Introduction
Spinal cord injury (SCI), which results from primary
and secondary injury mechanisms (1), is the most devastating complication
of spinal trauma, often resulting in progressive sensory and
functional damage. In addition to the severe physical and
psychological harm experienced by patients themselves, SCI also
imposes a huge economic burden on society (2). Despite years of research and
numerous attempts at various treatment strategies, the remedy for
paralysis remains elusive and current treatments are restricted to
the early administration of large doses of methylprednisolone and
immediate surgical intervention to alleviate spinal cord edema
(3,4). Recent advances in SCI research have
drawn attention to an array of novel experimental therapeutic
approaches (5).
Of all the potential strategies, bone marrow-derived
mesenchymal stem cells (BMSCs) appear to be a most promising
candidate for clinical application, since they have been
demonstrated to be helpful in the repair of injured spinal cords in
experimental animals (6–8). The administration of BMSCs, with
minimal delay, has been suggested as a way of attenuating secondary
injury and maximizing the volume of spared neurological tissue
(9). However, the homing process
of BMSCs is inefficient due to cell apoptosis, as well as
limitations in the cellular signals that modulate their recruitment
(10,11). It has been shown that the majority
of transplanted BMSCs are unable to reach the lesion site, and this
results in a limited therapeutic effect (12,13). Accumulating evidence has indicated
that the stromal cell-derived factor-1 (SDF-1)/CXC chemokine
receptor 4 (CXCR4) axis plays a crucial role in the recruitment of
BMSCs to lesion sites in animal models (14–17). It has been demonstrated that in
vitro-amplified BMSCs display a downregulation in the surface
expression of cardinal homing receptors, compromising CXCR4 and
their capacity to react to homing signals (18,19). Thus, an alternative strategy has
to be explored to enhance the therapeutic benefits of BMSCs.
Erythropoietin (EPO) is a glycoprotein that mediates
the differentiation and apoptosis of a number of non-hematopoietic
cells via the erythropoietin receptor (EPOR) (20). There is increasing evidence for
the different roles of EPO in various types of diseases, such as
calvarial defects (21), chronic
renal failure (22) myocardial
infarction (23), osteonecrosis
of the femoral head (24), spinal
cord injury (25,26), diabetes mellitus (27) and acute lung injury (28,29). The study by Zwezdaryk et al
(30) confirmed that EPOR is also
expressed on the surface of BMSCs. The study by Liu et al
(31) demonstrated that EPO
promotes the proliferative effects of BMSCs in an acute kidney
injury microenvironment and reverses their low secretory capacity.
Nair et al (32)
demonstrated the tremendous potential of EPO to mobilize BMSCs to
migrate to the defective bone tissue and to promote the osteogenic
differentiation of BMSCs.
On the basis of the above-mentioned findings, we
hypothesized that EPO may mobilize the BMSCs to migrate to the
lesion site following SCI and promote recovery; we hypothesized
that the SDF-1/CXCR4 axis plays an important role in this process.
Thus, in this study, we aimed to confirm this hypothesis and
elucidate the possible molecular mechanisms involved, in order to
provide new ideas for research into clinical treatments for
SCI.
Materials and methods
Animals
The present study was conducted using adult female
Sprague-Dawley (SD) rats (n=108; weighing 200–250 g). All rats used
in this study were purchased from the Animal Experiment Center of
Hubei University of Medicine (Wuhan, China). The study protocol was
approved by the Ethics Committee on Animal Experiments of Hubei
University of Medicine (Shiyan, China; protocol no. SYXK20 110008).
The rats were housed 3 per cage for approximately 1 week prior to
the start of the experiments, with free access to food and water
and maintained in a suitable environment at 21°C, 60% air humidity
and a 12-h light/dark cycle.
Creation of rat model of acute SCI
Following a baseline behavioral assessment, a model
of SCI was established in rats using the modified Allen's test, as
previously described (33). The
rats were anesthetized with an intraperitoneal injection of 10%
chloral hydrate (0.3 ml/100 g) following 12 h of pre-operative
fasting from food and water. Under sterile conditions, a dorsal
laminectomy was performed, centering on the T9 spinous process, and
the spinal cord was then exposed. Subsequently, each rat sustained
a contusive SCI from a Spinal Cord Impactor (W.M. Keck Center for
Collaborative Neuroscience, Rutgers, Piscataway, NJ, USA), with a
10 g impact rod vertically dropped from a height of 5 cm which then
impinged on the spinal cord in a circular zone with a 2 mm
diameter. The dwell time for each injury was 10 sec which was
sufficient to cause a moderate contusion. The release weight,
height of drop, and velocity of each SCI were determined using the
Spinal Cord Impactor software (version 7.5). The signs of the
successful infliction of SCI were as follows: the rat tails swung
spastically, there was a retraction of the lower limbs and a
torso-like flutter, and both lower extremities exhibited flaccid
paralysis. In the sham-operated group, the spinal cord was exposed
in the same manner, but without subjecting the rats to a contusive
SCI.
Derivation and culture of BMSCs
The BMSCs were isolated as previously described
(34). Briefly, another 10 SD
rats (weighing 120±20 g) were sacrificed following anesthesia with
an intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g).
Under aseptic conditions, the BMSCs were separated from the tibias
and femurs and all conterminal tissues were carefully removed. The
extracted BMSCs were centrifuged at 1,000 × g for 5 min and then
aseptically plated in Dulbecco's modified Eagle's medium (DMEM;
HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Invitrogen, Carlsbad, CA, USA), 2 ml glutamine and 100 U/ml
penicillin. The cells were incubated at 37°C, in 5% CO2
for 24 h and the culture medium was then replaced. This procedure
was performed every 72 h, through which the other non-adherent
cells were eliminated. The remaining BMSCs were examined everyday
with an inverted phase contrast microscope (Eclipse Ti-E/U/S;
Nikon, Tokyo, Japan) to verify that the cells were further
amplified. The BMSCs were subcultured at 1:3 every 4 days when they
reached approximately 80–90% confluence. Those used for
transplantation were subjected to trypsin preconditioning and
washed with phosphate-buffered saline (PBS). To trace the internal
migration of the BMSCs toward the lesion site following SCI,
transfection was carried out using a recombinant adenovirus
encoding green fluorescent protein (Ad-GFP) plasmid with
Lipofectamine 2000 (Cyagen Biosciences Inc., Santa Clara, CA, USA),
in accordance with the manufacturer's instructions. At 6 h after
transfection, the medium was exchanged with all nutrition culture
medium and transgene expression was examined 2 days later using a
fluorescence microscope (Eclipse Ti-E/U/S; Nikon).
Experimental groups and treatments
A total of 108 rats were randomly divided into 6
groups (n=18/group) as follows: i) the sham-operated group; ii) the
model control group; iii) the EPO group; iv) the BMSC group; v) the
BMSC + EPO group; and vi) the BMSC + EPO + AMD3100 group. Each
animal in the experimental groups was subjected to SCI as described
above. In the EPO group, recombinant human EPO (rhEPO; Beijing Four
Rings Bio-Pharmaceutical Co., Ltd., Beijing, China) was
administered at a distance of 2 mm cranially and then 2 mm caudally
from the site of injury (5×103 IU/kg) using a prefilled
syringe. In the BMSC group, 10 μl of BMSC suspension
containing approximately 3×104 cells was administered
via a microsyringe (Hamilton, Reno, NV, USA). In the BMSC + EPO
group, BMSCs and EPO were administered as described above. In the
BMSC + EPO + AMD3100 group, in addition to the injection of BMSCs
and EPO, AMD3100 (a chemokine receptor antagonist; Pfizer, Inc.,
New York, NY, USA) was administered in the same manner (5 mg/kg)
through a microsyringe (Hamilton). The AMD3100 dosage was selected
in accordance with a previous study (35). In the model control and
sham-operated groups, 10 μl of PBS was injected. Following
withdrawal of the microsyringe, the muscles and the skin were
stitched in respective layers.
Post-operative care
All the rats were administered penicillin
(3×104 U/kg) and an analgesic, sufentanil (0.05
μg/kg), for the first 3 days. The urinary bladders were
manually emptied by squeezing twice a day until the auto-urination
function was restored. Food and water were supplied in slender
drinking tubes by placing them at the bottom of the cage, until the
rats were able to lift their heads to reach the food placed at a
normal height/position in the cage. Rats diagnosed with bacterial
infections during the experiment were treated immediately with
cefazolin (10 mg/kg/day; Sandoz International GmbH, Holzkirchen,
Germany).
Neurological evaluation
Dual hind limb motor function was evaluated at 1, 3,
7, 14, 21 and 28 days post-SCI using the Basso-Beattie-Bresnahan
(BBB) Locomotor Rating Scale developed by Basso et al
(36). A 120×30 cm foam box with
a smooth bottom was manufactured for the assessment. The rats were
placed in the box 2 days pre-operatively, twice a day in order to
familiarize them with the environment. The dual hind limb motor
function was quantified using a scale ranging from 0 to 21, where 0
corresponds to no locomotor activity and 21 corresponds to normal
performance. A sensorimotor grid walk test was also conducted to
estimate the capacity of the rats to precisely control hind paw
placement, as previously described (37). The rats crawled freely on a
120×120 cm grid with 1.0 cm regularly spaced horizontal holes.
Slips were recorded when the animal misplaced the paw down through
the hole in the grid, until a maximum value of 20 missteps were
reached. The rats that could not perform with at least proficient
gait in the BBB test were unable to finish the walkway and were
classified according to the maximum value. To decrease the average
error and improve the accuracy, all evaluations were performed
using a double blind technique: two individuals observed from
different sides and recorded the scores independently, and the mean
values were then acquired.
Enzyme-linked immunosorbent assay
(ELISA)
Thoracic spinal cord segments (approximately
1-cm-thick) centered on the site of injury were serially collected
and then immediately homogenized at 1, 7 and 28 days
post-operation. The samples were centrifuged at 1,800 × g at 4°C
for 10 min, and subsequently, the detached spinal cord samples were
stored at −80°C for further research. The levels of cytokines,
including tumor necrosis factor-α (TNF-α) and SDF-1 levels were
measured using DuoSet ELISA kits (Elabscience Biotechnology Co.,
Ltd., Shanghai, China) in accordance with the manufacturer's
instructions. All analyses were carried out in duplicate, using the
proposed substrates, buffers and diluents. Each experiment was
repeated 4 times. The final outcomes were pooled as the average
concentration of cytokines.
Immunofluorescence assay
To identify the distribution of the BMSCs in the
injured spinal cord, an immunofluorescence assay was performed 2
weeks following surgery. On days 1, 7 and 28 following treatment, 6
animals from each group were randomly selected and were sacrificed
by anesthesia. The injured spinal cord tissues were carefully
extracted. At each time point, 4 specimens from each group were
fixed with 4% paraformaldehyde for 24 h and then stored at −80°C in
a constant temperature refrigerator for biological detection. Two
specimens were frozen and embedded in OCT (Leica Biosystems,
Shanghai, China), and then cut into 7-μm-thick longitudinal
cryostat sections. To reduce non-specific staining, the sections
were treated with diluted normal goat serum (Boster
Bio-Engineering, Wuhan, China) at room temperature for 30 min. To
stain the nuclei, 4′,6-diamidino-2-phenylindole (DAPI) was added
and followed by incubation in the dark for 5 min. The excess DAPI
was washed 4 times for 5 min with Tween-20 in PBS (PBST; Beyotime
Institue of Biotechnology, Beijing, China) following incubation.
The slices were then dried with absorbent paper and mounted with
anti-fluorescent quencher (SouthernBiotech, Wuhan, China).
Immunofluorescence was observed under a fluorescence microscope
(Olympus BX51; Olympus, Tokyo, Japan).
Transwell migration assay
Cellular migration was detected using Transwell
chambers, which were 6.5 mm in diameter with 8 μm
nitrocellulose pore filters (Amersham Biosciences, Piscataway, NJ,
USA). In the chemotaxis group, 200 μl of serum-free culture
medium containing 1×105 BMSCs were added to the upper
chambers, and 800 μl of DMEM containing 10 U/ml of rhEPO
(Beijing Four Rings Bio-Pharmaceutical Co., Ltd.) and 10% FBS were
added to the lower chambers. In the chemotaxis inhibition group,
200 μl of serum-free culture medium containing
1×105 BMSCs, which had been co-cultured with 10
μg/ml AMD3100 (Pfizer Compounds) at 37°C for 2 h, were added
to the upper chambers, and 800 μl of DMEM containing 10 U/ml
of rhEPO and 10% FBS were added to the lower chambers. In the
control group, 200 μl of serum-free culture medium
containing 1×105 BMSCs were added to the upper chambers,
and 800 μl of DMEM were added to the lower chambers.
Following incubation for 18 h, the non-migrated cells in the upper
chamber were cleared and the membranes were fixed with 4%
paraformaldehyde for 30 min. The migrated cells were stained with
5% crystal violet dye solution (Saichuang Technology, Wuhan, China)
for 20 min, washed with PBS, and then photographed under a
microscope (Eclipse Ti-E/U/S; Nikon).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay
The apoptotic index (AI) of the lesion area was
detected by TUNEL assay using an In Situ Cell Death
Detection kit (Roche Applied Science, Basel, Switzerland) according
to the manufacturer's instructions. The sections were dewaxed with
graded alcohols and then digested with protease K (20 μg/ml,
pH 7.4) at 25°C for 30 min. The sections were then incubated with
TUNEL reaction mixture, which contained 5 μl of enzyme
solution and 45 μl of fluorochrome-labeled solution at 37°C
without light for 60 min. After being washed with PBS 3 times, the
sections were incubated with DAPI (Beyotime Institue of
Biotechnology) for 30 min. Subsequently, the sections were dried
with absorbent paper and mounted with anti-fluorescent quencher
liquid (Southern Biotech, Birmingham, AL, USA) and then observed
under a fluorescence microscope (Eclipse Ti-E/U/S; Nikon).
Apoptotic nuclei with green fluorescence and DAPI-positive nuclei
with blue fluorescence were observed at ×400 magnification. The AI
was calculated as the percentage of TUNEL-positive cells among the
total number of nucleated cells.
Western blot analysis
The spinal cord samples were homogenized using
radioimmunoprecipitation lysis buffer (RIPA; Beyotime Institue of
Biotechnology) and then centrifuged at 18,000 × g for 15 min. The
supernatant containing 50 μg total protein was extracted for
electrophoresis on a 10% sodium dodecyl sulfate gel (SDS; Beyotime
Institute of Biotechnology) and then transferred onto
polyvinylidene fluoride membranes (PVDF; Millipore, Billerica, MA,
USA). After being blocked with Tris-buffered saline with Tween-20
(TBST; Biosciences, Shanghai, China), the PVDF membranes were
incubated at 4°C overnight with anti-CXCR4 (P61073) or anti-EPO
antibody (sc-80995; 1:200; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) or rabbit anti-GAPDH antibody (A300-639A; 1:200;
Xianzhi Biological Co. Ltd., Hangzhou, China) as primary
antibodies, followed by incubation with horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (A00098;
1:50,000; Santa Cruz Biotechnology, Inc.) at 26°C for 2 h. The
immunoreactive complexes were visualized using an ECL enhanced
detection kit (Thermo Fisher Scientific, Waltham, MA, USA) and
exposed to X-ray films. The densitometry of the bands was analyzed
using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville,
MD, USA). The expression of CXCR4 and EPO was then normalized to
GAPDH.
Statistical analysis
All data are expressed as the means ± standard
deviation (SD) and compared by one way analysis of variance
(ANOVA). All statistical analyses were conducted using Statistical
Product and Service Solutions (SPSS) version 19.0 software (IBM
Corp., Armonk, NY, USA), with a value of P<0.05 considered to
indicate a statistically significant difference.
Results
TNF-α and SDF-1 levels in rat spinal
cords
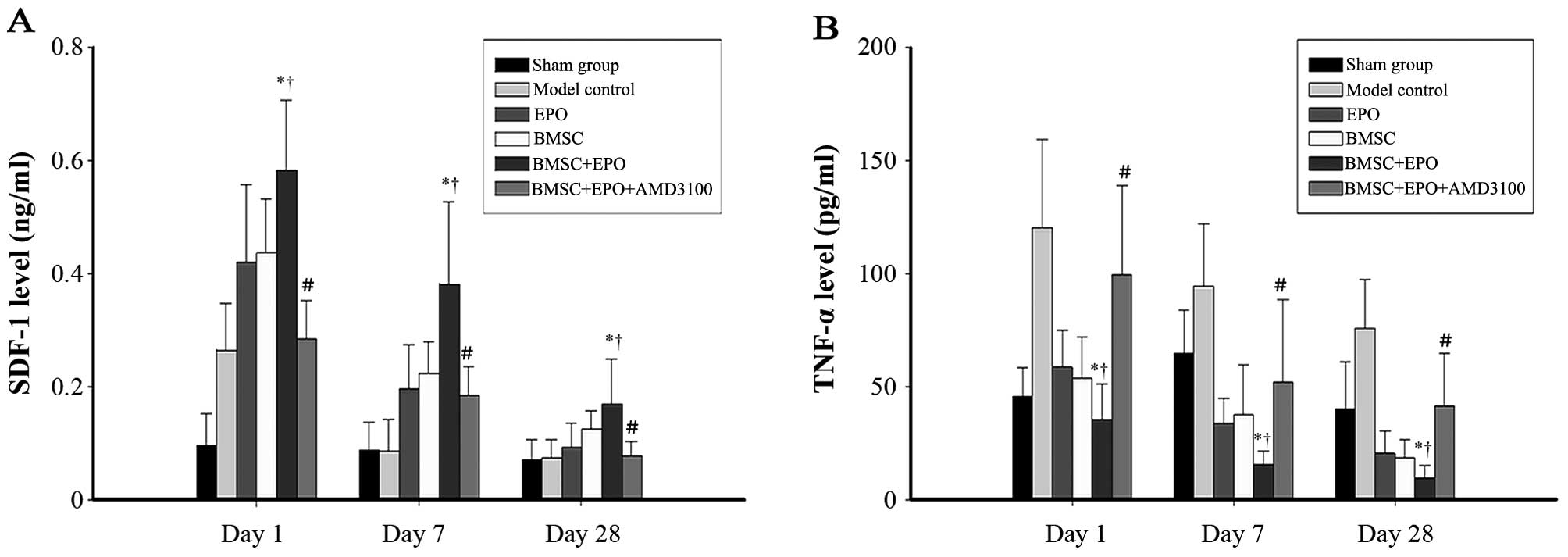
The levels of SDF-1 in the spinal cord increased
significantly following SCI (Fig.
1A). The SDF-1 levels in the BMSC + EPO group were
significantly higher compared with those in the sham-operated,
model control, EPO, BMSC and BMSC + EPO + AMD3100 groups
(P<0.05). However, no significant difference was observed
between the model control and BMSC + EPO + AMD3100 groups. On days
1, 7 and 28 post-SCI, the TNF-α levels (Fig. 1B) in the BMSC + EPO group were
significantly lower than those in the sham-operated, model control,
EPO, BMSC and the BMSC + EPO + AMD3100 groups (P<0.05). However,
the difference was not significant between the model control and
BMSC + EPO + AMD3100 groups (P>0.05).
The administration of BMSCs together with
EPO improves neurological recovery following SCI
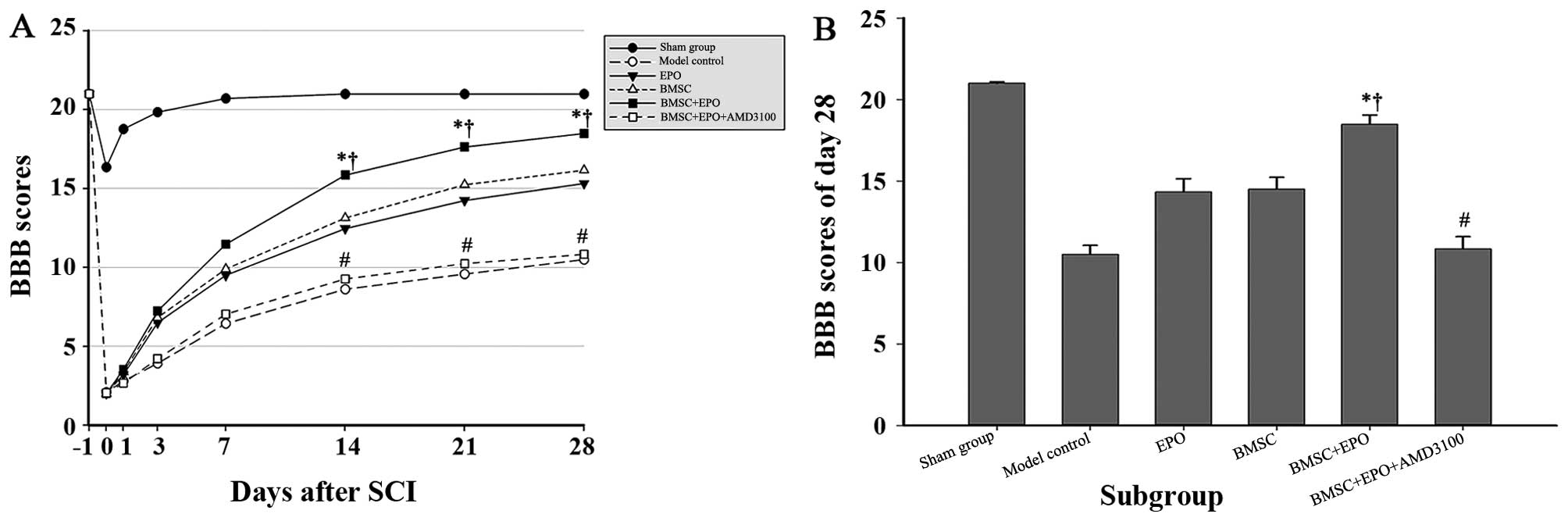
Dual hind limb loco-motor function was evaluated
using two complementary behavioural tests. The BBB motor rating
scale was adopted to evaluate the locomotor capacity of the animals
in each group up to 28 days post-operation (Fig. 2). Due to the effect of the
anesthesia, no significant difference was observed between the rats
subjected to SCI on day 1 post-operation. The mean BBB scores in
the sham-operated group declined slightly at the beginning, but
returned to normal values by day 7. Over time, the rats in the BMSC
+ EPO group obtained significantly higher BBB scores than those of
the rats in the model control and BMSC + EPO + AMD3100 group
(P<0.001) and those of the rats in the EPO and BMSC group
(P<0.05). However, no significant differences were observed
between the model control and BMSC + EPO + AMD3100 groups
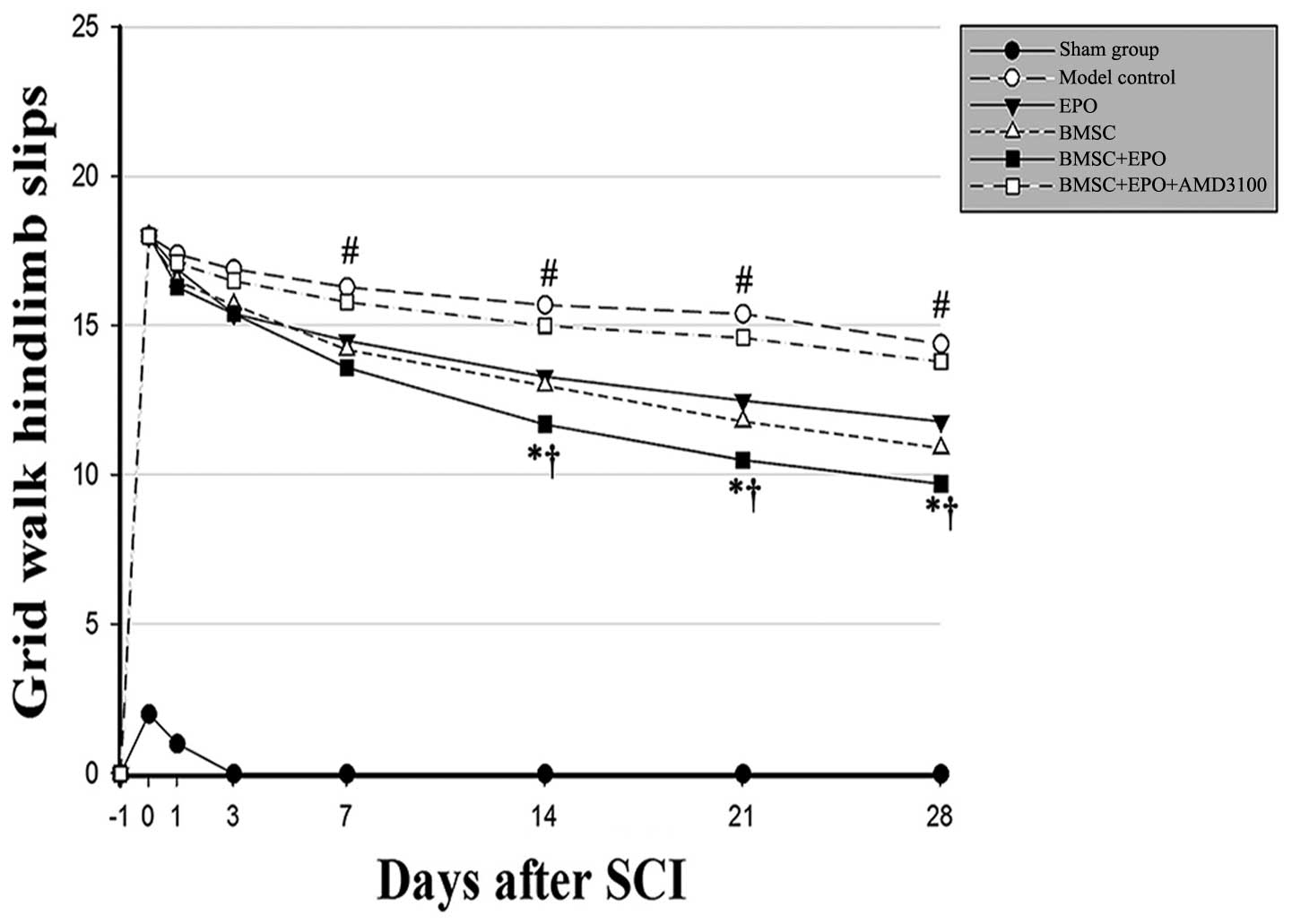
(P>0.05). The grid walk test of the hind limbs was applied to
estimate locomotor coordination and motor accuracy (Fig. 3). The animals administered the
BMSCs in conjunction with EPO exhibited progressive improvement in
hind limb control and paw placement accuracy in the grid walk test.
Moreover, the BMSC + EPO-treated rats experienced significantly
fewer hind limb slips from day 7 than the rats in the model control
and BMSC + EPO + AMD3100 groups (P<0.001) and the rats in the
EPO and BMSC group (P<0.05); however, no significant differences
were observed in these scores between the model control and the
BMSC + EPO + AMD3100 groups (P>0.05).
Detection of fluorescently-labeled cells
in injured spinal cords
The transplanted cells were detected in the injured
spinal cords 2 weeks following SCI (Fig. 4). GFP-labeled BMSCs were observed
and they were located at the lesion site in the BMSC and BMSC + EPO
groups. However, homologous cells were almost undetectable in the
sham-operated, model control, EPO and BMSC + EPO + AMD3100
groups.
 | Figure 4Localization of fluorescently-labeled
cells transplanted into the injured spinal cord. (A) The
sham-operated group, (B) model control, (C) EPO, (D) BMSC, (E) BMSC
+ EPO, and (F) BMSC + EPO + AMD3100 groups. GFP
fluorescently-labeled BMSCs (green) and DAPI positive nuclei (blue)
were observed at the lesion site following SCI. Bar, 400 μm.
EPO, erythropoietin; BMSC, bone marrow-derived mesenchymal stem
cell; GFP, green fluorescent protein; SCI, spinal cord injury. |
EPO induces BMSC migration in vitro
Our results (Fig.
5) revealed that EPO significantly promoted the migration of
BMSCs when compared with the control group (no EPO; P<0.001);
however, the EPO-induced cell migration was markedly inhibited when
the BMSCs were preconditioned with AMD3100 (P<0.001). No
significant differences were observed between the AMD3100 group and
the control group (P>0.05).
EPO reduces the AI in the lesion site
following SCI
The AIs at different time points following SCI in
each group are presented in Figs.
6 and 7. The quantity of
TUNEL-positive cells on day 1 following SCI showed no significant
difference between the model control, EPO, BMSC, BMSC + EPO and
BMSC + EPO + AMD3100 groups. On days 7 and 28 post-SCI, the AI in
the BMSC group was significantly lower than that in the model
control group (P<0.05) and the AI in the BMSC + EPO group was
significantly lower than that in the model control, EPO, BMSC and
BMSC + EPO + AMD3100 groups (P<0.05); however, the difference
between the model control and BMSC + EPO + AMD3100 groups was not
statistically significant (P>0.05). These data indicated that
EPO enhanced the anti-apoptotic effect of the BMSCs, which was
attenuated by AMD3100.
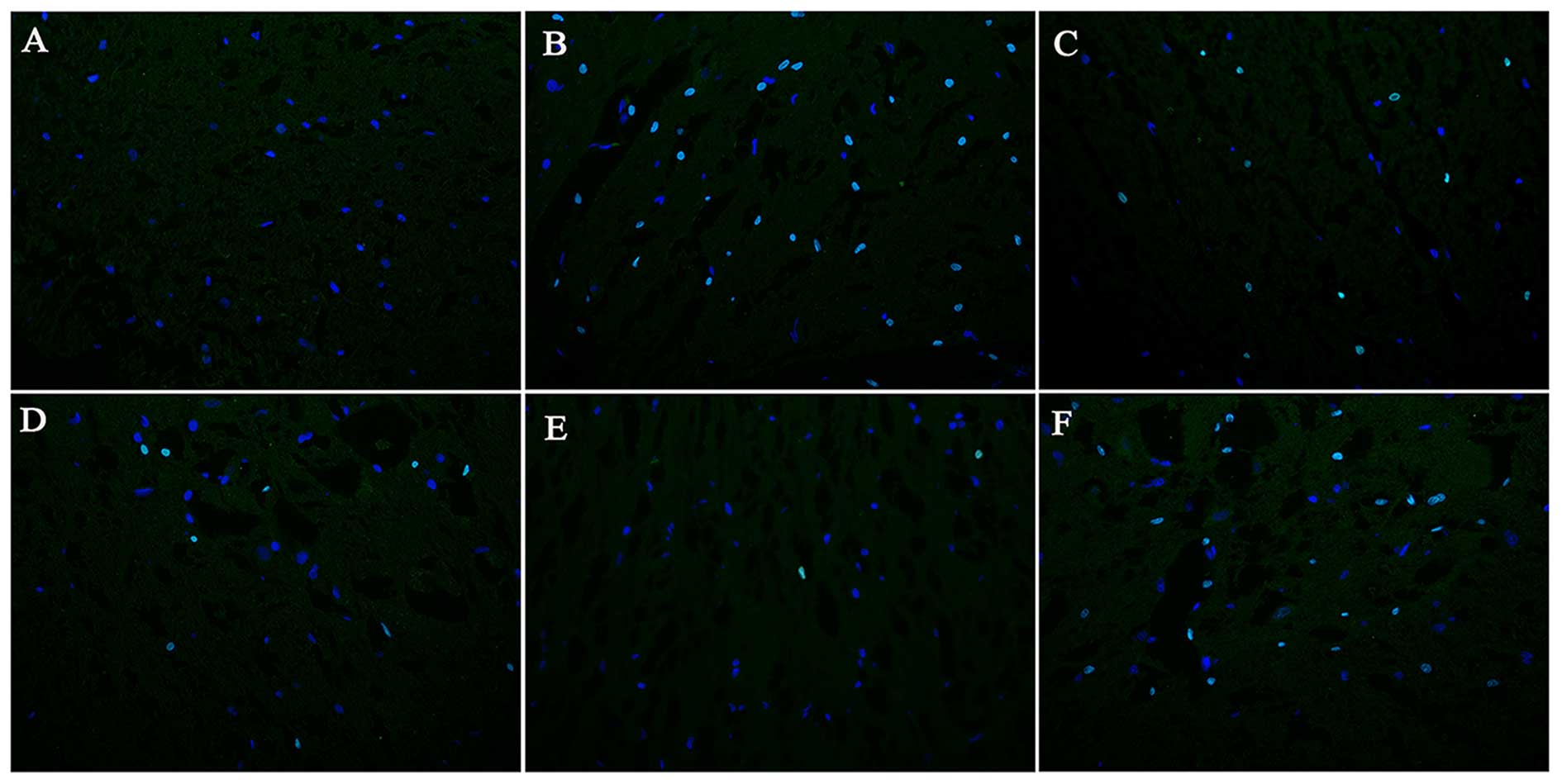 | Figure 6Apoptosis in the injured spinal cords
on day 7 following SCI (magnification, ×400). (A) The sham-operated
group, (B) model control, (C) EPO, (D) BMSC, (E) BMSC + EPO, (F)
BMSC + EPO + AMD3100 groups. The TUNEL-positive nuclei showed green
fluorescence and DAPI-positive nuclei showed blue fluorescence. The
number of TUNEL-positive cells was significantly lower in the BMSC
+ EPO group compared with the model control, EPO, BMSC, BMSC + EPO
and BMSC + EPO + AMD3100 groups. Bar, 400 μm. EPO,
erythropoietin; BMSC, bone marrow-derived mesenchymal stem cell;
SCI, spinal cord injury. |
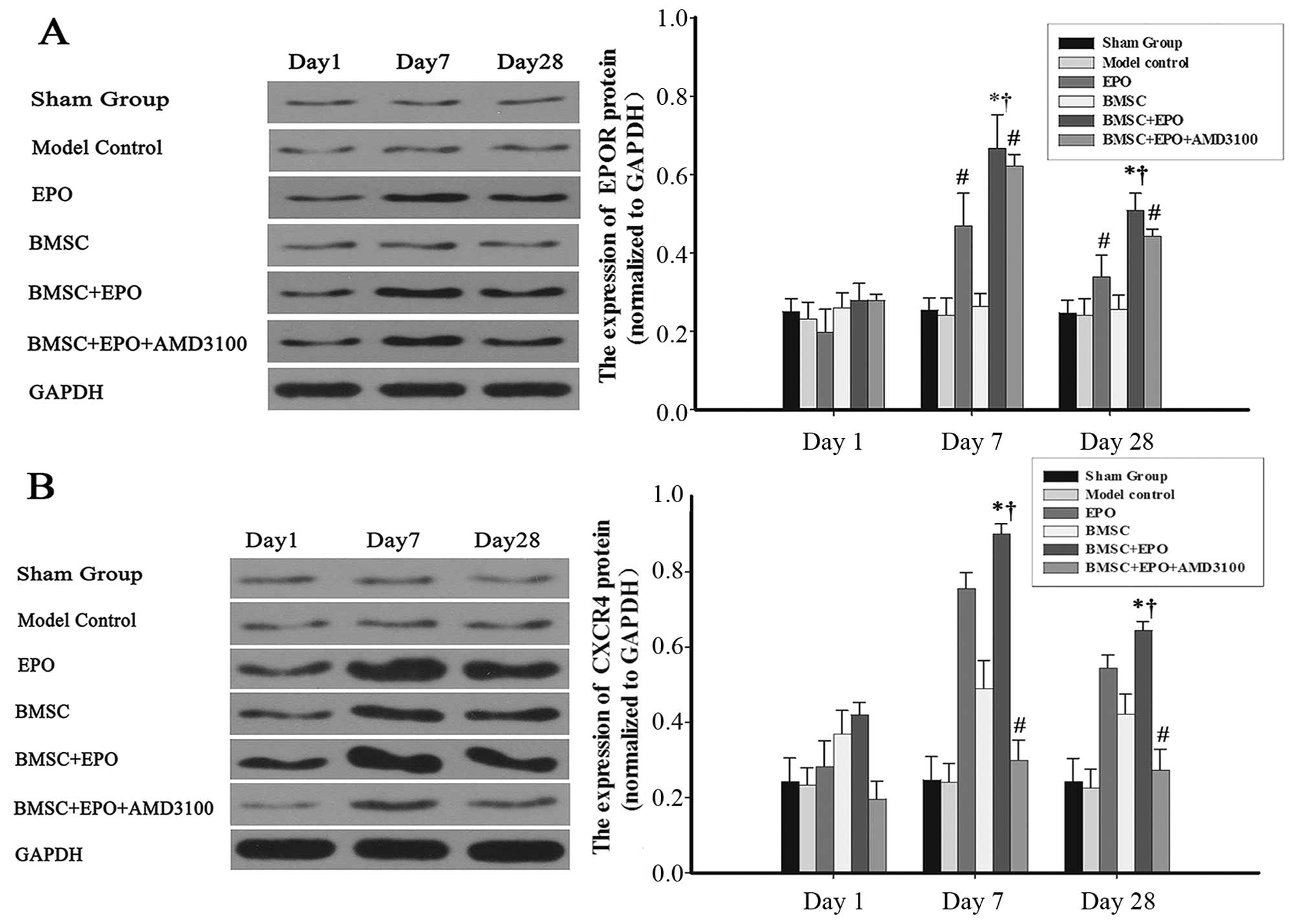
EPO enhances the protein expression of
CXCR4 in BMSCs
The protein expression levels of EPOR and CXCR4 were
measured by western blot analysis (Fig. 8). The protein expression of EPOR
increased with the administration of EPO and then gradually
decreased from day 7 to 28 (Fig.
8A). No significant differences were observed in EPOR protein
expression between the sham-operated, model control and BMSC
groups. The CXCR4 expression level in the EPO and BMSC + EPO groups
was consistent with the expression data obtaeind for EPOR; however,
this effect was antagonized with the administration of AMD3100
(Fig. 8B).
Discussion
With the development of transplantation and
regenerative medicine, an array of novel strategies have been
proposed to facilitate functional recovery following SCI. These
efforts have been principally focused on the attenuation of the
secondary delayed damage cascade that compounds a series of
pathophysiological courses which hinder repair following autologous
transplantation and remyelination (4,38).
The potential application of BMSCs in the treatment of SCI is being
widely investigated, yet it appears that this strategy alone is
inefficient, while a combined strategy seems to be more promising.
EPO, also known as red blood cell growth factor, is a glycoprotein
containing sialic acid with a molecular weight of 34 kDa. EPO
exerts its physiological effects by binding to its specific cell
surface receptor, EPOR (39). The
reason that EPO has attracted widespread attention is not only due
to its primary role in erythropoiesis, but also due to its effects
on the non-hematopoietic system, such as neurotrophic and
neuroprotective effects, and its regulatory role in embryonic
development (40). Over the past
two decades, a series of basic and clinical studies have been
carried out worldwide and it has been confirmed that EPO exerts
neuroprotective effects in acute SCI (41,42). Gorio et al (26) produced a model of acute thoracic
spinal cord contusion with an aneurysm clip, after which immediate
intraperitoneal injections of EPO were administered. Compared with
the control group, inflammation in the experimental group was
significantly inhibited, void formation in the spinal cord was
apparently reduced and motor function was significantly improved
(26). Celik et al
(43) created a spinal cord
ischemia reperfusion model using an abdominal aortic clamp, and
rhEPO was administered 48 h after modeling. The functional
neurological status in the rhEPO group was better compared with
that of the control group, and the apoptosis of motor neurons in
the rhEPO group was significantly reduced compared with the control
group (43). In addition to the
effects described above, EPO is a stem cell mobilization agent,
which mobilizes hematopoietic stem cells and BMSCs to participate
in the repair of a variety of tissues and organs (30,31). In the present study, we provide
evidence that EPO is able to mobilize BMSCs to migrate to the
lesion site following SCI and enhance their neuroprotective
effects.
The SDF-1/CXCR4 axis is of crucial importance in the
migration of BMSCs to the lesion sites, as it has been demonstrated
that the recruitment of BMSCs is terminated when the SDF-1/CXCR4
axis is impaired (14–17,44). Hence, the increased secretion of
SDF-1 at the lesion site promotes the homing of circulating
CXCR4-positive cells. BMSCs stimulated with SDF-1 express multiple
genes, 11 of which regulate cell migration (45). In a previous study, GFP-labeled
BMSCs were grafted into rats with unilateral mandibular distraction
osteogenesis and SDF-1 was found to promote the migration of BMSCs
in vivo and in vitro, and this effect was inhibited
by AMD3100, a CXCR4-blocking antibody (46). It has been demonstrated that CXCR4
mediates the migration of BMSCs to ischemic kidneys and this
CXCR4-mediated migration of BMSCs was inhibited by the CXCR4
antagonist, AMD3100 (47).
Moreover, the transplantation of BMSCs to the burn wound area in an
animal model promoted the epithelialization of the wound; however,
preconditioning with AMD3100 significantly inhibited the
mobilization of the BMSCs to the wound area (48). In another study on SCI, BMSCs were
administered by lumbar intrathecal injection 2 days after modeling,
and the results indicated that the SDF-1/CXCR4 axis participated in
the migration of BMSCs into the injured zone (49). Taken together, these findings
confirm that the association between locally generated SDF-1 and
its ligand CXCR4, expressed on the surface of BMSCs, plays a
crucial role in the homing of transplanted cells. This was
consistent with the findings of our study; we demonstrated that EPO
significantly upregulated the protein expression of CXCR4 in the
spinal cord samples and promoted the migration of BMSCs, whereas
these effects were markedly inhibited when the BMSCs were
co-transplanted with AMD3100.
There is evidence to confirm the involvement of
chemokines as migratory starting points in the trafficking of BMSCs
to the lesion site (50).
Modulating the detrimental environment can facilitate the
recruitment of BMSCs into the target region. The injured tissue
undergoes acute or chronic immunological responses, and BMSCs
migrating to these regions will come into contact with various
immunocytes in the local area. TNF-α has previously been
demonstrated to play a role in the upregulation of matrix
metalloproteinases (MMPs) in BMSCs, which strongly stimulates the
chemotactic migration of the cells through the extracellular matrix
(51). In a previous study, it
was confirmed that systemically transplanted regulatory T cells or
locally administered aspirin significantly enhanced the survival of
BMSCs and promoted bone regeneration through the inhibition of
interferon-γ (IFN-γ) and TNF-α in injured bone tissues (52). Another previous study demonstrated
that EPO inhibited the production of TNF by glial cells, which were
exposed to trimethyltin, an apoptosis-inducing toxin (53). In accordance with these findings,
in this study, we found that EPO significantly decreased thye
levels of TNF-α and increased the levels of SDF-1 in the injured
spinal cord, and these effects were attenuated by AMD3100.
Another possible mechanism is that EPO inhibits the
cell apoptosis (54). Apoptosis
is a significant element of the delayed secondary damage which
occurs following SCI. EPO has been documented to play a significant
role in preventing the apoptosis of neurons in a rat model of nerve
root crush injury (54). EPO has
also been shown to prevent spinal cord cell apoptosis following
acute traumatic injury in rats (55). Following transient spinal cord
ischemia, almost no apoptotic pattern was detectable (no TUNEL
labeling present) in EPO treated ventral horn motor neurons
(56), while in a model of crush
injury of the spinal nerve root, EPO prevented apoptosis in dorsal
root ganglion neurons (54). In
this study, we detected the AI in the injured spinal cords by the
TUNEL assay. The number of apoptotic cells in the spinal cords
significantly increased following SCI. The number of apoptotic
cells significantly decreased in the BMSC + EPO group compared with
the model control and BMSC groups, and this effect was attenuated
by AMD3100. This result indicated that EPO enhanced the
anti-apoptotic effect of the BMSCs by acting on the SDF-1/CXCR4
axis.
In the present study, two complementary behavioural
tests were adopted to evaluate and accurately estimate the recovery
of locomotor functions following SCI: the BBB Locomotor Rating
Scale and the grid walk test. In both tests, the animals in the
model control group showed spontaneous recovery following SCI,
which was interpreted by the pattern of the injury. Rats in the
BMSC + EPO and BMSC groups showed a more significant improvement in
the recovery of locomotor functions compared with the model control
group. Significant improvements in locomotor function were also
observed in the BMSC + EPO group compared with the BMSC group,
while no significant difference was observed between the model
control and BMSC + EPO + AMD3100 group. These outcomes demonstrated
that the administration of EPO, in conjunction with BMSCs, enhances
the neurological recovery of rats with SCI by acting on the
SDF-1/CXCR4 axis.
In conclusion, the findings of the present study
confirm that EPO mobilizes BMSCs to the lesion site following SCI
and enhances the anti-apoptotic effects of BMSCs by upregulating
the expression of the SDF-1/CXCR4 axis. EPO enhances the
therapeutic benefits of BMSCs and improves neurological outcomes
following SCI. Thus, the combined strategy of BMSC transplantation
with EPO therapy exhibits potential for the treatment of traumatic
SCI.
Acknowledgments
The authors appreciate the assistance provided by
Chen Shen and Yu Lin (Renmin Hospital of Wuhan University, Wuhan,
China) during the processing of this manuscript. This study was
financially supported by the Project of the Natural Science
Foundation of Hubei, China (grant no. 2013CFC035).
References
|
1
|
Martinez AM, Goulart CO, Ramalho BS,
Oliveira JT and Almeida FM: Neurotrauma and mesenchymal stem cells
treatment: From experimental studies to clinical trials. World J
Stem Cells. 6:179–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varma AK, Das A, Wallace G IV, Barry J,
Vertegel AA, Ray SK and Banik NL: Spinal cord injury: a review of
current therapy, future treatments, and basic science frontiers.
Neurochem Res. 38:895–905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bracken MB: Steroids for acute spinal cord
injury. Cochrane Database Syst Rev. 1:CD0010462012.PubMed/NCBI
|
|
4
|
Vawda R and Fehlings MG: Mesenchymal cells
in the treatment of spinal cord injury: current and future
perspectives. Curr Stem Cell Res Ther. 8:25–38. 2013. View Article : Google Scholar
|
|
5
|
Mothe AJ and Tator CH: Advances in stem
cell therapy for spinal cord injury. J Clin Invest. 122:3824–3834.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Himes BT, Neuhuber B, Coleman C, Kushner
R, Swanger SA, Kopen GC, Wagner J, Shumsky JS and Fischer I:
Recovery of function following grafting of human bone
marrow-derived stromal cells into the injured spinal cord.
Neurorehabil Neural Repair. 20:278–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neuhuber B, Timothy Himes B, Shumsky JS,
Gallo G and Fischer I: Axon growth and recovery of function
supported by human bone marrow stromal cells in the injured spinal
cord exhibit donor variations. Brain Res. 1035:73–85. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abrams MB, Dominguez C, Pernold K, Reger
R, Wiesenfeld-Hallin Z, Olson L and Prockop D: Multipotent
mesenchymal stromal cells attenuate chronic inflammation and
injury-induced sensitivity to mechanical stimuli in experimental
spinal cord injury. Restor Neurol Neurosci. 27:307–321.
2009.PubMed/NCBI
|
|
9
|
Novikova LN, Brohlin M, Kingham PJ,
Novikov LN and Wiberg M: Neuroprotective and growth-promoting
effects of bone marrow stromal cells after cervical spinal cord
injury in adult rats. Cytotherapy. 13:873–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stolzing A and Scutt A: Effect of reduced
culture temperature on antioxidant defences of mesenchymal stem
cells. Free Radic Biol Med. 41:326–338. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffmann J, Glassford AJ, Doyle TC,
Robbins RC, Schrepfer S and Pelletier MP: Angiogenic effects
despite limited cell survival of bone marrow-derived mesenchymal
stem cells under ischemia. Thorac Cardiovasc Surg. 58:136–142.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi T and Song SU: Immunomodulatory
properties of mesenchymal stem cells and their therapeutic
applications. Arch Pharm Res. 35:213–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galipeau J: The mesenchymal stromal cells
dilemma - does a negative phase III trial of random donor
mesenchymal stromal cells in steroid-resistant graft-versus-host
disease represent a death knell or a bump in the road? Cytotherapy.
15:2–8. 2013. View Article : Google Scholar
|
|
14
|
Cencioni C, Capogrossi MC and Napolitano
M: The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc
Res. 94:400–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu
H, Che Y, Ou L, Liu L and Kong D: SDF-1/CXCR4 axis modulates bone
marrow mesenchymal stem cell apoptosis, migration and cytokine
secretion. Protein Cell. 2:845–854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong J, Meng HB, Hua J, Song ZS, He ZG,
Zhou B and Qian MP: The SDF-1/CXCR4 axis regulates migration of
transplanted bone marrow mesenchymal stem cells towards the
pancreas in rats with acute pancreatitis. Mol Med Rep. 9:1575–1582.
2014.PubMed/NCBI
|
|
17
|
Ghadge SK, Mühlstedt S, Ozcelik C and
Bader M: SDF-1α as a therapeutic stem cell homing factor in
myocardial infarction. Pharmacol Ther. 129:97–108. 2011. View Article : Google Scholar
|
|
18
|
Yu J, Li M, Qu Z, Yan D, Li D and Ruan Q:
SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal
cells toward areas of heart myocardial infarction through
activation of PI3K/Akt. J Cardiovasc Pharmacol. 55:496–505.
2010.PubMed/NCBI
|
|
19
|
Wang Y, Deng Y and Zhou GQ:
SDF-1α/CXCR4-mediated migration of systemically transplanted bone
marrow stromal cells towards ischemic brain lesion in a rat model.
Brain Res. 1195:104–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koury MJ and Bondurant MC: Maintenance by
erythropoietin of viability and maturation of murine erythroid
precursor cells. J Cell Physiol. 137:65–74. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rölfing JH, Jensen J, Jensen JN, Greve AS,
Lysdahl H, Chen M, Rejnmark L and Bünger C: A single topical dose
of erythropoietin applied on a collagen carrier enhances calvarial
bone healing in pigs. Acta Orthop. 85:201–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teixeira M, Rodrigues-Santos P, Garrido P,
Costa E, Parada B, Sereno J, Alves R, Belo L, Teixeira F,
Santos-Silva A and Reis F: Cardiac antiapoptotic and
proproliferative effect of recombinant human erythropoietin in a
moderate stage of chronic renal failure in the rat. J Pharm
Bioallied Sci. 4:76–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weng S, Zhu X, Jin Y, Wang T and Huang H:
Protective effect of erythropoietin on myocardial infarction in
rats by inhibition of caspase-12 expression. Exp Ther Med.
2:833–836. 2011.
|
|
24
|
Chen S, Li J, Peng H, Zhou J and Fang H:
Administration of erythropoietin exerts protective effects against
glucocorticoid-induced osteonecrosis of the femoral head in rats.
Int J Mol Med. 33:840–848. 2014.PubMed/NCBI
|
|
25
|
Xiong M, Chen S, Yu H, Liu Z, Zeng Y and
Li F: Neuroprotection of erythropoietin and methylprednisolone
against spinal cord ischemia-reperfusion injury. J Huazhong Univ
Sci Technolog Med Sci. 31:652–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gorio A, Gokmen N, Erbayraktar S, Yilmaz
O, Madaschi L, Cichetti C, Di Giulio AM, Vardar E, Cerami A and
Brines M: Recombinant human erythropoietin counteracts secondary
injury and markedly enhances neurological recovery from
experimental spinal cord trauma. Proc Natl Acad Sci USA.
99:9450–9455. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi D, Schroer SA, Lu SY, Wang L, Wu X,
Liu Y, Zhang Y, Gaisano HY, Wagner KU, Wu H, et al: Erythropoietin
protects against diabetes through direct effects on pancreatic beta
cells. J Exp Med. 207:2831–2842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
MacRedmond R, Singhera GK and Dorscheid
DR: Erythropoietin inhibits respiratory epithelial cell apoptosis
in a model of acute lung injury. Eur Respir J. 33:1403–1414. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kakavas S, Demestiha T, Vasileiou P and
Xanthos T: Erythropoetin as a novel agent with pleiotropic effects
against acute lung injury. Eur J Clin Pharmacol. 67:1–9. 2011.
View Article : Google Scholar
|
|
30
|
Zwezdaryk KJ, Coffelt SB, Figueroa YG, Liu
J, Phinney DG, LaMarca HL, Florez L, Morris CB, Hoyle GW and
Scandurro AB: Erythropoietin, a hypoxia-regulated factor, elicits a
pro-angiogenic program in human mesenchymal stem cells. Exp
Hematol. 35:640–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu NM, Tian J, Wang WW, Han GF, Cheng J,
Huang J and Zhang JY: Effect of erythropoietin on mesenchymal stem
cell differentiation and secretion in vitro in an acute kidney
injury microenvironment. Genet Mol Res. 12:6477–6487. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nair AM, Tsai YT, Shah KM, Shen J, Weng H,
Zhou J, Sun X, Saxena R, Borrelli J Jr and Tang L: The effect of
erythropoietin on autologous stem cell-mediated bone regeneration.
Biomaterials. 34:7364–7371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kwon BK, Oxland TR and Tetzlaff W: Animal
models used in spinal cord regeneration research. Spine.
27:1504–1510. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng W, Bivalacqua TJ, Chattergoon NN,
Jeter JR Jr and Kadowitz PJ: Engineering ex vivo-expanded marrow
stromal cells to secrete calcitonin gene-related peptide using
adenoviral vector. Stem Cells. 22:1279–1291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bobadilla M, Sainz N, Abizanda G, Orbe J,
Rodriguez JA, Páramo JA, Prósper F and Pérez-Ruiz A: The CXCR4/SDF1
axis improves muscle regeneration through MMP-10 activity. Stem
Cells Dev. 23:1417–1427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang S, Wu Z, Chiang P, Fink DJ and Mata
M: Vector-mediated expression of erythropoietin improves functional
outcome after cervical spinal cord contusion injury. Gene Ther.
19:907–914. 2012. View Article : Google Scholar :
|
|
38
|
McDonald JW and Sadowsky C: Spinal-cord
injury. Lancet. 359:417–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Noguchi CT, Wang L, Rogers HM, Teng R and
Jia Y: Survival and proliferative roles of erythropoietin beyond
the erythroid lineage. Expert Rev Mol Med. 10:e362008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paschos N, Lykissas MG and Beris AE: The
role of erythropoietin as an inhibitor of tissue ischemia. Int J
Biol Sci. 4:161–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grasso G, Sfacteria A, Passalacqua M,
Morabito A, Buemi M, Macrì B, Brines ML and Tomasello F:
Erythropoietin and erythropoietin receptor expression after
experimental spinal cord injury encourages therapy by exogenous
erythropoietin. Neurosurgery. 56:821–827; discussion 821–827. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qi C, Xu M, Gan J, Yang X, Wu N, Song L,
Yuan W and Liu Z: Erythropoietin improves neurobehavior by reducing
dopaminergic neuron loss in a 6 hydroxydopamine induced rat model.
Int J Mol Med. 34:440–450. 2014.PubMed/NCBI
|
|
43
|
Celik M, Gökmen N, Erbayraktar S,
Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E,
Cerami A and Brines M: Erythropoietin prevents motor neuron
apoptosis and neurologic disability in experimental spinal cord
ischemic injury. Proc Natl Acad Sci USA. 99:2258–2263. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kitaori T, Ito H, Schwarz EM, Tsutsumi R,
Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T and Nakamura T:
Stromal cell-derived factor 1/CXCR4 signaling is critical for the
recruitment of mesenchymal stem cells to the fracture site during
skeletal repair in a mouse model. Arthritis Rheum. 60:813–823.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stich S, Haag M, Häupl T, Sezer O, Notter
M, Kaps C, Sittinger M and Ringe J: Gene expression profiling of
human mesenchymal stem cells chemotactically induced with CXCL12.
Cell Tissue Res. 336:225–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jaerve A, Schira J and Müller HW: Concise
review: the potential of stromal cell-derived factor 1 and its
receptors to promote stem cell functions in spinal cord repair.
Stem Cells Transl Med. 1:732–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu N, Patzak A and Zhang J:
CXCR4-overexpressing bone marrow-derived mesenchymal stem cells
improve repair of acute kidney injury. Am J Physiol Renal Physiol.
305:F1064–F1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu C, Yong X, Li C, Lü M, Liu D, Chen L,
Hu J, Teng M, Zhang D, Fan Y and Liang G: CXCL12/CXCR4 axis
promotes mesenchymal stem cell mobilization to burn wounds and
contributes to wound repair. J Surg Res. 183:427–434. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fan DY, Liu Y, Xu FC, et al: CXCL12/CXCR4
biology axis effects on the repair of spinal cord injury with bone
marrow mesenchymal stem cells. J Clin Rehabil Tissue Eng Res.
15:6651–6656. 2011.
|
|
50
|
Karp JM and Leng Teo GS: Mesenchymal stem
cell homing: the devil is in the details. Cell Stem Cell.
4:206–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ries C, Egea V, Karow M, Kolb H, Jochum M
and Neth P: MMP-2, MT1-MMP, and TIMP-2 are essential for the
invasive capacity of human mesenchymal stem cells: differential
regulation by inflammatory cytokines. Blood. 109:4055–4063. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen
C, Xu X, Yang R, Chen W, Wang S and Shi S: Mesenchymal stem
cell-based tissue regeneration is governed by recipient T
lymphocytes via IFN-γ and TNF-α. Nat Med. 17:1594–1601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Villa P, Bigini P, Mennini T, Agnello D,
Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman
TR, et al: Erythropoietin selectively attenuates cytokine
production and inflammation in cerebral ischemia by targeting
neuronal apoptosis. J Exp Med. 198:971–975. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sekiguchi Y, Kikuchi S, Myers RR and
Campana WM: ISSLS prize winner: Erythropoietin inhibits spinal
neuronal apoptosis and pain following nerve root crush. Spine.
28:2577–2584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Arishima Y, Setoguchi T, Yamaura I, Yone K
and Komiya S: Preventive effect of erythropoietin on spinal cord
cell apoptosis following acute traumatic injury in rats. Spine.
31:2432–2438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Knabe W, Sirén AL, Ehrenreich H and Kuhn
HJ: Expression patterns of erythropoietin and its receptor in the
developing spinal cord and dorsal root ganglia. Anat Embryol
(Berl). 210:209–219. 2005. View Article : Google Scholar
|