Introduction
The acronym TORCH was proposed in 1971 by Nahmias
et al (1) to denote 4
congenital infections which affect fetuses and newborns, namely
(T)oxoplasmosis (TOX; caused by Toxoplasma gondii
infection), (R)ubella virus (RV; also known as German Measles),
(C)ytomegalovirus (CMV) and (H)erpes simplex virus (HSV) types 1
and 2 (HSV-1/2). These diseases are common causes of congenital
infections which lead to a syndrome that includes one or more of
the following clinical symptoms: low birth weight, prematurity,
purpura, jaundice, anaemia, microcephaly, hydrocephaly, cerebral
calcification, chorioretinitis, cataracts, microphthalmia and
pneumonitis (2–6). As more infections that cause similar
clinical symptoms became recognised, the 'O' in the acronym came to
stand for 'Other' pathogens.
TORCH infections now include under 'Other'
pathogens, such as syphilis, parvovirus B19, enterovirus, hepatitis
B and human immunodeficiency virus (HIV). Primary infections with
TORCH pathogens, including those in pregnant women, are usually
subclinical and, even if symptomatic, lack specificity in terms of
their symptoms and signs (7,8).
Most importantly, the pathogens can be vertically transmitted to
the fetus through the placenta after maternal primary infection.
This may result in spontaneous abortion, prematurity and fetal or
neonatal infections (9,10). The World Health Organization (WHO)
has estimated that >100,000 infants are born with congenital
rubella syndrome (CRS) each year (11). Moreover, an association between
infection with HIV and infection with TORCH pathogens has been
demonstrated (12,13). Existing strategies to protect
against, or to treat TORCH infections during pregnancy are, thus
far, limited (7). Therefore, the
prevention and early diagnosis of TORCH infections is a matter of
great importance for pregnant women (14).
Currently, the identification of primary TORCH
infections in pregnant women is achieved by the detection of TORCH
pathogen-specific immunoglobulin G (IgG) and/or immunoglobulin M
(IgM) antibodies. Of these, TORCH-specific IgM antibodies have been
shown to be an indicator of acute and recent TORCH pathogen
infection, as the IgM antibody can be detected within 2 weeks of
infection (15). IgM antibody can
be detected by enzyme-linked immunosorbent assay (ELISA) with a
relatively high level of specificity and sensitivity. However, the
lack of adequate laboratory infrastructures and the time-consuming
nature of the procedures constitute obstacles to the rapid
detection and diagnosis.
Point-of-care testing (POCT) is now emerging as an
excellent and effective method which can circumvent these
above-mentioned issues (16–19). The substantial progress made in
research on lateral-flow immunochromatographic assay (LFIA) and
nanomaterials has contributed to their increasing application in
the detection of proteins and pesticide residues and for the
diagnosis of disease (20–25).
In previous studies, a series of ligands, such as poly(acrylic
acid) (PAA), poly-L-lysine and polystyrene were attached to the
nanoparticles, and this markedly influenced particle behaviour and
spatial distribution (26–28).
This significantly impacted on the performance of the LFIA
detection system.
TORCH-pathogens have been detected using various
methods (29–32). In the present study,
poly(methacrylic acid) (PMAA)-modified gold magnetic nanoparticles
(GoldMag) were applied in the LFIA system for the simultaneous
detection of 4 different TORCH pathogens, notably TOX, RV, CMV and
HSV-2.
Materials and methods
Reagents and materials
Gold magnetic nanoparticles were purchased from
Xi'an GoldMag Nanobiotech Co., Ltd. (Xi'an, China). Mouse
anti-human IgM (μ-chain) monoclonal antibody was purchased from
HyTest Ltd. (Turku, Finland). Toxoplasma antigen was purchased from
Ruislip Biotech Ltd. (Hillingdon, UK). Purified rubella antigen,
CMV-M concentrate antigen and HSV-2 antigen were obtained from
Meridian Life Science, Inc. (Saco, ME, USA). Goat anti-mouse IgG
serum was purchased from Solarbio Co., Ltd. (Beijing, China).
Bovine serum albumin (BSA), triglyceride, hemoglobin and bilirubin
were obtained from Sigma-Aldrich (St. Louis, MO, USA). All
chemicals were of analytical-reagent grade and were used as
received without further purification. Ultrapure water was used in
the experiments.
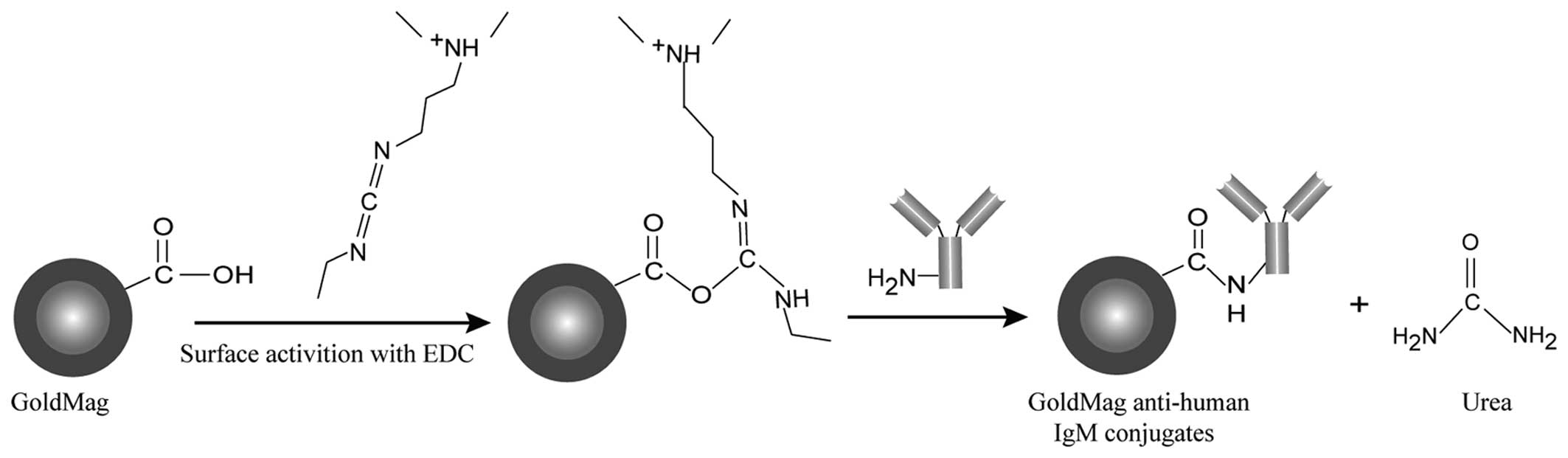
Preparation of GoldMag and mouse
anti-human IgM (μ-chain) monoclonal antibody conjugation
GoldMag were prepared according to the method
described in our previous study (24). As shown in Fig. 1, the mouse anti-human IgM
(μ-chain) monoclonal antibody (anti-human IgM) covalently bonded on
the surface of GoldMag using 1-ethyl-3-(3-dimethylaminopropyl)
carbodiimide (EDC) as a linker. The preparation of the conjugates
was carried out as follows: firstly, 1 mg of particles was
equilibrated in 1 ml of borate saline (BS) buffer (0.02 M) in a
tube, and 15 µl of EDC (5 mg/ml) were added dropwise. The
mixture was further processed by ultrasonication for 20 min.
Anti-human IgM antibody was added to the EDC-activated particles
and incubated for 1 h. Subsequently, the GoldMag anti-human IgM
complex was separated magnetically and the supernatant (solution
contained unbound anti-human IgM) was removed. The conjugation
efficiency was calculated by determining the concentration of
anti-IgM antibody in the solution before and after coupling using
the BCA protein assay method. Secondly, in order to cover the
non-specific binding sites, the GoldMag anti-human IgM complex was
further blocked with 3% (w/v) BSA and 5% (v/v) calf serum in BS
buffer, and incubated for another 40 min. After washing with BS
buffer containing 0.05% (v/v) Tween-20, the GoldMag anti-human IgM
complex was magnetically separated and finally suspended in 200
µl BS suspension buffer containing 1% (w/v) BSA. These
functional gold magnetic nanoparticles were stored at 4°C and were
ready to use.
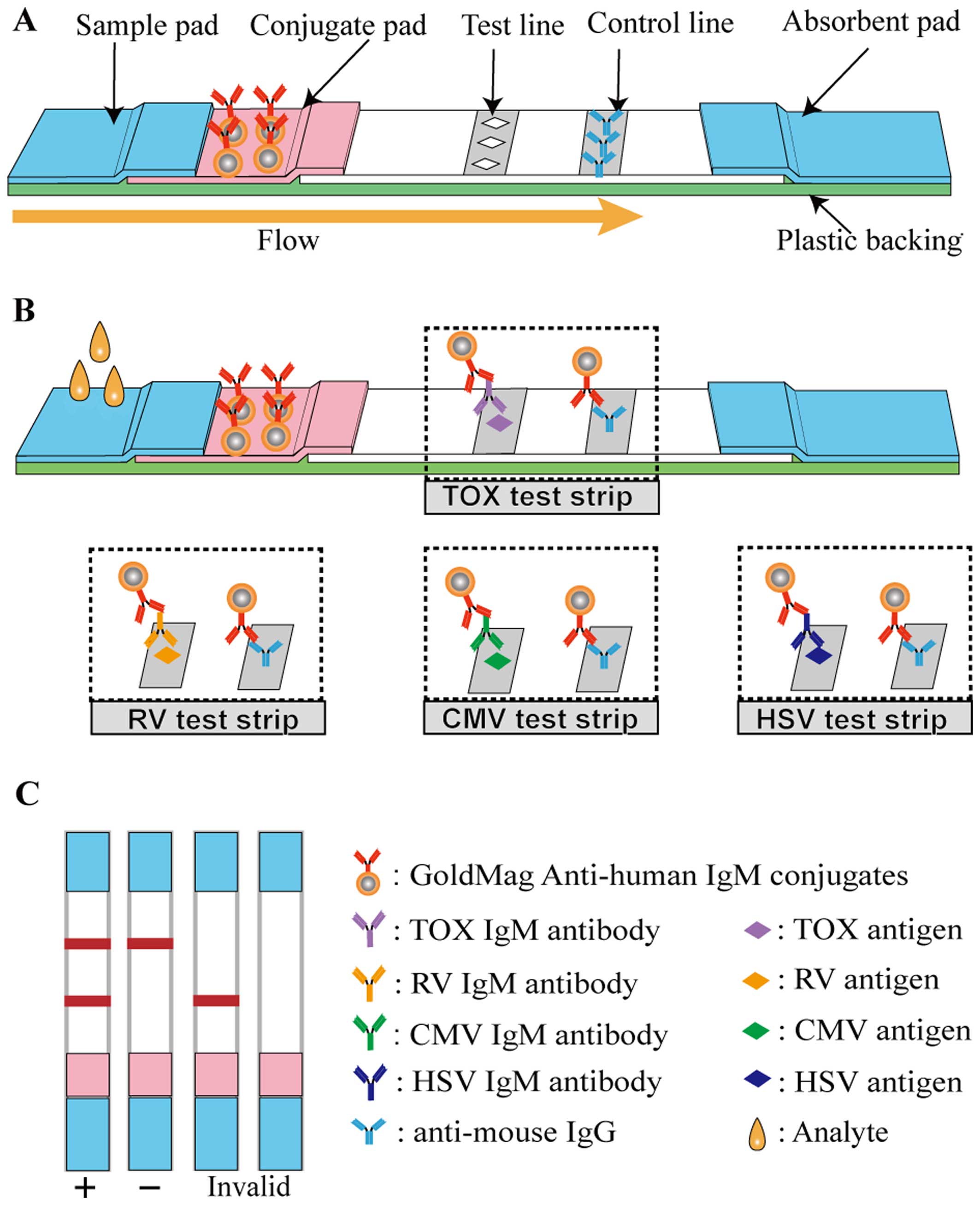
LFIA device
The structure of the LFIA strip is illustrated in
Fig. 2A. The 4×40-mm lateral flow
immunochromatographic strip consists of 5 components, namely a
sample pad, a conjugate pad, a nitrocellulose (NC) membrane, an
absorbent pad and a plastic backing. Some elements were treated
before the device was constructed. The sample pad was saturated
with BS buffer containing 0.5% (v/v) rabbit anti-human IgG serum,
and the conjugate pad was immersed in ultrapure water containing
0.2% (v/v) Triton X-100, 0.2% (v/v) Tween-20 and 0.8% (w/v)
sucrose. Subsequently, a solution of 5 mg/ml of the GoldMag
anti-human IgM conjugates, prepared as described above, was sprayed
onto the conjugate pad using a Biodot AirJet dispensing apparatus
(BioDot, Irvine, CA, USA). TORCH antigens and goat anti-mouse IgG
(2 mg/ml) were respectively sprayed on the NC membrane to form a
test line (T-line) and a control line (C-line). Finally, the whole
LFIA device was assembled after all the aforementioned components
had dried fully.
A schematic diagram of the method for TORCH-IgM
antibody detection is illustrated in Fig. 2B, and Fig. 2C depicts possible detection
results. More specifically, 60 µl of human sera, which
possibly contained TORCH-IgM were dropped onto the sample pad. The
solution migrated toward the absorbent pad via capillary force and
rehydrated the GoldMag anti-human IgM conjugates. If the testing
sera contained TORCH-IgM antibodies, the GoldMag anti-human IgM
probes recognised and captured them. When the complexes reached the
T-line, the immobilised TORCH antigens interacted with the captured
TORCH-IgM antibodies, and when they reached the C-line, the
immobilised goat anti mouse IgG antibody (anti-mouse IgG) captured
the excess GoldMag anti-human IgM probes. Thus, 2 characteristic
red bands were observed within 15 min. However, healthy sera
without a TORCH-IgM antibody led to a red band on the C-line.
Evaluation of the TORCH-LFIA system
The sensitivity, repeatability, reproducibility and
specificity of the TORCH-LFIA system were evaluated (negative sera
were used as controls in all experiments). First, 60 µl
standard TORCH-IgM positive control sera purchased from SeraCare
Life Sciences, Inc. (Milford, MA, USA) were used to test the
sensitivity of the system. The repeatability was then investigated
by testing the same TORCH samples (60 µl) 10 times with the
same batch of GoldMag-based test strips (the concentrations of TOX
IgM, RV IgM, CMV IgM and HSV IgM were 13.7, 42.5, 32 Au/ml and 2.93
S/CO, respectively). The reproducibility was determined by testing
the same TORCH samples with 3 batches of GoldMag-based test strips.
For interference testing, one sample of TORCH-negative and one of
TORCH-positive serum were split and individually spiked with 33
mg/ml triglycerides, 0.2 mg/ml bilirubin and 5 mg/ml hemoglobin
samples (the concentrations of TOX, RV, CMV, HSV IgM were 13.7, 44,
49.7 Au/ml and 3.5 S/CO, respectively). The results from 60
µl spiked samples were compared with those from 60 µl
non-spiked serum samples. For cross-reactivity assays, 60 µl
TOX-positive serum (the concentration of TOX IgM was 25.3 Au/ml),
RV-positive serum (the concentration of RV IgM was 76.1 Au/ml),
CMV-positive serum (the concentration of CMV IgM was 76.1 Au/ml)
and HSV-2 positive serum (the concentration of HSV IgM was 2.67
S/CO) were assessed (these 4 serum samples contained only one
specific TORCH pathogen each as opposed to 2 or more TORCH
pathogens). All results were presented in the form of optical
images, which were captured using a digital camera D7000 (Nikon,
Tokyo, Japan).
Detection of human serum samples with
TORCH-LFIA strips
Human serum samples which were positive for TOX
(n=3), CMV (n=14), HSV-2 (n=19) and RV (n=5) were obtained from
Xiangya Hospital (Changsha, China). Negative serum samples (n=121)
were collected from The Second Affiliated Hospital of Shaanxi
University of Chinese Medicine (Shaanxi, China). They were then
re-tested using our LFIA strips. This study was approved by the
Ethics Committee of the National Engineering Research Center for
Miniaturized Detection Systems, Xi'an, China.
Results
Conjugation of GoldMag with targeted
moieties
GoldMag particles were conjugated to anti-human IgM
antibody in order to construct probes for the detection of
TORCH-IgM antibodies. Dynamic light scattering was used to monitor
the distribution of the hydrodynamic size of the gold magnetic
nanoparticles before and after conjugation with anti-human IgM
antibody. As shown in Fig. 3A,
the hydrodynamic size of the antibody-conjugated particles, as
compared to the corresponding GoldMag, was increased by
approximately 18 nm (from 64.8±0.73 to 82.7±0.34 nm). The
reasonable increment of the hydrodynamic size after the conjugation
suggests that the antibody molecules were effectively coupled to
the particles (33). This
conclusion was further confirmed by UV visible spectroscopy. The
characteristic absorption peak of the GoldMag was 532±0.53 nm
(Fig. 3B). It shifted slightly to
536±0.61 nm when the anti-human IgM antibody was conjugated to the
GoldMag, which indicates that the surface chemical structure of the
GoldMag changed from one with a non-antibody layer to that with an
antibody layer, as previously described (34).
To achieve optimal performance, experimental
parameters involved in the preparation of the GoldMag anti-human
IgM conjugates were systematically optimised, including the pH
value of the coupling buffer and the amount of anti-human IgM
antibody added. Fig. 4A
illustrates the determination of the optimum pH value of the
coupling buffer. The amount of conjugate formed by GoldMag and
anti-human IgM was higher under neutral conditions than under
alkaline conditions. The addition of a coupling solution at pH 7.0
resulted in the highest conjugation efficiency. Therefore, pH 7.0
was selected as the optimal pH for the preparation of the GoldMag
anti-human IgM conjugates.
To effectively utilise the antibody, the amount of
anti-human IgM antibody added to 1 mg of GoldMag was optimised.
Fig. 4B demonstrates that
increasing the amount of anti-human IgM led to a gradual increase
in the amount of antibody conjugated to the GoldMag. However, when
the amount of anti-human IgM reached 150 µg, the addition of
more anti-human IgM antibody no longer increased the quantity of
the conjugate. This indicated that the saturation of anti-human IgM
antibody to the GoldMag was reached when the amount of conjugated
antibody was approximately 94 µg. Therefore, the optimum
amount of anti-human IgM antibody added was 150 µg for 1 mg
GoldMag.
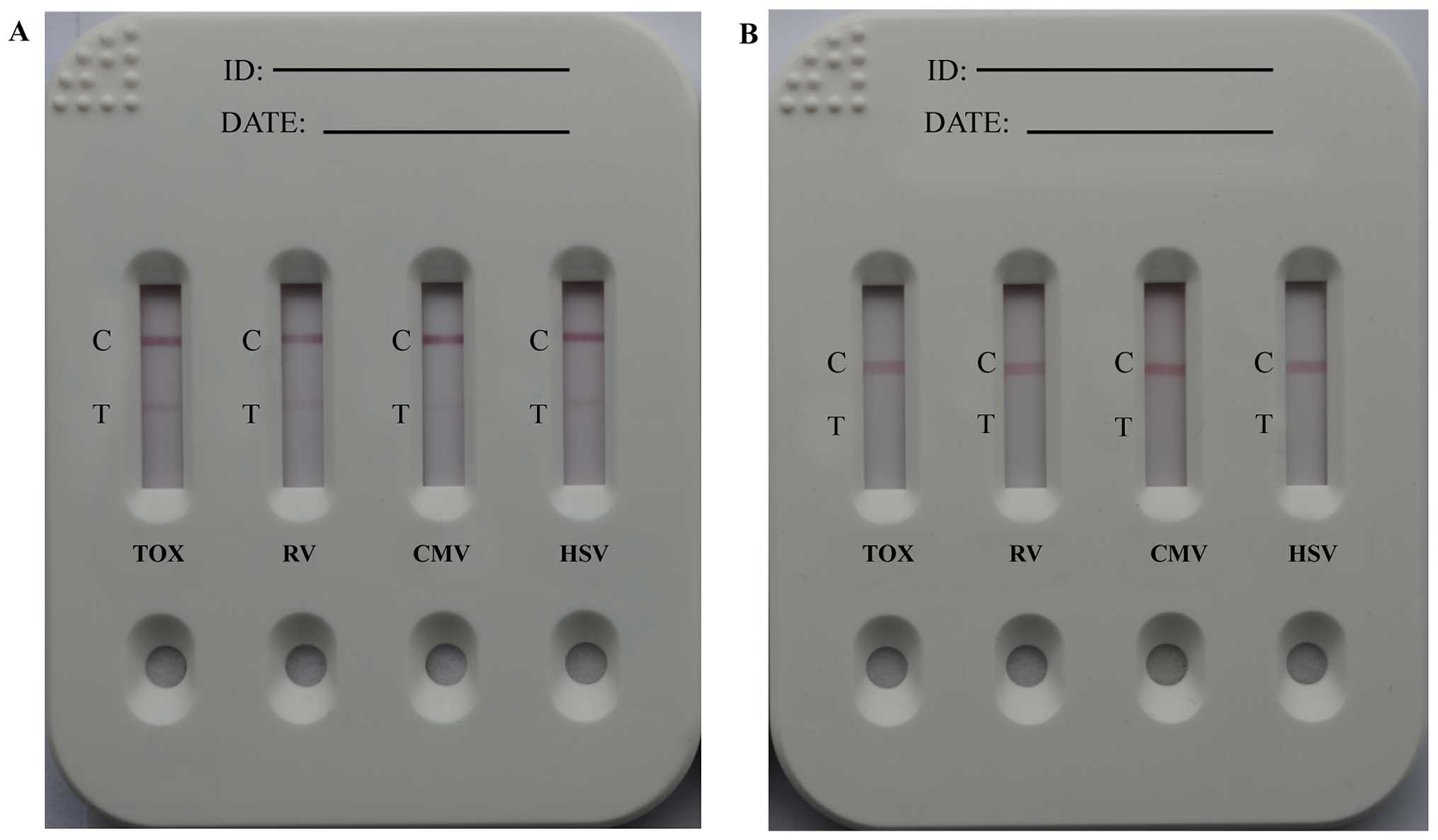
Sensitivity of TORCH IgM LFIA test
strips
A standard TORCH-IgM positive control serum sample
was used to compare the sensitivity of the GoldMag-based LFIA to a
commercial colloidal gold-based immunoassay for the detection of
TORCH IgM antibodies. Fig. 5
demonstrates that the GoldMag-based TORCH LFIA had a higher
sensitivity than the colloidal gold-based LFIA.
Repeatability and reproducibility of
measurements with the TORCH IgM LFIA test strips
The repeatability and reproducibility of the
GoldMag-LFIA system were investigated. The colour intensity of the
T-line on the test strips remained constant for all 4 pathogens:
TOX, RV, CMV and HSV (Fig. 6).
This indicates that within each batch of the GoldMag-LFIA system,
the results are perfectly repeatable. Furthermore, Fig. 7 shows that the colour intensity of
the T-lines of 3 different batches of the GoldMag-based test strips
was constant, which confirms a high level of reproducibility of the
LFIA.
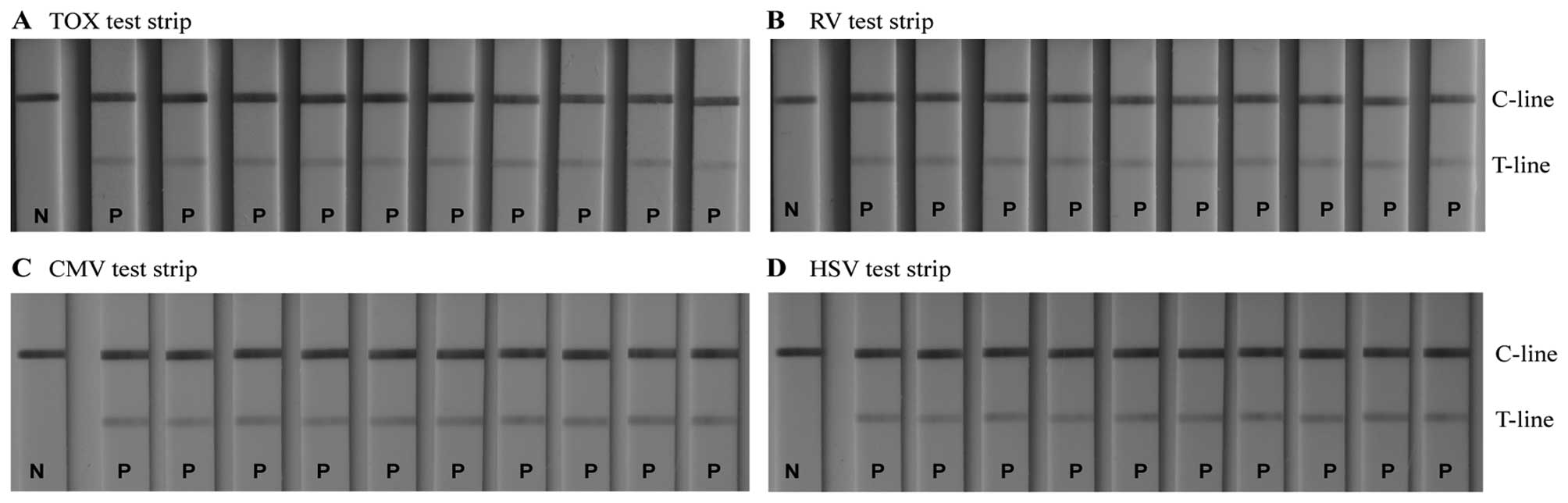
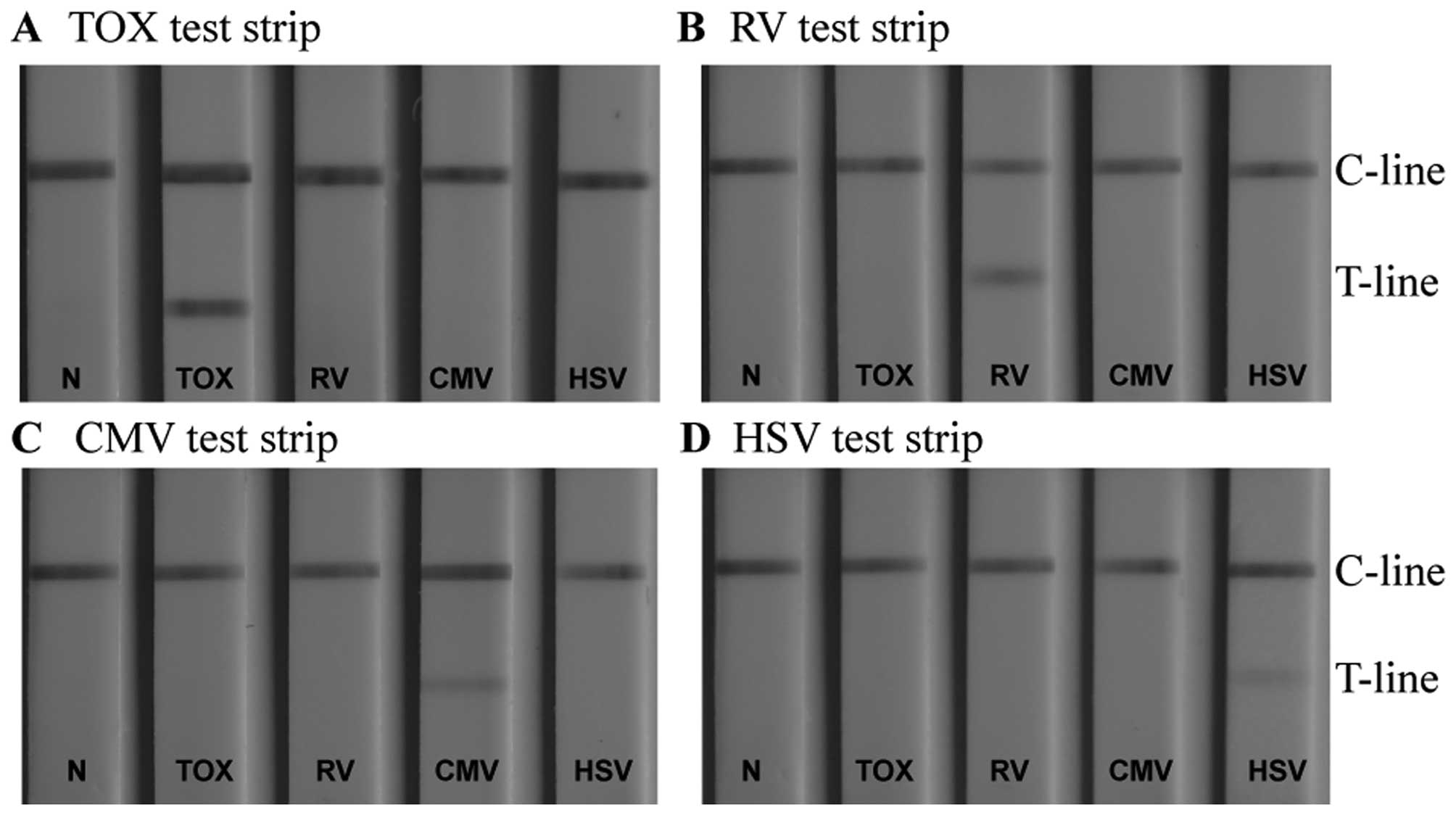
Specificity of TORCH IgM LFIA test
strips
Detecting whether a patient suffers from an
infection with one pathogen or from an infection with multiple
TORCH pathogens is of great significance for accurate diagnosis, as
it permits us to take appropriate therapeutic measures. It has been
shown that different TORCH pathogens have a number of common
antigenic determinants (33). If
cross-reactivity occurs during the diagnostic determination, it
leads to non-specific signals at the T-line, and thus to
false-positive results. This has a negative impact on the
diagnosis, and thus on the treatment of a TORCH infectious disease.
It is therefore essential for each serum analysis to use a
conjugate that captures only the specific antigen. This antigen is
immobilised on the T-line of the 4 LFIA strips. In our study, to
investigate the existence of possible cross reactivity, 4 serum
samples, each containing antigens of one specific TORCH pathogen
only, were respectively titrated to the sample pad of the same type
of LFIA detection device (negative sera samples were used as
controls). A particular test strip detected only the corresponding
sample (Fig. 8). Other sera
produced no observable binding at the T-line, indicating that each
assay was specific for its own pathogen. Cross-reactivity between
the assays did not occur.
It is also essential to assess whether common and
potentially interfering substances in the sample have an impact on
the test results. Therefore, TORCH IgM-negative and -positive serum
samples, spiked with a number of potentially interfering
substances, were added to the sample pad of the devices (untreated
TORCH IgM-negative and -positive sera samples were used as
controls). Sufficiently high concentrations of the interfering
substances were spiked with the serum samples [triglycerides (33
mg/ml), bilirubin (0.2 mg/ml) and hemoglobin (5 g/l)] in an attempt
to elicit a response in the test zone of the corresponding assay.
The results of the interference experiments are illustrated in
Fig. 9. The TORCH IgM negative
serum samples, whether control or spiked with potentially
interfering substances, demonstrated no non-specific reaction in
any of the assays (the test zones remained colourless). Similarly,
the TORCH IgM positive serum samples, whether control or spiked
with potentially interfering substances exhibited the same colour
intensity in the test zones. These results indicate that no
interference occured on the TORCH LFIA test strips.
 | Figure 9Evaluation of the possible effects of
potentially interfering substances on the test results: (A)
interference test on Toxoplasmosis (TOX) strips. (B) Interference
test on rubella virus (RV) strips. (C) Interference test on
cytomegalovirus (CMV) strips. (D) Interference test on herpes
simplex virus (HSV) strips. N, negative serum; N+T, negative serum
with 33 mg/ml triglyceride; N+B, negative serum with 0.2 mg/ml
bilirubin; N+H, negative serum with 5 g/l hemoglobin; P, positive
serum; P+T, positive serum with 33 mg/ml triglyceride; P+B,
positive serum with 0.2 mg/ml bilirubin; P+H, positive serum with 5
g/l hemoglobin; C-line, control line; T-line, test line. |
Detection of human sera samples with
TORCH LFIA strips
Using GoldMag-based TORCH LFIA devices, 3 TOX, 5 RV,
14 CMV and 19 HSV-2 seropositive samples obtained from Xiangya
Hospital and 121 negative sera samples were re-examined. The
results indicated 100% specificity and 100% sensitivity. This
demonstrates that the GoldMag-based immunoassay has great potential
for use in the clinical diagnosis of human serum samples, and the
results are considered highly accurate and reliable.
Discussion
The TORCH pathogens, including TOX, RV, CMV and HSV
pose a serious threat to pregnant women and their fetuses.
Unfortunately, existing strategies used to protect against, or to
treat, TORCH infections during pregnancy are, thus far, limited
(7). Therefore, a rapid and
simple method for the early diagnosis of TORCH infections would
allow for the better management of TORCH-infected diseases.
POCT has attracted considerable interest due to the
fact that it is a rapid, time-saving and cost-effective menthod. In
this study, a LFIA system was established using GoldMag
nanoparticles instead of colloidal gold nanoparticles, which are
widely used in conventional LFIAs. The gold magnetic nanoparticles
modified by PMAA have carboxyl groups on their surfaces, and they
covalently link with amino groups on the mouse anti-human IgM
(μ-chain) monoclonal antibody after the carboxyl moieties have been
activated by EDC. The procedure is a chemical coupling process, and
the conjugated gold magnetic nanoparticles and antibodies are
hardly influenced by physical effects. Moreover, the carboxyl
moieties provide a more steric, ion-independent stabilization, and
a hydrophilic surface layer for downstream applications (26). Compared with commercial colloidal
gold-based LFIA strips, the PMAA-modified gold magnetic
nanoparticle-based LFIA strips exhibited higher sensitivity.
Moreover, no interference with triglycerides, hemoglobin or
bilirubin was observed, and no cross-reactivity was noted among the
4 pathogens, suggesting that these strips have a high specificity
for the IgM antibody of each of the TORCH pathogens examined.
The experimental parameters involved in the
preparation of the GoldMag anti-human IgM conjugates, such as the
pH value of the coupling buffer and the amount of antibody added,
influenced the conjugation efficiency of the antibody and the
stability of the conjugates. The conjugation efficiency of the
antibody was highest when the pH of the coupling solution was 7.0
(Fig. 4A). This result is
attributable to the charge of the anti-human IgM at different pH
values. The saturation of anti-human IgM antiobdy to GoldMag was
reached when 150 µg of anti-human IgM was added (Fig. 4B). Adding greater quantities of
anti-human IgM antibody did not lead to a signifi-cant difference
in the quantity of anti-human IgM conjugated. This effect is
related to the additional adsorption of antibodies and the
occurrence of steric hindrance when there is a high density of
antibodies on the nanoparticle surface (35,36).
In conclusion, in this study, we successfully
developed a gold magnetic nanoparticle conjugate-based lateral flow
assay for the detection of TORCH IgM antibodies with high levels of
sensitivity and specificity. The results imply that this method is
sufficiently sensitive to detect TORCH antibodies in clinical
samples. Additionally, the 4 test strips were assembled in plastic
cases, thus making the detection method more convenient than others
currently in use. Our GoldMag anti-human IgM conjugates can be
applied for the detection of 4 different pathogens, as they were
able to capture any IgM antibody that appeared in an immune
response. The LFIA system that we established can therefore be
further developed for utilization in the detection of other
IgM-specific antibodies, including those for recently identified
autoimmune diseases. Our TORCH LFIA method has great potential for
future use in the simultaneous detection of several pathogens on a
single LFIA device. This could be accomplished by spraying several
pathogen antigens onto the NC membrane to form several T-lines.
Efforts to establish a TORCH LFIA device capable of simultaneously
determining the 4 TORCH pathogens, TOX, RV, CMV and HSV, are
currently underway.
Acknowledgments
This study was supported by the Project of
Prevention and Treatment of Major Infectious Diseases (no.
2013ZX10004804008) and the National Natural Science Foundation of
China (no. 31200749).
Abbreviations:
|
PMAA
|
poly(methacrylic acid) sodium salt
|
|
BS
|
borate saline
|
|
EDC
|
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
|
|
TOX
|
toxoplasmosis
|
|
RV
|
rubella virus
|
|
CMV
|
cytomegalovirus
|
|
HSV
|
herpes simplex virus
|
|
POCT
|
point-of-care testing
|
|
LFIA
|
lateral flow immunochromatographic
assay
|
|
GoldMag
|
PMAA-modified gold magnetic
nanoparticles
|
References
|
1
|
Nahmias AJ, Walls KW, Stewart JA, Herrmann
KL and Flynt WJ: The ToRCH complex-perinatal infections associated
with toxoplasma and rubella, cytomegol- and herpes simplex viruses.
Pediatr Res. 5:405–406. 1971. View Article : Google Scholar
|
|
2
|
Stegmann BJ and Carey JC: TORCH
Infections. Toxoplasmosis, Other (syphilis, varicella-zoster,
parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes
infections. Curr Womens Health Rep. 2:253–258. 2002.PubMed/NCBI
|
|
3
|
Newton ER: Diagnosis of perinatal TORCH
infections. Clin Obstet Gynecol. 42:59–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams Waldorf KM and McAdams RM: Influence
of infection during pregnancy on fetal development. Reproduction.
146:R151–R162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu F, Sternberg MR, Kottiri BJ, McQuillan
GM, Lee FK, Nahmias AJ, Berman SM and Markowitz LE: Trends in
herpes simplex virus type 1 and type 2 seroprevalence in the United
States. JAMA. 296:964–973. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nigro G, Adler SP, La Torre R, Best AM,
Congenital Cytomegalovirus and Collaborating Group: Passive
immunization during pregnancy for congenital cytomegalovirus
infection. N Engl J Med. 353:1350–1362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Delorme-Axford E, Sadovsky Y and Coyne CB:
The placenta as a barrier to viral infections. Annu Rev Virol.
1:133–146. 2014. View Article : Google Scholar
|
|
8
|
Corey L and Wald A: Maternal and neonatal
herpes simplex virus infections. N Engl J Med. 361:1376–1385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown ZA, Selke S, Zeh J, Kopelman J,
Maslow A, Ashley RL, Watts DH, Berry S, Herd M and Corey L: The
acquisition of herpes simplex virus during pregnancy. N Engl J Med.
337:509–515. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soper DE: Congenital cytomegalovirus
infection: An obstetrician's point of view. Clin Infect Dis.
57(Suppl 4): S171–S173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Santis M, Cavaliere AF, Straface G and
Caruso A: Rubella infection in pregnancy. Reprod Toxicol.
21:390–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mwaanza N, Chilukutu L, Tembo J, Kabwe M,
Musonda K, Kapasa M, Chabala C, Sinyangwe S, Mwaba P, Zumla A and
Bates M: High rates of congenital cytomegalovirus infection linked
with maternal HIV infection among neonatal admissions at a large
referral center in sub-Saharan Africa. Clin Infect Dis. 58:728–735.
2014. View Article : Google Scholar
|
|
13
|
Celum C, Wald A, Lingappa JR, Magaret AS,
Wang RS, Mugo N, Mujugira A, Baeten JM, Mullins JI, Hughes JP, et
al: Partners in Prevention HSV/HIV Transmission Study Team:
Acyclovir and transmission of HIV-1 from persons infected with
HIV-1 and HSV-2. N Engl J Med. 362:427–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wallon M, Peyron F, Cornu C, Vinault S,
Abrahamowicz M, Kopp CB and Binquet C: Congenital toxoplasma
infection: Monthly prenatal screening decreases transmission rate
and improves clinical outcome at age 3 years. Clin Infect Dis.
56:1223–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rorman E, Zamir CS, Rilkis I and Ben-David
H: Congenital toxoplasmosis - prenatal aspects of Toxoplasma gondii
infection. Reprod Toxicol. 21:458–472. 2006. View Article : Google Scholar
|
|
16
|
Niemz A, Ferguson TM and Boyle DS:
Point-of-care nucleic acid testing for infectious diseases. Trends
Biotechnol. 29:240–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chan CP, Mak WC, Cheung KY, Sin KK, Yu CM,
Rainer TH and Renneberg R: Evidence-based point-of-care
diagnostics: Current status and emerging technologies. Annu Rev
Anal Chem (Palo Alto, Calif). 6:191–211. 2013. View Article : Google Scholar
|
|
18
|
Akanda MR, Joung HA, Tamilavan V, Park S,
Kim S, Hyun MH, Kim MG and Yang H: An interference-free and rapid
electrochemical lateral-flow immunoassay for one-step
ultrasensitive detection with serum. Analyst (Lond). 139:1420–1425.
2014. View Article : Google Scholar
|
|
19
|
Pöhlmann C, Dieser I and Sprinzl M: A
lateral flow assay for identification of Escherichia coli by
ribosomal RNA hybridisation. Analyst (Lond). 139:1063–1071. 2014.
View Article : Google Scholar
|
|
20
|
Xu H, Mao X, Zeng Q, Wang S, Kawde AN and
Liu G: Aptamer-functionalized gold nanoparticles as probes in a
dry-reagent strip biosensor for protein analysis. Anal Chem.
81:669–675. 2009. View Article : Google Scholar
|
|
21
|
Xia X, Xu Y, Zhao X and Li Q: Lateral flow
immunoassay using europium chelate-loaded silica nanoparticles as
labels. Clin Chem. 55:179–182. 2009. View Article : Google Scholar
|
|
22
|
Kobys VL, Konovalenko VF, Repina NV,
Golovko TS, Gulak LO, Tarasova TO, Zaharycheva EV and Matyushok OF:
Treatment of large osteosarcoma in children: new approach. Exp
Oncol. 35:105–108. 2013.PubMed/NCBI
|
|
23
|
Song C, Liu Q, Zhi A, Yang J, Zhi Y, Li Q,
Hu X, Deng R, Casas J, Tang L and Zhang G: Development of a lateral
flow colloidal gold immunoassay strip for the rapid detection of
olaquindox residues. J Agric Food Chem. 59:9319–9326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elingarami S, Deng Y, Fan J, Zhang Y and
He N: NEIL-2 single nucleotide polymorphism genotyping using single
base extension on core-shell
Fe3O4@SiO2@Au magnetic
nanoparticles and Association of the Genotypes with Gastric Cancer
Risk in Northern Jiangsu (China). Sci Adv Mater. 6:899–907. 2014.
View Article : Google Scholar
|
|
25
|
Jiang H, Zeng X, He N, Deng Y, Lu G and Li
K: Preparation and biomedical applications of gold-coated magnetic
nanocomposites. J Nanosci Nanotechnol. 13:1617–1626. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang D, Ma J, Zhang Q, Li N, Yang J, Raju
PA, Peng M, Luo Y, Hui W, Chen C and Cui Y: Polyelectrolyte-coated
gold magnetic nanoparticles for immunoassay development: Toward
point of care diagnostics for syphilis screening. Anal Chem.
85:6688–6695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JY, Zhang Q, Emrick T and Crosby AJ:
Nanoparticle Alignment and Repulsion during Failure of Glassy
Polymer Nanocomposites. Macromolecules. 39:7392–7396. 2006.
View Article : Google Scholar
|
|
28
|
Liu C, Jia Q, Yang C, Qiao R, Jing L, Wang
L, Xu C and Gao M: Lateral flow immunochromatographic assay for
sensitive pesticide detection by using Fe3O4
nanoparticle aggregates as color reagents. Anal Chem. 83:6778–6784.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reiter-Owona I, Petersen E, Joynson D,
Aspöck H, Dardé ML, Disko R, Dreazen O, Dumon H, Grillo R, Gross U,
et al: The past and present role of the Sabin-Feldman dye test in
the serodi-agnosis of toxoplasmosis. Bull World Health Organ.
77:929–935. 1999.
|
|
30
|
Jiang S, Hua E, Liang M, Liu B and Xie G:
A novel immunosensor for detecting toxoplasma gondii-specific IgM
based on goldmag nanoparticles and graphene sheets. Colloids Surf B
Biointerfaces. 101:481–486. 2013. View Article : Google Scholar
|
|
31
|
Laderman EI, Whitworth E, Dumaual E, Jones
M, Hudak A, Hogrefe W, Carney J and Groen J: Rapid, sensitive, and
specific lateral-flow immunochromatographic point-of-care device
for detection of herpes simplex virus type 2-specific
immunoglobulin G antibodies in serum and whole blood. Clin Vaccine
Immunol. 15:159–163. 2008. View Article : Google Scholar :
|
|
32
|
Leruez-Ville M, Vauloup-Fellous C, Couderc
S, Parat S, Castel C, Avettand-Fenoel V, Guilleminot T,
Grangeot-Keros L, Ville Y, Grabar S and Magny JF: Prospective
identification of congenital cytomegalovirus infection in newborns
using real-time polymerase chain reaction assays in dried blood
spots. Clin Infect Dis. 52:575–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jans H, Liu X, Austin L, Maes G and Huo Q:
Dynamic light scattering as a powerful tool for gold nanoparticle
bioconjugation and biomolecular binding studies. Anal Chem.
81:9425–9432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Dai Q, Austin L, Coutts J, Knowles
G, Zou J, Chen H and Huo Q: A one-step homogeneous immunoassay for
cancer biomarker detection using gold nanoparticle probes coupled
with dynamic light scattering. J Am Chem Soc. 130:2780–2782. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Safenkova I, Zherdev A and Dzantiev B:
Factors influencing the detection limit of the lateral-flow
sandwich immunoassay: a case study with potato virus X. Anal
Bioanal Chem. 403:1595–1605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thobhani S, Attree S, Boyd R, Kumarswami
N, Noble J, Szymanski M and Porter RA: Bioconjugation and
characterisation of gold colloid-labelled proteins. J Immunol
Methods. 356:60–69. 2010. View Article : Google Scholar : PubMed/NCBI
|