Introduction
Mesenchymal stem cells (MSCs) are multipotent stem
cells derived from the mesoderm, and possess potent proliferative
potential, and the capacity for self-renewal and multilineage
differentiation (1–3). MSCs grown in vitro are
capable of differentiating into a number of cell types including
osteoblasts, chondrocytes and adipocytes (4,5).
MSCs are of great interest to researchers for their potential
applications in the field of regenerative medicine and tissue
engineering (6). Prolonged
passaging of the in vitro culture environment is a
prerequisite for acquiring a suitable number of MSCs for use in
cell therapy. The process may, however, lead to adverse effects on
the physiological properties of MSCs, such as stemness,
proliferation and differentiation potency (6). In addition, during long-term in
vitro culture, MSCs easily develop cellular senescence,
consequently further limiting the number of cell doublings
(7). It is therefore important to
understand the mechanisms of the senescence of MSCs to reverse or
prevent the aging processes in these cells.
In normal somatic cells, each cell division is
associated with the shortening of telomeric DNA at the end of each
chromosome, which leads to the cessation of replication, and the
eventual arrest of cell growth and proliferation (8–10).
The activation of telomerase is responsible for extending telomere
length at the end of chromosomes, which helps prevent telomere
erosion and inhibit replicative senescence in vitro
(11,12). Human telomerase reverse
transcriptase (hTERT) is a catalytic subunit of human telomerase
(13,14), which provides the reverse
transcriptase activity needed to maintain the length of the
telomere (15). It has been shown
that overexpression of hTERT increases the cell lifespan of
ameloblastoma cells (16), human
fibroblasts (17),
adipose-derived stem cells (18)
and endothelial cells (19) in
vitro. Several studies have demonstrated that the modification
of human MSCs with the hTERT gene generates cells with an improved
ability for proliferation and cell renewal that retain their
potential to differentiate into osteocytes, adipocytes,
chondrocytes and gingival epithelial cell lines (20–25). However, the detailed role and the
underlying molecular mechanisms of action of hTERT in MSCs remain
largely unknown. Therefore, in the present study, we examined the
effects of hTERT expression on the proliferation, apoptosis and
senescence of rat MSCs, as well as the underlying molecular
mechanisms.
Materials and methods
Isolation and culture of MSCs
The MSCs used in the present study were obtained
from 17- to 18-month old male Sprague Dawley rats (Tonghua
Laboratory Animal Center, Beijing, China). The isolation and
culture of the MSCs were performed as previously described
(26,27). In brief, the MSCs were isolated
from the bone marrow of the femurs and tibias of SD rats by
inserting a 21-gauge needle into the shaft of the bone and flushing
it with α-modified Eagle's medium (α-MEM; Invitrogen, Carlsbad, CA,
USA) and cultured for 1–2 days. Non-adherent cells were then
removed, and adherent cells representing MSCs were washed twice
with phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO,
USA). The cells were then incubated for 7–10 days in DMEM medium
supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT,
USA) to reach confluence and were extensively propagated for
further experiments. The culture medium was replaced every 3–4 days
and the cells were passaged at 70–80% confluence. The morphology of
MSCs at passages 3, 7, 9 and 12 was observed under an IX51 inverted
microscope (Olympus Corp., Tokyo, Japan). MSCs at passage 3 were
harvested and resuspended in culture medium at a density of
1×106 cells/ml. The surface markers of MCS were examined
using flow cytometry and 4 antibodies against rat surface antigens
(CD29, CD45, CD71 and CD90; BD Biosciences, San Jose, CA, USA).
Senescence-associated β-galactosidase
(SA-β-gal) staining
The cells (4×104) were seeded into 6-well
plates. After 48 h, the cells were washed twice with PBS and fixed
for 10 min. After removing the fixative, the cells were washed
twice with PBS and stained with the staining solution provided by
the β-Galactosidase Reporter Gene Staining kit (Sigma-Aldrich) for
12 h at 37°C, and the percentage of β-galactosidase-positive cells
was then determined by randomly counting 5 fields on a phase
contrast microscope (Olympus Corp.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the MSCs using TRIzol
reagent (Invitrogen). The RNA was reverse-transcribed into cDNA
using a PrimeScript™ RT Reagent kit according to the manufacturer's
instructions (Takara Bio, Dalian, China). Quantitative PCR (qPCR)
was carried out using SYBR-Green Real-Time PCR Master Mix (Toyobo
Co., Ltd., Osaka, Japan) and qPCR amplification equipment. The PCR
reactions were prepared in duplicate and heated to 95°C for 5 min
followed by 40 cycles of denaturation at 95°C for 15 sec, annealing
at 54°C for 30 sec, and extension at 72°C for 30 sec. Standard
curves (cycle threshold values vs. template concentration) were
prepared for each target gene and the endogenous reference (GAPDH)
in each sample. The quantification of the samples was carried out
using LightCycler software version 3.5 (Roche, Mannheim, Germany)
with the 2−ΔΔCT method. The sequences of the hTERT and
GAPDH primers were consistent with those of our previous study
(28): hTERT forward,
5′-GGAGCAAGTTGCAAAGCATTG-3′ and reverse,
5′-TCCCACGACGTAGTACATGTT-3′; GAPDH forward, 5′-TG
TGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′.
Detection of telomerase activity and
measurement of telomere length
Telomerase activity was determined using the
telomeric repeat amplification protocol (TRAP) with the TeloTAGGG
PCR enzyme-linked immunosorbent assay (ELISA) kit≈(Roche) according
to the manufacturer's instructions.
Genomic DNA from the cultured cells was isolated
using the High Pure PCR Template Preparation kit (Roche) and
telomere length was estimated using the TeloTAGGG Telomere Length
assay kit (Roche) as previously described (28).
Cell proliferation assay
The MSCs were seeded in a 96-well plate at a density
of 1×04 cells/well. Cell proliferation was determined at
the indicated time points using the Cell Counting kit-8 (CCK-8;
Dojindo Laboratories, Kumamoto, Japan) according to the
manufacturer's instructions. The absorbance was read using an ELISA
plate reader (Thermo Labsystems, Vantaa, Finland) at 450 nm.
Plasmid construction and
transfection
The plasmid, pGCsilencer-siRNA-hTERT (pSi-hTERT),
encoding siRNA specific to hTERT was constructed as previously
described (28). The plasmid
pCI-neo-hTERT (phTERT) containing the hTERT coding region was
kindly provided by Professor Ximin Guo (Academy of Military Medical
Sciences, Beijing, China). The plasmids, pSi-hTERT and phTERT, were
transiently transfected into the MSCs at passage 9 (late passage)
using Lipofectamine™ 2000 reagent (Invitrogen) according to the
manufacturer's instructions. The transfection efficiency was
evaluated by RT-qPCR and western blot analysis following
transfection with various plasmids. Untransfected cells were used
as controls in this experiment and the following experiments.
Western blot analysis
The cells were harvested by centrifugation, and the
cell pellet was resuspended in lysis buffer (Sigma-Aldrich)
containing proteinase inhibitors and incubated on ice for 30 min.
Following centrifugation at 14,000 × g for 30 min at 4°C, the
supernatant containing total cell extract was collected, and the
concentrations of total cellular protein were determined using the
Bradford protein assay (Bio-Rad Laboratories, Marnes-la-Coquette,
France). Equal amounts of protein (20 µg) were separated by
10% gradient sodium dodecyl sulfate-polyacrylamide electrophoresis
(SDS-PAGE) gels and transferred onto polyvinylidene fluoride (PVDF)
membranes (Millipore, Billerica, CA, USA) using the Trans-Blot
Turbo Transfer system (Bio-Rad Laboratories). The membranes were
incubated in blocking buffer (TBST containing 5% skim milk) for 1 h
at room temperature to block non-specific protein binding and then
incubated with the following primary antibodies overnight at −4°C:
anti-hTERT (1:2,000; Cat. no. Sc-7204; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), anti-GAPDH (1:5,000; Cat. no. 3683),
anti-PI3K (1:2,000; Cat. no. 4249), anti-phosphorylated (p)-PI3K
(Tyr458, 1:1,500; Cat. no. 4228), anti-AKT (1:1,000; Cat. no. 2920)
and anti-p-AKT (Ser473; 1:500; Cat. no. 4000; all from Cell
Signaling Technology - New England Biolabs, Hitchin, UK). Following
3 washes with TBST, the membranes were incubated with goat
anti-mouse IgG (1:5,000; Cat. no. Sc-2005) or goat anti-rabbit
(1:5,000; Cat. no. Sc-2004; both from Santa Cruz Biotechnology,
Inc.) horseradish peroxidase (HRP) diluted in blocking buffer for 1
h. Antibody binding was visualized using an enhanced
chemiluminescence (ECL) western blotting detection system (Amersham
Biosciences, Piscataway, NJ, USA). GAPDH was used for the
normalization of protein loading and protein expression was
measured by quantifying the density of immunoblots adjusted to
GAPDH using image analysis software 3.1 Image J (Bio-Rad
Laboratories).
Cell cycle analysis
The cells (1×105 cells/10-cm dish in
diameter) were harvested by trypsinization, fixed with a
fluorescence-activated cell sorting (FACS) lysing solution (BD
Biosciences) and permeabilized with a FACS permeabilization
solution (BD Biosciences). After being washed with PBS, the cells
were resuspended in staining solution [containing 200 µg/ml RNase A
and 20 µg/ml propidium iodide staining solution (Merck,
Whitehouse Station, NJ, USA)], incubated for 30 min, and analyzed
for their DNA content using a FACScan flow cytometer (BD
Biosciences).
Apoptosis assay
The detection of apoptotic cells was performed on
cytospin preparations using the TUNEL assay according to the
manufacturer's instructions (In Situ Cell Death Detection
kit, AP; Roche Molecular Biochemicals, Mannheim, Germany) after the
MSCs at passage 3 were treated with the indicated plasmid. The
number of apoptotic cells was counted under a IX51 inverted
microscope (Olympus Corp.) and averaged from 3 visual fields.
Treatment with LY294002
LY294002, a PI3K inhibitor, was obtained from
Sigma-Aldrich. MSCs at passage 9 (late passage; 5×104
cells/well) were seeded into each well of a 24-well plate.
Subsequently, 0.6 µM LY294002 was added and the cells were
cultured for 48 h in DMEM (Invitrogen) supplemented with FBS at
37°C in a humidified atmosphere containing 5% CO2. PI3K,
p-PI3K, AKT and p-AKT protein expression was then determined by
western blot analysis.
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Comparisons between 2 groups were made using the Student's
t-test. Statistical differences among more than 2 groups were
assessed by one-way analysis of variance (ANOVA). GraphPad Prism
software version 6.01 (GraphPad Software, San Diego, CA, USA) was
used for statistical analyses. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Characterization of MSCs at different
passages
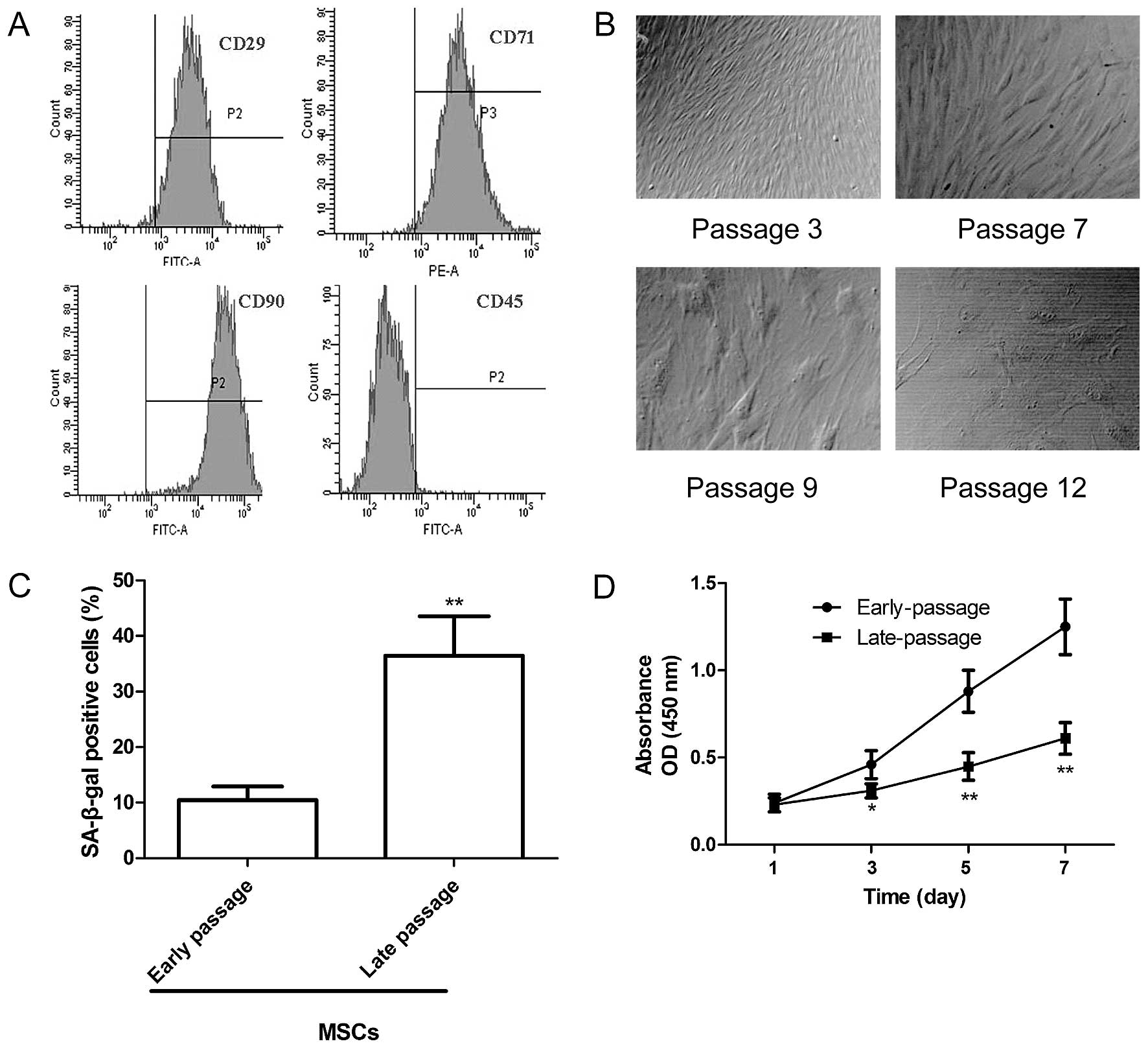
High-purity MSCs were isolated and obtained by
density gradient centrifugation, adherence selection and the
monoclonal culture system. The immunophenotype of the MSCs obtained
from the Sprague-Dawley rats was assessed by flow cytometry using
specific cell surface antigens. The MSCs were positive for the
expression of the MSCs markers CD29, CD71 and CD90 but negative for
CD45 expression (Fig. 1A). In
addition, the morphological characteristics of the MSCs at
different passages were observed under an inverted microscope. As
shown in Fig. 1B, at passage 3,
the cells had a whirlpool or fish-like shape. At passage 7, the
MSCs became larger in size with an enlarged rough endoplasmic
reticulum, microvilli desquamate, with many secondary lysosomes,
suggesting that the MSCs entered senescence. At passage 9, large
parts of the microvilli disappeared and the enlargement of the
rough endoplasmic reticulum was more obvious. At passage 12, the
MSCs had lost their normal shape, and karyopyknosis and
heterochromatin were observed. To ascertain alterations in the
senescence of the MSCs at different passages, SA-β-gal activity was
analyzed. Compared with the early-passage MSCs (passage 3), the
percentage of SA-β-gal-positive cells and the staining intensity
increased significantly in the late-passage MSCs (passage 9;
Fig. 1C). In addition, we
assessed the proliferation of MSCs at different passages by CCK-8
assay. We found that the proliferation of the late-passage MSCs
decreased significantly compared with that of the early-passage
MSCs (Fig. 1D). These data
indicate an increase in the senescene of MSCs at a late
passage.
Senescence-associated alterations in
hTERT expression, and in telomerase activity and telomere length in
MSCs
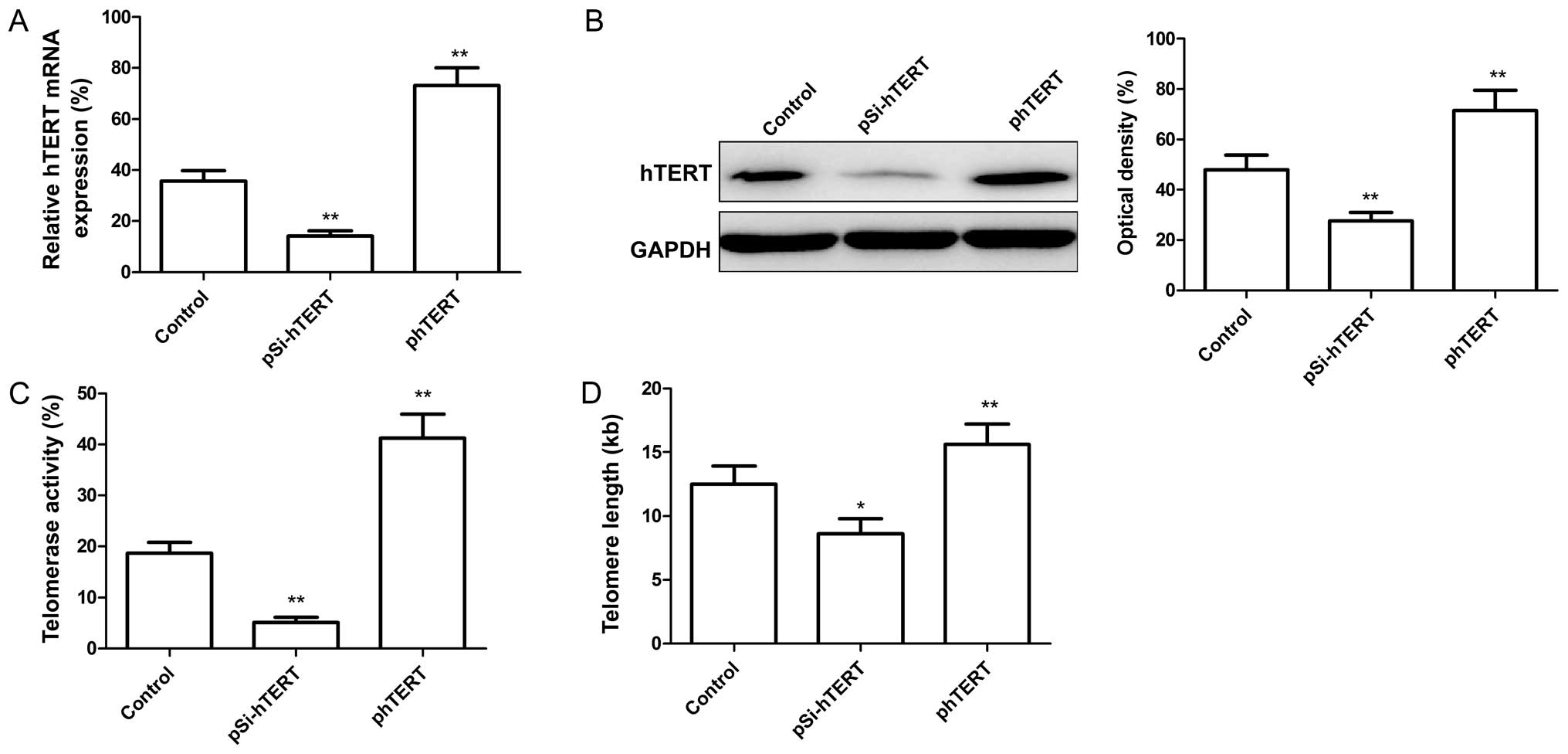
To determine whether hTERT expression is altered in
MSCs at different passages, we measured the hTERT expression levels
by RT-qPCR and western blot analysis. Our results revealed that not
only was the mRNA expression level of hTERT in the late-passage
MSCs lower than that in the early-passage MSCs (Fig. 2A), but the protein expression
level of hTERT was also significantly decreased in the late-passage
MSCs compared to the early-passage MSCs (Fig. 2B). In addition, we evaluated
telomerase activity and telomere length in the early- and
late-passage MSCs. We discovered that telomerase activity (Fig. 2C) and telomere length (Fig. 2D) were significantly decreased in
the late-passage MSCs compared with the early-passage MSCs.
hTERT modulates telomere length and
telomerase activity in MSCs
It has been demonstrated that telomere attrition
triggers telomere dysfunction, induces DNA damage and,
consequently, cellular senescence (29). We wished to determine whether
hTERT expression regulates telomerase activity. For this purpose,
the MSCs at passage 9 were transfected with the plasmids, pSi-hTERT
(for the downregulation of hTERT), and phTERT (for the upregulation
of hTERT). The hTERT mRNA and protein expression levels were then
measured by RT-qPCR and western blot analysis, respectively. Our
results revealed that the hTERT mRNA and protein expression levels
decreased significantly following transfection with pSi-hTERT
(Fig. 3A and B), whereas these
levels significantly increased folloiwng transfection with phTERT
(Fig. 3A and B). In addition,
telomerase activity and telomere length in the MSCs were
determined. We found that the downregulation of hTERT significantly
reduced telomerase activity and telomere length in the MSCs
(Fig. 3C and D), whereas the
upregulation of hTERT significantly increased telomerase activity
and telomere length in the MSCs (Fig.
3C and D).
hTERT modulates the senescence,
proliferation, cell cycle and apoptosis of MSCs
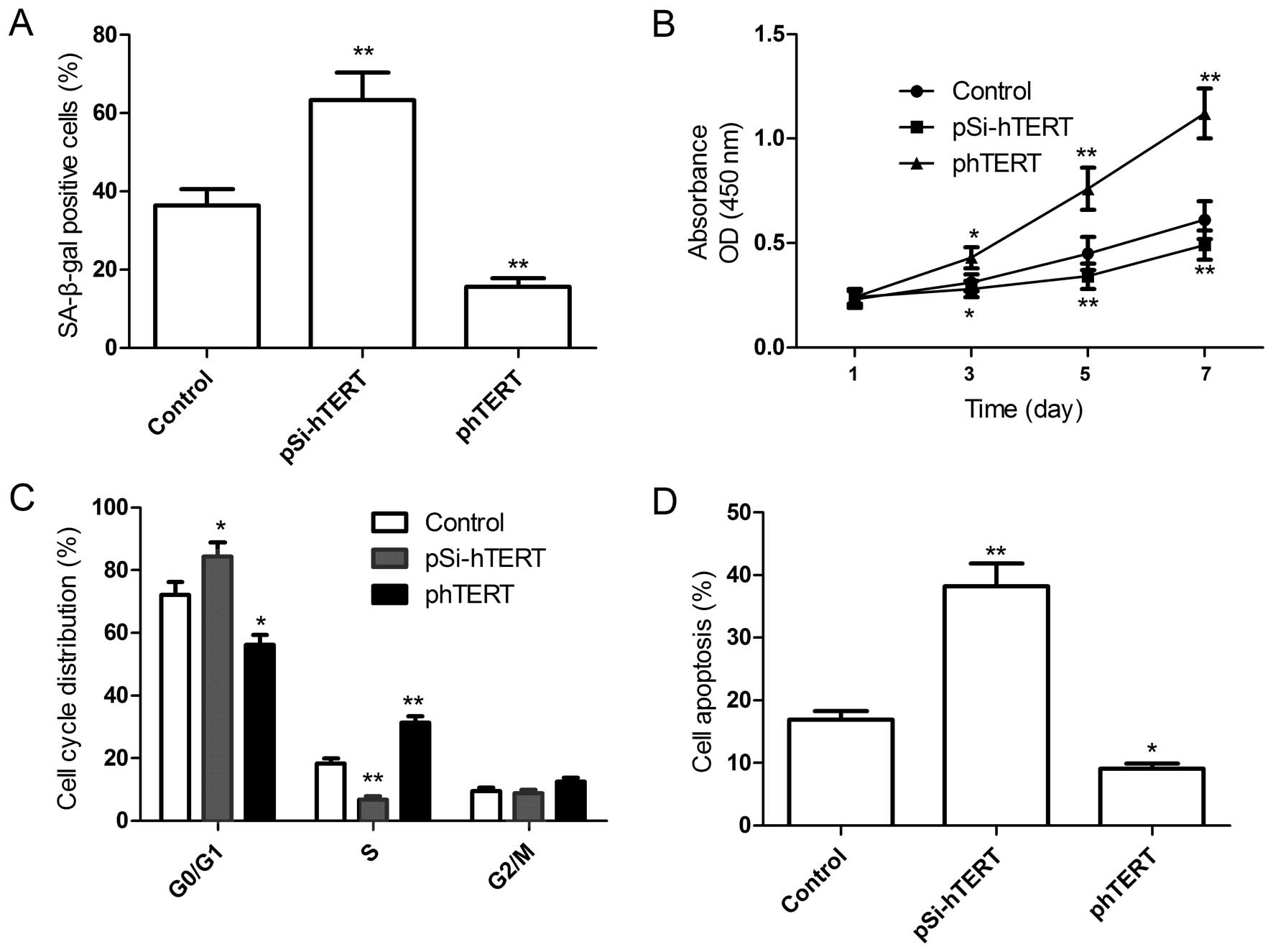
To further determine the role of hTERT in regulating
the senescence, proliferation, cell cycle and apoptosis of MSCs,
the plasmids, pSi-hTERT or phTERT, were transfected into the MSCs
at passage 9 (late passage), and the senescence, proliferation,
cell cycle and apoptosis of the MSCs were then determined. Compared
with the control group, the downregulation of hTERT induced
cellular senescence (Fig. 4A;
shown by an increase in the number of SA-β-gal-positive cells),
decreased cell proliferation (Fig.
4B) and the percentage of cells in the S phase (Fig. 4C) and increased the percentage of
apoptotic MSCs (Fig. 4D).
However, the upregulation of hTERT significantly decreased the
number of senescent cells (Fig.
4A), increased cell proliferation (Fig. 4B) and the percentage of cells in
the S phase (Fig. 4C) and
decreased the percentage of apoptotic late-passage MSCs (Fig. 4D). Taken together, these results
suggest that hTERT overexpression prevents the replicative
senescence of MSCs.
hTERT modulates the activation of the
PI3K/AKT signaling pathway in MSCs
It has been demonstrated that the PI3K/AKT pathway
plays a role in stem cell self-renewal, maintenance and
differentiation (30). In a
recent study of ours, we demonstrated that the downregulation of
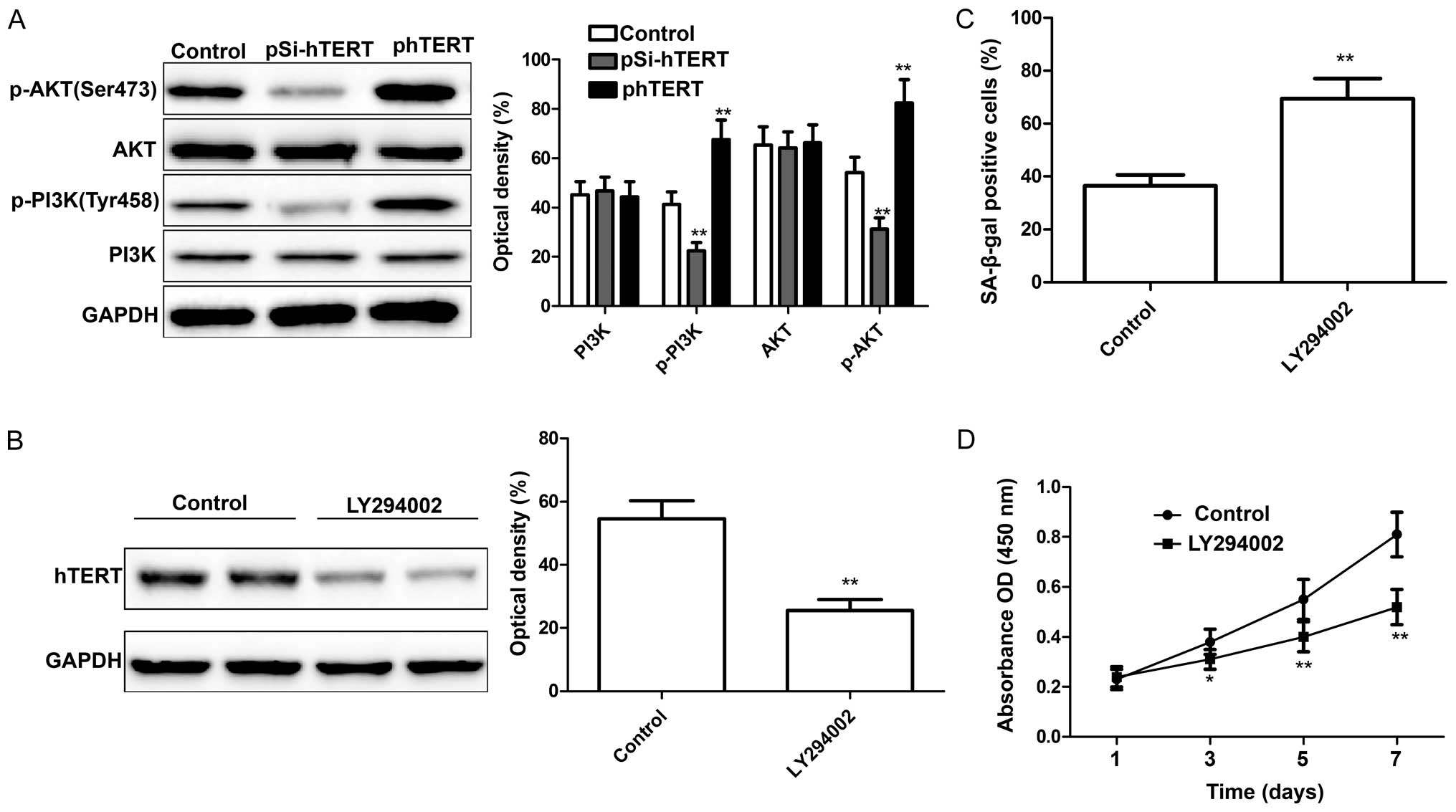
hTERT inhibited the activation of the PI3K/AKT pathway (28). In the present study, we wished to
determine whether hTERT modulates the activation of the PI3K/AKT
pathway in MSCs. At 24 h following transfection with pSi-hTERT or
phTERT, the expression levels of PI3K, p-PI3K, AKT and p-AKT were
measured by western blot analysis. We found that the downregulation
of hTERT significantly decreased p-PI3K and p-AKT expression in the
MSCs, whereas the upregulation of hTERT significantly increased
p-PI3K and p-AKT expression in the MSCs; no changes were observed
in the levels of total PI3K and AKT in either group (Fig. 5A). To further determine whether
the actiation of the PI3K/AKT signaling pathway is associated with
the hTERT expression level in MSCs, the MSCs were treated with
LY294002 (Sigma-Aldrich), a PI3K inhibitor, and the hTERT
expression levels were then measured by western blot analysis. The
results revealed that LY294002 significantly inhibited hTERT
expression (Fig. 5B). In
addition, the proliferation and senescence of the MSCs were
determined following treatment with LY294002. The results revealed
that treatment with LY294002 led to a significant increase in
cellular senescence (Fig. 5C; as
shown by an increase in the number of SA-β-gal-positive cells) and
a decreased in the proliferation of MSCs (Fig. 5D). These findings suggest that
hTERT mediates the senescence of MSCs through the PI3K/AKT
signaling pathway.
Discussion
MSCs are one of the most promising resources for
cell and gene therapy for osteogenesis imperfecta, the tissue
engineering of cartilage and bone and post-transplant immune
reconstitution due to their versatile plasticity in vitro
and in vivo (32).
However, their clinical application and basic research is limited
as primary MSCs have a limited lifespan. It is crucial to
understand the mechanisms regulating cellular senescence, so that
ways can be found to extend the lifespan of MSCs.
Cellular senescence is a complex process that, thus
far, remains largely unknown. Previous studies have demonstrated
that telomere attrition triggers telomere dysfunction, and induces
DNA damage, leading to cellular senescence (29,33). Telomere attrition may thus be
closely related to impaired telomerase activity and telomere length
as the course of the telomeric DNA elongation is dependent on
telomerase catalysis and may results in a reduction in telomerase
activity in MSC (34). It has
previously been demonstrated that if telomere shortening is not
balanced by elongation, it leads to cell death, cellular senescence
or abnormal cell proliferation (35). In the present study, we found that
the long-term in vitro culture of MSCs led to cellular
senescence, and telomerase activity and telomere length were both
decreased in the late-passage MSCs compared with the early-passage
MSCs. These findings suggested that telomeres play a key role in
the senescence of long-term cultured MSCs.
It has been demonstrated that the ectopic expression
of hTERT, the catalytic component of telomerase, leads to telomere
elongation and extends the lifespan of a number of cell types
(11–13). The upregulation of hTERT in MSCs
has been shown to enhance their stem-like properties without
affecting their potential to differentiate into osteocytes,
adipocytes and chondrocytes (20–25). Consistent with these results, in
the present study, we found that the downregulation of hTERT by
siRNA markedly decreased telomere length and telomerase activity in
the MSCs, whereas the overexpression of hTERT increased telomere
length and telomerase activity in the MSCs. The downregulation of
hTERT led to a decrease in the proliferation and an increase in the
number of senescent and apoptotic MSCs, whereas the upregulation of
hTERT led to an increase in cell proliferation and a decrease in
the number of senescent and apoptotic MSCs.
It was well known that AKT (also known as PKB), a
serine/threonine protein kinase, plays a central role in regulating
cell survival, metabolism and protein synthesis through the
phosphorylation of its numerous substrates (36). Previous studies have shown that
AKT plays a role in the self-renewal, maintenance and
differentiation of several types of stem cells, including
pluripotent stem cells (31),
neuronal stem cells (37) and
epithelial stem cells (38). In
addition, AKT is able to phosphorylate hTERT and activate
telomerase activity (39). Two
putative AKT phosphorylation sites within hTERT (serine residues at
227 and 824) have been identified. It has been reported that AKT
enhances telomerase activity through the phosphorylation and
nuclear translocation of hTERT (40,41). In the present study, we found that
the downregulation of hTERT significantly inhibited p-PI3K and
p-AKT expression in the MSCs, whereas the upregulation of hTERT
significantly increased p-PI3K and p-AKT expression in the MSCs.
This pathway was blocked with LY294002 (PI3K inhibitor), which led
to a decrease in hTERT expression, increase in cellular senescence
and a decrease in the proliferation of MSCs. Our findings reveal a
previously unknown regulatory mechanism of hTERT; namely that hTERT
mediates the senescence of MSC through the PI3K/AKT signaling
pathway.
In conclusion, in the present study, we demonstrated
that hTERT expression, telomerase activity and telomere length were
decreased in late-passage MSCs compared to early-passage MSCs. The
upregulation of hTERT in the late-passage MSCs prevented cellular
senescence and apoptosis, increased cell proliferation, and
increased telomerase activity and telomere length. We also
demonstrated that the upregulation of hTERT activates the PI3K/AKT
signaling pathway. and that the inhibition of the activation of the
PI3K/AKT signaling pathway with LY294002 led to a decrease in hTERT
expression, an increase in cellular senescence and a decrease in
the proliferation of MSCs. These findings suggest that hTERT
mediates the senescence of MSCs through the PI3K/AKT signaling
pathway.
Acknowledgments
The present study was supported by the Scientific
Research Project of Jilin Provincial Bureau of Health (2013ZC005;
2013Z028); the Jilin Provincial Science and Technology Projects
(20130101130JC); the Norman Bethune Program of Jilin University
(2012204); and The Project-sponsored by SRF for ROCS, SEM, and The
Project-Basic Study by Jilin University.
References
|
1
|
Bayati V, Hashemitabar M, Gazor R,
Nejatbakhsh R and Bijannejad D: Expression of surface markers and
myogenic potential of rat bone marrow- and adipose-derived stem
cells: a comparative study. Anat Cell Biol. 46:113–121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakitani S, Saito T and Caplan AI:
Myogenic cells derived from rat bone marrow mesenchymal stem cells
exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Javazon EH, Colter DC, Schwarz EJ and
Prockop DJ: Rat marrow stromal cells are more sensitive to plating
density and expand more rapidly from single-cell-derived colonies
than human marrow stromal cells. Stem Cells. 19:219–225. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beyer Nardi N and da Silva Meirelles L:
Mesenchymal stem cells: isolation, in vitro expansion and
characterization. Handb Exp Pharmacol. 174:249–282. 2006.
View Article : Google Scholar
|
|
5
|
Brighton CT and Hunt RM: Early
histological and ultrastructural changes in medullary fracture
callus. J Bone Joint Surg Am. 73:832–847. 1991.PubMed/NCBI
|
|
6
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J and Pei M: Cell senescence: A
challenge in cartilage engineering and regeneration. Tissue Eng
Part B Rev. 18:270–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allsopp RC, Vaziri H, Patterson C,
Goldstein S, Younglai EV, Futcher AB, Greider CW and Harley CB:
Telomere length predicts replicative capacity of human fibroblasts.
Proc Natl Acad Sci USA. 89:10114–10118. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Broccoli D, Young JW and de Lange T:
Telomerase activity in normal and malignant hematopoietic cells.
Proc Natl Acad Sci USA. 92:9082–9086. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Counter CM, Avilion AA, LeFeuvre CE,
Stewart NG, Greider CW, Harley CB and Bacchetti S: Telomere
shortening associated with chromosome instability is arrested in
immortal cells which express telomerase activity. EMBO J.
11:1921–1929. 1992.PubMed/NCBI
|
|
11
|
Vaziri H and Benchimol S: Reconstitution
of telomerase activity in normal human cells leads to elongation of
telomeres and extended replicative life span. Curr Biol. 8:279–282.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bodnar AG, Ouellette M, Frolkis M, Holt
SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S and
Wright WE: Extension of life-span by introduction of telomerase
into normal human cells. Science. 279:349–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greider CW and Blackburn EH: A telomeric
sequence in the RNA of Tetrahymena telomerase required for telomere
repeat synthesis. Nature. 337:331–337. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng J, Funk WD, Wang SS, Weinrich SL,
Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al:
The RNA component of human telomerase. Science. 269:1236–1241.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kassem M, Abdallah BM, Yu Z, Ditzel N and
Burns JS: The use of hTERT-immortalized cells in tissue
engineering. Cytotechnology. 45:39–46. 2004. View Article : Google Scholar
|
|
16
|
Tao Q, Lv B, Qiao B, Zheng CQ and Chen ZF:
Immortalization of ameloblastoma cells via reactivation of
telomerase function: Phenotypic and molecular characteristics. Oral
Oncol. 45:e239–e244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morales CP, Holt SE, Ouellette M, Kaur KJ,
Yan Y, Wilson KS, White MA, Wright WE and Shay JW: Absence of
cancer-associated changes in human fibroblasts immortalized with
telomerase. Nat Genet. 21:115–118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rajamani K, Lin YC, Wen TC, Hsieh J, Subeq
YM, Liu JW, Lin PC, Harn HJ, Lin SZ and Chiou TW: The
antisenescence effect of trans-cinnamaldehyde on adipose-derived
stem cells. Cell Transplant. 24:493–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Chang E, Cherry AM, Bangs CD, Oei
Y, Bodnar A, Bronstein A, Chiu CP and Herron GS: Human endothelial
cell life extension by telomerase expression. J Biol Chem.
274:26141–26148. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bischoff DS, Makhijani NS and Yamaguchi
DT: Constitutive expression of human telomerase enhances the
proliferation potential of human mesenchymal stem cells. Biores
Open Access. 1:273–279. 2012. View Article : Google Scholar
|
|
21
|
Huang G, Zheng Q, Sun J, Guo C, Yang J,
Chen R, Xu Y, Wang G, Shen D, Pan Z, et al: Stabilization of
cellular properties and differentiation mutilpotential of human
mesenchymal stem cells transduced with hTERT gene in a long-term
culture. J Cell Biochem. 103:1256–1269. 2008. View Article : Google Scholar
|
|
22
|
Kobune M, Kawano Y, Ito Y, Chiba H,
Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, et al:
Telomerized human multipotent mesenchymal cells can differentiate
into hematopoietic and cobblestone area-supporting cells. Exp
Hematol. 31:715–722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piper SL, Wang M, Yamamoto A, Malek F, Luu
A, Kuo AC and Kim HT: Inducible immortality in hTERT-human
mesenchymal stem cells. J Orthop Res. 30:1879–1885. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moffatt-Jauregui CE, Robinson B, de Moya
AV, Brockman RD, Roman AV, Cash MN, Culp DJ and Lamont RJ:
Establishment and characterization of a telomerase immortalized
human gingival epithelial cell line. J Periodontal Res. 48:713–721.
2013.PubMed/NCBI
|
|
25
|
Yang YX, Miao ZC, Zhang HJ, Wang Y, Gao JX
and Feng MF: Establishment and characterization of a human
telomerase catalytic subunit-transduced fetal bone marrow-derived
osteoblastic cell line. Differentiation. 75:24–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scutt A and Bertram P: Bone marrow cells
are targets for the anabolic actions of prostaglandin E2 on bone:
induction of a transition from nonadherent to adherent osteoblast
precursors. J Bone Miner Res. 10:474–487. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galderisi U, Helmbold H, Squillaro T,
Alessio N, Komm N, Khadang B, Cipollaro M, Bohn W and Giordano A:
In vitro senescence of rat mesenchymal stem cells is accompanied by
downregulation of stemness-related and DNA damage repair genes.
Stem Cells Dev. 18:1033–1042. 2009. View Article : Google Scholar
|
|
28
|
Shi YA, Zhao Q, Zhang LH, Du W, Wang XY,
He X, Wu S and Li YL: Knockdown of hTERT by siRNA inhibits cervical
cancer cell growth in vitro and in vivo. Int J Oncol. 45:1216–1224.
2014.PubMed/NCBI
|
|
29
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Follo MY, Manzoli L, Poli A, McCubrey JA
and Cocco L: PLC and PI3K/Akt/mTOR signalling in disease and
cancer. Adv Biol Regul. 57:10–16. 2015. View Article : Google Scholar
|
|
31
|
Yoon KA, Cho HS, Shin HI and Cho JY:
Differential regulation of CXCL5 by FGF2 in osteoblastic and
endothelial niche cells supports hematopoietic stem cell migration.
Stem Cells Dev. 21:3391–3402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stewart JA, Chaiken MF, Wang F and Price
CM: Maintaining the end: Roles of telomere proteins in
end-protection, telomere replication and length regulation. Mutat
Res. 730:12–19. 2012. View Article : Google Scholar :
|
|
34
|
Serakinci N, Graakjaer J and Kolvraa S:
Telomere stability and telomerase in mesenchymal stem cells.
Biochimie. 90:33–40. 2008. View Article : Google Scholar
|
|
35
|
Fajkus J, Simícková M and Maláska J:
Tiptoeing to chromosome tips: facts, promises and perils of today's
human telomere biology. Philos Trans R Soc Lond B Biol Sci.
357:545–562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dimitrova V and Arcaro A: Targeting the
PI3K/AKT/mTOR signaling pathway in medulloblastoma. Curr Mol Med.
15:82–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chell JM and Brand AH:
Nutrition-responsive glia control exit of neural stem cells from
quiescence. Cell. 143:1161–1173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sewell GW, Marks DJ and Segal AW: The
immunopathogenesis of Crohn's disease: a three-stage model. Curr
Opin Immunol. 21:506–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang SS, Kwon T, Kwon DY and Do SI: Akt
protein kinase enhances human telomerase activity through
phosphorylation of telomerase reverse transcriptase subunit. J Biol
Chem. 274:13085–13090. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kawagoe J, Ohmichi M, Takahashi T, Ohshima
C, Mabuchi S, Takahashi K, Igarashi H, Mori-Abe A, Saitoh M, Du B,
et al: Raloxifene inhibits estrogen-induced up-regulation of
telomerase activity in a human breast cancer cell line. J Biol
Chem. 278:43363–43372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kimura A, Ohmichi M, Kawagoe J, Kyo S,
Mabuchi S, Takahashi T, Ohshima C, Arimoto-Ishida E, Nishio Y,
Inoue M, et al: Induction of hTERT expression and phosphorylation
by estrogen via Akt cascade in human ovarian cancer cell lines.
Oncogene. 23:4505–4515. 2004. View Article : Google Scholar : PubMed/NCBI
|