Introduction
Peyronie's disease (PD) is a localized fibrotic
condition of the tunica albuginea (TA) that is often characterized
by the presence of a fibrous plaque containing an excessive amount
of collagen, and by fibroblast proliferation, which leads to penile
deformity and curvature, as well as potential erectile dysfunction
(1). It has been hypothesized
that PD arises as a result of a disordered wound-healing response
to repetitive micro-injuries of the TA (2).
Previous studies involving patients with PD have
demonstrated that the transformation of TA-derived fibroblasts
(TAFs) into α-smooth muscle actin (αSMA)-positive myofibroblasts is
key to the development of fibrous plaques and that this process is
promoted by the accumulation of transforming growth factor-β1
(TGF-β1) (3,4). During the wound-healing process,
myofibroblasts secrete extracellular matrix (ECM) components
(particularly collagen) and contract by using smooth muscle
actinmyosin complexes to speed up wound repair. These cells undergo
apoptosis after the healing process is complete (5). However, the persistence of
myofibroblasts leads to ECM expansion and enhanced contractility,
which is observed in several fibrotic diseases. In PD, the
excessive secretion of ECM components and the concurrent
contraction of myofibroblasts result in the respective development
of fibrous plaques and penile curvature (6,7).
The Smad-dependent and Smad-independent signaling pathways are both
involved in the activation of myofibroblasts, which occurs through
the interaction between TGF-β1 and its receptor (TGF-β1R) (8). Activated TGF-β1R then phosphorylates
Smad2 and Smad3, which in turn form a complex with Smad4. These
activated Smad complexes translocate to the nucleus where they
regulate the transcription of target genes and accelerate the
production of collagen (9).
Additionally, the Smad-independent ras homolog gene family, member
A (RhoA)/Rho-associated, coiled-coil containing protein kinase
(ROCK) pathway is a primary regulator of myofibroblast shape,
polarity and contraction through the modulation of actin
polymerization, actomyosin contractility, cell adhesion and
microtubule dynamics (10).
Therefore, preventing the transformation of fibroblasts into
myofibroblasts may be a useful strategy for suppressing the TA
fibrotic-remodeling events that ultimately lead to PD.
Previous studies have demonstrated that the
development of human fibrotic diseases differs according to gender:
women are generally more resistant to fibrosis than men (11). For example, Yang et al
demonstrated that pre-menopausal women have a lower risk of
developing severe liver fibrosis compared to men and
post-menopausal women (12). An
increasing body of evidence has demonstrated that estrogen inhibits
the activation of fibroblasts and fibrogenesis in a number of
organs, including the liver (13), heart (14), lungs (15) and kidneys (16). However, the specific details of
how estrogen effects the fibrotic process or fibroblast
transformation remain controversial. Novotny et al found
that estrogen receptor (ER) agonists induced the transformation of
fibroblasts into myofibroblasts and increased the production of ECM
components in ovariectomized rats (17). Therefore, the effects of estrogen
on the fibrotic disease process may be related to the type of organ
or tissue affected.
To the best of our knowledge, no research studies
have been conducted to date to investigate the mechanisms through
which estrogen affects the pathological and physiological process
of PD. Therefore, the aim of the present study was to determine the
specific effects of estrogen [17β-estradiol (E2)] on the functions
of cultured TAFs and to explore the mechanisms of action of TGF-β1
in TAFs in vitro. Additionally, we sought to determine
whether E2 regulates the secretion of collagen through the
TGF-β-Smad signaling pathway and/or the contraction of
myofibroblasts through the RhoA-ROCK signaling pathway.
Materials and methods
Cell culture
In independent experiments, 3 male Sprague-Dawley
rats (from the Animal Feeding Center of Nanjing Medical University,
Jiangsu, China) were used to isolate the TAFs. Under 4% chloral
hydrate anesthesia, penile tissue was harvested to isolate the TAFs
and then all rats were euthanized. Primary TAFs were isolated,
cultured and identified as previously described (18); cells at passage 8 or less were
used in the experiments. Briefly, TAFs from Sprague-Dawley rats
(Animal Feeding Center of Nanjing Medical University, Jiangsu,
China) were cultured in low-glucose Dulbecco's modified Eagle's
medium (DMEM), supplemented with 10% fetal bovine serum (FBS; both
from Gibco, Carlsbad, CA, USA) and 1% antibiotics (100 mg/l
streptomycin and 100 U/ml penicillin) at 37°C in a humidified 95%
air/5% CO2 environment. When the TAFs reached 80%
confluence, they were routinely digested by 0.25% trypsin and then
passaged at a 1:2 dilution. All procedures were approved by the
Institutional Animal Care and Use Committee of Nanjing University.
The TAFs were then cultured in serum-free medium and treated with
E2 (Sigma, St. Louis, MO, USA) at a concentration of 1–100 nM.
TGF-β1 (5–10 ng/ml; Sigma) was added at the same time as E2.
Various concentrations of TGF (0, 5 and 10 ng/ml) and E2 (0, 10 and
100 nM) were used to explore the dose-dependent response. Finally,
we found that 5 ng/ml of TGF induced the conversion of TAFs into
myofibroblasts and that 10 nM of E2 effectively attenuated this
process in our repeated independent experiments. Moreover, this
dose is consistent with that used in previous research (19–21). The control cells were exposed to
the vehicle (DMEM; referred to untreated or normal control). The
cells were then grown for either 24 h or for corresponding amounts
of time.
Collagen gel contraction assay
Collagen gels were prepared using 2 mg/ml of rat
tail collagen I (Wobio, Nanjing, China) that was neutralized with 1
M NaOH and supplemented with DMEM. Gels contained 2–3 mg/ml of rat
tail collagen in 0.5-ml aliquots; the amount of collagen used
depended upon the experimental protocol employed. The TAFs were
seeded at a density of 3×105 cells/ml in microtiter
plates that were lubricated with FBS. Following lubrication, 0.5 ml
of the final collagen gel was incubated at 37°C in a humidified 95%
air/5% CO2 environment for 24 h. Images were acquired
using an Odyssey Scanning System (LI-COR Biosciences, Lincoln, NE,
USA), and the surface areas were quantified using ImageJ software
(NIH, Bethesda, MD, USA).
Measurement of hydroxyproline
concentration
Hydroxyproline was used to measure the total
collagen secreted into the medium according to the method described
in the study by Woessner (22)
and the protocol included in the hydroxyproline kit (A030-1;
Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Duplicate 300-µl aliquots of medium were taken from each
sample and transferred to microtiter plates, and the absorbance of
each was determined at 550 nm by spectrophotometry. The
hydroxyproline concentrations of the sample pending to be tested
were calculated using a linear standard curve and are presented as
µg/ml cell culture medium.
Western blot analysis
Cellular proteins were obtained from the TAFs.
Western blot analysis of these proteins was performed as previously
described (23). Briefly, the
TAFs were washed twice with phosphate-buffered saline (PBS) and
then lysed in RIPA buffer (Sigma). Total protein concentrations
were measured using bicinchoninic acid (BCA) reagent (Beyotime
Biotech, Jiangsu, China). Proteins were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
were then electrophoretically transferred onto polyvinylidene
fluoride (PVDF) membranes. The membranes were incubated at 37°C for
1 h in blocking solution containing 5% non-fat milk in TBST. The
membranes were then incubated at 4°C overnight with the relevant
primary antibodies [anti-αSMA (SAB5500002), anti-Smad2
(SAB2103328), anti-phosphorylated (p-)Smad2 (SAB4300251),
anti-Smad4 (SAB4200405), anti-RhoA (WH0000387M3), anti-ras-related
C3 botulinum toxin substrate 1 (Rac1; SAB4300461), anti-ROCK1
(R6028), anti-ROCK2 (R8653) and anti-GAPDH (G9295); all from Sigma]
at a dilution of 1:1,000. After washing the membranes with TBST,
they were then incubated with the secondary antibody (horseradish
peroxidase conjugated goat anti-rabbit/mouse IgG; Wuhan Boster
Biological Technology Ltd., Wuhan, China) at room temperature for 2
h. The band densities were detected using enhanced
chemiluminescence (ECL) reagents (Beyotime Biotech) and an Odyssey
Scanning System (LI-COR Biosciences). We then used ImageJ software
to quantify the expression levels of the target proteins by
calculating the ratio of the mean intensity of each target protein
relative to that of GAPDH.
Immunofluorescence staining
Immunofluorescence staining for αSMA was performed
using the cultured TAFs as previously described (23). Briefly, the TAFs on the coverslips
were fixed with 4% paraformaldehyde, washed 3 times with
pre-chilled PBS and permeabilized with 3% bovine serum albumin
(BSA) in PBS containing 0.3% Triton X-100 for 30 min at room
temperature. Following this, the TAFs were incubated with primary
antibody (anti-αSMA) overnight at 4 °C. After being washed 3 times
with PBS, the TAFs were incubated with 0.1% DAPI and secondary
Rhodamine Red-X labeled antibody (Invitrogen/Life Technologies,
Carlsbad, CA, USA) at a dilution of 1:500 for 60 min at room
temperature, after which they were again washed with PBS. The
coverslips were then transferred onto glass slides and imaged using
a fluorescence microscope (Olympus, Tokyo, Japan).
Statistical analysis
All data were obtained from at least 3 individual
experiments. The experimental results are expressed as the mean
values ± standard deviation (SD). Statistical analysis was
performed by one-way analysis of variance (ANOVA) using Microsoft
Excel 2010 and GraphPad software. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
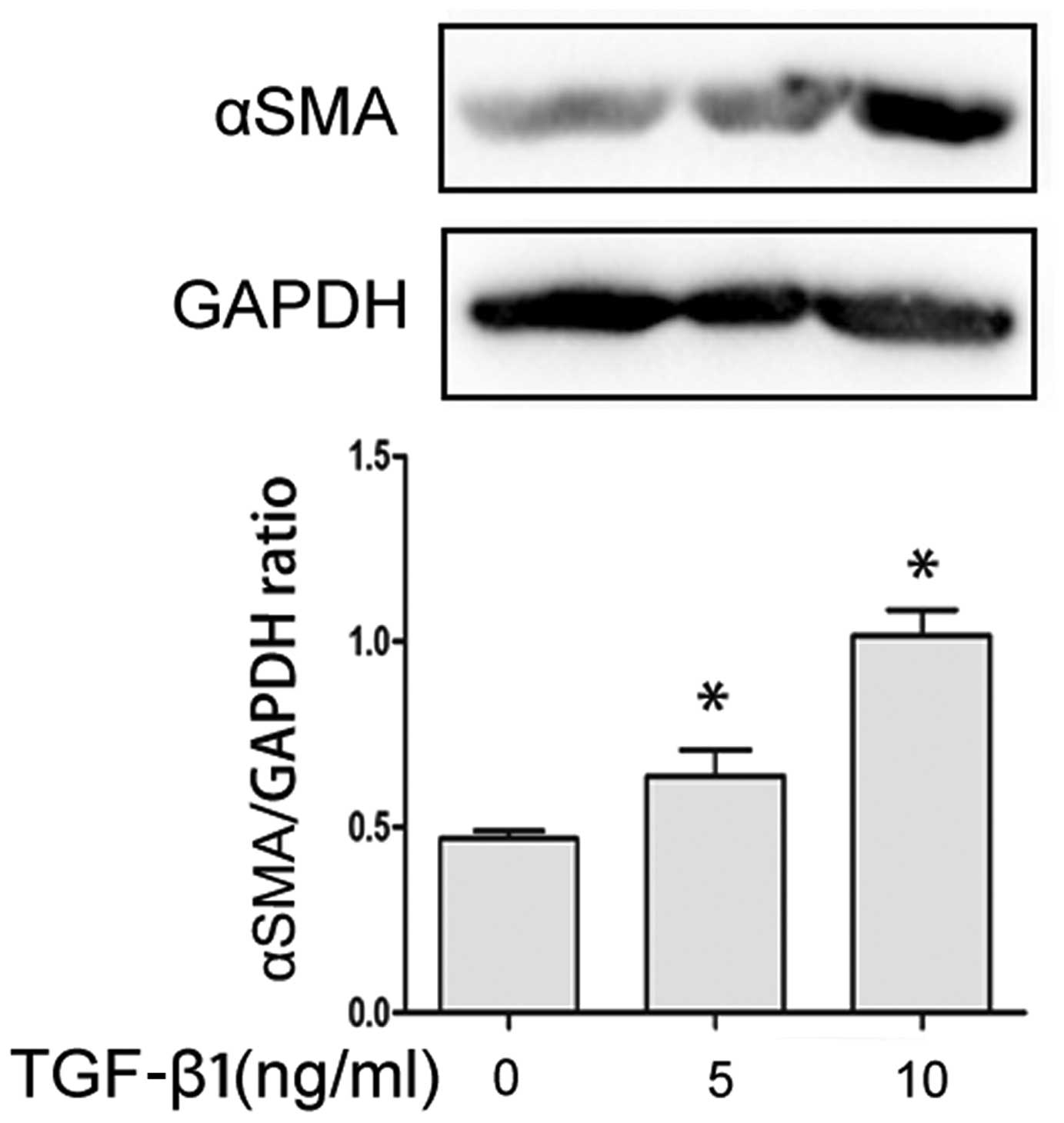
TGF-β1 promotes the transformation of
TAFs into myofibroblasts
In independent experiments with TAFs obtained from 3
different Sprague-Dawley rats, TGF-β1 significantly increased the
expression of αSMA (a myofibroblast marker) in a
concentration-dependent manner compared to the TAFs which were not
treated with TGF-β1 and exposed to the vehicle (1% DMEM). The above
results were obtained by western blot analysis (Fig. 1).
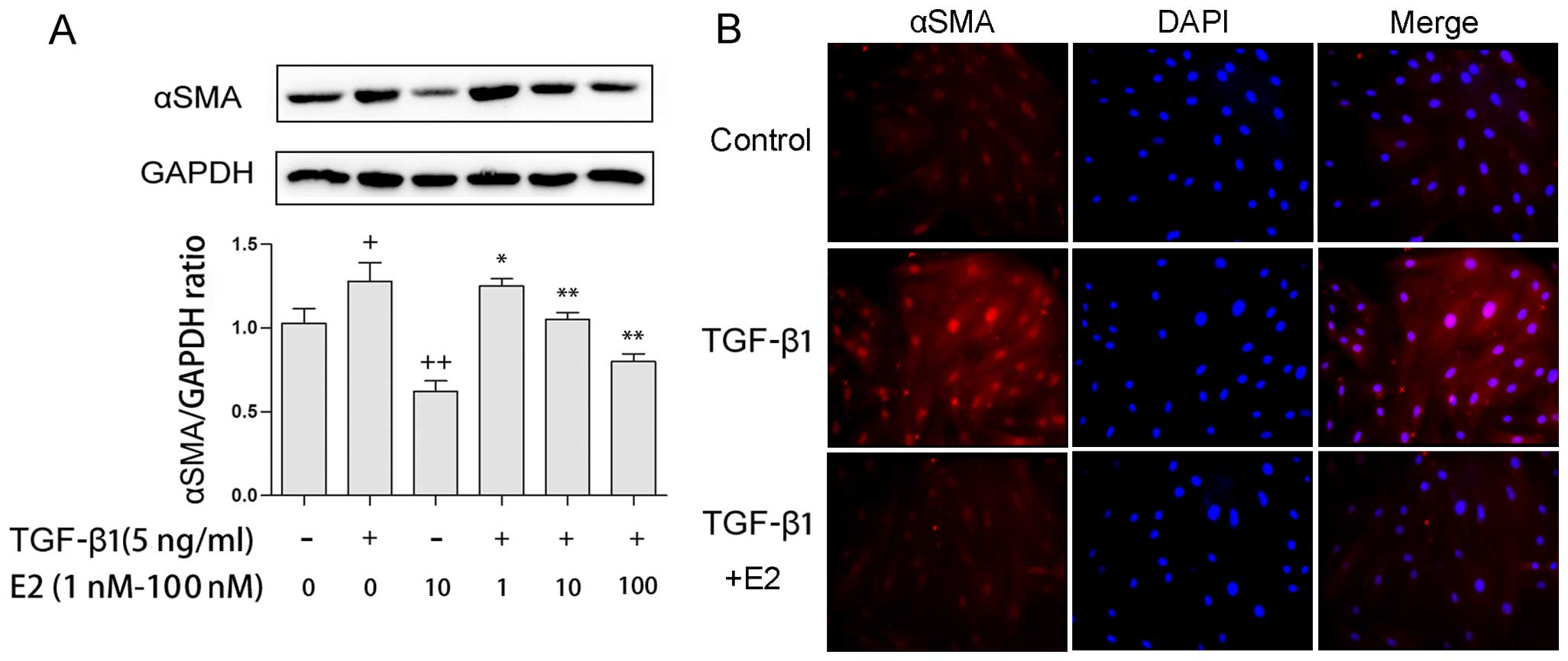
Supplementation of E2 attenuates the
TGF-β1-induced expression of αSMA in TAFs
Since the expression of αSMA is a hallmark indicator
that can be used to identify myofibroblasts, the effects of E2 on
TGF-β1-induced αSMA protein levels were measured by western blot
analysis and the immunofluorescence staining of TAFs. Treatment
with E2 (1–100 nM) reduced the expression of αSMA in a
dose-dependent manner in both the presence and absence of TGF-β1 (5
ng/ml; Fig. 2A). Consistent with
these results, treatment with 10 nM E2 reduced αSMA protein
expression in both the presence and absence of TGF-β1, as
determined by immunofluorescence staining (Fig. 2B).
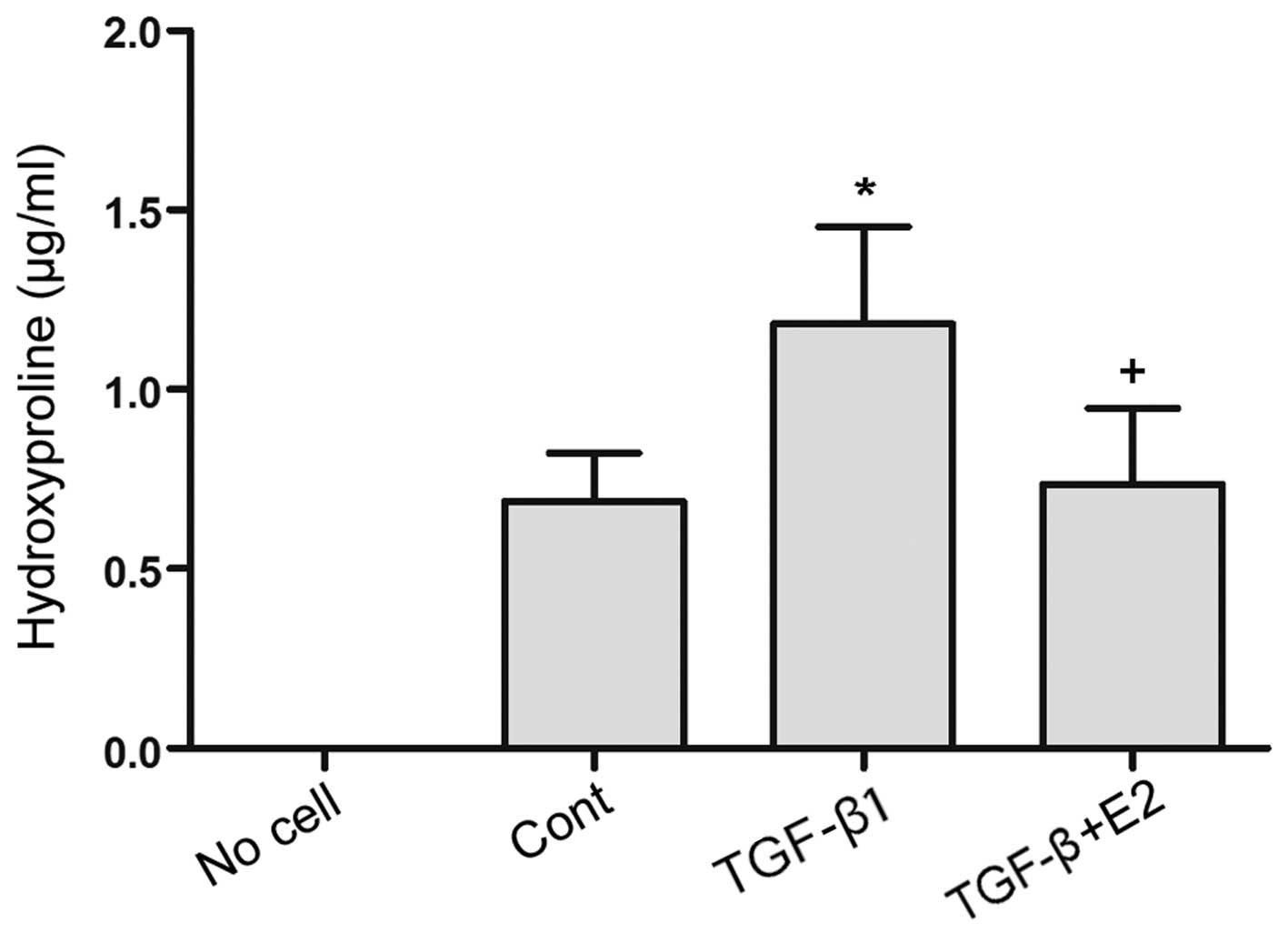
E2 reduces the levels of TGF-β1-induced
hydroxyproline in TAFs
Hydroxyproline is generally considered to be an
amino acid specific to collagen proteins, and it can therefore be
used to measure fibrosis (22).
As shown in Fig. 3, stimulation
with TGF-β1 (5 ng/ml) led to elevated levels of hydroxyproline in
the TAFs compared to the controls (P<0.01). We then examined the
effects of E2 on the TGF-β1-mediated induction of collagen
secretion in TAFs and revealed that treatment with E2 (10 nM)
suppressed the TGF-β1-induced increase in hydroxyproline levels in
these cells (P<0.01).
TGF-β1-induced collagen gel contraction
is suppressed by E2
We carried out a collagen gel contraction assay to
analyze the inhibitory effects of E2 on the contractile process.
The TAFs mixed with E2 were applied to collagen gels and incubated
with or without TGF-β1 for 24 h. The collagen gels were prepared as
follows: i) normal control; ii) collagen gel with 5 ng/ml TGF-β1;
and iii) collagen gel with 5 ng/ml TGF-β1 and 10 nM E2. TGF-β1
significantly enhanced gel contraction compared to the vehicle
control (P<0.001; Fig. 4).
Although E2 significantly suppressed gel contraction at a
concentration of 10 nM (P<0.001) in the presence of TGF-β1 (5
ng/ml), it did not completely reverse this contraction (P<0.01;
Fig. 4).
E2 decreases TGF-β1-induced Smad
signaling
In exploring the molecular mechanisms underlying the
negative role of E2 in relation to the functioning of TAFs, we
discovered that the levels of p-Smad2 were enhanced in the TAFs
following stimulation with TGF-β1 (5 ng/ml); this effect was
attenuated following incubation with increasing doses of E2 (1–100
nM; Fig. 5A). Additionally, no
significant differences in the total protein levels of Smad2 and
Smad4 were noted between the TAFs incubated with TGF-β1 or the TAFs
treated with E2 (Fig. 5A and
B).
The TGF-β1-induced activation of the
Rho/ROCK pathway is inhibited by E2
The Rho/ROCK signaling pathway promotes cell
contraction and migration and is a major effector of fibrotic
disease (24–26). We thus performed an in
vitro experiment in which the cells were pre-treated with
TGF-β1 (5 ng/ml) for 24 h and then treated with 10 nM E2 for 0, 2,
6, 24, 48 and 72 h. The expression of RhoA decreased after 2 h and
was completely depleted by 24 h (Fig.
5C). To elucidate the molecular mechanisms responsible for
this, we examined the expression of proteins of the Rho/ROCK
signaling pathway, which include RhoA, Rac1, ROCK1 and ROCK2
(Fig. 5D). We found that only
RhoA was upregulated after 24 h of stimulation with TGF-β1 (5
ng/ml). It should be noted that the expression levels of RhoA and
ROCK were decreased in the TAFs following incubation with E2 in a
dose-dependent manner compared to the TAFs which received only
TGF-β1 and that obvious changes to the total protein levels of
Rac1, ROCK1 and ROCK2 were demonstrated (Fig. 5D). These results suggest that E2
suppresses the TGF-β1-induced activation of the RhoA/ROCK2
signaling pathway.
Discussion
PD is characterized by fibrosis of the TA. TGF-β is
crucial to tissue regeneration and remodeling in the TA, as has
been demonstrated in previous in vivo and in vitro
studies (27,28). Estrogen is a type of sex hormone
that is present in both men and women and is found in particularly
high levels in women of reproductive age. The major functions of
estrogen include the development of female secondary sexual
characteristics, the thickening of the endometrium, and other
aspects of menstrual cycle regulation (29,30). In males, estrogen regulates
certain functions of the reproductive system that play an important
role in the maturation of sperm (31) and may be necessary for a healthy
libido (32). Previous studies
have suggested that exposure to estrogen has long-term benefits,
preventing the progression of fibrotic disease (11–15). However, there is little detailed
information currently available as to the mechanisms through which
estrogen affects PD. TAFs have been shown to be important to the
pathological process of PD. Thus, in the present study, we examined
the effects of estrogen (E2) on the activation of cultured TAFs
in vitro, as well as the possible mechanisms involved. Our
results revealed the following: i) TGF-β1 increased αSMA expression
in cultured TAFs in a concentration-dependent manner, and the
maximal protein expression of αSMA was noted in the TAFs treated
with 10 ng/ml TGF-β1 for 24 h; ii) E2 partially inhibited the
transformation of TAFs into myofibroblasts; iii) E2 attenuated the
TGF-β1-induced collagen production, as well as the contraction of
myofibroblasts; iv) the phosphorylation of Smad2 was enhanced in
the TAFs following stimulation with TGF-β1 and was significantly
suppressed following exposure to E2; v) TGF-β1 stimulation
increased the expression of RhoA and ROCK2, although the expression
of these proteins was decreased following treatment with E2.
TAFs represent the largest class of cells that make
up the normal TA, and the phenotypic transformation of TAFs into
myofibroblasts is the main characteristic of PD. Under abnormal
conditions, the persistence of myofibroblasts can facilitate
fibrosis, which results in the structural remodeling of the penis
(33). Therefore, preventing
myofibroblast transformation is a potential therapeutic strategy to
limit the fibrotic transformation of the TA. Myofibroblasts are
highly active cells that express αSMA. In this study, we employed
western blot analysis and immunofluorescence staining and found
that E2 inhibited TAF activation, which was indicated by a
dose-dependent decrease in αSMA expression (Figs. 1 and 2). Additionally, we found that activated
myofibroblasts synthesized and secreted increased levels of
collagen proteins and possessed contractile function similar to
smooth muscle cells. We demonstrated that TGF-β1 elevated the level
of hydroxyproline (an amino acid that found only in collagen) in
the TAF supernatant and that E2 suppressed this elevation (Fig. 3). The results that we obtained
using cultured TAFs confirmed the beneficial effects of E2, which
have also been demonstrated in previous studies (34,35). We also found that TGF-β1
significantly enhanced the contraction of primary TAFs in a
collagen gel contraction assay and that the addition of E2 partly
negated this effect (Fig. 4).
Therefore, we suggest that the appropriate supplementation of E2 is
an effective strategy for the treatment of PD.
Smad proteins are thought to play an important role
in regulating intracellular responses to TGF-β1. Following the
TGF-β1-induced phosphorylation of Smad2 and Smad3, these proteins
have been shown to localize to the nucleus and form a complex with
Smad4, which mediates collagen expression (36–38). In accordance with these
observations, our study demonstrated that TGF-β1 induced Smad2
phosphorylation in TAFs. However, we also found that E2
significantly suppressed the expression of p-Smad2 in TAFs exposed
to TGF-β1. Additionally, the expression of Smad4 was reduced in the
TAFs treated with a combination of E2 and TGF-β1 compared to those
treated with TGF-β1 alone. These findings indicate that E2 blocks
the TGF-β1-induced production of p-Smad2 in TAFs and inhibits their
secretion of collagen. We suggest a possible mechanism to explain
these findings, which is that E2 attenuated TGF-β1-induced Smad2
phosphorylation and therefore inhibited Smad complex formation
through interactions with the G protein-coupled estrogen receptor
(GPER).
RhoA, a member of the Ras homolog gene family is a
small guanosine triphosphate (GTP)ase known to regulate the actin
cytoskeleton during the formation of stress fibers. Previous
studies have suggested that the small GTP-binding protein Rho and
its downstream targets, ROCK (including ROCK1 and ROCK2), play
important roles in numerous fibrotic diseases (39). By phosphorylating the myosin light
chain (MLC), Rho GTPase also induces Ca2+ sensitization
during the contraction of various cells (40). It is well known that the Rho/ROCK
pathway is a TGF-β-Smad independent pathway (8). In agreement with these
aforementioned studies these results, we found that the RhoA/ROCK2
pathway became was activated in TAFs treated with TGF-β1, which
then enhanced myofibroblast contractility. By contrast, E2 clearly
hindered the TGF-β1-induced upregulation of RhoA and ROCK2
proteins. However, our results demonstrated that the expression
levels of Rac1 and ROCK1 in TAFs were not markedly decreased when
TGF-β1 was supplemented with E2. This discovery indicates that E2
hinders the ability of TGF-β1 to contract TAFs through the
modulation of the RhoA-ROCK2 signaling pathway. Moreover, it has
previously been revealed that the inhibition of the RhoA/ROCK
pathway results in the relaxation of smooth muscle in the penis
(41), thereby enhancing erectile
dysfunction, which is a common symptom of PD.
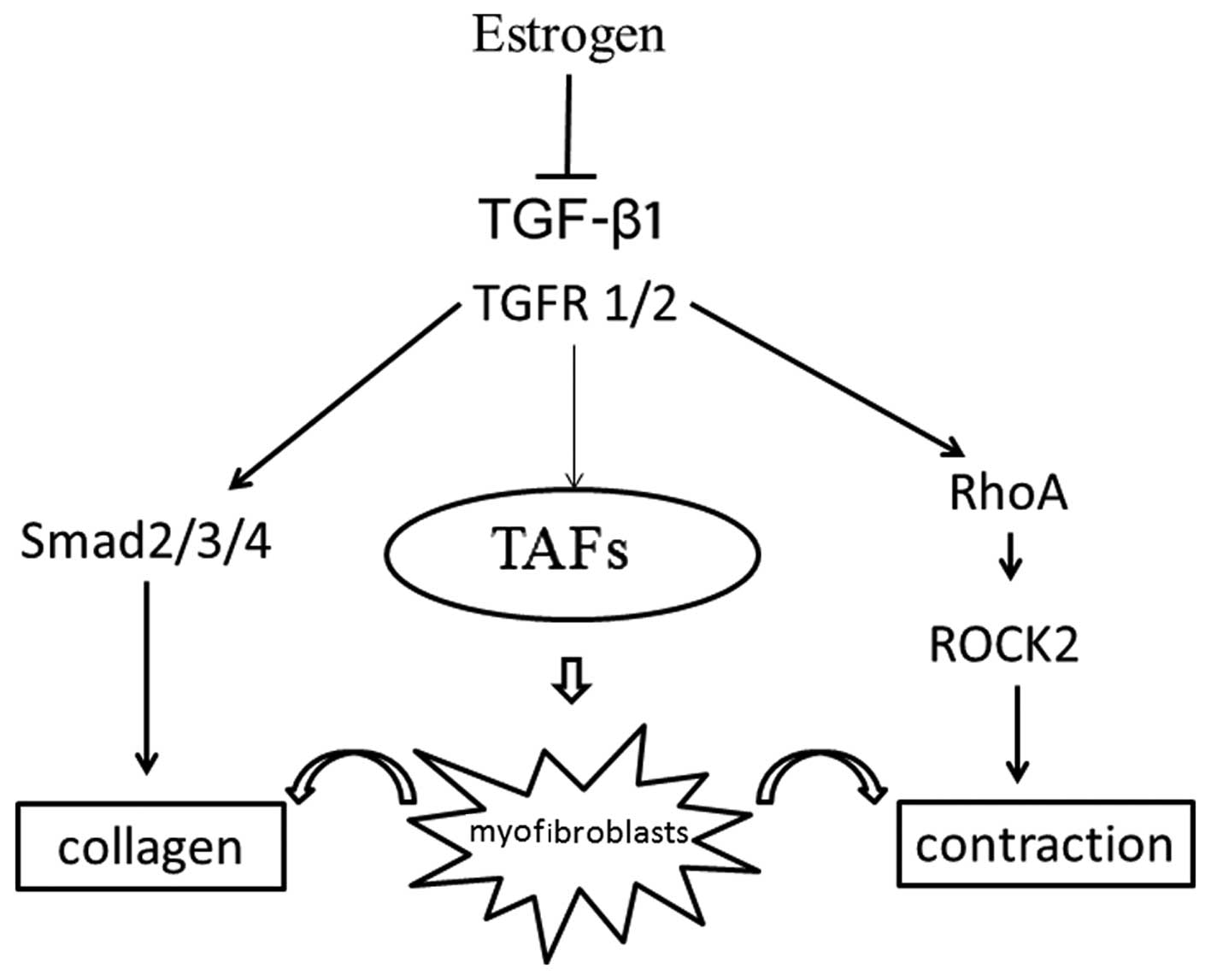
In conclusion, the findings of the present study
demonstrate that E2 inhibits the transformation of TAFs into
myofibroblasts, reduces the expression of collagen through the
modulation of the TGF-β1-Smad signaling pathway, and suppresses the
contraction of myofibroblasts through the modulation of the
RhoA-ROCK2 signaling pathway (Fig.
6). These results suggest that E2 attenuates the development of
PD. However, further studies are warranted to elucidate the
detailed mechanisms of action of estrogen in PD in vitro and
in vivo.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81170563 and
81270694).
Abbreviations:
|
E2
|
17β-estradiol
|
|
TAFs
|
tunica albuginea-derived
fibroblasts
|
|
PD
|
Peyronie's disease
|
|
αSMA
|
α-smooth muscle actin
|
|
ECM
|
extracellular matrix
|
|
TGF-β1
|
transforming growth factor-β1
|
References
|
1
|
Langston JP and Carson CC III: Peyronie's
disease: review and recent advances. Maturitas. 78:341–343. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jarow JP and Lowe FC: Penile trauma: an
etiologic factor in Peyronie's disease and erectile dysfunction. J
Urol. 158:1388–1390. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Sakka AI, Hassoba HM, Chui RM,
Bhatnagar RS, Dahiya R and Lue TF: An animal model of
Peyronie's-like condition associated with an increase of
transforming growth factor beta mRNA and protein expression. J
Urol. 158:2284–2290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valente EGA, Vernet D, Ferrini MG, Qian A,
Rajfer J and Gonzalez-Cadavid NF: L-arginine and phosphodiesterase
(PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic
plaque and related fibroblast cultures. Nitric Oxide. 9:229–244.
2003. View Article : Google Scholar
|
|
5
|
Powell DW, Mifflin RC, Valentich JD, Crowe
SE, Saada JI and West AB: Myofibroblasts. I. Paracrine cells
important in health and disease. Am J Physiol. 277:C1–C9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gelbard M: Myofibroblasts and
mechanotransduction: do forces in the tunica albuginea contribute
to Peyronie's disease? J Sex Med. 5:2974–2976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vernet D, Ferrini MG, Valente EG, Magee
TR, Bou-Gharios G, Rajfer J and Gonzalez-Cadavid NF: Effect of
nitric oxide on the differentiation of fibroblasts into
myofibroblasts in the Peyronie's fibrotic plaque and in its rat
model. Nitric Oxide. 7:262–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Derynck R and Zhang YE: Smad-dependent and
Smad- independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li JH, Zhu HJ, Huang XR, Lai KN, Johnson
RJ and Lan HY: Smad7 inhibits fibrotic effect of TGF-Beta on renal
tubular epithelial cells by blocking Smad2 activation. J Am Soc
Nephrol. 13:1464–1472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schofield AV and Bernard O: Rho-associated
coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem
Mol Biol. 48:301–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Martino V, Lebray P, Myers RP, Pannier
E, Paradis V, Charlotte F, Moussalli J, Thabut D, Buffet C and
Poynard T: Progression of liver fibrosis in women infected with
hepatitis C: long-term benefit of estrogen exposure. Hepatology.
40:1426–1433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang JD, Abdelmalek MF, Pang H, Guy CD,
Smith AD, Diehl AM and Suzuki A: Gender and menopause impact
severity of fibrosis among patients with nonalcoholic
steatohepatitis. Hepatology. 59:1406–1414. 2014. View Article : Google Scholar :
|
|
13
|
Smyk DS, Rigopoulou EI, Pares A, Billinis
C, Burroughs AK, Muratori L, Invernizzi P and Bogdanos DP: Sex
differences associated with primary biliary cirrhosis. Clin Dev
Immunol. 2012:6105042012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pedram A, Razandi M, O'Mahony F, Lubahn D
and Levin ER: Estrogen receptor-beta prevents cardiac fibrosis. Mol
Endocrinol. 24:2152–2165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morani A, Barros RPA, Imamov O, Hultenby
K, Arner A, Warner M and Gustafsson JA: Lung dysfunction causes
systemic hypoxia in estrogen receptor beta knockout
(ERbeta−/−) mice. Proc Natl Acad Sci USA. 103:7165–7169.
2006. View Article : Google Scholar
|
|
16
|
Dixon A and Maric C: 17beta-Estradiol
attenuates diabetic kidney disease by regulating extracellular
matrix and transforming growth factor-beta protein expression and
signaling. Am J Physiol Renal Physiol. 293:F1678–F1690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Novotný M, Vasilenko T, Varinská L,
Smetana K Jr, Szabo P, Sarišský M, Dvořánková B, Mojžiš J, Bobrov
N, Toporcerová S, et al: ER-α agonist induces conversion of
fibroblasts into myofibroblasts, while ER-β agonist increases ECM
production and wound tensile strength of healing skin wounds in
ovariectomised rats. Exp Dermatol. 20:703–708. 2011. View Article : Google Scholar
|
|
18
|
Ahuja SK, Sikka SC and Hellstrom WJG:
Stimulation of collagen production in an in vitro model for
Peyronie's disease. Int J Impot Res. 11:207–212. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Illsley MC, Peacock JH, McAnulty RJ and
Yarnold JR: Increased collagen production in fibroblasts cultured
from irradiated skin and effect of TGF beta(1)- clinical study. Br
J Cancer. 83:650–654. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong M and Mudera V: Feedback inhibition
of high TGF-beta1 concentrations on myofibroblast induction and
contraction by Dupuytren's fibroblasts. J Hand Surg Br. 31:473–483.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar A, Ruan M, Clifton K, Syed F, Khosla
S and Oursler MJ: TGF-β mediates suppression of adipogenesis by
estradiol through connective tissue growth factor induction.
Endocrinology. 153:254–263. 2012. View Article : Google Scholar :
|
|
22
|
Woessner JF Jr: The determination of
hydroxyproline in tissue and protein samples containing small
proportions of this amino acid. Arch Biochem Biophys. 93:440–447.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Z, Wang C, Shi C, Sun F, Xu X, Qian W,
Nie S and Han X: Activated Wnt signaling induces myofibroblast
differentiation of mesenchymal stem cells, contributing to
pulmonary fibrosis. Int J Mol Med. 33:1097–1109. 2014.PubMed/NCBI
|
|
24
|
Lauriol J, Keith K, Jaffré F, Couvillon A,
Saci A, Goonasekera SA, McCarthy JR, Kessinger CW, Wang J, Ke Q, et
al: RhoA signaling in cardiomyocytes protects against
stress-induced heart failure but facilitates cardiac fibrosis. Sci
Signal. 7:ra1002014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji H, Tang H, Lin H, Mao J, Gao L, Liu J
and Wu T: Rho/Rock cross-talks with transforming growth
factor-β/Smad pathway participates in lung fibroblast-myofibroblast
differentiation. Biomed Rep. 2:787–792. 2014.PubMed/NCBI
|
|
26
|
Manickam N, Patel M, Griendling KK, Gorin
Y and Barnes JL: RhoA/Rho kinase mediates TGF-β1-induced kidney
myofibroblast activation through Poldip2/Nox4-derived reactive
oxygen species. Am J Physiol Renal Physiol. 307:F159–F171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Castiglione F, Hedlund P, Van der Aa F,
Bivalacqua TJ, Rigatti P, Van Poppel H, Montorsi F, De Ridder D and
Albersen M: Intratunical injection of human adipose tissue-derived
stem cells prevents fibrosis and is associated with improved
erectile function in a rat model of Peyronie's disease. Eur Urol.
63:551–560. 2013. View Article : Google Scholar
|
|
28
|
Gonzalez-Cadavid NF and Rajfer J:
Experimental models of Peyronie's disease. Implications for new
therapies. J Sex Med. 6:303–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McKenna TJ: Oestrogens for women: the
risk/benefit ratio. Ir Med J. 80:219–221. 1987.PubMed/NCBI
|
|
30
|
Studd J and Zamblera D: Estrogen therapy
in women over 60 years of age. Gynecol Endocrinol. 8:191–196. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hess RA, Bunick D, Lee KH, Bahr J, Taylor
JA, Korach KS and Lubahn DB: A role for oestrogens in the male
reproductive system. Nature. 390:509–512. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hill RA, Pompolo S, Jones MEE, Simpson ER
and Boon WC: Estrogen deficiency leads to apoptosis in dopaminergic
neurons in the medial preoptic area and arcuate nucleus of male
mice. Mol Cell Neurosci. 27:466–476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gonzalez-Cadavid NF and Rajfer J:
Mechanisms of Disease: new insights into the cellular and molecular
pathology of Peyronie's disease. Nat Clin Pract Urol. 2:291–297.
2005. View Article : Google Scholar
|
|
34
|
Wu M, Han M, Li J, Xu X, Li T, Que L, Ha
T, Li C, Chen Q and Li Y: 17beta-estradiol inhibits angiotensin
II-induced cardiac myofibroblast differentiation. Eur J Pharmacol.
616:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Imaoka M, Jindo T and Takasaki W: The
process and development mechanism of age-related fibrosis in the
pancreatic islets of Sprague-Dawley rats: Immunohistochemical
detection of myofibroblasts and suppression effect by estrogen
treatment. J Toxicol Pathol. 26:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsuo SE, Fiore APZP, Siguematu SM, Ebina
KN, Friguglietti CU, Ferro MC, Kulcsar MA and Kimura ET: Expression
of SMAD proteins, TGF-beta/activin signaling mediators, in human
thyroid tissues. Arq Bras Endocrinol Metabol. 54:406–412. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan S-M, Wang J, Hu X-N, Li D-M and Jing
H: Transforming growth factor-β/Smad signaling function in the
aortopathies. Rev Bras Cir Cardiovasc. 26:393–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong C, Li H-J, Chang S, Liao HJ, Zhang
ZP, Huang P and Tang HH: A disintegrin and metalloprotease with
thrombos-pondin motif 2 may contribute to cirrhosis in humans
through the transforming growth factor-β/SMAD pathway. Gut Liver.
7:213–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thumkeo D, Watanabe S and Narumiya S:
Physiological roles of Rho and Rho effectors in mammals. Eur J Cell
Biol. 92:303–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fukata Y, Kimura K, Oshiro N, Saya H,
Matsuura Y and Kaibuchi K: Association of the myosin-binding
subunit of myosin phosphatase and moesin: dual regulation of moesin
phos-phorylation by Rho-associated kinase and myosin phosphatase. J
Cell Biol. 141:409–418. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sopko NA, Hannan JL and Bivalacqua TJ:
Understanding and targeting the Rho kinase pathway in erectile
dysfunction. Nat Rev Urol. 11:622–628. 2014. View Article : Google Scholar : PubMed/NCBI
|