Introduction
Hepatocellular carcinoma (HCC) is a prevalent
malignancy and the most common malignant tumor of the liver. It
requires an adequate blood supply to support its growth in
vivo. Vasculogenic mimicry (VM) is an alternative pathway for
the blood supply. VM is formed by tumor cells that mimic
endothelial cells to form cell extracellular matrix-rich channels
[periodic acid Schiff (PAS)-positive], which are sinusoidal
structures that surround clusters of tumor cells. Studies have
demonstrated that tumors, including HCC, may develop alternative
pathways, such as VM and mosaic vessels (1,2).
Hypoxia has an important role in various functional
responses in tumor cells, including apoptosis and angiogenesis
(3,4). A previous study has demonstrated
that hypoxia is critical in VM formation (5). Hypoxia can directly upregulate the
expression of HIF-1 (SETD2), and HIF-1 can further combine to the
promoter of Twist1 and promote its transcript expression. Twist1 is
a basic helix-loop-helix transcription factor that can form homo-
or hetero-dimers and bind the Ndel E-box element and activate or
repress its target genes. Previous evidence has revealed the
important role of Twist1 in cancer metastasis, as shown by its
overexpression in human cancers, induction of
epithelial-mesenchymal transition (EMT) and association with a more
malignant phenotype (6).
Previous studies identified that Bmi1 is directly
regulated by the EMT regulator Twist1 (7). Bmi1 is a polycomb group family
member that maintains self-renewal and is frequently overexpressed
in human cancers (8). Twist1 and
Bmi1 are mutually essential in promoting cell stemness, and the
co-overexpression of Twist1 and Bmi1 can promote the
tumor-initiating capability of cancer cells (7). This functional connection between
Twist1 and Bmi1 provides a novel insight into the common mechanism
mediating EMT and cancer stemness.
Although hypoxia has been reported to promote VM
(5), the underlying mechanism
remains unclear. In the present study, we hypothesized that hypoxia
may lead to Twist1 and Bmi1 upregulation, and the interaction
between Twist1 and Bmi1 may promote VM formation by inducing EMT
and cell stemness.
Materials and methods
Plasmid
The plasmid pEGFP-Twist1 was as previously described
(9). The HepG2 cells were
transfected with pEGFP-Twist1 or pcDNA control vector
(pcDNA-negative).
Small interfering RNAs (siRNAs) encoding oligos
against human Twist1 were as previously described (9). A non-silencing small interfering RNA
sequence (target sequence, AATTCT CCGAACGTGTCACGT) was used as the
negative control, as previously described (10). siRNA was used to block the Twist1
expression in Bel7402 cells.
Western blot analysis
Cells were washed using phosphate-buffered saline
(PBS), and 10% sodium dodecyl sulfate (SDS) was used to lyse the
cells. The whole cell lysates were resolved via SDS-polyacrylamide
gel electrophoresis and transferred onto polyvinylidene fluoride
membranes (Millipore, Temecula, CA, USA). The membranes were
blocked with skimmed milk powder and incubated with primary
antibodies, followed by incubation with a secondary antibody, goat
anti-rabbit (ZF-2301), goat anti-mouse (ZF-2305) or rabbit
anti-goat (ZF-2305) immunoglobulin G-horseradish peroxidase
(1:2,000; Zhongshan Chemical Co., Beijing, China). Protein
expression was measured using an enhanced chemiluminescence
detection kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
The primary antibodies for Twist1 (SC-15393; 1:200), E-cadherin
(SC-7870; 1:200), Oct4 (SC-8629; 1:200) and cluster of
differentiation 44 (CD44; SC-53298; 1:200) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), Bmi1 (Ab14389;
1:1,000), vimentin (42011; 1:500; Epitomics), vascular
endothelial-cadherin (VE-cadherin; AB-33168; 1:200) and β-actin
(ab8226; 1:2,000) were purchased from Abcam (Cambridge, MA, USA),
and fms-related tyrosine kinase 1 (FLT1; RB-1527; 1:200) and kinase
insert domain receptor (KDR; RB-1526; 1:200) were purchased from
Thermo Fisher Scientific (Waltham, MA, USA).
Cell culture and hypoxia treatment in
vitro
Human liver cell lines HepG2 and Bel7402 were
purchased from the American Type Culture Collection (Manassas, VA,
USA). These cells were cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS;
Invitrogen, Carlsbad, CA, USA). Cobalt chloride (CoCl2)
was used to simulate hypoxic conditions. Cells were seeded in
dishes or plates at 300 cells/mm2 and grew for 24 h in
complete medium. The medium was removed, and the cells were washed
with PBS. The cells were treated with 100 µM
CoCl2 and were subsequently incubated for different
times as required.
3D culture assay
VM formation in vitro was evaluated using 3D
culture. In the 3D culture assay, Matrigel (BD Biosciences, San
Jose, CA, USA) was thawed at 4°C and 30 µl was quickly added
to each well of a 96-well plate and allowed to solidify for 12 h at
37°C in a humidified 5% CO2 incubator. Tumor cells in
complete medium were subsequently seeded onto the gel and incubated
at 37°C for 24 h. The formation of capillary-like structures was
observed under a phase-contrast microscope (magnification, ×200).
Each experiment was performed in triplicate.
Wound-healing assay
In wound-healing assays, different groups of cells
were plated in 24-well culture plates and allowed to grow for 24 h.
A micropipette was used to create a straight scratch in the center
of each well. Cell migration ability was assessed by measuring the
movement of cells into the scratch. The migration rate (MR) was
monitored after 12, 24, 36 and 48 h. The following formula was used
to calculate the MR at different time-points: MR = (d – d′)/d (d is
the length of the wound at 0 h, and d′ is the length at other
different time-points).
Invasion assay
In the invasion assay, 1×105 cells in 100
µl of DMEM without FBS were seeded in the upper 24 wells
coated with Matrigel matrix (1 mg/ml; BD Biosciences) containing
polyethylene terephthalate filters with 8-µm porosity
(Invitrogen). The lower chamber was filled with medium containing
10% FBS. The cells were incubated for 48 h and the non-invading
cells on the upper surface of the membrane were removed. The cells
that invaded through the Matrigel matrix and attached to the bottom
surface of the membrane were fixed by methanol and stained by 0.5%
crystal violet. The number of invading cells was counted using an
inverted light microscope (Nikon, Tokyo, Japan).
Sphere culture assay
The sphere culture method was as described
previously (11). Briefly, single
HCC cells were seeded in 12-well plates coated with
poly(2-hydroxyethylmeth-acrylate) (poly-HEMA; Sigma-Aldrich, St.
Louis, MO, USA) at a density of 1×104 viable cells/ml in
a serum-free medium (DMEM-F12 1: 1 media; Gibco, Grand Island, NY,
USA) supplemented with 1xB27 (Invitrogen), 10 ng/ml epidermal
growth factor and 20 ng/ml fibroblast growth factor 2 (both from
Peprotech, Rocky Hill, NY, USA), and 1% pen/strep (Invitrogen). The
culture medium was replaced or supplemented with additional growth
factors every 3 days.
Clone formation assay
In the clone formation assay, each well of a 6-well
plate was seeded with 1,000 cells. The plates were incubated at
37°C and 5% CO2 for 12 days, fixed with methanol and
stained with 0.5% crystal violet. The number of clones was counted
under the microscope.
Murine xenograft model
A total of 40 five-week-old male BALB/c nude mice
were purchased from HuaFukang Biological Technology Co., Ltd,
Beijing, China. All the studies on mice were carried out in
accordance with the Guidelines of the Laboratory Animal of Tianjin
Medical University (Tianjin, China). The mice were divided into two
groups; the HepG2 and Bel7402 groups. All the animals received
anesthesia with 2% pentobarbital sodium (60 mg/kg) prior to
surgery. The overall surgical mortality rate was 0%. Through a
1–1.5-cm incision in the right groin, the femoral artery was
carefully separated from the vein and nerve fiber, and ligated by
8–0 silk with a needle placed along the vessel during ligation. The
needles were 0.25 mm in diameter and were removed following
ligation. The left limb was opened without femoral artery ligation
as a control. Subsequently, 107 cells suspended in 0.1
ml of PBS were injected intramuscularly into both sides. The mice
were monitored, and tumor sizes were measured daily using a Vernier
caliper. The following formula was used to calculate tumor volume:
Tumor volume = 1/2ab2 (a is the length and b is the
width of the tumor). When the observation was complete, the mice
were sacrificed. Tumors were harvested and fixed by formalin.
Immunohistochemistry (IHC) and
endomucin/PAS double-staining
Prior to immunostaining, 4-µm paraffin
sections were deparaffinized in xylene and rehydrated by a graded
series of aqueous ethanol solutions. Endogenous peroxidase activity
was blocked with 3% hydrogen peroxide in 100% methanol for 30 min
at room temperature. The sections were pretreated in a microwave,
blocked and incubated using a series of antibodies. The staining
systems used in the present study were PicTure PV6000 (Zhongshan
Chemical Co.). In endomucin/PAS double-staining, the sections were
washed with running water for 5 min and were subsequently incubated
with PAS for 15 min after IHC for endomucin was performed. Finally,
the sections were counterstained with hematoxylin, dehydrated and
mounted. PBS was used instead of the primary antibodies for the
negative control. The results were quantified according to the
method described by Bittner et al (12).
Statistical analysis
All the data in the study were evaluated with SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). Student's t-test was
performed to determine differences between two groups in cell
functional assays. Paired t-test was performed to compare the
hypoxia and control groups in vivo. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of hypoxia in vitro
Hypoxia results in Twist1-Bmi1
upregulation
HepG2 and Bel7402 cells were used in the study to
verify the association of hypoxia with VM in HCC cells in
vitro. HepG2 and Bel7402 cells were cultured under hypoxic
conditions, and Twist1 and Bmi1 protein expression levels were
detected by western blot analysis after 0, 12, 24, 36, 48 or 60 h
of hypoxia. The two groups demonstrated a significant increase in
Twist1 and Bmi1 expression with time under hypoxia (Fig. 1A).
 | Figure 1Effects of hypoxia and transfection
on hepatocellular carcinoma cell lines. (A) Protein expression
levels of Twist1, Bmi1, stem cell, EMT and VM-associated markers in
HepG2 and Bel7402 cells after different incubation times of hypoxia
(0, 12, 24, 36, 48 and 60 h). β-actin was used as loading control.
The two groups demonstrated a significant increase in Twist1 and
Bmi1 expression with time under hypoxia. The expression of
E-cadherin in the two cell lines was downregulated and the
expression of vimentin in HepG2 was upregulated by hypoxia,
indicative of EMT. Vimentin expression was not observed in Bel7402
cells. The expression of stem cell markers and VM-associated
markers was also promoted by hypoxia. (B) Effect of hypoxia on VM
formation in HCC cells cultured on Matrigel matrix (magnification,
×100; bars, 100 µm). HepG2 cells did not form typical
tube-like structures, whereas Bel7402 cells formed a modest level
of tube formation. After hypoxia was administered, VM formation was
significantly induced. The arrows show the tube-like structures in
the 3D culture. (C) Western blot results showed that the Bmi1
expression level was upregulated following Twist1 transfection and
downregulated following siTwist1 transfection. The results also
revealed that vimentin expression was increased and E-cadherin
expression was decreased in the HepG2 cells following Twist1/Bmi1
upregulation. By contrast, E-cadherin expression was increased in
Bel7402 cells following Twist1/Bmi1 downregulation. The stem cell
marker CD44 and Oct4 expression was also upregulated by Twist1/Bmi1
upregulation and downregulated by Twist1/Bmi1 downregulation. CD44,
cluster of differentiation 44; EMT, epithe lial-mesenchymal
transition; HCC, hepatocellular carcinoma; VM, vasculogenic
mimicry. |
Hypoxia induces EMT and cell
stemness
Accompanied with Twist1/Bmi1 upregulation, the
expression of E-cadherin in the two cells lines was downregulated
and the expression of vimentin in HepG2 was upregulated by hypoxia,
indicative of EMT (Bel7402 cells did not show any vimentin
expression; Fig. 1A). The
expression of stem cell markers was also promoted by hypoxia. Stem
cell marker Oct4 (OCT3/4, POU5F1) is a member of the POU
transcription factor family, and it has a critical role in
maintaining cell stemness (13).
CD44 is a major extracellular matrix adhesion molecule that is
widely used as a cancer stem cell marker in various types of
cancer, including HCC (14,15). Western blot analysis showed that
hypoxia increased the expression of CD44 and Oct4 in HepG2 and
Bel7402 cells (Fig. 1A).
Hypoxia promotes VM formation ability
of HCC cells
The expression of VM-associated markers VE-cadherin,
FLT1 and KDR were also elevated by hypoxia (Fig. 1A). The ability of VM formation on
Matrigel of HepG2 and Bel7402 cells under normoxia and cells that
underwent 48 h of hypoxia was assessed. Hypoxia promoted VM
formation in the HepG2 and Bel7402 cells (Fig. 1B).
Twist1-Bmi1 cooperation is a critical
factor in VM formation Bmi1 expression is regulated by Twist1
The endogenous expression of Twist1 in the HepG2
cell line was significantly lower compared with the Bel7402 cell
line. Thus, HepG2 cells were infected with Twist1 plasmid and the
Bel7402 cells with siTwist1 plasmid. Western blot analysis showed
that Bmi1 expression was upregulated in HepG2-Twist1 cells and
down-regulated in Bel7402-siTwist1 cells (Fig. 1C), confirming that Bmi1 expression
was regulated by Twist1.
Upregulation of Twist1 and Bmi1
induces EMT and cell stemness
Western blot analysis showed that in HepG2 cells,
the upregulation of Twist1 promoted vimentin expression and
inhibited E-cadherin expression, indicative of EMT. In Bel7402
cells, the downregulation of Twist1 promoted E-cadherin expression.
The expression levels of stem cell marker CD44 and Oct4 were
upregulated in HepG2-Twist1 cells and downregulated in
Bel7402-siTwist1 cells (Fig.
1C).
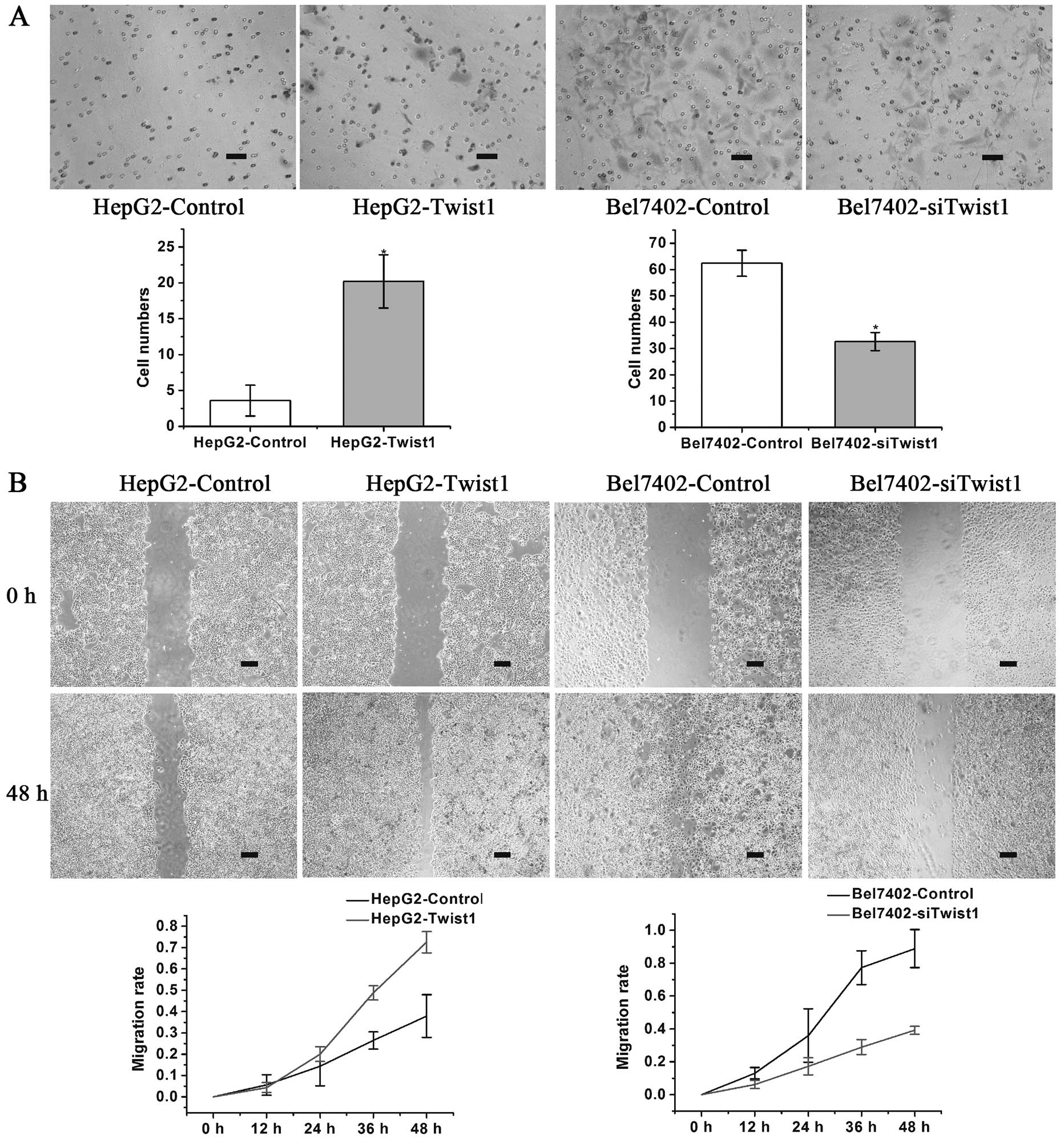
The tumor cells that exhibited the ability to
undergo EMT also had a greater ability to invade and metastasize.
The HepG2-control, HepG2-Twist1, Bel7402-control and
Bel7402-siTwist1 cells were subsequently analyzed for functional
changes in invasion and migration ability. The in vitro
invasion assay demonstrated that upregulation of Twist1 in HepG2
cells significantly promoted cell invasion, while down-regulation
of Twist1 in Bel7402 cells significantly inhibited cell invasion
(Fig. 2A). In the wound-healing
assay, the MR at the terminal phase (48 h) increased in the
HepG2-Twist1 group and decreased in the Bel7402-siTwist1 group
compared with the control groups (Fig. 2B).
Sphere culture and clone formation assays were
performed to evaluate cell stemness. In the sphere culture assay,
HepG2-control, HepG2-Twist1, Bel7402-control and Bel7402-siTwist1
cells were seeded in plates coated with poly-HEMA at a density of
1×104 cells/ml using a commonly used suspension culture
containing 1% methylcellulose. After 4–7 days of culture, a viable
sphere formation was observed in the HepG2-Twist1 group, however,
no spheres were formed in the HepG2-control group (Fig. 3A). Neither Bel7402-control nor
Bel7402-siTwist1 group formed spheres (data not shown). In the
clone formation assay, Twist1 upregulation in HepG2 cells resulted
in the significant promotion of cell colony formation, while in
Bel7402 cells, Twist1 downregulation significantly inhibited colony
formation (Fig. 3B).
Expression levels of Twist1 and Bmi1
influences VM formation
Western blot analysis showed that the expression
levels of VM-associated markers, VE-cadherin, FLT1 and KDR, were
upregulated in HepG2-Twist1 cells and downregulated in
Bel7402-siTwist1 cells (Fig. 3C).
The formation of capillary-like structures was assessed by the 3D
culture assay. HepG2-control cells could not form any tube-like
structures, while HepG2-Twist1 formed typical tube-like structures
on the surface of the Matrigel medium. Bel7402-control cells showed
modest tube-like structures while Bel7402-siTwist1 cells showed no
tube-like structures (Fig.
3D).
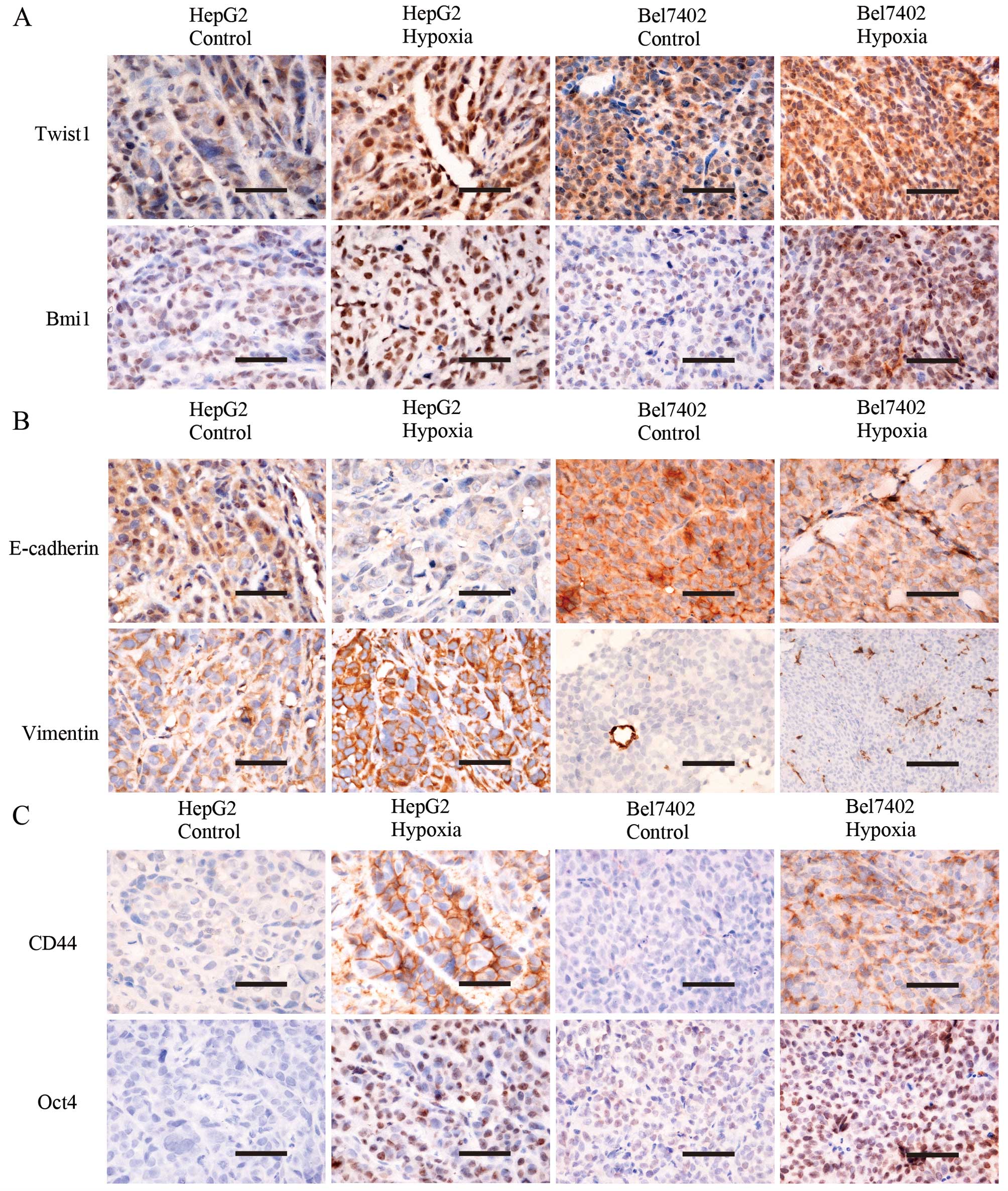
Effect of hypoxia in vivo
Hypoxia promotes Twist1 and Bmi1
expression and induces EMT and cell stemness in vivo
An incomplete femoral artery ligation murine model
was established to investigate the effects of hypoxia in
vivo. A total of 14 mice carried tumors in the bilateral
hindlimbs in the HepG2 group and there were 7 mice in the Bel7402
groups. Using IHC, Twist1, Bmi1, EMT marker and stem cell marker
expression was examined in the engrafted tumors in the HepG2
control, HepG2 hypoxia, Bel7402 control, and Bel7402 hypoxia
groups. Cells exhibited positive staining for Twist1 in the nucleus
and cytoplasm, Bmi1 in the nucleus, E-cadherin in the plasma
membrane, vimentin in the plasma membrane and cytoplasm, CD44 in
the plasma membrane, and Oct4 in the nucleus (Fig. 4). The expression of Twist1 and
Bmi1 expression in the hypoxia group was stronger compared with
that in the control group (P<0.05; Fig. 4A). The expression of E-cadherin
was downregulated and the expression of vimentin was upregulated in
the hypoxia group (tumor cells in Bel7402-engrafted tumors showed
no vimentin expression) (P<0.05; Fig. 4B). The expression of stem cell
marker CD44 and Oct4 was also stronger in the hypoxia group
compared with that in the control group in the HepG2- and
Bel7402-engrafted tumors (P<0.05; Fig. 4C).
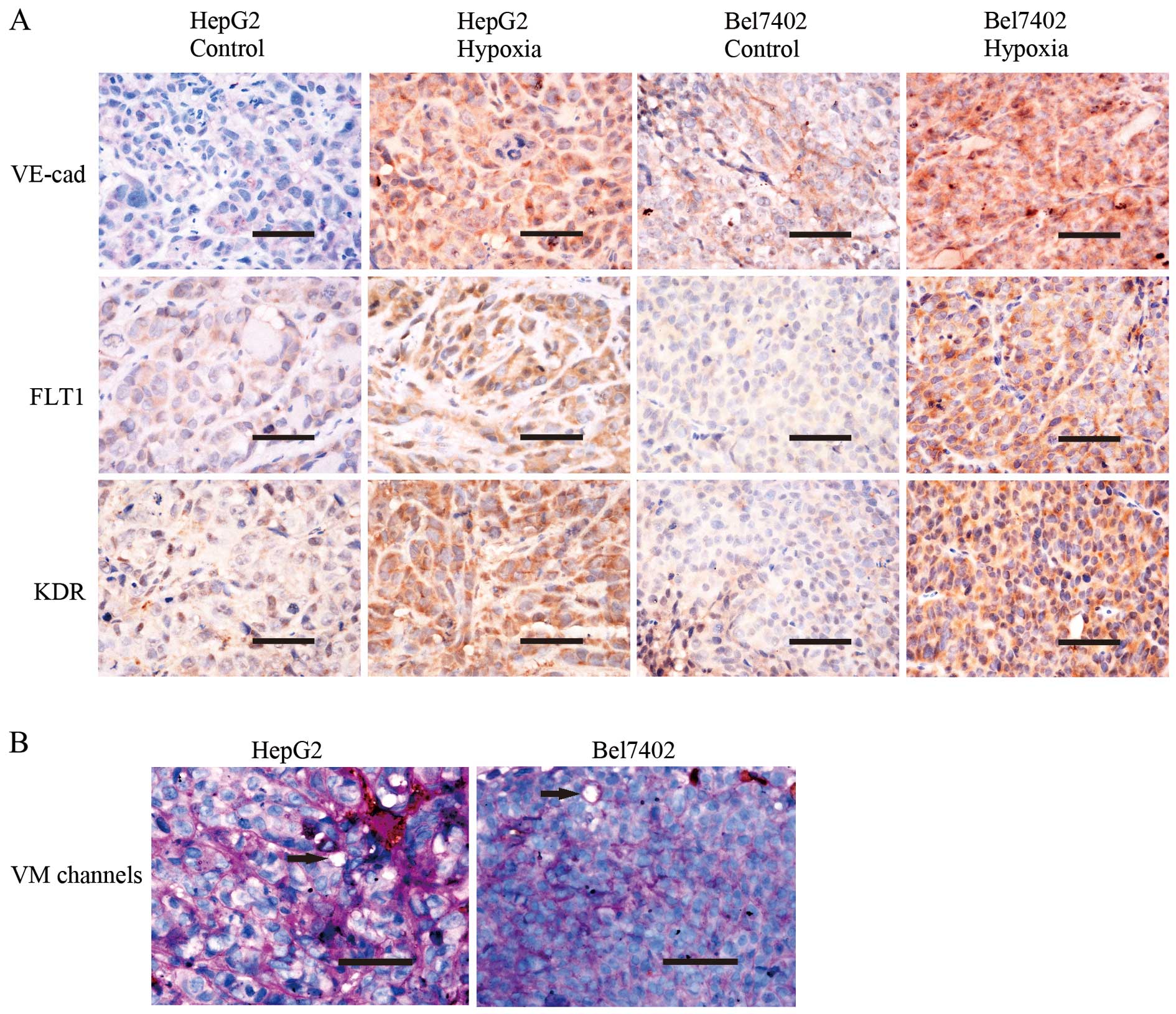
Hypoxia promotes VM in vivo
The expression levels of VM-associated markers,
including VE-cadherin, FLT1 and KDR, were examined using IHC
staining (Fig. 5A). The control
and hypoxia groups contained plasma membrane VE-cadherin-,
cytoplasmic VEGFR1- and cytoplasmic VEGFR2-positive tumor cells.
Tumors in the hypoxia group expressed more of these proteins
compared with the control group (P<0.05). Using endomucin/PAS
double-staining, VM channels (Fig.
5B) were observed in the engrafted tumors. VM channels were
formed by tumor cells lined with PAS-positive substances, and these
cells were negative for endomucin. Hypoxia promoted VM formation.
The HepG2 hypoxia group had 5.14±1.505 VM channels, whereas the
control group had 3.00±0.756 VM channels (P<0.05). The Bel7402
hypoxia group had 3.29±0.700 VM channels, whereas the control group
had 1.43±0.495 VM channels (P<0.05).
Discussion
Hypoxia has a pivotal role in the microenvironment,
and a hypoxic microenvironment could affect tumor angio-genesis,
metastasis, metabolism and the survival of tumor cells (5,16,17). CoCl2 is a
hypoxia-mimicking reagent that is commonly used in hypoxia studies
in vitro (18,19). It mimics hypoxic conditions by
inhibiting the activity of prolyl hydroxylase, a critical enzyme in
the oxygen-sensing pathway (20).
In the present study, CoCl2 was used in cell cultures to
simulate constant hypoxia. In the in vivo experiments,
incomplete femoral artery ligation was performed in nude mice to
create hypoxic conditions, as serious gangrene could occur
following complete femoral artery ligation and excision in the
hindlimb of nude mice (21).
Hypoxia has been proved to promote VM formation
(5). VM was first reported in
highly aggressive uveal melanoma in 1999 by Maniotis et al
(22). VM has been found in HCC
samples, and studies have reported that VM is associated with
metastasis and a shorter survival period in HCC (23). Tumor cells that formed the unique
structure of VM channels were directly exposed to the bloodstream,
so they could easily metastasize to distant sites by entering the
microcirculation. This discovery may explain the elevated risk of
metastasis, tumor recurrence and shorter survival period in
patients with VM-positive HCC. Hypoxia is a crucial factor in VM
formation (5,9).
The EMT regulator Twist1 has been reported to
regulate the expression of Bmi1 directly, which provides a novel
insight into the association between EMT and cancer stemness
(7). The Twist1-Bmi1 link was
responsible for inducing EMT and cell stemness under hypoxia
conditions. EMT is a pivotal developmental program that is often
activated in cancer development (24,25). A number of changes may occur to
epithelial cells during EMT, including loss of epithelial
characteristics, acquisition of mesenchymal phenotypes, and ability
to invade, resist apoptosis and disseminate. Twist1 is an EMT
regulator that regulates EMT in various tumors (26,27), and can be upregulated by hypoxia.
Twist1 can repress E-cadherin and promote mesenchymal markers
expression (28). Bmi1 is
directly regulated by Twist1. Twist1 and Bmi1 are mutually
essential in promoting cell stemness, and the co-overexpression of
Twist1 and Bmi1 can promote the tumor-initiating capability of
cancer cells (7). The present
study also showed that the expression of CD44 and Oct4 was
upregulated following Twist1-Bmi1 cooperation. Oct4 has an
important role in maintaining stemness (29). CD44 is a major extracellular
matrix adhesion molecule that is widely used as a cancer stem cell
marker in various cancer types, including HCC (14,15). Bmi1 has been reported to be
co-expressed with CD44 in cancer stem cells (CSCs) (30,31). Thus, the present study showed that
cell stemness was induced by hypoxia. Stemness and EMT are
interconnected processes. Twist1/Bmi1-induced stemness may be an
important section of the EMT progress. The epithelial cells may
first obtain high levels of stemness, and subsequently, they can
differentiate toward mesenchymal-like cells. Ectopic expression of
Bmi1 in normal nasopharyngeal epithelial cells is sufficient to
cause EMT (32). A recent
breakthrough in metastasis studies demonstrated that induction of
EMT also generates cells with stem-like properties (33,34). The mutual promotion of these two
processes is the basis of VM formation.
The present study demonstrated increased VM
formation following the induction of EMT and cell stemness. EMT and
stemness can induce VM formation. Twist1 can induce EMT, and EMT
can contribute to tumor cell plasticity; the general mechanism
behind this phenomenon involves inhibiting E-cadherin transcription
caused by Twist1 interacting with its promoter and further
induction of the expression of downstream mesenchymal markers
(6). This process is
characteristic of VM formation, which involves tumor cells
mimicking endothelial cells, a type of mesenchymal cell, similar to
the EMT process. Tumor cell stemness is associated with VM
formation; CSCs can undergo asymmetric cell division, which results
in tumor cells that promote angiogenesis (35). CSCs may express an endothelial
phenotype and form vessel-like networks, thereby mimicking the
pattern of vascular networks in the embryo (36,37). Tumor cells expressing high
stemness can exist in HCC, and this stem cell population is
responsible for VM formation (38). The present study indicated that
Twist1-Bmi1 cooperation induced VM formation through induction of
EMT and tumor cell stemness. Increased expression of VM-associated
markers and VM formation was observed following induced EMT and
stemness in vitro and in vivo.
In conclusion, the present study demonstrated that
the effects of hypoxia on VM formation in HCC cells involved the
Twist1-Bmi1 connection, which induced EMT and stemness. Studies on
VM mechanisms could help us develop appropriate drugs to inhibit
tumor growth and metastasis by suppressing VM. Inhibitors could be
specially designed to inhibit Twist1, Bmi1 or their associated
markers.
Abbreviations:
|
CoCl2
|
cobalt chloride
|
|
CSCs
|
cancer stem cells
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HCC
|
hepatocellular carcinoma
|
|
IHC
|
immunohistochemistry
|
|
MR
|
migration rate
|
|
PAS
|
periodic acid Schiff
|
|
VE-cadherin
|
vascular endothelial-cadherin
|
|
VM
|
vasculogenic mimicry
|
Acknowledgments
The present study was supported by grants from the
Key Project of the National Natural Science Foundation of China
(no. 81230050), the National Natural Science Foundation of China
(nos. 81172046, 81173091 and 81201791), the Key Project of the
Tianjin Natural Science Foundation (no. 12JCZDJC23600), and the
Natural Science Foundation of Tianjin Education Commission (no.
20120103).
References
|
1
|
Bai XL, Zhang Q, Ye LY, Liang F, Sun X,
Chen Y, Hu QD, Fu QH, Su W, Chen Z, et al: Myocyte enhancer factor
2C regulation of hepatocellular carcinoma via vascular endothelial
growth factor and Wnt/β-catenin signaling. Oncogene. Oct
20–2014.Epub ahead of print. View Article : Google Scholar
|
|
2
|
Deng JH and Li HZ: Vasculogenic mimicry
and mosaic vessels and targeted therapy in renal cell carcinoma.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 33:462–467. 2011.In Chinese.
PubMed/NCBI
|
|
3
|
Zhu P, Ning Y, Yao L, Chen M and Xu C: The
proliferation, apoptosis, invasion of endothelial-like epithelial
ovarian cancer cells induced by hypoxia. J Exp Clin Cancer Res.
29:1242010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan L, Li J, Yu Z, Dang X and Wang K:
Hypoxia-inducible factor prolyl hydroxylase inhibitor prevents
steroid-associated osteonecrosis of the femoral head in rabbits by
promoting angiogenesis and inhibiting apoptosis. PLoS One.
9:e1077742014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J,
Sun T, Wang J, Sun R and Liu Y: Hypoxia promotes vasculogenic
mimicry formation by inducing epithelial-mesenchymal transition in
ovarian carcinoma. Gynecol Oncol. 133:575–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin Q, Xu Y, He T, Qin C and Xu J: Normal
and disease-related biological functions of Twist1 and underlying
molecular mechanisms. Cell Res. 22:90–106. 2012. View Article : Google Scholar :
|
|
7
|
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY,
Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siddique HR and Saleem M: Role of BMI1, a
stem cell factor, in cancer recurrence and chemoresistance:
Preclinical and clinical evidences. Stem Cells. 30:372–378. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun T, Sun BC, Zhao XL, Zhao N, Dong XY,
Che N, Yao Z, Ma YM, Gu Q, Zong WK, et al: Promotion of tumor cell
metastasis and vasculogenic mimicry by way of transcription
coactivation by Bcl-2 and Twist1: A study of hepatocellular
carcinoma. Hepatology. 54:1690–1706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW,
Che N, Wang XH, Du J, Liu YX and Sun BC: Expression and functional
significance of Twist1 in hepatocellular carcinoma: Its role in
vasculogenic mimicry. Hepatology. 51:545–556. 2010. View Article : Google Scholar
|
|
11
|
Wong CE, Paratore C, Dours-Zimmermann MT,
Rochat A, Pietri T, Suter U, Zimmermann DR, Dufour S, Thiery JP,
Meijer D, et al: Neural crest-derived cells with stem cell features
can be traced back to multiple lineages in the adult skin. J Cell
Biol. 175:1005–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bittner M, Meltzer P, Chen Y, Jiang Y,
Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, et
al: Molecular classification of cutaneous malignant melanoma by
gene expression profiling. Nature. 406:536–540. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nichols J, Zevnik B, Anastassiadis K, Niwa
H, Klewe-Nebenius D, Chambers I, Schöler H and Smith A: Formation
of pluripotent stem cells in the mammalian embryo depends on the
POU transcription factor Oct4. Cell. 95:379–391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Angeloni V, Tiberio P, Appierto V and
Daidone MG: Implications of stemness-related signaling pathways in
breast cancer response to therapy. Semin Cancer Biol. 31:43–51.
2015. View Article : Google Scholar
|
|
15
|
Chen R, Dong Y, Xie X, Chen J, Gao D, Liu
Y, Ren Z and Cui J: Screening candidate metastasis-associated genes
in three-dimensional HCC spheroids with different metastasis
potential. Int J Clin Exp Pathol. 7:2527–2535. 2014.PubMed/NCBI
|
|
16
|
Wong CC, Kai AK and Ng IO: The impact of
hypoxia in hepato-cellular carcinoma metastasis. Front Med.
8:33–41. 2014. View Article : Google Scholar
|
|
17
|
Marie-Egyptienne DT, Lohse I and Hill RP:
Cancer stem cells, the epithelial to mesenchymal transition (EMT)
and radioresistance: Potential role of hypoxia. Cancer Lett.
341:63–72. 2013. View Article : Google Scholar
|
|
18
|
Bae S, Jeong HJ, Cha HJ, Kim K, Choi YM,
An IS, Koh HJ, Lim DJ, Lee SJ and An S: The hypoxia-mimetic agent
cobalt chloride induces cell cycle arrest and alters gene
expression in U266 multiple myeloma cells. Int J Mol Med.
30:1180–1186. 2012.PubMed/NCBI
|
|
19
|
Bauer N, Liu L, Aleksandrowicz E and Herr
I: Establishment of hypoxia induction in an in vivo animal
replacement model for experimental evaluation of pancreatic cancer.
Oncol Rep. 32:153–158. 2014.PubMed/NCBI
|
|
20
|
Salnikow K, Donald SP, Bruick RK,
Zhitkovich A, Phang JM and Kasprzak KS: Depletion of intracellular
ascorbate by the carcinogenic metals nickel and cobalt results in
the induction of hypoxic stress. J Biol Chem. 279:40337–40344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang Y, Park C, Kim D, Seong CM, Kwon K
and Choi C: Unsorted human adipose tissue-derived stem cells
promote angiogenesis and myogenesis in murine ischemic hindlimb
model. Microvasc Res. 80:310–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao
X, Zhang W and Hao X: Vasculogenic mimicry is associated with high
tumor grade, invasion and metastasis, and short survival in
patients with hepatocellular carcinoma. Oncol Rep. 16:693–698.
2006.PubMed/NCBI
|
|
24
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raimondi C, Gianni W, Cortesi E and
Gazzaniga P: Cancer stem cells and epithelial-mesenchymal
transition: Revisiting minimal residual disease. Curr Cancer Drug
Targets. 10:496–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biroccio A, Candiloro A, Mottolese M,
Sapora O, Albini A, Zupi G and Del Bufalo D: Bcl-2 overexpression
and hypoxia synergistically act to modulate vascular endothelial
growth factor expression and in vivo angiogenesis in a breast
carcinoma line. FASEB J. 14:652–660. 2000.PubMed/NCBI
|
|
27
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koo BS, Lee SH, Kim JM, Huang S, Kim SH,
Rho YS, Bae WJ, Kang HJ, Kim YS, Moon JH, et al: Oct4 is a critical
regulator of stemness in head and neck squamous carcinoma cells.
Oncogene. 34:2317–2324. 2015. View Article : Google Scholar
|
|
30
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and
characterization of ovarian cancer-initiating cells from primary
human tumors. Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu
LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al: The polycomb group
protein Bmi-1 represses the tumor suppressor PTEN and induces
epithelial-mesenchymal transition in human nasopharyngeal
epithelial cells. J Clin Invest. 119:3626–3636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bjerkvig R, Johansson M, Miletic H and
Niclou SP: Cancer stem cells and angiogenesis. Semin Cancer Biol.
19:279–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong J, Zhao Y, Huang Q, Fei X, Diao Y,
Shen Y, Xiao H, Zhang T, Lan Q and Gu X: Glioma stem/progenitor
cells contribute to neovascularization via transdifferentiation.
Stem Cell Rev. 7:141–152. 2011. View Article : Google Scholar
|
|
37
|
Monzani E and La Porta CA: Targeting
cancer stem cells to modulate alternative vascularization
mechanisms. Stem Cell Rev. 4:51–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun D, Sun B, Liu T, Zhao X, Che N, Gu Q,
Dong X, Yao Z, Li R, Li J, et al: Slug promoted vasculogenic
mimicry in hepatocellular carcinoma. J Cell Mol Med. 17:1038–1047.
2013. View Article : Google Scholar : PubMed/NCBI
|