Introduction
Hepatocellular carcinoma (HCC) is the sixth most
widespread human cancer worldwide and the third most frequent cause
of cancer-related mortality (1).
HCC largely stems from hepatitis B viral (HBV) and hepatitis C
viral (HCV) infections, together with other risk factors, such as
aflatoxin, cirrhosis, alcohol consumption, non-alcoholic fatty
liver disease, diabetes and tobacco. Considerable evidence has
shown that the incidence of HCC in association with viral infection
has increased in recent years. Among the viral infections, HCV was
attributed to ~33% of total liver cancer studies in developing
countries and ~20% of cancers reported in developed countries
(2–5). Surgical therapies, such as
hepatectomy and liver transplantation, are only effective for early
stage HCC, and systemic chemotherapy remains the main approach for
the majority of patients. However, current chemotherapeutics and
associated therapies, including antitumor antibiotics, hormones,
alkylating and antimetabolic agents, have a low specificity for
tumor cells, thereby causing adverse reactions and resulting in
drug resistance, which restricts their use. Antiviral treatments
can improve liver function and reduce the occurrence of liver
disease in advanced stages to improve conditions for comprehensive
therapy (6). Therefore, antiviral
therapy may be a novel strategy for the treatment of HCC.
The tricyclic symmetric amine amantadine is an
antiviral drug used to treat influenza A, which blocks the M2
proton channel and inhibits viral reproduction. Amantadine can also
treat Parkinson's disease by increasing the release of dopamine or
postponing dopamine metabolism (7–10).
Previous studies have indicated that amantadine has a protective
role in mitochondrial dysfunction and oxidative stress mediated by
HCV protein expression and treats chronic HCV infection by
inhibiting p7 cation channel activity (11,12). Additionally, emerging evidence
suggests that antiviral treatments can reduce the risk and prolong
the survival rate of HCC patients with chronic hepatitis B and C
(13,14). With regards to these findings,
antiviral treatments, such as amantadine, represent novel
therapeutic approaches for HCC.
The aim of the present study was to determine
whether amantadine exerts significant anticancer effects on HCC
cells and elucidate the mechanisms by which it produces these
effects. Therefore, the effects of amantadine on cell cycle-related
genes and proteins, including cyclin D1, cyclin E and CDK2, and
apoptosis via modulation of Bax and Bcl-2 were investigated.
Results of the present study will help to validate whether
amantadine can be used as a novel therapeutic agent for treatment
of liver cancer.
Materials and methods
Cell lines and reagents
Human HCC cell lines (HepG2 and SMMC-7721) and
normal hepatocellular cells (L02 cells) were obtained from the Key
Laboratory of Environment and Gene Related to Diseases, Ministry of
Education (Xi'an Jiaotong University Health Science Center, Xi'an,
China). HepG2 and SMMC-7721 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM), while L02 cells were cultured in
RPMI-1640 (both from HyClone, Logan, UT, USA). DMEM and RPMI-1640
were supplemented with 10% inactivated fetal bovine serum and 1%
penicillin/streptomycin (both from HyClone). Cells were incubated
in a 37°C humidified atmosphere with 5% CO2. Amantadine
hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Cell growth assay
Cell viability was determined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich, USA) colorimetric assay. HepG2, SMMC-7721 and L02
cells were seeded in 96-well plates (5×103 cells/well)
and treated with various concentrations of amantadine (0, 1, 2, 5,
10, 25, 50 and 100 µg/ml) for 24, 48 and 72 h. At the end of
the treatment, 20 µl of a 5 mg/ml MTT solution was added to
each well and incubated with cells for 4 h at 37°C. Dimethyl
sulfoxide (150 µl; Sigma-Aldrich) was added to dissolve the
formazan crystals, and the absorbance at 490 nm was recorded using
a Microplate Reader (FLUOstar OPTIMA; BMG Labtech, Offenburg,
Germany) to calculate cell viability.
Cell cycle analysis
Cell cycle analysis was performed by propidium
iodide (PI) staining according to the manufacturer's instructions
(KeyGEN, Nanjing, China). Cells were seeded in 6-well plates
(2×105 cells/well) and treated with various
concentrations of amantadine (0, 10, 25, 50 and 75 µg/ml)
for 48 h. Cells were subsequently harvested and washed with cold
phosphate-buffered saline (PBS) followed by fixing in cold 70%
ethanol overnight at 4°C. The next day, fixed cells were washed
with cold PBS and incubated with 100 µl RNaseA (100
µg/ml) for 30 min at 37°C. Cells were subsequently stained
with 400 µl PI for 30 min at 4°C in the dark. Stained cells
were examined by flow cytometry (Becton-Dickinson, Franklin Lakes,
NJ, USA).
Apoptosis assay
Double staining with Annexin V-fluorescein
isothiocyanate (FITC) and PI was used to assess cellular apoptosis
using an apoptosis detection kit (BD Bioscience, Franklin Lakes,
NJ, USA). HepG2 and SMMC-7721 cells were incubated with various
concentrations of amantadine (0, 10, 25, 50 and 75 µg/ml)
for 48 h and were collected by centrifugation at 100 × g for 5 min
and washed with ice-cold PBS. Cells were resuspended in 100
µl 1X binding buffer and mixed with 5 µl of Annexin
V-FITC and 5 µl of PI for 15 min at room temperature (RT) in
the dark. Another 400 µl of 1X binding buffer was added to
samples for flow cytometry analysis (Becton-Dickinson).
Western blotting
Cell cycle and apoptosis-related proteins were
evaluated by western blot analysis. Anti-Bax (Cat. no. ab32503),
anti-cyclin D1 (Cat. no. ab134175), anti-cyclin E (Cat. no.
ab33911) and anti-CDK2 (Cat. no. ab32147) primary antibodies were
purchased from AbCam (Cambridge, MA, USA), while anti-Bcl-2 (Cat.
no. GTX100064) and anti-β-actin (Cat. no. CW0096) primary
antibodies were purchased from GeneTex (Wuhan, China) and CW
Biotech (Beijing, China) respectively. Horseradish
peroxidase-conjugated goat anti-mouse (Cat. no. ZB2305) and
anti-rabbit (Cat. no. ZB2301) secondary antibodies were obtained
from Zsbio (Beijing, China). All antibodies were diluted with 5%
skimmed milk in PBS containing 0.1% Tween-20. HepG2 and SMMC-7721
cells were exposed to different concentrations of amantadine (0,
10, 25, 50 and 75 µg/ml) for 48 h prior to collection.
Cellular protein samples were separated on a 12% sodium dodecyl
sulfate-polyacrylamide gel and wet transferred to polyvinylidene
fluoride membranes. Blots were blocked with 5% skimmed milk in PBS
containing 0.1% Tween-20 for 2 h and were subsequently probed with
the appropriate primary antibody overnight at 4°C. The next day,
blots were washed with PBS containing 0.1% Tween-20 and were
incubated with horseradish peroxidase-conjugated goat anti-mouse or
anti-rabbit immunoglobulin G (1:5,000) for 1 h at room temperature.
Proteins were detected with ECL™ western blot detection reagents
(Millipore, Darmstadt, Germany) according to the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from HepG2 and SMMC-7721
cells following amantadine treatment (0, 10, 25, 50 and 75
µg/ml) for 48 h using an RNA Fast 200 kit (Pioneer
Biotechnology, Inc., Shanghai, China); cDNA was obtained using a
cDNA synthesis kit (Takara, Shiga, Japan). Primer sequences were as
follows: Bcl-2 forward, 5′-CCG GAT CAC CAT CTG AAG AG-3′ and
reverse, 5′-AGG GCA AAG AAA TGC AAG TG-3′; Bax forward, 5′-ATG GGC
TGG ACA TTG GAC-3′ and reverse, 5′-GGG ACA TCA GTC GCT TCA GT-3′;
cyclin D1 forward, 5′-GTG TAT CGA GAG GCC AAA GG-3′ and reverse,
5′-GCA ACC AGA AAT GCA CAG AC-3′; cyclin E forward, 5′-CTG GAT GTT
GAC TGC CTT GA-3′ and reverse, 5′-ATG TCG CAC CAC TGA TAC CC-3′;
CDK2 forward, 5′-CAG GAT GTG ACC AAG CCA GT-3′ and reverse, 5′-TGA
GTC CAA ATA GCC CAA GG-3′; and GAPDH forward, 5′-AGG TCC ACC ACT
GAC ACG TT-3′ and reverse, 5′-GCC TCA AGA TCA TCA GCA AT-3′. The
RT-qPCR reaction mixtures were prepared following the
manufacturer's instructions (Takara) and performed using a Bio-Rad
iQ5 real-time PCR system. Experiments were performed in triplicate,
and the data were calculated using the ΔΔCt method.
Statistical analysis
The data are expressed as mean ± standard error of
the mean and were analyzed using one-way analysis of the variance
followed by least significant difference correction for multiple
comparisons tests. All the figures were generated using GraphPad
Prism v.5.01 (GraphPad Software Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Amantadine selectively inhibits HCC cell
growth
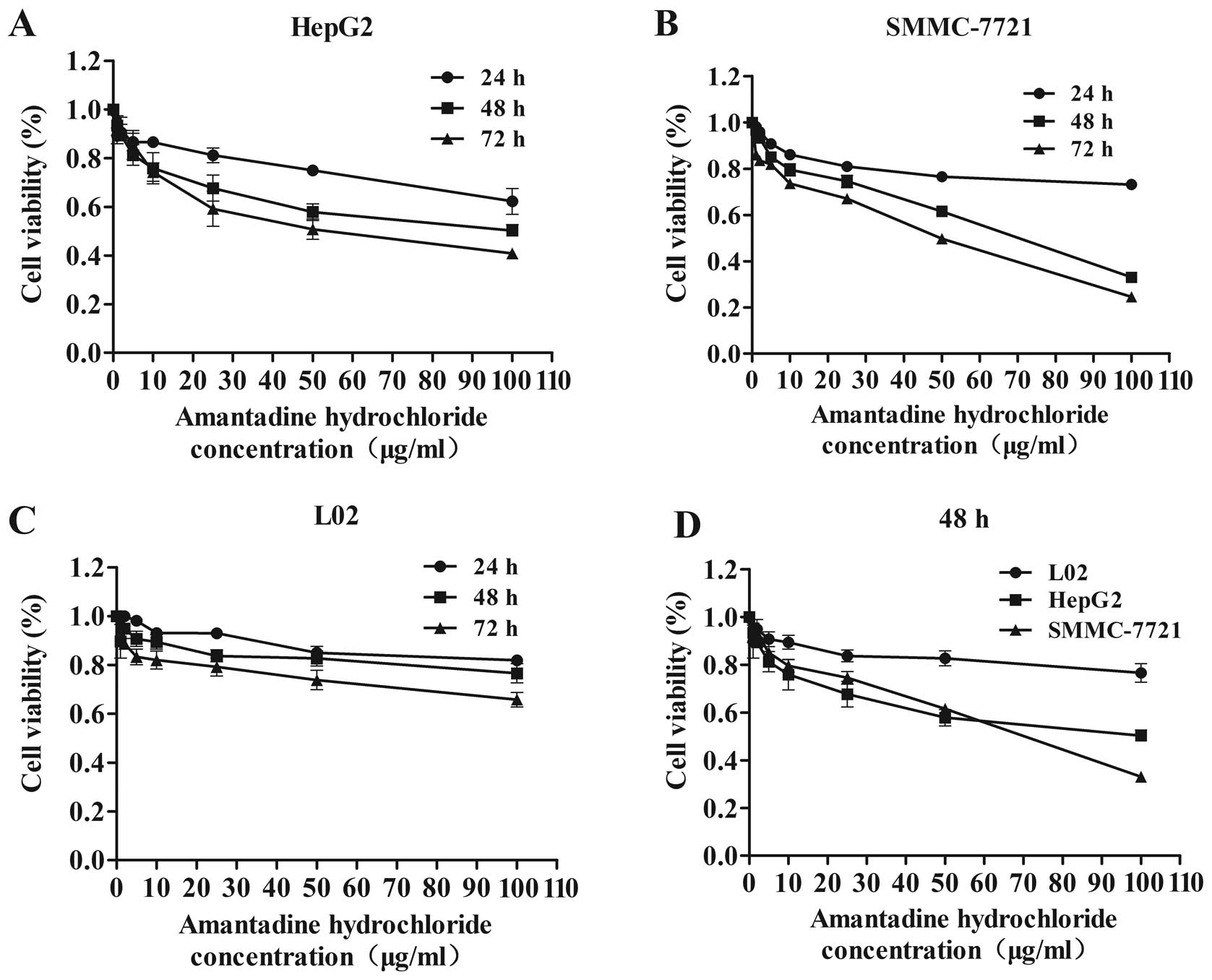
To detect the anti-proliferative effect of
amantadine, HepG2, SMMC-7721 and L02 cells were treated with a
variety of amantadine concentrations (0, 1, 2, 5, 10, 25, 50 and
100 µg/ml) for 24, 48 and 72 h, and were analyzed by the MTT
assay. Although amantadine reduced the viability of HepG2,
SMMC-7721 and L02 cells, this reduction was greater in the HCC
cells (HepG2 and SMMC-7721) compared to the control (L02) cells
(Fig. 1). Amantadine inhibited
cellular proliferation in a time- and dose-dependent manner in
HepG2 and SMMC-7721 cells. Following 48 or 72 h amantadine
exposure, cell growth was significantly inhibited relative to 24 h
treatment. These results indicated that amantadine may be a
promising therapeutic agent for HCC.
Amantadine induces G0/G1 phase cell cycle
arrest
The effect of amantadine on cell cycle distribution
was examined by flow cytometry to investigate the mechanisms by
which it reduced tumor cell viability. Incubation with 10, 25, 50
and 75 µg/ml amantadine for 48 h significantly increased the
population of HepG2 and SMMC-7721 cells in the G0/G1 phase in a
dose-dependent manner. Amantadine (10, 25, 50 and 75 µg/ml)
treatment led to a significant decrease in the number of HepG2
cells in the S phase (Fig. 2). In
addition, the population of SMMC-7721 cells in the S phase was
markedly decreased at 25, 50 and 75 µg/ml amantadine
(Fig. 3).
Amantadine induces apoptosis of HCC
cells
In order to investigate whether amantadine had an
effect on cellular apoptosis, its potential proapoptotic activity
was examined in HepG2 and SMMC-7721 cells by flow cytometry using
Annexin V-FITC and PI staining. After 48 h exposure to 0, 10, 25,
50 or 75 µg/ml amantadine, the percentage of apoptotic HepG2
and SMMC-7721 cells (early- and late-stage apoptosis) markedly
increased in a dose-dependent manner. Following amantadine
treatment at 10 to 75 µg/ml for 48 h, the percentages of
apoptotic cells were markedly increased from 8.4% in non-treated
control cells to 10.9, 13.1, 20.7 and 27.5% in HepG2, respectively
(Fig. 4). In SMMC-7721 cells, the
apoptosis index increased from 6.7% in non-treated control cells,
to 7.6, 13.1, 21.1 and 31.9% in the amantadine-pretreated cells
(10, 25, 50 and 75 µg/ml, respectively) (Fig. 5).
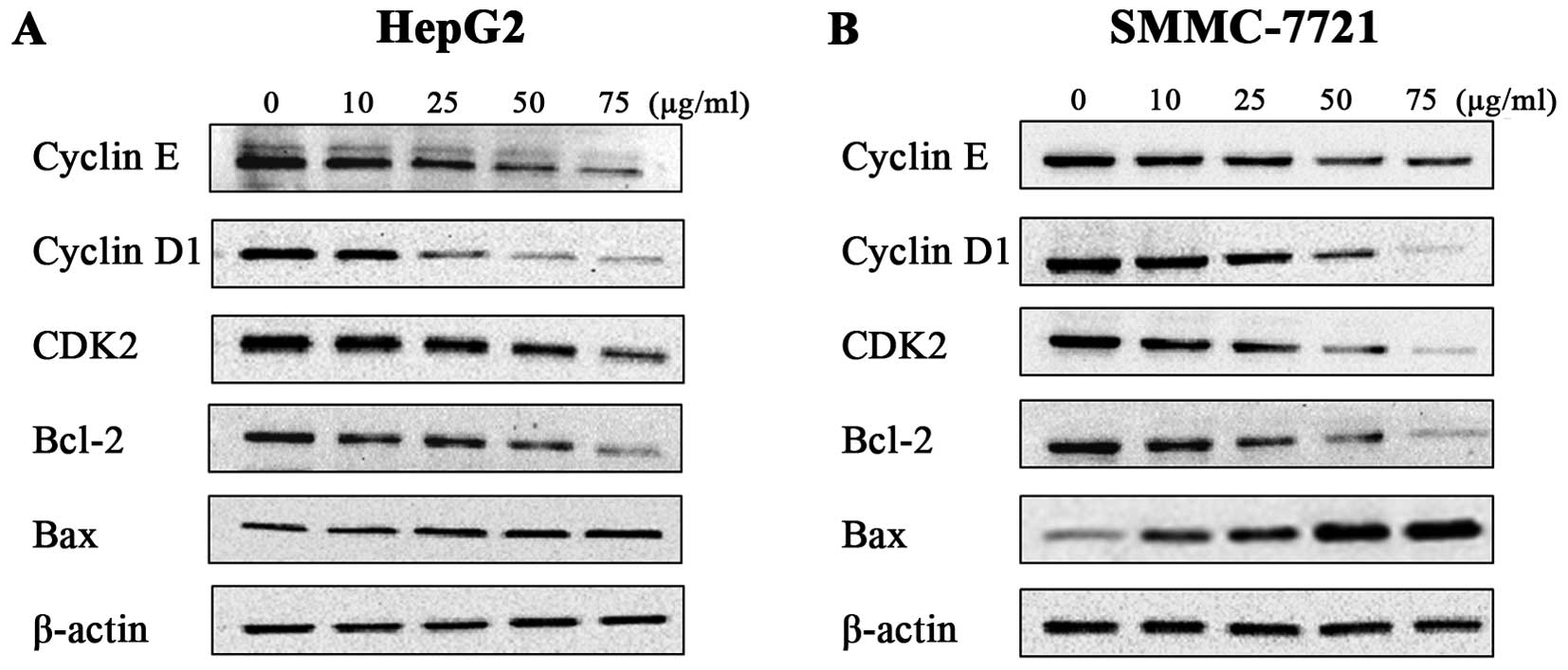
Amantadine regulates the expression of
cell cycle- and apoptosis-related proteins and genes
To further define the effects of amantadine on cell
cycle regulation and apoptosis, the expression levels of the genes
(Fig. 6) and proteins (Fig. 7) involved in cell cycle regulation
and the apoptosis pathway were examined in HepG2 and SMMC-7721
cells. The cyclin E-CDK2 complex and cyclin D1 are critical
regulatory factors in the G1/S phase cell cycle transition. After
48 h incubation with amantadine, HepG2 and SMMC-7721 cells showed
downregulation of cyclin D1, cyclin E and CDK2 in comparison to the
control group. The cyclin D1, cyclin E and CDK2 genes were
similarly decreased compared to the control. These results
confirmed the flow cytometry results demonstrating the
amantadine-induced G0/G1 phase cell cycle arrest.
Western blotting was used to validate the changes in
the protein levels of apoptotic regulators Bcl-2 (antiapoptotic)
and Bax (proapoptotic); downregulation of the Bcl-2/Bax ratio is a
known molecular switch initiating apoptosis. The results showed
that a decrease in Bcl-2 levels was accompanied by an increase of
Bax levels in HepG2 and SMMC-7721 cells treated with amantadine (0,
10, 25, 50 and 75 µg/ml) for 48 h. In addition, RT-qPCR
revealed an increase in Bax and decrease in Bcl-2 genes. Thus, the
Bcl-2/Bax ratios in HepG2 and SMMC-7721 cells were lower compared
to the control cells, suggesting induction of apoptosis by
amantadine.
Discussion
To the best of our knowledge, this is the first
study to investigate the anticancer effects of amantadine on HCC
in vitro. Amantadine could exert its antitumor properties by
markedly inhibiting cellular proliferation and inducing apoptosis
in the HCC cell lines (HepG2 and SMMC-7721), with less
proliferative inhibition of normal hepatocellular (L02) cells.
Further studies revealed that amantadine could inhibit cell growth
by modulating cyclin D1, cyclin E and CDK2 and inducing apoptosis
via regulation of Bax and Bcl-2.
The development and progression of HCC is a
multistage process involving regulation of genes that are crucial
to cell cycle control, cell growth, apoptosis and cell migration
(15). Transformation and
uncontrolled cell growth caused by cell cycle dysregulation are
some of the fundamental biological features of malignancy. The cell
cycle is regulated by signaling pathways mediated by different
cyclins and CDKs. Cyclins positively regulate cell cycle
progression and function by forming a complex with CDKs (16). Cyclin D1 acts as a growth sensor
and provides a link between mitogenic stimuli and the cell cycle.
Mutated cyclin D1 expression has been identified in numerous human
cancers (17,18). Cyclin E is one of the main
limiting factors of G1/S phase transition, which has a crucial role
in cellular proliferation; overexpression of cyclin E can
accelerate G1 phase procession of the cell (19). CDK2 is a Ser/Thr kinase and CDK2
induces downstream processes by phosphorylating selected proteins
during G1/S phase transition. Cyclin E complexes with CDK2 to
regulate the progression of cells from G1 into the S phase
(17). In the present study, flow
cytometric analysis clearly revealed that amantadine significantly
arrested the two HCC cell lines in the G0/G1 phase. Furthermore,
western blotting and RT-qPCR demonstrated that the
amantadine-induced G0/G1 phase cell cycle arrest was closely
associated with a marked downregulation in the protein and gene
levels of cyclin E, cyclin D1 and CDK2, suggesting that inhibiting
proliferation is a main anticancer mechanism of amantadine.
Apoptosis is essential to cell growth and has an
important role in oncogenesis. Apoptosis has long been regarded as
a barrier to carcinogenesis (20)
and its induction is crucial to the suppression of tumorigenesis.
The present study showed that amantadine markedly increased the
percentage of apoptotic cells in the two HCC cell lines. Bcl-2
(antiapoptotic) and Bax (proapoptotic) are two critical regulators
of cellular apoptosis (21).
Overexpression of Bcl-2 results in apoptotic resistance, whereas
overexpression of Bax increases apoptosis. The ratio of Bcl-2/Bax
is vital for determining whether cells undergo apoptosis (22–24). In the present study, Bcl-2
expression significantly decreased with the increased expression of
Bax following amantadine treatment, thereby reducing the ratios of
Bcl-2/Bax in HepG2 and SMMC-7721 cells and further confirming the
flow cytometry results. Thus, the present data indicate that
amantadine induces apoptosis by regulating the expression of Bcl-2
and Bax.
In conclusion, the present results revealed that the
HCC cell lines, HepG2 and SMMC-7721, were highly sensitive to
growth suppression by amantadine, which is associated with cell
cycle arrest and apoptosis induction. Amantadine exerts its
anticancer effects by downregulating the expression of cyclin E,
cyclin D1 and CDK2, influencing cell cycle progression, and
inducing apoptosis by increasing the level of proapoptotic Bax and
decreasing antiapoptotic Bcl-2 levels. Thus, the present study
provides insight into a new prospective HCC therapeutic. Further
studies investigating amantadine suppression of tumor cell
proliferation and induction of apoptosis in vivo are clearly
warranted, in addition to examination of the precise mechanisms
associated with the antitumor effects of amantadine.
Acknowledgments
The present study was supported by funds from the
National Natural Science Foundation of China (grant no. 81170176),
the Scientific Research Foundation for the Returned Overseas
Chinese Scholars, State Education Ministry (grant no. 2012-08), the
Shaanxi Province Science and Technology Plan Project (grant no.
2014KTCL03-10), and the Specialized Research Fund for the Doctoral
Program of Higher Education (grant no. 20130201130008).
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davis GL, Alter MJ, El-Serag H, Poynard T
and Jennings LW: Aging of hepatitis C virus (HCV)-infected persons
in the United States: A multiple cohort model of HCV prevalence and
disease progression. Gastroenterology. 138:513–521. 521.e511–516.
2010. View Article : Google Scholar
|
|
5
|
Parkin DM: The global health burden of
infection-associated cancers in the year 2002. Int J Cancer.
118:3030–3044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye S: Expert consensus on antiviral
therapy to treat hepatitis B/C virus-related hepatocellular
carcinoma. Zhonghua Gan Zang Bing Za Zhi. 22:321–326. 2014.In
Chinese. PubMed/NCBI
|
|
7
|
Balgi AD, Wang J, Cheng DY, Ma C, Pfeifer
TA, Shimizu Y, Anderson HJ, Pinto LH, Lamb RA, DeGrado WF, et al:
Inhibitors of the influenza A virus M2 proton channel discovered
using a high-throughput yeast growth restoration assay. PLoS One.
8:e552712013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Das K: Antivirals targeting influenza A
virus. J Med Chem. 55:6263–6277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cady SD, Schmidt-Rohr K, Wang J, Soto CS,
Degrado WF and Hong M: Structure of the amantadine binding site of
influenza M2 proton channels in lipid bilayers. Nature.
463:689–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JY, Oh S, Kim JM, Kim JS, Oh E, Kim
HT, Jeon BS and Cho JW: Intravenous amantadine on freezing of gait
in Parkinson's disease: A randomized controlled trial. J Neurol.
260:3030–3038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quarato G, Scrima R, Ripoli M, Agriesti F,
Moradpour D, Capitanio N and Piccoli C: Protective role of
amantadine in mitochondrial dysfunction and oxidative stress
mediated by hepatitis C virus protein expression. Biochem
Pharmacol. 89:545–556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
StGelais C, Tuthill TJ, Clarke DS,
Rowlands DJ, Harris M and Griffin S: Inhibition of hepatitis C
virus p7 membrane channels in a liposome-based assay system.
Antiviral Res. 76:48–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lai CL and Yuen MF: Prevention of
hepatitis B virus-related hepatocellular carcinoma with antiviral
therapy. Hepatology. 57:399–408. 2013. View Article : Google Scholar
|
|
14
|
Kimer N, Dahl EK, Gluud LL and Krag A:
Antiviral therapy for prevention of hepatocellular carcinoma in
chronic hepatitis C: Systematic review and meta-analysis of
randomised controlled trials. BMJ Open. 2:22012. View Article : Google Scholar
|
|
15
|
Xiao F, Zhang W, Chen L, Chen F, Xie H,
Xing C, Yu X, Ding S, Chen K, Guo H, et al: MicroRNA-503 inhibits
the G1/S transition by downregulating cyclin D3 and E2F3 in
hepatocellular carcinoma. J Transl Med. 11:1952013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lamb R, Lehn S, Rogerson L, Clarke RB and
Landberg G: Cell cycle regulators cyclin D1 and CDK4/6 have
estrogen receptor-dependent divergent functions in breast cancer
migration and stem cell-like activity. Cell Cycle. 12:2384–2394.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murray AW: Recycling the cell cycle:
Cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Youn M-J, Kim JK, Park SY, Kim Y, Kim SJ,
Lee JS, Chai KY, Kim HJ, Cui MX, So HS, et al: Chaga mushroom
(Inonotus obliquus) induces G0/G1 arrest and apoptosis in human
hepatoma HepG2 cells. World J Gastroenterol. 14:511–517. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang RA, Li QL, Li ZS, Zheng PJ, Zhang HZ,
Huang XF, Chi SM, Yang AG and Cui R: Apoptosis drives cancer cells
proliferate and metastasize. J Cell Mol Med. 17:205–211. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Zhu Y, He H, Lou L, Ye W, Chen Y and
Wang J: Synergistically killing activity of aspirin and histone
deacetylase inhibitor valproic acid (VPA) on hepatocellular cancer
cells. Biochem Biophys Res Commun. 436:259–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsumoto H, Wada T, Fukunaga K, Yoshihiro
S, Matsuyama H and Naito K: Bax to Bcl-2 ratio and Ki-67 index are
useful predictors of neoadjuvant chemoradiation therapy in bladder
cancer. Jpn J Clin Oncol. 34:124–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oshikawa T, Okamoto M, Ahmed SU, Tano T
and Sato M: The relationship between gene expression of Bcl-2 and
Bax and the therapeutic effect in oral cancer patients. Gan To
Kagaku Ryoho. 33:1723–1725. 2006.In Japanese.
|
|
24
|
Adhya AK, Srinivasan R and Patel FD:
Radiation therapy induced changes in apoptosis and its major
regulatory proteins, Bcl-2, Bcl-XL, and Bax, in locally advanced
invasive squamous cell carcinoma of the cervix. Int J Gynecol
Pathol. 25:281–287. 2006. View Article : Google Scholar : PubMed/NCBI
|