increased under normal conditions. However, when negative control
cells were cultured in the osteogenic-inducing media, a decrease in
cell proliferation was observed, when compared with that of the ANX
A1-KD cells. A previous study proposed that the proliferation of
ANX A1+/+ cells were inhibited by dexamethasone in a
concentration-dependent manner (0.01–1 μM), whereas the ANX
A1−/− cells were not significantly affected at these
dexamethasone concentrations (21). In addition, one study demonstrated
that the addition of osteogenic-inducing media to human BM-MSCs
inhibited cell proliferation (33). The MTT data obtained in the
current study indicates that the negative control BM-MSCs
over-expressing the ANX A1 protein have a reduced proliferation
rate from day 5 to 7, this reduction is likely to be associated
with the over-expression of ANX A1 and the osteogenic medium, which
contains 1 μM dexamethasone. Therefore, it is proposed that,
under the exposure of osteogenic-inducing media, downregulation of
ANX A1 may protect cells from the effect of osteogenic
medium-induced inhibition of cell proliferation.
In conclusion, the present study revealed that the
knockdown of ANX A1 in BM-MSCs significantly effects the
proliferation rate under osteogenic-inducing media treatment.
Additionally, silencing ANX A1 using shRNA significantly inhibits
the phosphorylation of ERK1/2 during osteogenesis and results in
reduced osteogenic differentiation of BM-MSCs. These data identify
that ANX A1-mediated ERK1/2 activation is involved in BM-MSC
proliferation and osteogenic differentiation. Furthermore, these
results may have important implications with regard to bone
development and remodeling.
Acknowledgments
The authors would like to thank Professor Zhao for
providing the scrambled lentiviral-shRNA particles. The present
study was supported primarily by the Natural Science Foundation of
China (grant no. 81460297).
References
|
1
|
Odabas S, Elçin AE and Elçin YM: Isolation
and characterization of mesenchymal stem cells. Methods Mol Biol.
1109:47–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valenti MT, Garbin U, Pasini A, Zanatta M,
Stranieri C, Manfro S, Zucal C and Dalle Carbonare L: Role of
ox-PAPCs in the differentiation of mesenchymal stem cells (MSCs)
and Runx2 and PPARγ2 expression in MSCs-like of osteoporotic
patients. PLoS One. 6:e203632011. View Article : Google Scholar
|
|
3
|
Cheng SL, Shao JS, Charlton-Kachigian N,
Loewy AP and Towler DA: MSX2 promotes osteogenesis and suppresses
adipogenic differentiation of multipotent mesenchymal progenitors.
J Biol Chem. 278:45969–45977. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim LH and Pervaiz S: Annexin 1: The new
face of an old molecule. FASEB J. 21:968–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De BK, Misono KS, Lukas TJ, Mroczkowski B
and Cohen S: A calcium-dependent 35-kilodalton substrate for
epidermal growth factor receptor/kinase isolated from normal
tissue. J Biol Chem. 261:13784–13792. 1986.PubMed/NCBI
|
|
6
|
Bai XF, Ni XG, Zhao P, Liu SM, Wang HX,
Guo B, Zhou LP, Liu F, Zhang JS, Wang K, et al: Overexpression of
annexin 1 in pancreatic cancer and its clinical significance. World
J Gastroenterol. 10:1466–1470. 2004.PubMed/NCBI
|
|
7
|
Masaki T, Tokuda M, Ohnishi M, Watanabe S,
Fujimura T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M, et
al: Enhanced expression of the protein kinase substrate annexin in
human hepatocellular carcinoma. Hepatology. 24:72–81.
1996.PubMed/NCBI
|
|
8
|
Lecona E, Barrasa JI, Olmo N, Llorente B,
Turnay J and Lizarbe MA: Upregulation of Annexin A1 expression by
butyrate in human colon adenocarcinoma cells: Role of p53, NF-Y,
and p38 mitogen-activated protein kinase. Mol Cell Biol.
28:4665–4674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrella A, Festa M, Ercolino SF, Zerilli
M, Stassi G, Solito E and Parente L: Annexin-1 downregulation in
thyroid cancer correlates to the degree of tumor differentiation.
Cancer Biol Ther. 5:643–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen D, Nooraie F, Elshimali Y, Lonsberry
V, He J, Bose S, Chia D, Seligson D, Chang HR and Goodglick L:
Decreased expression of Annexin A1 is correlated with breast cancer
development and progression as determined by a tissue microarray
analysis. Hum Pathol. 37:1583–1591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garcia Pedrero JM, Fernandez MP, Morgan
RO, Herrero Zapatero A, Gonzalez MV, Suarez Nieto C and Rodrigo JP:
Annexin A1 down-regulation in head and neck cancer is associated
with epithelial differentiation status. Am J Pathol. 164:73–79.
2004. View Article : Google Scholar
|
|
12
|
Rodrigo JP, García-Pedrero JM, González
MV, Fernández MP, Suárez C and Herrero A: Expression of Annexin A1
in normal and chronically inflamed nasal mucosa. Arch Otolaryngol
Head Neck Surg. 130:211–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bizzarro V, Fontanella B, Franceschelli S,
Pirozzi M, Christian H, Parente L and Petrella A: Role of Annexin
A1 in mouse myoblast cell differentiation. J Cell Physiol.
224:757–765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Damazo AS, Moradi-Bidhendi N, Oliani SM
and Flower RJ: Role of annexin 1 gene expression in mouse
craniofacial bone development. Birth Defects Res A Clin Mol
Teratol. 79:524–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salasznyk RM, Klees RF, Hughlock MK and
Plopper GE: ERK signaling pathways regulate the osteogenic
differentiation of human mesenchymal stem cells on collagen I and
vitronectin. Cell Commun Adhes. 11:137–153. 2004. View Article : Google Scholar
|
|
16
|
Alldridge LC, Harris HJ, Plevin R, Hannon
R and Bryant CE: The annexin protein lipocortin 1 regulates the
MAPK/ERK pathway. J Biol Chem. 274:37620–37628. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huo XF and Zhang JW: Annexin1 regulates
the erythroid differentiation through ERK signaling pathway.
Biochem Biophys Res Commun. 331:1346–1352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kassis I, Zangi L, Rivkin R, Levdansky L,
Samuel S, Marx G and Gorodetsky R: Isolation of mesenchymal stem
cells from G-CSF-mobilized human peripheral blood using fibrin
microbeads. Bone Marrow Transplant. 37:967–976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YJ, Lee JY, Lee SJ, Chung C-P and
Park YJ: Alpha-adrenergic blocker mediated osteoblastic stem cell
differentiation. Biochem Biophys Res Commun. 416:232–238. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alldridge LC and Bryant CE: Annexin 1
regulates cell proliferation by disruption of cell morphology and
inhibition of cyclin D1 expression through sustained activation of
the ERK1/2 MAPK signal. Exp Cell Res. 290:93–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Croxtall JD, Gilroy DW, Solito E,
Choudhury Q, Ward BJ, Buckingham JC and Flower RJ: Attenuation of
glucocorticoid functions in an Anx-A1−/− cell line.
Biochem J. 371:927–935. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mei Y, Bian C, Li J, Du Z, Zhou H, Yang Z
and Zhao RC: miR-21 modulates the ERK-MAPK signaling pathway by
regulating SPRY2 expression during human mesenchymal stem cell
differentiation. J Cell Biochem. 114:1374–1384. 2013. View Article : Google Scholar
|
|
23
|
Mu D, Gao Z, Guo H, Zhou G and Sun B:
Sodium butyrate induces growth inhibition and apoptosis in human
prostate cancer DU145 cells by up-regulation of the expression of
Annexin A1. PLoS One. 8:e749222013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gavins FN and Hickey MJ: Annexin A1 and
the regulation of innate and adaptive immunity. Front Immunol.
3:3542012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002.PubMed/NCBI
|
|
26
|
Zeng W, Yan Y, Zhang F, Zhang C and Liang
W: Chrysin promotes osteogenic differentiation via ERK/MAPK
activation. Protein Cell. 4:539–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yi C, Liu D, Fong CC, Zhang J and Yang M:
Gold nanoparticles promote osteogenic differentiation of
mesenchymal stem cells through p38 MAPK pathway. ACS Nano.
4:6439–6448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SH, Choi YR, Park MS, Shin JW, Park
KD, Kim SJ and Lee JW: ERK 1/2 activation in enhanced osteogenesis
of human mesenchymal stem cells in poly(lactic-glycolic acid) by
cyclic hydrostatic pressure. J Biomed Mater Res A. 80:826–836.
2007. View Article : Google Scholar
|
|
29
|
Castro-Caldas M, Mendes AF, Duarte CB and
Lopes MC: Dexamethasone-induced and estradiol-induced CREB
activation and annexin 1 expression in CCRF-CEM lymphoblastic
cells: Evidence for the involvement of cAMP and p38 MAPK. Mediators
Inflamm. 12:329–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jaiswal RK, Jaiswal N, Bruder SP,
Mbalaviele G, Marshak DR and Pittenger MF: Adult human mesenchymal
stem cell differentiation to the osteogenic or adipogenic lineage
is regulated by mitogen-activated protein kinase. J Biol Chem.
275:9645–9652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Almawi WY, Saouda MS, Stevens AC, Lipman
ML, Barth CM and Strom TB: Partial mediation of glucocorticoid
antiproliferative effects by lipocortins. J Immunol. 157:5231–5239.
1996.PubMed/NCBI
|
|
32
|
Schlaepfer DD and Haigler HT: Expression
of annexins as a function of cellular growth state. J Cell Biol.
111:229–238. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng SL, Yang JW, Rifas L, Zhang SF and
Avioli LV: Differentiation of human bone marrow osteogenic stromal
cells in vitro: Induction of the osteoblast phenotype by
dexamethasone. Endocrinology. 134:277–286. 1994.PubMed/NCBI
|