Introduction
Cardiovascular disease (CVD) is the leading cause of
mortality globally, with the majority of patients succumbing to
ischemic heart disease. Acute myocardial infarction (AMI) is
associated with the risk of cardiac failure and mortality (1–3).
The mainstay of current therapies for AMI is re-perfusion of the
infarcted area via thrombolysis, angioplasty or bypass-grafting.
However, re-perfusion following ischemia/hypoxia induces further
cardiomyocyte death (4). Over the
past decade, bone marrow stem cell (BMSC) therapy has been shown to
be safe in experimental animal models and AMI patients, and it is
widely accepted that BMSCs improve cardiac function (5,6).
BMSCs have the capacity to multiply and differentiate into new
blood-vessel cells or to enhance mobilization of resident cardiac
stem cells, predominantly via a paracrine mechanism (7,8).
However, the efficacy of this approach is not sufficient to achieve
cardiac repair.
Several pre-clinical studies have suggested that
genetic strategies can improve BMSC survival and differentiation
(9,10). Further studies have indicated that
transplantation of gene-modified stem cells may be beneficial in
mediating substantial functional recovery after AMI (11,12). However, achieving a high rate of
cell survival after transplantation into an infarcted heart remains
a challenge. Thus, it is necessary to reinforce BMSCs against the
damaging environment, including high levels of pro-apoptotic
factors, incurred as a result of ischemia and to improve the
efficacy of BMSC therapy.
Serum response factors (SRF) play an important role
in the regulation of nearly every known smooth muscle-specific gene
via binding to the sequence [CC(A/T)6GG], termed a serum
response element (SRE) (13,14). Studies have indicated that
myocardin-related transcription factor-A (MRTF-A) is abundant in
tissues from newborn and adult rats, elevates SRF-driven
transcription and enhances the expression of target genes (13,15) that can contribute to cardiac and
neuronal protection under various forms of stress (16,17).
The protective effects of MRTF-A against heart
disease have been demonstrated in previous studies (18–20). Certain studies have indicated that
MRTF-A regulates myofibroblast activation and fibrosis in response
to the renin-angiotensin system and post-MI remodeling (20). Of note, normalization of activated
myocardin signaling in the ventricular myocardium during the
mid-stages of the development of heart failure can improve impaired
ventricular function (21). Thus,
MRTF-A is likely to be a key regulator of cardiac function.
In the present study, adult rat BMSCs were
genetically modified to overexpress MRTF-A with the aim of
protecting cardiomyocytes against hydrogen peroxide-induced damage
and enhancing their resistance to ischemic conditions following
transplantation in a rat model of AMI. It was hypothesized that
genetic modification of BMSCs with regard to MRTF-A protects BMSCs
against the ischemic environment and improves their viability in
the early post-transplantation period, thereby enhancing cardiac
functional recovery after AMI.
Materials and methods
Animals
Male Sprague-Dawley rats (aged 8–12 weeks, 250–280
g, n=48) were obtained from the Experimental Animal Center of
Zhejiang Province (Zhejiang, China). Rats were housed at a constant
room temperature of 22°C under a 12-h light/dark cycle with free
access to food and drinking water. Housing facilities and all
experimental protocols were performed according to the National
Institutes of Health (NIH) Guide for the Care and Use of Laboratory
Animals (NIH Publication no. 85-23, revised 1996), and approved by
the Animal Care and Use Committee of Zhejiang University School of
Medicine, which adopts the abovementioned NIH guidelines.
Sprague-Dawley rats were divided into four groups
(six rats in each group): Sham group (sham), left thoracotomy
without coronary artery ligation; AMI model group (AMI), acute
myocardial infarction model; BMSCs treatment group
[lv(−)-BMSCs], AMI model rats treated with empty vector;
MRTF-A-modified BMSCs treatment group (MRTF-A-BMSCs), AMI model
rats treated with MRTF-A-modified BMSCs.
AMI model and BMSC transplantation
Animals were anesthetized with pentobarbital (50
mg/kg body weight; Roche, Basel, Switzerland). All rats underwent
thoracotomy at the fifth left intercostal space, the pericardium
was opened and a loose 00 braided silk suture was placed around the
left anterior descending coronary artery ~1–2 mm below its origin.
To facilitate the successive removal of the suture, a small silicon
ring was inserted in the silk thread below the knot. The chest was
then closed with a silk suture to minimize heart displacement,
taking care to leave the ends of the coronary suture threads
emerging from the surgical wound (11,22). Immediately after ligation, a
31-gauge needle was used to inject 6×106 MRTF-A-BMSCs
[injected at six sites (23)]
into the anterior and lateral aspects of the viable myocardium
bordering the infarction. Twelve AMI model rats were administered
an equal volume of Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA) via the same route served as
medium controls. Twelve rats were injected with the empty
vector-transfected BMSCs. Animals were sacrificed at 1 week
post-transplantation.
Primary cardiomyocyte culture
The primary cardiomyocytes were prepared from
newborn Sprague-Dawley rats as previously described (24). Briefly, post-natal day-0 primary
cardiomyocytes were dissociated with 0.25% pancreatic enzymes
(Sigma-Aldrich) and cultured at a density of 2×106/plate
in 35-mm plates. The primary cardiomyocytes were cultured in 90%
DMEM, 10% fetal bovine serum (FBS; Gibco-Invitrogen, Carlsbad, CA,
USA) and 100 U/ml penicillin/0.1 mg/ml streptomycin
(Sigma-Aldrich).
Isolation, culture and expansion of
BMSCs
BMSCs were prepared according to previous methods
(25,26). Male Sprague-Dawley rats were
sacrificed by cervical dislocation, femurs and tibias were removed
and carefully flushed with DMEM-low glucose (DMEM-LG; HyClone
Laboratories, Inc., Logan, UT, USA) using a 0.45-mm syringe needle
until the bones became pale. The released cells were discarded and
the bones were dissected into fragments of 1–3 mm3 and
digested with collagenase II (0.5 mg/ml; Sigma-Alrich) for 1–2 h in
a shaking incubator at 37°C and a shaking speed of 200 rpm. The
collagenase was de-activated by dilution with DMEM-LG containing
10% FBS. The digested bone fragments were also washed by
centrifuging twice for 5 min at 100 × g followed by culture in a
60-mm dish (Costar, Corning Inc., Corning, NY, USA) with DMEM-LG
containing 10% FBS and penicillin/streptomycin at 37°C in a 5%
CO2 humidified incubator. To isolate putative BMSCs,
after 72 h of culture, non-adherent cells and tissue debris were
removed with phosphate-buffered saline (PBS; HyClone Laboratories),
and adherent cells were maintained. On reaching 70–80% confluence,
these adherent cells were detached from the plate by digestion with
0.25% (wt/v) trypsin/0.02% (wt/v) EDTA (Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA) for 2–3 min and the cells were
re-plated. The medium was replaced every 2–3 days.
Identification of BMSCs by flow
cytometric analysis
At passage three, the cultured BMSCs were detached
from the plates by trypsin-EDTA digestion. Aliquots of cells
(1×106) were washed with cold PBS and re-suspended in
100 µl PBS per Eppendorf tube and stained with fluorescein
isothiocyanate-conjugated anti-mouse CD45 (Becton-Dickinson, San
Jose, CA, USA), phycoerythrin (PE)-conjugated anti-mouse CD34
(eBioScience, San Diego, CA, USA) and CD90 (eBioScience) antibodies
at a concentration of 2 µg/ml at 4°C for 30 min. One tube of
unstained cells was prepared as a control for the staining
antibodies. Data were acquired using a BD FACScalibur fluorescence
activated cell sorting cytometer (BD Biosciences, San Jose, CA,
USA) and analyzed using CellQuest software (BD Accuri C6 Software
1.0.264.21; BD Biosciences).
Transfection of BMSCs with lentiviral
vector
The lentiviral vector encoding MRTF-A was obtained
from ShengGong (ShenGong, Shanghai, China). Briefly, BMSCs
(4×105 cells/well) at passage three were plated in a
six-well plate (Costar) 24 h prior to transfection. BMSCs were
incubated with 5 µl recombinant lentivirus (1×108
particles/ml) for at least 24 h in minimal culture medium
containing polybrene (final concentration, 8 mg/l; Sigma-Aldrich).
At 24, 48, 72 and 96 h after the first infection, lentiviral
transduction of BMSCs was assessed by western blot analysis.
Unbound virus was removed and replaced with fresh complete medium.
The cells were then incubated for a further five days prior to
H2O2 treatment. BMSCs were infected with 5
µl of the empty lentiviral vector (1×108
particles/ml) as a control. The multiplicity of infection was
10.
Hydrogen peroxide treatment and cell
viability assay
BMSCs (1.2×106) were treated with
H2O2 (200 µM at 37°C for 3 h; Beyotime
Institute of Biotechnology, Haimen, China) after recombinant
lentiviral infection. In addition to this, the cardiomyocytes were
treated with BMSCs for 2 h (BMSCs vs. cardiomyocytes is 1:1) and 50
µM H2O2 were then added to the cell
mixture. BMSC and cardiomyocyte apoptosis was assayed by Annexin
V-PE/7-actinomycin D (7ADD) staining (BD-559763; BD Biosciences)
and analyzed by flow cytometry.
Histopathology
Ηematoxylin and eosin (H&E) staining was adopted
to examine the histological alterations in the heart tissue.
Briefly, the heart tissues preserved in 4% paraformaldehyde were
dehydrated using Carnoy's fluid and then used to prepare
paraffin-embedded sections. After being stained with H&E
(Beyotime Institute of Biotechnology), the heart histomorphology of
the tree shrews was observed and photographed using an Olympus
imaging system (CX31; Olympus Optical Co., Ltd, Tokyo, Japan).
Western blot analysis
Cell pellets were lysed in 0.1 ml of RIPA buffer.
Total cellular proteins were extracted using a Total Protein
Extraction kit (Beyotime Institute of Biotechnology), quantified
usi ng the Bicinchoninic Acid Protein assay kit (Beyotime Institute
of Biotechnology), separated by 10% SDS-PAGE (Beyotime Institute of
Biotechnology) and transferred onto nitrocellulose membranes
(Millipore, Billerica, MA, USA) at 200 mA. The membranes were
blocked with 5% skimmed milk, incubated with anti-B-cell lymphoma 2
(Bcl-2) (monoclonal; 1:2,000 dilution; cat no. sc-7382; Santa Cruz
Biotechnology, Dallas, TX, USA), anti-MRTF-A (monoclonal; 1:5,000
dilution; cat no. sc-398675; Santa Cruz Biotechnology) and
anti-β-actin (monoclonal; 1:5,000 dilution; cat no. sc-8432; Santa
Cruz Biotechnology) antibodies, and, after washing with 0.1%
Tween-20 in TBS, were incubated with
horseradish-peroxidase-conjugated secondary antibodies for 1.5 h at
room temperature (1:10,000; Beyotime Institute of Biotechnology).
The bands were then evaluated by densitometric analysis using a
Desaga Cab UVIS scanner and Desaga ProViDoc software (Desaga GmbH,
Wiesloch, Germany). Enhanced chemiluminescence detection of the
target protein was performed with a computerized image processing
system (ImageQuant 1.19; Molecular Dynamics, Sunnyvale, CA, USA)
and exposed using an X-ray film.
Statistical analyses
All values are expressed as the means ± standard
deviation and analyzed by SPSS Statistical software (SPSS
Statistics 22.0; SPSS Inc., Stanford, CA, USA). One-way analysis of
variance with Scheffe's post-hoc test for unequal sample sizes was
used to compare numeric data among the four experimental groups.
Datasets consisting of two groups only were compared using the
unpaired Student's t-tests. A level of P<0.05 was considered to
indicate statistical significance.
Results
Identification of BMSCs and infection
with lentivirus
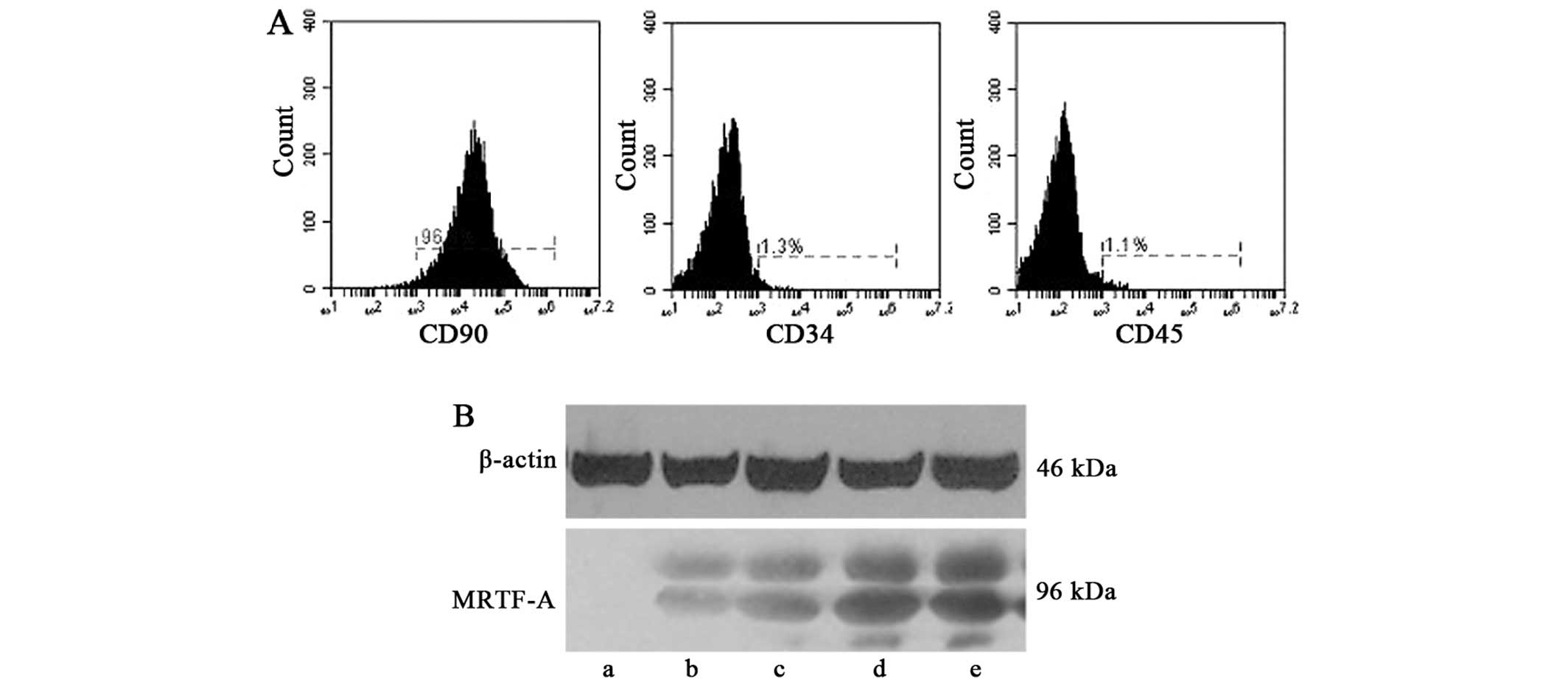
The adherent BMSCs at passage three exhibited a
surface antigen phenotype consistent with those previously reported
(27); CD90-positive and CD34-
and CD45-negative. Flow cytometric analysis was employed to
determine surface marker expression (Fig. 1A). The cultured BMSCs were devoid
of t he BMSC-specific markers CD34 and CD45, while high expression
of CD90 was observed.
BMSCs at passage three were infected with 5
µl recombinant lentivirus (1×108 particles/ml).
At 24, 48, 72 and 96 h after the first infection, lentiviral
transduction of BMSCs was confirmed by western blot analysis
(Fig. 1B). A similar protocol was
used for preparing negative control BMSCs transfected with empty
vector. Successfully transfected BMSCs were used for
transplantation into the rat model of AMI.
MRTF-A overexpression enhances BMSC
survival
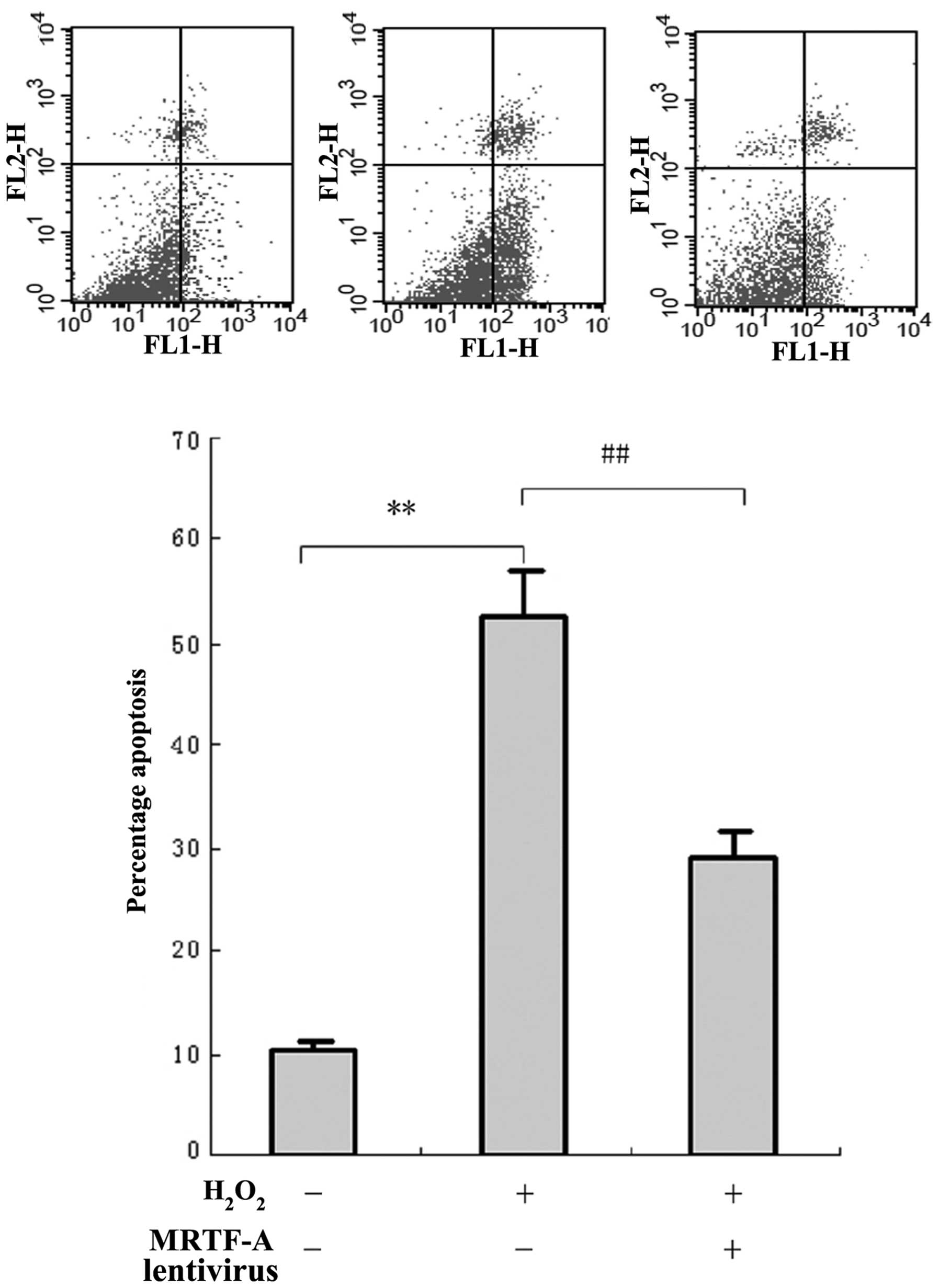
To investigate the resistance of MRTF-A to cellular
injury, BMSCs were exposed to H2O2 and cell
viability was evaluated by flow cytometric analysis of Annexin
V-PE/7ADD staining (Fig. 2A).
BMSCs displayed morphological changes typical of apoptosis and
necrosis after H2O2-mediated cellular injury.
However, the number of apoptotic and necrotic cells in the MRTF-A
group (27.9±5.2%) was significantly lower compared with that in the
control (52.6±6.9%; P<0.01). This indicated that MRTF-A
overexpression in the lentivirus-infected BMSCs partially prevented
the apoptosis induced by cellular injury.
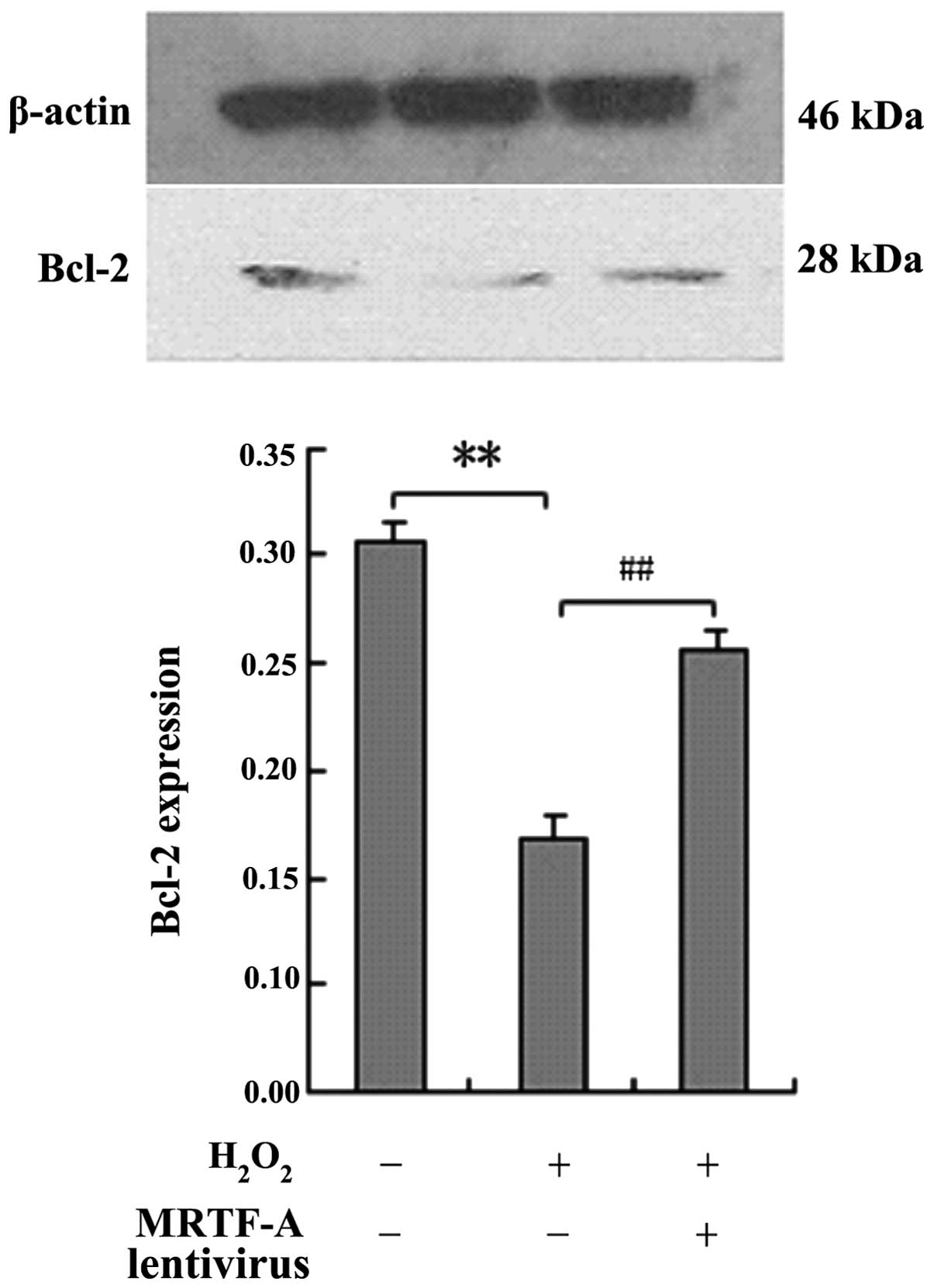
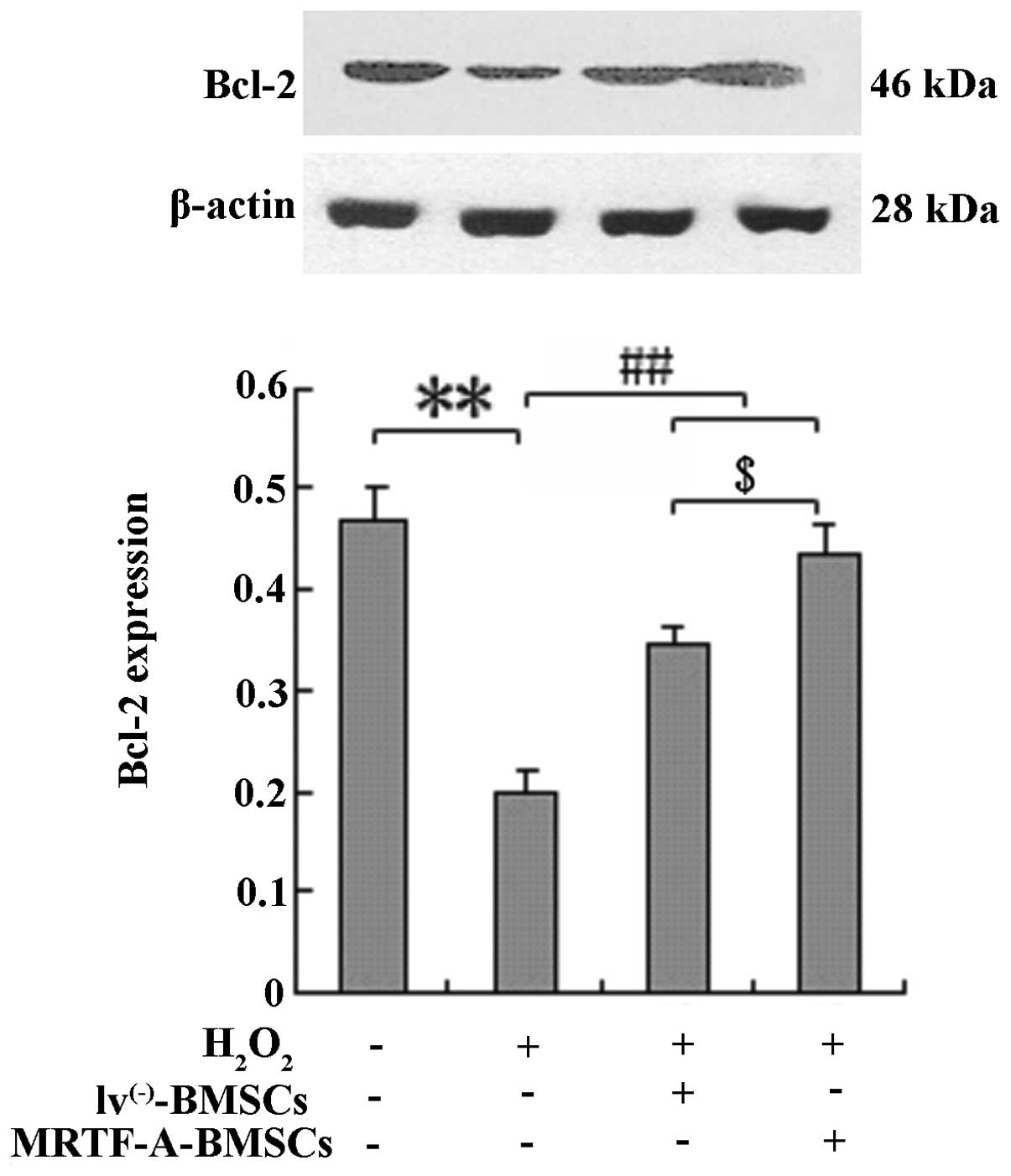
BMSCs were exposed to H2O2 and
Bcl-2 protein expression was analyzed by western blot analysis to
elucidate whether the protective effects of MRTF-A are mediated by
upregulated expression of the apoptosis-associated protein Bcl-2
(Fig. 3). Compared with the
control cells, MRTF-A-overexpressing BMSCs displayed enhanced Bcl-2
expression following treatment with H2O2
(P<0.01).
MRTF-A-overexpressing BMSCs exert
increased protective effects on cardiomyocyte viability in an ex
vivo model of cellular injury
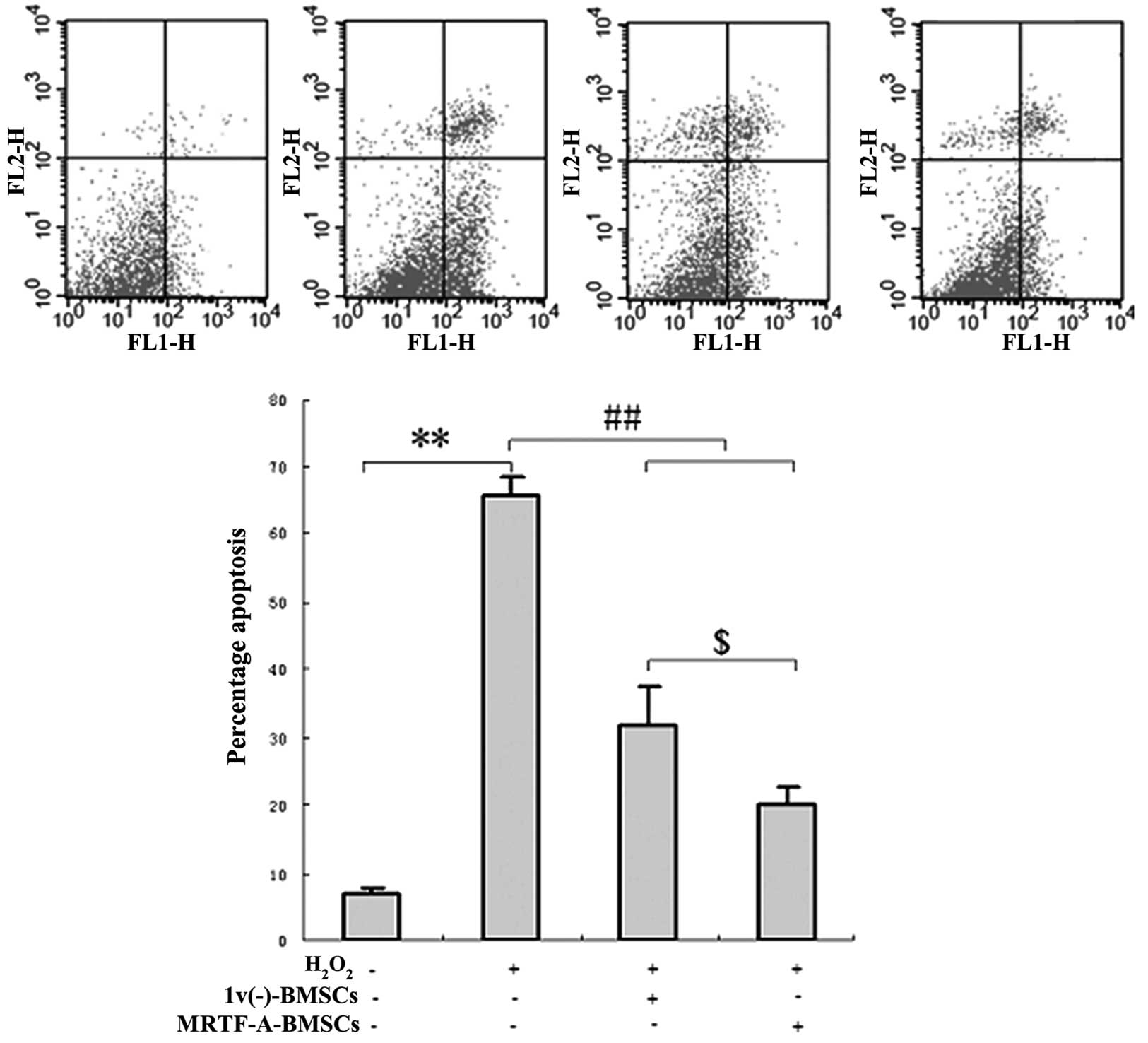
To determine the protective effects of
MRTF-A-modified BMSCs on cardiomyocyte viability in an ex
vivo model of cellular injury, primary cardiomyocytes were
co-cultured with lv(−)-BMSCs or with MRTF-A-BMSCs in
six-well plates. After five days, the cardiomyocytes were exposed
to hydrogen peroxide and analyzed by flow cytometry. After exposure
to hydrogen peroxide, the proportion of apoptotic cells was
significantly reduced in the primary cardiomyocytes co-cultured
with lv(−)-BMSCs (32.75±6.21%) or MRTF-A-BMSCs
(21.51±5.44%) compared with that of the primary cardiomyocytes
cultured in isolation (66.83±7.58; P<0.05) (Fig. 4).
Consistent with these results, after hydrogen
peroxide exposure, the expression of the apoptosis-associated
protein Bcl-2 was significantly increased in the primary
cardiomyocytes co-cultured with lv(−)-BMSCs or
MRTF-A-BMSCs compared with that detected in the primary
cardiomyocytes cultured in isolation (P<0.01; Fig. 5). Furthermore, the protein
expression of Bcl-2 in the primary cardiomyocytes co-cultured with
MRTF-A-BMSCs following hydrogen peroxide exposure was obviously
increased compared with that in cardiomyocytes co-cultured with
lv(−)-BMSCs (Fig.
5).
MRTF-A-overexpressing BMSCs prevent
cardiac damage after AMI
The effect of MRTF-A overexpression on the efficacy
of bone marrow stem cell-based therapy was evaluated in a rat model
of AMI. Immediately after ligation of the coronary artery,
6×106 MRTF-A-BMSCs were injected into the infarction
area. Animals were sacrificed at 1 week after cell transplantation
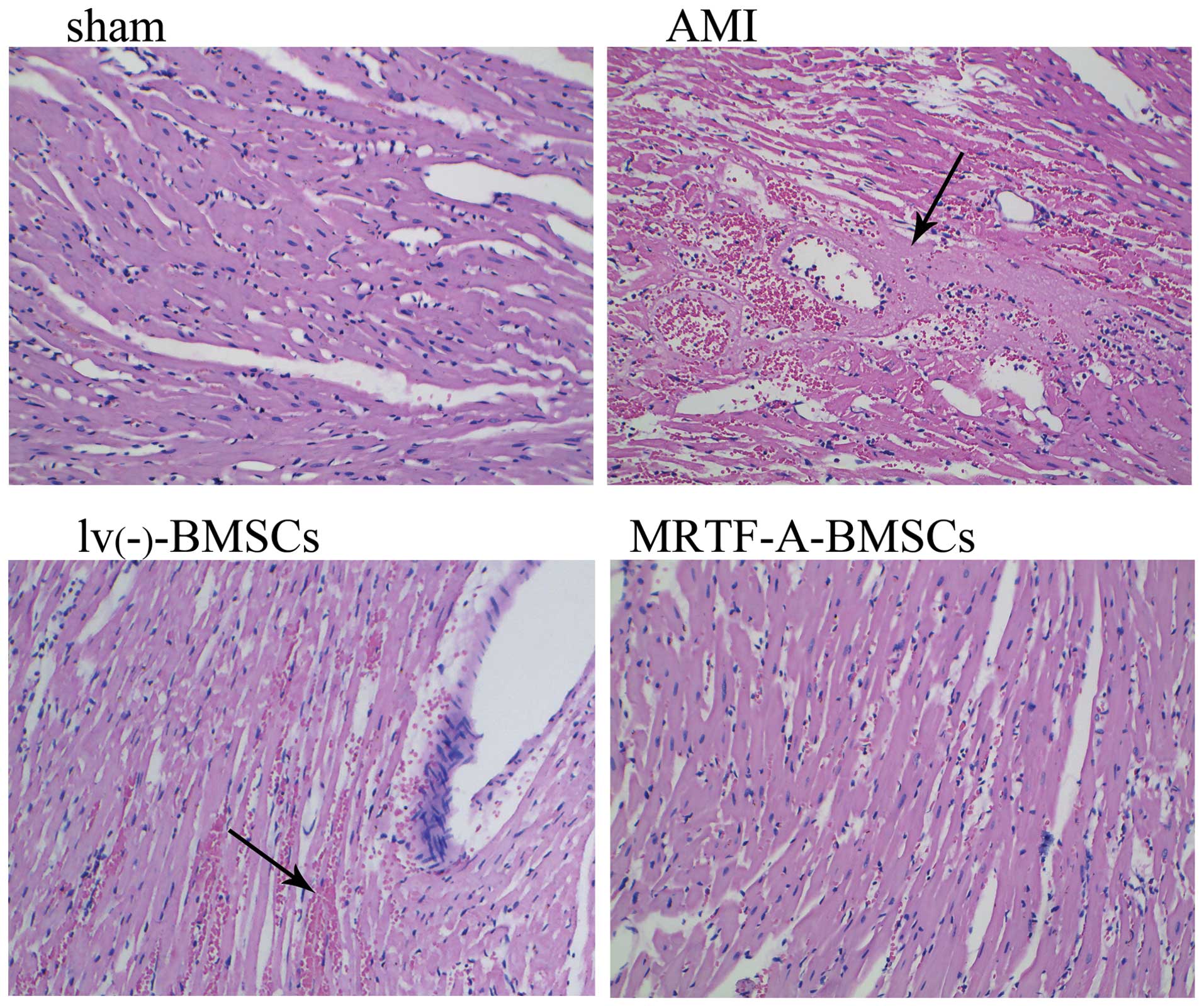
and the extent of myocardial injury was evaluated in
H&E-stained sections of myocardial tissue (Fig. 6). While no cardiac damage was
apparent in the sections from the sham group, extensive damage was
detected in those from the AMI group. The damaged myocardial tissue
displayed marked cardiomyocyte necrosis and a number of red cells.
The damage to cardiomyocytes induced by AMI was effectively
improved after transplantation of lv(−)-BMSCs or
MRTF-A-BMSCs, with obviously reduced necrosis and numbers of red
cells observed in the myocardial tissue sections, compared to those
from the AMI group. Of note, fewer red blood cells were observed in
the MRTF-A-BMSCs group compared with those in the
lv(−)-BMSCs group.
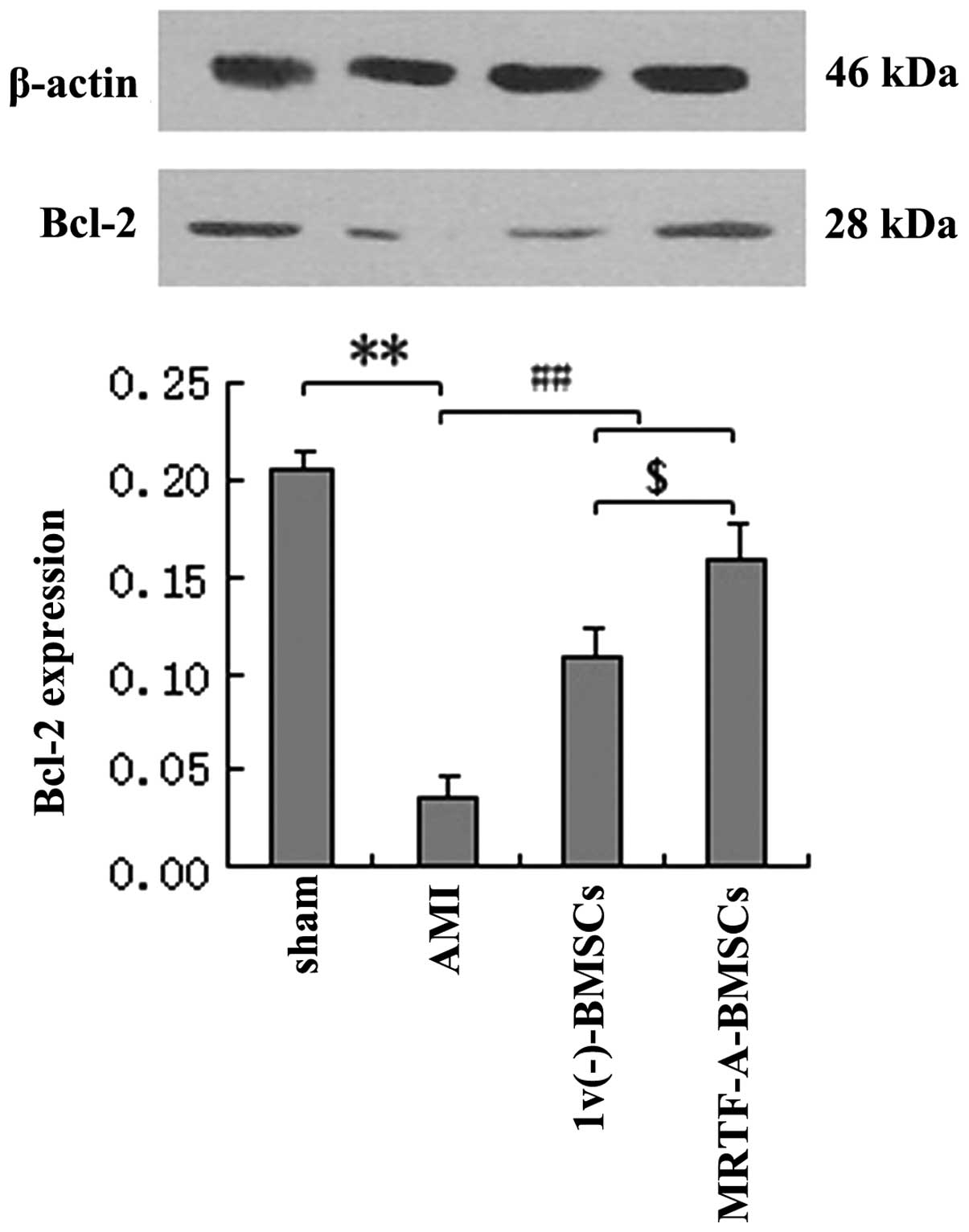
In addition, the expression levels of Bcl-2 protein
in myocardial tissues after AMI following transplantation of
lv(−)-BMSCs or MRTF-A-BMSCs were assessed. Bcl-2
expression in the AMI group was significantly decreased compared
with that in the sham group (P<0.01), while the levels of Bcl-2
protein in the lv(−)-BMSC and MRTF-A-BMSC groups were
significantly increased compared with those in the AMI group
(P<0.01) (Fig. 7). In
addition, the expression of Bcl-2 after MRTF-A-BMSC transplantation
was significantly greater than that after lv(−)-BMSC
transplantation.
Discussion
Although it is promising to use BMSCs in cell-based
therapy (CBT) for AMI, BMSC survival is poor following
transplantation into the heart following AMI. This may limit the
functional improvements associated with this approach (28) and therefore, strategies to enhance
BMSC survival are critically required for the clinical translation
of this CBT.
MRTF-A, as a co-factor of SRF that is required for
gene expression, has been implicated in myocardial survival
(20). The present study
investigated the ability of BMSC transplantation alone and in
combination with and MRTF-A overexpression to suppress
cardiomyocyte injury induced by hypoxia-ischemia.
Initially, the BMSCs were isolated from male
Sprague-Dawley rats and were subsequently cultured and expanded.
Immunophenotyping analysis of BMSC surface markers revealed that
these cells exhibited a surface antigen phenotype consistent with
those reported previously (CD90-positive; CD34-and CD45-negative)
(27). One of the significant
challenges in the clinical application of BMSC-based therapy is the
low survival of transplanted cells in vivo, particularly in
the heart following AMI (29).
The results of the present study showed that overexpression of
MRTF-A in BMSCs partially prevented hydrogen peroxide-induced
apoptosis in primary cardiomyocytes ex vivo and enhanced
Bcl-2 expression in BMSCs, suggesting that MRTF-A may enhance the
survival of BMSCs.
The modulation of apoptosis-associated gene
expression by MRTF-A may be involved in the enhanced survival and
protective effects of MRTF-A-BMSCs on cardiomyocytes. Upregulation
of Bcl-2 had an important role in mediating the beneficial effects
of CBT with MRTF-A-BMSCs. Western blot analysis showed that Bcl-2
was upregulated in MRTF-A- modified BMSCs. Furthermore,
H2O2-induced apoptosis was ameliorated in
primary cardiomyocytes co-cultured with MRTF-A-modified BMSCs,
indicating that the apoptosis-associated pathways were partially
inhibited by the reduction of Bcl-2 expression.
Further experiments showed that transplantation of
MRTF-A-BMSCs markedly improved these parameters, indicating that
MRTF-A enhances the efficacy of CBT with BMSCs. Lentivirus-mediated
transduction and stable expression of MRTF-A in BMSCs improved the
efficiency of CBT, demonstrating that the effect of MRTF-A is
independent of lentiviral transduction. Thus, the results of the
present study suggested that ex vivo modification of BMSCs
with signaling molecules, such as MRTF-A, can improve their
therapeutic potential in CBT.
In the present study, the effect of MRTF-A regarding
the extension of the survival of grafted BMSCs under ischemia was
identified in vitro and in vivo. It was confirmed
that overexpression of MRTF-A reduced BMSC death and apoptosis
induced by hydrogen peroxide exposure. Furthermore, in vivo
transplantation of MRTF-A-BMSCs enhanced the survival of myocardial
tissue in a rat model of AMI. These observations suggested that
overexpression of MRTF-A in BMSCs may be of significant value in
improving the efficacy of stem cell therapy following a broad range
of cardiac diseases.
Acknowledgments
The present study was supported by the Medical
Science and Technology Plan Projects of Zhejiang Province (no.
2012KYA149), and the Major Social Development Project of the
Science and Technology Agency of Zhejiang Province (no.
2012C03SA1E0003).
References
|
1
|
Sabbah HN and Sharov VG: Apoptosis in
heart failure. Prog Cardiovasc Dis. 40:549–562. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mill JG, Stefanon I, dos Santos L and
Baldo MP: Remodeling in the ischemic heart: the stepwise
progression for heart failure. Braz J Med Biol Res. 44:890–898.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takemura G and Fujiwara H: Role of
apoptosis in remodeling after myocardial infarction. Pharmacol
Ther. 104:1–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeong H, Yim HW, Cho Y, Park HJ, Jeong S,
Kim HB, Hong W and Kim H: The effect of rigorous study design in
the research of autologous bone marrow-derived mononuclear cell
transfer in patients with acute myocardial infarction. Stem Cell
Res Ther. 4:822013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fisher SA, Dorée C, Brunskill SJ, Mathur A
and Martin-Rendon E: Bone marrow stem cell treatment for ischemic
heart disease in patients with no option of revascularization: A
systematic review and meta-analysis. PLoS One. 8:e646692013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gnecchi M, He H, Liang OD, et al:
Paracrine action accounts for marked protection of ischemic heart
by Akt-modified mesenchymal stem cells. Nat Med. 11:367–368. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Behfar A, Zingman LV, Hodgson DM, Rauzier
J-M, Kane GC, Terzic A and Pucéat M: Stem cell differentiation
requires a paracrine pathway in the heart. FASEB J. 16:1558–1566.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams AR, Suncion VY, McCall F, Guerra
D, Mather J, Zambrano JP, Heldman AW and Hare JM: Durable scar size
reduction due to allogeneic mesenchymal stem cell therapy regulates
whole-chamber remodeling. J Am Heart Assoc. 2:e0001402013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forest VF, Tirouvanziam AM, Perigaud C,
Fernandes S, Fusellier MS, Desfontis JC, Toquet CS, Heymann MF,
Crochet DP and Lemarchand PF: Cell distribution after intracoronary
bone marrow stem cell delivery in damaged and undamaged myocardium:
Implications for clinical trials. Stem Cell Res Ther. 1:42010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Ma N, Ong LL, et al: Bcl-2
engineered MSCs inhibited apoptosis and improved heart function.
Stem Cells. 25:2118–2127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das H, George JC, Joseph M, et al: Stem
cell therapy with overexpressed VEGF and PDGF genes improves
cardiac function in a rat infarct model. PLoS One. 4:e73252009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pipes GC, Creemers EE and Olson EN: The
myocardin family of transcriptional coactivators: Versatile
regulators of cell growth, migration, and myogenesis. Genes Dev.
20:1545–1556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miano JM: Serum response factor: toggling
between disparate programs of gene expression. J Mol Cell Cardiol.
35:577–593. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Chang PS, Wang Z, Sutherland L,
Richardson JA, Small E, Krieg PA and Olson EN: Activation of
cardiac gene expression by myocardin, a transcriptional cofactor
for serum response factor. Cell. 105:851–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mikhailov AT and Torrado M: In search of
novel targets for heart disease: Myocardin and myocardin-related
transcriptional cofactors. Biochem Res Int. 2012:9737232012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao XL, Hu XM, Hu JQ and Zheng WX:
Myocardin-related transcription factor-A promoting neuronal
survival against apoptosis induced by hypoxia/ischemia. Brain Res.
1385:263–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Latif N, Sarathchandra P, Chester AH and
Yacoub MH: Expression of smooth muscle cell markers and
co-activators in calcified aortic valves. Eur Heart J.
36:1335–1345. 2015. View Article : Google Scholar
|
|
19
|
Liao XH, Wang N, Liu QX, Qin T, Cao B, Cao
DS and Zhang TC: Myocardin-related transcription factor-A induces
cardiomyocyte hypertrophy. IUBMB Life. 63:54–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Small EM, Thatcher JE, Sutherland LB,
Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara
K and Olson EN: Myocardin-related transcription factor-a controls
myofibroblast activation and fibrosis in response to myocardial
infarction. Circ Res. 107:294–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parmacek MS: Myocardin-related
transcription factor-A: mending a broken heart. Circ Res.
107:168–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo J, Li HZ, Wang LC, Zhang WH, Li GW,
Xing WJ, Wang R and Xu CQ: Increased expression of calcium-sensing
receptors in atherosclerosis confers hypersensitivity to acute
myocardial infarction in rats. Mol Cell Biochem. 366:345–354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao SL, Zhang YJ, Li MH, Zhang XL and
Chen SL: Mesenchymal stem cells with overexpression of midkine
enhance cell survival and attenuate cardiac dysfunction in a rat
model of myocardial infarction. Stem Cell Res Ther. 5:372014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sacherer M, Sedej S, Wakuła P, et al:
CONTICA investigators: JTV519 (K201) reduces sarcoplasmic reticulum
Ca2+ leak and improves diastolic function in
vitro in murine and human non-failing myocardium. Br J Pharmacol.
167:493–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai BZ, Meng FY, Zhu SL, et al: Arsenic
trioxide induces the apoptosis in bone marrow mesenchymal stem
cells by intracellular calcium signal and caspase-3 pathways.
Toxicol Lett. 193:173–178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao YS, Ding H, Xie XT and Zhang CQ:
Osteogenic induction protects rat bone marrowederived mesenchymal
stem cells against hypoxia-induced apoptosis in vitro. J Surg Res.
184:873–879. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ning J, Li C, Li H and Chang J: Bone
marrow mesenchymal stem cells differentiate into urothelial cells
and the implications for reconstructing urinary bladder mucosa.
Cytotechnology. 63:531–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gnecchi M, Danieli P and Cervio E:
Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol.
57:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clifford D, Fisher S, Brunskill S, Doree
C, Mathur A, Watt S and Martin-Rendon E: Stem cell treatment for
acute myocardial infarction. Cochrane Database Syst Rev.
2:CD0065362012.PubMed/NCBI
|