Introduction
Angiogenesis, the process of new vessels forming
from preexisting microvasculature, is essential for tumor growth
and metastasis, and is controlled by the balance between
proangiogenic factors and angiogenesis inhibitors (1–4).
Identifying the proangiogenic factors and inhibitors, and
characterizing their contribution to endothelial cell motility and
adhesion will aid the understanding of the angiogenesis process
mechanism, but also provide potential candidates for developing
numerous possible antiangiogenic therapeutic strategies targets for
cancer.
Human immediate early response 2 (IER2, also
known as Chx1 or ETR101, a homologous gene in rat and
mouse for pip92), a member of the immediate early
responsible genes, was initially identified and cloned from the
human myeloid leukemia cell line HL-60 (5), and found to be rapidly or
transiently upregulated by extracellular stimuli, such as growth
factors, cytokines, 12-O-tetradecanoylphorbol-13-acetate and
certain pathogen infections (5–11).
Human IER2 consists of 223 amino acids and is rich in proline,
serine and arginine, and 76% of the amino acid residues
encompassing the whole region are identical between human IER2 and
mouse pip92. IER2 contains two putative nuclear localization
signals and multiple phosphorylation sites, suggesting that IER2
may be a nuclear protein and require post-translational
modifications. Currently, IER2 has been characterized as a putative
nuclear protein that served function as a fibroblast growth factor
intracellular binding protein 1-interacting partner (12), and as a transcription factor or
transcriptional co-activator for the human myo-inositol 1-phosphate
synthase gene involved in the regulation of cellular responses
(9,12). Furthermore, data from a previous
study indicated that IER2 is involved in the regulation of tumor
progression and metastasis (13),
although the precise role and signaling mechanism involved remain
elusive. In our previous study, microarray was performed to analyze
the gene expression profile in response to basic fibroblast growth
factor (bFGF), which is a proangiogenic factor (14), at selective time points during
different phases of human dermal microvascular endothelial cells
morphogenesis (15), and
stimulation of HMVECs growing in fibrin matrices by bFGF led to a
significant upregulation of IER2 within 24 h and a subsequently
significant downregulation between 24 and 72 h (data not shown),
which prompted the evaluation of whether IER2 participates in the
regulation of the endothelial cell morphogenesis and
angiogenesis.
The aim of the present study was to investigate the
role of IER2 in human endothelial cell motility, cell-matrix
adhesion and in vitro capillary-like structures formation.
To the best of our knowledge, this is the first demonstration that
IER2 may be required for endothelial cell morphogenesis and in
vitro angiogenesis.
Materials and methods
Cell lines and culture conditions
Human umbilical vein endothelium cells (HUVECs) were
purchased from AllCells (Shanghai, China) and cultured in 0.5%
gelatin-coated dishes in endothelial cell growth medium (EGM;
AllCells) according to the manufacturer's instruction, and the
passage numbers of the HUVECs were maintained between passage 3 and
8. Human embryonic kidney (HEK) 293T cells, obtained from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China), were grown in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 µg/ml streptomycin in a humidified incubator at 37°C
with 5% CO2.
Plasmid construction and lentivirus
production
Recombinant lentiviruses encoding IER2 (LV-IER2) or
control lentivirus (LV-CTL) were generated in HEK293T cells by
co-transfecting the pEZ-Lv105-IER2 vector or pEZ-Lv105-null with
the Lenti-Pac™ HIV Expression Packaging kit obtained from
GeneCopoeia, Inc. (Rockville, MD, USA) according to the
manufacturer's instruction. For lentiviral shRNA vector
construction, three different shRNA corresponding to human
IER2 (GenBank accession: NM_004907) were designed as
follows: shR-1 forward, 5′-CCGGGA CACAATCAGCCGAGAAGTT CTCGAG
AACTTCTCGGCTGATTGTG
TCTTTTTG-3′ and reverse, 5′-AATTCAAAAAGA CACAATCAGCCGAGAAGTT CTCGAG
AACTTCTCGGCTGATTGTG
TC-3′; shR-2 forward, 5′-CCGGCTACTTT CACATTCTCAAGTT
CTCGAGAACTTGAGAATGTGAAAGTAGT TTTTG-3′ and reverse,
5′-AATTCAAAAACTACTTTC ACATTCTCAAGTTCTCGAG AACTTGAGAATGTGAA AGT AG-3′; and
shR-3 forward, 5′-CCGGGA TCTACTTT CACATTCTCAA CTCGAG
TTGAGAATGTGAAAGTAGA
TCTTTTT G-3′ and reverse, 5′-AATTCAAAAAGA TCTA CTTTCACATTCTCAA CTCGAG
TTGAGAATGTGAAA
GTAGATC -3′. The underlined sections refer to sequences
corresponding to human IER2 (GenBank accession no.
NM_004907) shRNA targets. The oligonucleotides were synthesized,
annealed and cloned into as cloned into the lentiviral expression
vector pGV115 obtained from Shanghai Genechem Co., Ltd., (Shanghai,
China) at the AgeI and EcoRI sites. Recombinant
lentiviruses (LV-shR-1, LV-shR-2 and LV-shR-3) were produced in
HEK293T cells by co-transfection of the lentiviral shRNA vectors
with the packaging vectors pHelper 1.0 and pHelper 2.0 purchased
from Shanghai Genechem using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's instruction. The
lentiviruses were collected, purified and concentrated, and the
infectious titers were determined by Shanghai Genechem.
Non-silencing control shRNA lentivirus (LV-shCTL) was also obtained
from Shanghai Genechem, and performed as the empty vectors for
knockdown gene expression. For lentiviral transduction, HUVECs were
seeded in the 0.5% gelatin-coated 6-well culture plates, and were
subsequently infected with the indicated lentiviruses at a
multiplicity of infection of 30 in the presence of polybrene (5
µg/ml), and cultured for >96 h in EGM. The green
fluorescence protein, co-expressed in the lentivirus-transduced
cells, served as a selection marker to indicate the successfully
transduced HUVECs.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HUVECs using the RNA
isolator Total RNA extraction reagent (Vazyme Biotech Co., Ltd.,
Nanjing, China), according to the manufacturer's instruction, and
reverse transcribed using the HiScript First Strand cDNA Synthesis
kit (Vazyme Biotech). RT-qPCR was performed on an ABI 7500
real-time PCR system (Applied Biosystems, Foster City, CA, USA)
using the AceQ® qPCR SYBR® Green Master mix
kit (Vazyme Biotech Co., Ltd.) according to the manufacturer's
instructions. To discriminate between endogenous versus exogenous
IER2, the following were designed: Primers I sense, 5′-TGG
TGA AAC TGG GCC AAT CT-3′ and antisense, 5′-AAG AAT CCA CCG CAC GAA
AG-3′, which spanned the 3′-untranslated region (UTR) of the
IER2 gene and only recognized the endogenous mRNA of
IER2, and primers II sense, 5′-CCA AAG TCA GCC GCA AAC GA-3′
and antisense, 5′-TTT CTT CCA GAC GGG CTT TCT TGC-3′, which spanned
the coding region and recognized the endogenous and exogenous
IER2 gene. Primers for GAPDH were sense, 5′-GCA CCG
TCA AGG CTG AGA AC-3′ and antisense, 5′-TGG TGA AGA CGC CAG TGG
A-3′. The expression of the IER2 mRNA was normalized to
GAPDH mRNA, and the fold changes in the expression of
IER2 were evaluated by the 2−ΔΔCT method.
Western blot analysis
HUVECs were washed with ice-cold phosphate-buffered
saline (PBS) and lysed with a total protein extraction kit
including Protease Inhibitor mix (Vazyme Biotech Co., Ltd.).
Protein concentrations were determined using the Bradford protein
quantification kit (Vazyme Biotech Co., Ltd.). Total protein was
separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and
transferred to polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). Following blocking in PBS-Tween-20 containing
5% non-fat dried milk, the membranes were incubated at 4°C
overnight with mouse polyclonal anti-IER2 (Cat. no. ab168980;
1:1,000; Abcam, Cambridge, MA, USA), rabbit monoclonal anti-focal
adhesion kinase (FAK; Cat. no. 13009), rabbit polyclonal
anti-pY397FAK (Cat. no. 3283; 1:1,000; Cell Signaling Technology,
Danvers, MA, USA) or mouse monoclonal anti-GAPDH (Cat. no. KC-5G4;
1:1,000; KangChen Bio-tech, Inc., Shanghai, China) primary
antibodies, followed by incubation for 2 h at room temperature with
horseradish peroxidase-conjugated goat anti-mouse immu-noglobulin G
(IgG; Cat. no. KC-MM-035) or goat anti-rabbit IgG (Cat. no.
KC-RB-035) antibodies (1:2,000; KangChen Bio-tech, Inc.), and
visualized using the Pierce ECL Plus Western Blotting Substrate kit
(Thermo Fisher Scientific, Rockford, IL, USA).
Cell viability analysis
Cell viability assay was performed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylterazolium bromide (MTT)
and the Cell Counting kit-8 (CCK-8; Obio Technology, Shanghai,
China) assays. For the MTT assay, 100 µl of HUVECs
(3×104 cells/well) suspended in EGM were plated in the
96-well culture plates pre-coated with 0.5% gelatin. After
incubation at 37°C for 24 h, 20 µl of MTT solution (5 mg/ml;
Sigma St. Louis, MO, USA) was added to each well, and the samples
were incubated for another 4 h at 37°C. Subsequently, the MTT
solution was removed and 100 µl of dimethyl sulfoxide was
added to dissolve the formazan crystals followed by absorbance
measurement at 570 nm using a multifunctional microplate reader
(BioTek Instruments, Winooski, VT, USA). The CCK-8 assay was also
performed according to the manufacturer's instructions. After
incubation at 37°C for 24 h, 10 µl of CCK-8 solution was
added to each well and incubated for another 1 h at 37°C, and the
absorbance at 450 nm was measured.
Cell migration and invasion analysis
The Transwell migration assay was used to evaluate
the capacity of HUVEC migration. In brief, HUVECs were
serum-starved overnight and 200 µl of 1×105 cells
were seeded in the upper chamber (with 8.0-µm pore size;
Costar, Corning, NY, USA) of the 24-well Transwell, which was
subsequently placed into the lower chamber containing 600 µl
of EGM, and allowed to migrate toward the underside of the insert
for 24 h at 37°C. Cells on the upper surface of the inserts were
gently removed, and the cells on the lower surface of the filter
were fixed with methanol and stained with 1% crystal violet. The
number of migrated cells in each chamber was counted in ≥5 randomly
selected fields (magnification, ×200), and images were captured
under a light phase contrast microscopy equipped with a digital
image capturing system. The cell invasion assay was also performed
in the Transwell chambers with the minor modification that the
transwell inserts were pre-coated with 100 µg/ml Matrigel
(BD Biosciences, San Jose, CA, USA), and 2×105 cells
were plated into the upper chambers.
Cell-matrix adhesion analysis
The cell-matrix adhesion assay was performed in
96-well culture plates pre-coated with 10 µg/ml collagen
type I (BD Biosciences) overnight at 4°C. After blocking with 1%
heat-denatured bovine serum albumin in PBS at 37°C for ≥1 h, HUVECs
(2×105 cells/well) were added to each well and incubated
at 37°C for 1 h. Unbound cells were gently removed and attached
cells were quantified by the CCK-8 assay.
In vitro capillary-like tube formation
analysis
To test the angiogenic activity, the capillary-like
tube formation by HUVECs on Matrigel was performed in vitro.
In brief, a pre-chilled 96-well culture plate was coated with 50
µl of Matrigel, which was previously thawed at 4°C
overnight, and allowed to polymerize for ≥1 h at 37°C. HUVECs
(3×104 cells/well) were plated in coated wells and
incubated at 37°C for 24 h. The tube formation was observed using
an inverted phase contrast microscope and analyzed with the ImageJ
software (http://imagej.nih.gov/ij/). Only the
complete ring structures created by HUVECs were counted as
tubes.
Immunofluorescence analysis
HUVECs were seeded in coverslips coated with 0.5%
gelatin in 24-well plates and incubated in EGM at 37°C for 24 h,
and subsequently were fixed with 4% paraformaldehyde for 30 min,
permeabilized with 0.5% Triton X-100 for 10 min, and blocked with
Image-iT FX Signal Enhancer (Invitrogen) for 30 min. Afterwards
cells were washed twice with PBS and subsequently incubated with
the rhodamine-conjugated phalloidin (Sigma) for 1 h at room
temperature. The coverslips were washed thoroughly with PBS,
mounted on glass slides and analyzed under a fluorescent microscope
equipped with a digital image capturing system.
Statistical analysis
Data are expressed as mean ± standard deviation.
Statistical significance was determined by the Student's t-test,
and P<0.05 was considered to indicate a statistically
significant difference.
Results
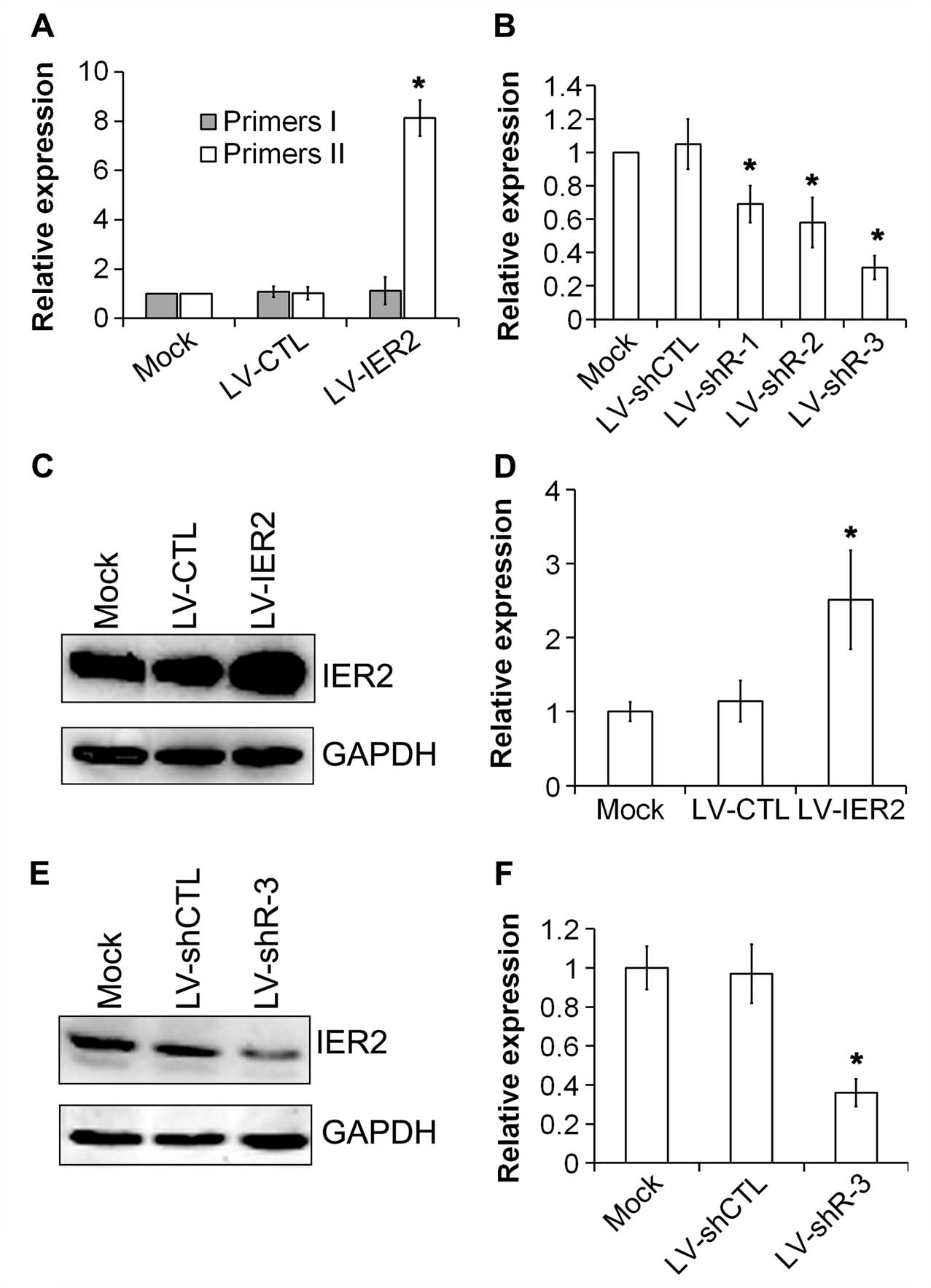
IER2 expression in HUVECs
Initially, the expression of IER2 in HUVECs
after indicated lentiviruses transduction was examined. As shown in
Fig. 1, the levels of endogenous
and the total (endogenous plus exogenous) IER2 mRNA was
measured by RT-qPCR using primers I and II, respectively.
Overexpression of IER2 mRNA (total IER2 mRNA) was
observed in LV-IER2-transduced HUVECs, and the levels of endogenous
IER2 mRNA were induced, but not significantly (Fig. 1A), suggesting that LV-IER2
infection did not affect the expression of endogenous IER2.
However, the knockdown of IER2 expression by LV-shR-3 was
achieved with the most potent inhibition at the mRNA levels
(Fig. 1B). Data from the western
blot assay showed that IER2 was constitutively expressed in the
transduced empty vectors (LV-CTL and LV-shCTL), and the
untransduced control HUVECs (mock) (Fig. 1C and E), and upregulation and
efficient inhibition of the IER2 protein were shown in LV-IER2- and
LV-shR-3-transduced HUVECs, respectively (Fig. 1C-F). These data indicated that
lentiviruses were successfully trans-duced into HUVECs, and that
IER2 was overexpressed in LV-IER2-transduced HUVECs, and the
most efficient inhibition of IER2 was shown in
LV-shR-3-transduced cells.
IER2 regulates endothelial cell migration
and invasion
Results from the Transwell cell migration analysis
showed that depletion of IER2 in HUVECs by infection with LV-shR
significantly reduced the number of the motile cells, while
overexpression of IER2 by LV-IER2 infection significantly promoted
the HUVECs migration (Fig. 2A and
C) in comparison with empty vector-transduced cells and the
mock. Similar effects of IER2 knockdown and overexpression on the
HUVECs invasion were also shown in the Transwell cell invasion
analysis (Fig. 2B and D).
Furthermore, the alteration in cell motility induced by knockdown
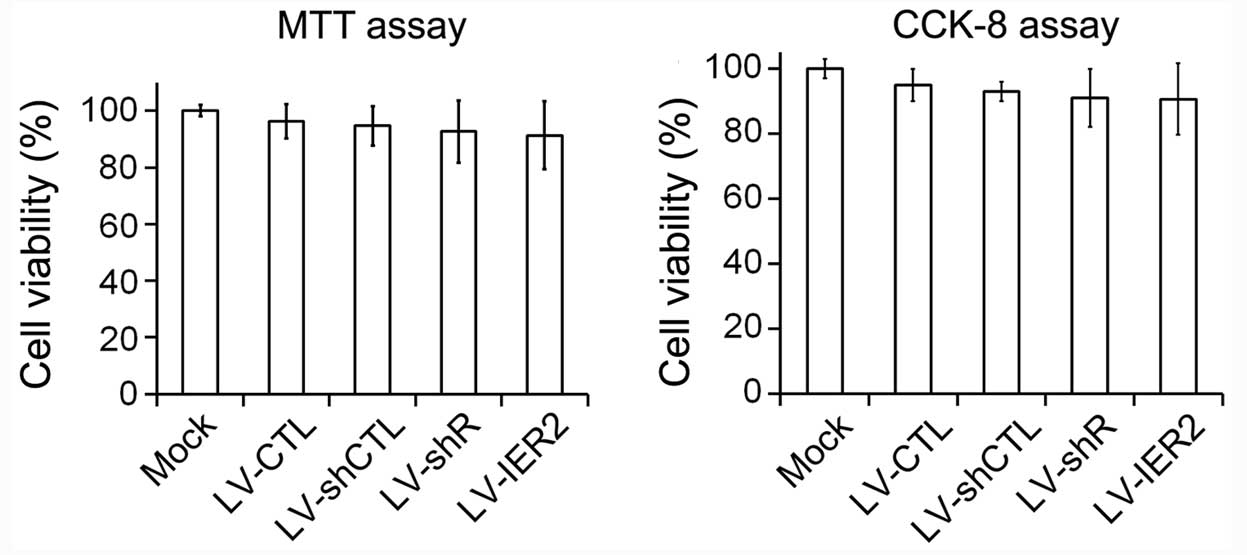
and overexpression of IER2 did not appear to be due to decreased
cell viability. As shown in Fig.
3, knockdown and overexpression of IER2 had a non-significant
decrease on the cell viability compared to that of empty
vector-transduced cells and the mock. These results demonstrated
that depletion and overexpression of IER2 modulated the general
capacity of endothelial cell motility, and the endogenous
IER2 expression may be required for endothelial cell
migration and invasion.
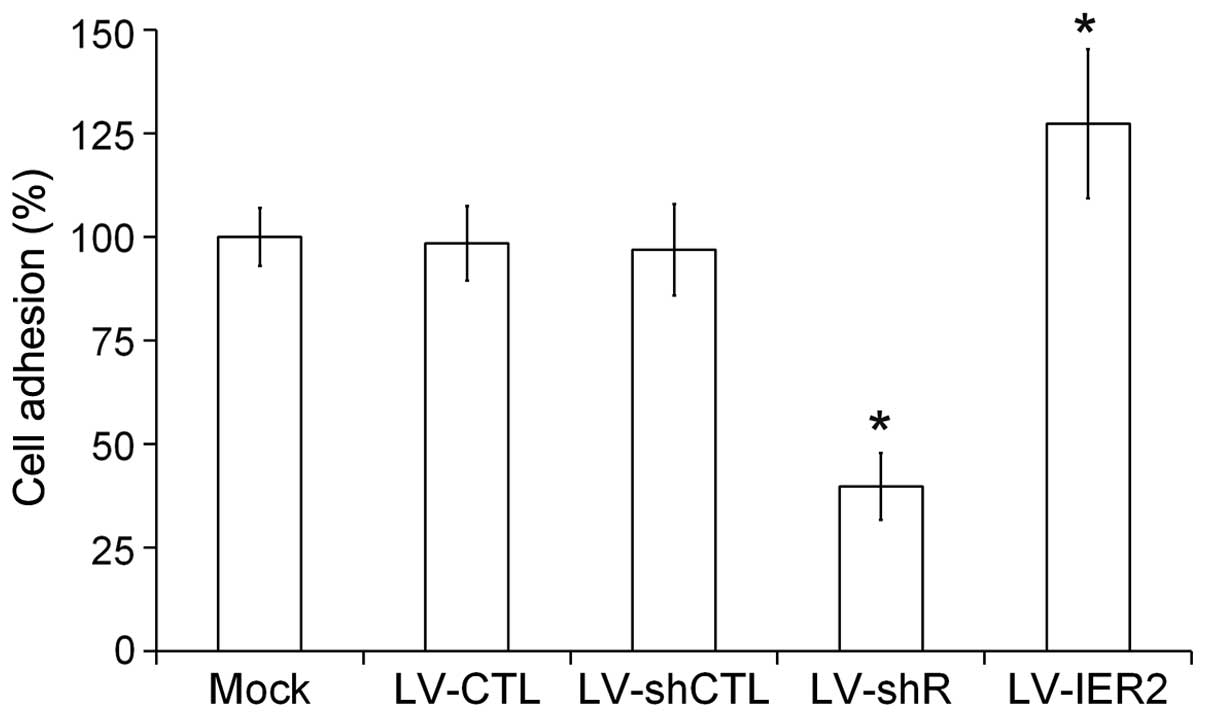
IER2 regulates HUVECs adhesion onto the
collagen type I matrix
Endothelial cell adhesion onto the extracellular
matrix has a central role in endothelial cell migration and
angiogenesis (16,17). To examine the role of IER2 in the
cell-matrix adhesion, HUVECs were seeded in collagen type I-coated
96-well plates and incubated at 37°C for 1 h. As shown in Fig. 4, LV-shR infection caused a
significant reduction of HUVECs adhesion onto collagen type I,
while LV-IER2 infection promoted HUVECs cell adhesion onto the
matrix compared to those in the empty vector-transduced cells and
the mock, suggesting that IER2 may be an important regulator of
endothelial cell adhesion to the matrix, and the endogenous IER2
expression may be required for endothelial cell adhesion to the
matrix and motility.
IER2 regulates the capillary tube
formation in vitro of HUVECs via a FAK-dependent mechanism
Endothelial cells plated on the extracellular matrix
may rapidly attach and form capillary-like tube structures
(18). To examine the role of
IER2 in endothelial cell differentiation into capillary-like
structures, the in vitro tube formation assay was performed.
As indicated in Fig. 5A and B, a
spontaneous differentiation of HUVECs into capillary-like tubes was
identified in the mock, empty vector- and LV-IER2-transduced
HUVECs, particularly in LV-IER2-transduced cells, while knockdown
of IER2 clearly disrupted the HUVECs capillary morphogenesis,
suggesting that IER2 may act as a regulator in HUVECs
differentiation into capillary-like structures in vitro. As
endothelial FAK expression and activity have been implicated as the
important modulators of angiogenesis (4–16,19), the western blot assay was
subsequently performed to evaluate the effects of IER2 on the FAK
expression, the phosphorylation of which is known to regulate
cell-matrix adhesion during cell migration. As shown in Fig. 5C and D, knockdown of IER2 in
HUVECs by LV-shR infection clearly downregulated the
phosphorylation level of FAK at Y397 (pY397FAK), while upregulation
of pY397FAK was shown in LV-IER2-transduced HUVECs, and no
significant effects on the level of total FAK were observed in
HUVECs with either knockdown or overexpression of IER2. LV-CTL or
LV-shCTL transduction in HUVECs did not cause a significant
alteration of pY397FAK and total FAK (data not shown). Taken
together, the data suggested that IER2 may regulate the endothelial
cell-matrix adhesion, cell migration and invasion during in
vitro capillary tube formation of HUVECs, possibly via a FAK
involved manner. Studies of the effects of IER2 on the regulation
of the FAK-mediated signal pathway during angiogenesis are under
way.
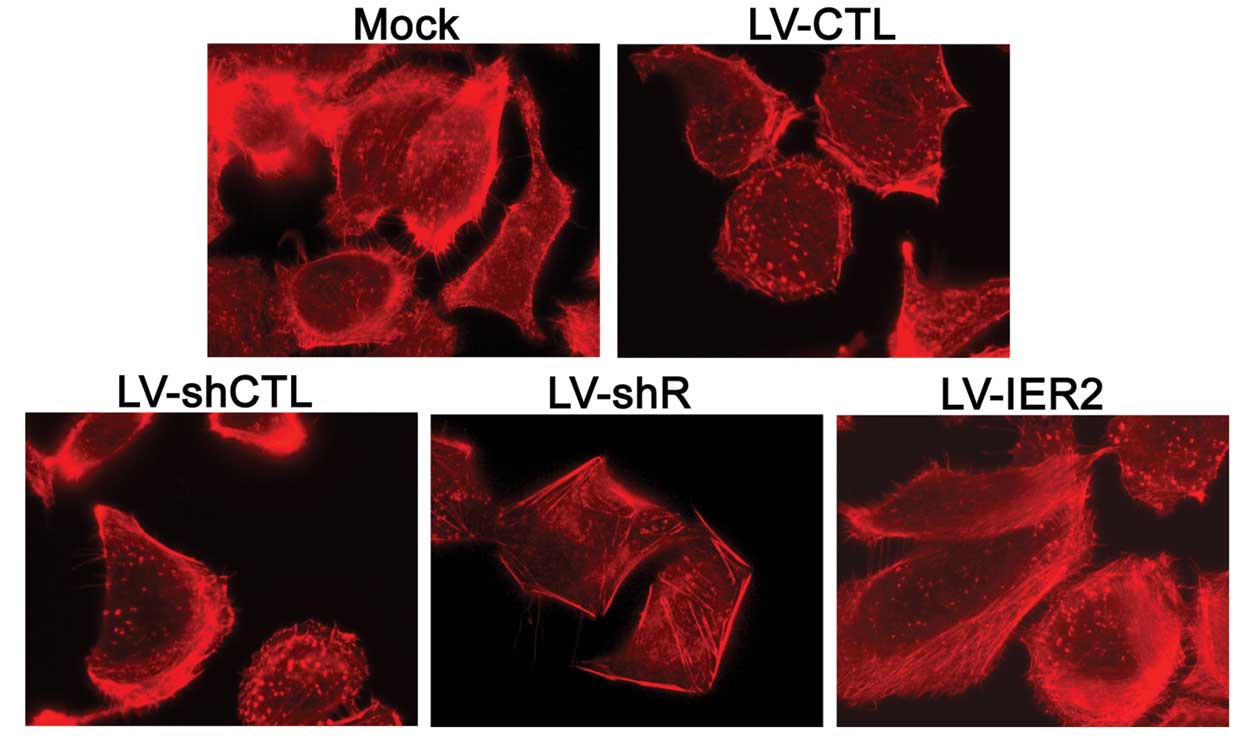
IER2 regulates the actin cytoskeleton
rearrangement
Considering endothelial cell migration, adhesion and
capil-lary morphogenesis require dynamic remodeling of the actin
microfilaments, whether IER2 could regulate the actin cytoskeleton
arrangement in HUVECs was further examined by staining with the
rhodamine-conjugated phalloidin. As shown in Fig. 6, the formation of the filopodia
and lamel-lipodia at the leading edge were shown in the mock, empty
vector- and LV-IER2-transduced HUVECs, particularly in
LV-IER2-transduced cells, while IER2 depletion clearly disrupted or
inhibited the formation of the filopodia and lamellipodia at the
leading edge of the HUVECs. These data suggested that the altered
cell motility, cell-matrix adhesion and the capillary tube
formation of HUVECs caused by IER2 depletion and overexpression may
be the consequence of the regulation of the actin cytoskeleton
rearrangement.
Discussion
In the present study, the HUVEC cells were used to
study the role of IER2 in cell motility, matrix adhesion and
capillary-like tube formation in vitro, which are the key
steps for angiogenesis, and provided the first evidence for a role
of IER2 as a potential regulator for endothelial cell morphogenesis
and in vitro angiogenesis.
Our previous study reported gene expression profile
analysis of the human microvascular endothelial cells during
capillary morphogenesis (15),
and identified a significant alteration of IER2 expression at
selective time-points (data not shown), suggesting that IER2 may be
involved in the regulation of the endothelial cell morphogenesis
and angiogenesis. As endothelial cell motility has an important
role in the maintenance of vascular function (20), whether IER2 regulates endothelial
cell migration and invasion was initially evaluated. The present
study demonstrated that IER2 was constitutively expressed in
HUVECs, and overexpression of IER2 promoted HUVECs migration and
invasion as shown by the Transwell cell migration and invasion
assays, respectively. To determine whether the increased cell
motility is due to the exogenous IER2 and not the endogenous IER2,
depletion of IER2 in HUVECs was performed by infection with LV-shR,
which directed against the 3′-UTR of the IER2 mRNA and only
knocked down the endogenous IER2. Knockdown of the endogenous IER2
by LV-shR infection significantly decreased the HUVECs migration
and invasion. This result was in accordance with another study
showing that IER2 promoted cell motility and tumor metastasis
(13). These findings suggested
that knockdown and overexpression of IER2 modulated the general
capacity of endothelial cell motility, and the endogenous IER2
expression may be required for endothelial cell migration and
invasion.
The present study further demonstrated that
silencing of IER2 expression resulted in a significant
reduction of HUVECs adhesion onto the collagen type I substrate,
while overexpression of IER2 increased the adhesive capacity of
HUVECs to collagen type I. As endothelial cell-matrix adhesion is a
principal requirement for cell migration during angiogenesis
(21), these data clearly
indicated that IER2 may be an important regulator of endothelial
cell migration and adhesion to the substratum, which are critical
features of endothelial cell differentiation into capillary
tube-like structures and vessel formation. To test whether
endogenous IER2 expression is required for endothelial cell
differentiation into capillary-like structures, the in vitro
tube formation assay was performed. Inhibition of IER2 expression
in HUVECs significantly decreased the capillary tube-like
structures formation. These findings provided the first evidence
that IER2 may be a novel regulator in the modulation of angiogenic
key events. During angiogenesis, endothelial cells sense the motile
stimuli by filopodia, extend protrusions or lamellipodia at the
leading edge, migrate and aggregate in the cell-matrix adhesion to
form vessels structures (16,22). In the present study, inhibition of
IER2 clearly disrupted or decreased the formation of the filopodia
and lamellipodia at the leading edge of the HUVECs, which was in
agreement to the decrease of cell motility, cell-matrix adhesion
and in vitro tube-like structure formation, as mentioned
above.
Considering that endothelial FAK expression and
activity are essential for cell motility, cell-matrix adhesion and
actin polymerization (4–16,19), and the phosphorylation of FAK at
Y397 is known to be the first step for the activation of the
FAK-mediated signal pathway to regulate cell-matrix adhesion and
migration (23,24), the effects of IER2 on the FAK
expression were evaluated. Depletion of IER2 expression
significantly downregulated the level of pY397FAK in HUVECs, while
over-expression upregulated the level of pY397FAK. Studies of the
effects of IER2 expression on the regulation of other molecular
levels and activity in the FAK-mediated signaling pathway during
angiogenesis are under way. Taken together, these findings
suggested that IER2 regulates endothelial cell motility, adhesion
on collagen type I matrix and the capillary tube formation as the
result of the regulation of the actin cytoskeleton rearrangement
presumably via a FAK-dependent mechanism.
In conclusion, the present study demonstrated that
IER2 is constitutively expressed in HUVECs, and is essential for
endothelial cell motility, adhesion on collagen type I matrix and
the capillary tube formation in vitro. IER2 regulated
endothelial cell motility, cell-matrix adhesion and the capillary
tube formation of HUVECs as the result of modulation of the
remodeling of actin cytoskeleton presumably through a FAK-dependent
mechanism. Although further study is required to identify potential
partners or targets of IER2 in regulating FAK activity, the results
presented may provide a novel therapeutic target to inhibit
angiogenesis.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (grant no. 81172278) and the
Innovation and Entrepreneurship Training Program of Jiangsu College
Students (grant no. 201311117060Y).
References
|
1
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29(Suppl 16): 15–18. 2002.
View Article : Google Scholar
|
|
2
|
Birdsey GM, Dryden NH, Amsellem V,
Gebhardt F, Sahnan K, Haskard DO, Dejana E, Mason JC and Randi AM:
Transcription factor Erg regulates angiogenesis and endothelial
apoptosis through VE-cadherin. Blood. 111:3498–3506. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iruela-Arispe ML and Dvorak HF:
Angiogenesis: A dynamic balance of stimulators and inhibitors.
Thromb Haemost. 78:672–677. 1997.PubMed/NCBI
|
|
4
|
Cabrita MA, Jones LM, Quizi JL, Sabourin
LA, McKay BC and Addison C: Focal adhesion kinase inhibitors are
potent anti-angiogenic agents. Mol Oncol. 5:517–526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimizu N, Ohta M, Fujiwara C, Sagara J,
Mochizuki N, Oda T and Utiyama H: Expression of a novel immediate
early gene during 12-O-tetradecanoylphorbol-13-acetate-induced
macrophagic differentiation of HL-60 cells. J Biol Chem.
266:12157–12161. 1991.PubMed/NCBI
|
|
6
|
Deng YJ, Huang ZX, Zhou CJ, Wang JW, You
Y, Song ZQ, Xiang MM, Zhong BY and Hao F: Gene profiling involved
in immature CD4+ T lymphocyte responsible for systemic
lupus erythematosus. Mol Immunol. 43:1497–1507. 2006. View Article : Google Scholar
|
|
7
|
Shen QY and Zheng SS: Identification of
genes differentially expressed in monocyte-derived dendritic cells
with 1á, 25-dihydroxyvitamin D3 using cDNA arrays. J Zhejiang Univ
SCI. 5:222–225. 2004. View Article : Google Scholar
|
|
8
|
Zeng F, Hon CC, Sit WH, Chow KY, Hui RK,
Law IK, Ng VW, Yang XT, Leung FC and Wan JM: Molecular
characterization of Coriolus versicolor PSP-induced apoptosis in
human promyelotic leukemic HL-60 cells using cDNA microarray. Int J
Oncol. 27:513–523. 2005.PubMed/NCBI
|
|
9
|
Takaya T, Kasatani K, Noguchi S and Nikawa
J: Functional analyses of immediate early gene ETR101 expressed in
yeast. Biosci Biotechnol Biochem. 73:1653–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Ma S, Li B, Fink T, Zachar V,
Takahashi M, Cuttichia J, Tsui LC, Ebbesen P and Liu X:
Transcriptional activation of immediate-early gene ETR101 by human
T-cell leukaemia virus type I Tax. J Gen Virol. 84:3203–3214. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hess S, Rheinheimer C, Tidow F, Bartling
G, Kaps C, Lauber J, Buer J and Klos A: The reprogrammed host:
Chlamydia trachomatis-induced up-regulation of glycoprotein 130
cytokines, transcription factors, and antiapoptotic genes.
Arthritis Rheum. 44:2392–2401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong SK and Dawid IB: FGF-dependent
left-right asymmetry patterning in zebrafish is mediated by Ier2
and Fibp1. Proc Natl Acad Sci USA. 106:2230–2235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neeb A, Wallbaum S, Novac N,
Dukovic-Schulze S, Scholl I, Schreiber C, Schlag P, Moll J, Stein U
and Sleeman JP: The immediate early gene Ier2 promotes tumor cell
motility and metastasis, and predicts poor survival of colorectal
cancer patients. Oncogene. 31:3796–3806. 2012. View Article : Google Scholar
|
|
14
|
Bayless KJ and Davis GE: The Cdc42 and
Rac1 GTPases are required for capillary lumen formation in
three-dimensional extracellular matrices. J Cell Sci.
115:1123–1136. 2002.PubMed/NCBI
|
|
15
|
Sun XT, Zhang MY, Shu C, Li Q, Yan XG,
Cheng N, Qiu YD and Ding YT: Differential gene expression during
capillary morphogenesis in a microcarrier-based three-dimensional
in vitro model of angiogenesis with focus on chemokines and
chemokine receptors. World J Gastroenterol. 11:2283–2290. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuo MW, Wang CH, Wu HC, Chang SJ and
Chuang YJ: Soluble THSD7A is an N-glycoprotein that promotes
endothelial cell migration and tube formation in angiogenesis. PLoS
One. 6:e290002011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Otrock ZK, Mahfouz RA, Makarem JA and
Shamseddine AI: Understanding the biology of angiogenesis: Review
of the most important molecular mechanisms. Blood Cells Mol Dis.
39:212–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kubota Y, Kleinman HK, Martin GR and
Lawley TJ: Role of laminin and basement membrane in the
morphological differentiation of human endothelial cells into
capillary-like structures. J Cell Biol. 107:1589–1598. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braren R, Hu H, Kim YH, Beggs HE,
Reichardt LF and Wang R: Endothelial FAK is essential for vascular
network stability, cell survival, and lamellipodial formation. J
Cell Biol. 172:151–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ettenson DS and Gotlieb AI: Endothelial
wounds with disruption in cell migration repair primarily by cell
proliferation. Microvasc Res. 48:328–337. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eccles SA: Parallels in invasion and
angiogenesis provide pivotal points for therapeutic intervention.
Int J Dev Biol. 48:583–598. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fraley SI, Feng Y, Krishnamurthy R, Kim
DH, Celedon A, Longmore GD and Wirtz D: A distinctive role for
focal adhesion proteins in three-dimensional cell motility. Nat
Cell Biol. 12:598–604. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Nimwegen MJ and van de Water B: Focal
adhesion kinase: A potential target in cancer therapy. Biochem
Pharmacol. 73:597–609. 2007. View Article : Google Scholar
|