Introduction
Oxidative stress resulting from an imbalance between
system-generating and scavenging reactive oxygen species (ROS) is
the pathological basis of a number of chronic diseases. Low levels
of ROS are scavenged effectively by the antioxidant defense system
of cells. However, under conditions of oxidative stress, the
excessive accumulation of ROS causes destructive and irreversible
damage to cellular components, including nucleic acids, proteins
and lipids, as well as to other macromolecules, which ultimately
results in cell death (1,2). As a result, the induction of
antioxidant enzymes is one of the most important determinants of
cytoprotective effects against oxidative stress.
Nuclear factor erythroid 2-related factor 2 (Nrf2),
a regulator of the antioxidant response, plays a critical role in
protecting cells against oxidative stress. Under basal conditions,
Nrf2 is sequestered and inactivated in the cytoplasm by binding to
its inhibitor protein, Kelch-like ECH-associated protein 1 (Keap1),
which functions as an adaptor for Cullin 3 (Cul3)-based E3 ligase
in order to regulate the proteasomal degradation of Nrf2 (3,4).
When the complex is disrupted by exposure to various stimuli, free
Nrf2 subsequently translocates into the nucleus, where it
sequentially binds to the antioxidant response element (ARE)
(5,6). This results in a cytoprotective
response, which is characterized by the induction of the gene
expression of phase II enzymes. This response involves the
induction of heme oxygenase-1 (HO-1) and NAD(P)H:quinone
oxidoreductase 1 (NQO1), as well as decreased sensitivity to
oxidative stress-induced damage (3,7).
Recent studies have indicated that the Nrf2 protein may be
phosphorylated by several signal transduction pathways, including
mitogen-activated protein kinases (MAPKs), phosphatidylinositol
3-kinase (PI3K)/Akt and protein kinase C (8–10).
In this way, Nrf2 dissociates from Keap1 and translocates to the
nucleus, where it activates the ARE region of promoters for
numerous cytoprotective genes.
Certain toxic substances that are harmful to the
human body are contained in raw materials used for food, and in
order to discover new functional substances in the raw materials of
food that humankind has long ingested, previous research has
concentrated on such substances (11). In particular, for the prevention
and treatment of diverse diseases, including, but not limited to,
metabolic disorders, cancer, cardiovascular disease and Alzheimer's
disease, caused by oxidative stress, rather than using artificially
synthesized compounds, food derived from natural products can be a
more useful potential therapy.
Garlic (Allium sativum L., Alliaceae) has
been used as a food additive and herbal medicine for over 5,000
years, and is one of the earliest-documented herbs to be used for
the maintenance of health and the treatment of disease. Previous
studies have examined the close association between garlic intake
and the occurrence of disease (12,13). Garlic is known for its production
of organosulphur compounds, as well as steroid saponins. Although
organosulphur compounds, which are the major antioxidant components
of garlic extract, have scavenging free radical properties and
reduce lipid peroxidation, they are unstable and give rise to
transformed products (14,15).
However, garlic saponins are more stable and thus are more suitable
for cooking and storage, and have been found to be involved in
various pharmacological activities (16–20). Previous studies have proven that
garlic saponins are a potent antioxidant, protecting cells by
reducing ROS production in response to oxidative stress (18,19,21). For example, Luo et al
(22) confirmed that garlic
saponins functions as antioxidants to protect rat pheochromocytoma
PC12 cells from the direct damage of hypoxia-induced ROS and exert
protective effects through redox-sensitive signaling pathways
mediated by ROS. These studies also hypothesized that Nrf2/ARE
activation may be an important pathway for the activation of the
catalase that is induced following treatment with garlic saponins.
However, to the best of our knowledge, no study to date has
suggested that garlic saponins may act both as an antioxidant for
the direct elimination of ROS and as a signaling molecule for the
activation of Nrf2/ARE. As a result, in this study, we aimed to
investigate the antioxidant effects of garlic saponins.
The aim of the present study was to further examine
the intracellular pathways involved in order to determine whether
garlic saponins are able to activate Nrf2 and induce the expression
of its downstream target genes in mouse-derived C2C12 myoblasts
stimulated with hydrogen peroxide (H2O2).
Materials and methods
Cell culture and treatment with garlic
saponins
C2C12 myoblasts obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) were grown in
Dulbecco's modified Eagle's medium (DMEM; WelGENE Inc., Daegu,
Korea), supplemented with 10% fetal bovine serum (FBS) and 100
µg/ml penicillin/streptomycin antibiotics (WelGENE Inc.) in
a humidified 5% CO2 atmosphere at 37°C. For the
preparation of the crude garlic saponins, an improved method was
used for saponin extraction based on a previous study (22). Garlic was collected around Namhae
city (Gyeongsangnam-do, Korea); the bulbs were peeled, washed and
chopped before being stored at −20°C. The frozen samples were
lyophilized and homogenized using a grinder before extraction. The
samples were extracted twice with methanol by refluxing at 80°C for
2 h. The methanol extract was then suspended in water and
partitioned sequentially with n-hexane, chloroform, ethyl
acetate and n-butanol. Subsequently, the water-saturated
n-butanol fraction was evaporated to dryness in a vacuum.
The crude saponins recovered in this process were loaded onto a
Diaion® HP-20 MCI gel (Sigma-Aldrich Chemical Co., St.
Louis, MO, USA). The sugar residues were then removed with 40%
CH3OH. The fractions were eluted with 60–80%
CH3OH, collected, and then dried to obtain the garlic
saponins. The saponins were then dissolved in dimethyl sulfoxide
(DMSO, Sigma-Aldrich Chemical Co.) and adjusted to final
concentrations using complete DMEM prior to use.
Cell viability assay
Cell viability was measured based on the formation
of blue formazan, which was metabolized from colorless
3-(4.5-dimethylthiazol-2-yl)-2.5 diphenyltetrazolium bromide (MTT;
Sigma-Aldrich Chemical Co.) by mitochondrial dehydrogenases. These
are active only in live cells. Briefly, the C2C12 cells were seeded
in 6-well plates at a density of 1×105 cells per well.
After 24 h of incubation, the cells were treated with the specified
concentrations of garlic saponins in the absence or presence of
H2O2 and/or zinc protoporphyrin IX (ZnPP;
Sigma-Aldrich Chemical Co.) and N-acetyl-L-cysteine (NAC;
Sigma-Aldrich Chemical Co.) for the specified duration. MTT working
solution was then added to the culture plates following by
continuous incubation at 37°C. Three hours later, the supernatant
was removed, and the formation of formazan was measured at 540 nm
using an enzyme-linked immunosorbent assay (ELISA) plate reader
(Dynatech Laboratories, Chantilly, VA, USA). Control cells were
supplemented with complete medium containing 0.05% DMSO (vehicle
control). The inhibitory effect on cell growth was assessed as the
percentage of cell viability, where the vehicle-treated cells were
considered 100% viable.
Measurement of ROS production
The intracellular accumulation of ROS was determined
using the fluorescent probes, 2′,7′-dichlorodihydrofluorescein
diacetate (H2DCFDA; Molecular Probes, Eugene, OR, USA).
In order to monitor ROS generation, the cells were incubated with
10 µM H2DCFDA for 20 min at room temperature in
the dark. ROS production in the cells was monitored using a flow
cytometer (Becton Dickinson, San Jose, CA, USA) using CellQuest Pro
software, as previously described (23).
Comet assay (single-cell gel
electrophoresis)
Comet assay, a sensitive and rapid technique for
detection of DNA damage in individual cells, was performed as
previously described (24).
Briefly, harvested individual cells were mixed with molten low melt
agarose and spread on a fully-frosted microscopic slide pre-coated
with 1% normal melting agarose. The embedded cells were then lysed
using lysis solution and treated with alkaline solution to relax
and denature the DNA. Subsequently, electrophoresis of the samples
was carried out under alkaline condition at 25 V and 300 mA for 20
min. Following electrophoresis, the slides were washed, stained
with 20 µg/ml propidium iodide (PI; Sigma-Aldrich Chemical
Co.), and were then examined under a fluorescence microscope (Carl
Zeiss, Jena, Germany).
Protein extraction, electrophoresis and
western blot analysis
Western blot analysis and protein extraction were
performed as previously described (24). In brief, the cells were lysed, and
then equal amounts of cell lysates were separated on sodium dodecyl
sulfate (SDS)-polyacrylamide gels and transferred onto
nitrocellulose membranes (Schleicher & Schuell Bioscience,
Inc., Keene, NH, USA). The membranes were probed with specific
antibodies for 1 h and incubated with the diluted enzyme-linked
secondary antibodies (Amersham Co., Arlington Heights, IL, USA).
The proteins were visualized using an enhanced chemiluminescence
(ECL) detection system (Amersham Co.) according to the
manufacturer's instructions. The primary antibodies used in this
study were as follows: γH2AX (1:500, CS #2577; rabbit polyclonal,
Cell Signaling Technology, Inc., Danvers MA, USA), p-γH2AX (1:500,
CS #9718S; rabbit polyclonal, Cell Signaling Technology, Inc.),
Nrf2 (1:500, SC-13032; rabbit polyclonal, Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), p-Nrf2 (1:500, ab76026; rabbit
monoclonal, Abcam, Inc., Cambridge, UK), HO-1 (1:500, SC-136960;
mouse monoclonal, Santa Cruz Biotechnology, Inc.), Keap1 (1:1,000,
SC-33569; rabbit polyclonal, Santa Cruz Biotechnology, Inc.), NQO-1
(1:1,000, SC-16464; goat polyclonal, Santa Cruz Biotechnology,
Inc.), TrxR1 (1:1,000, SC-28321; mouse monoclonal, Santa Cruz
Biotechnology, Inc.), ERK (1:1,000, SC-154; rabbit polyclonal,
Santa Cruz Biotechnology, Inc.), p-ERK (1:500, #9106S; mouse
monoclonal, Cell Signaling Technology, Inc.), p38 (1:1,000, SC-535;
rabbit polyclonal, Santa Cruz Biotechnology, Inc.), p-p38 (1:500,
#9211S; rabbit polyclonal, Cell Signaling Technology, Inc.), JNK
(1:1,000, #9252S; rabbit polyclonal, Cell Signaling Technology,
Inc.), p-JNK (1:500, #9255S; mouse monoclonal, Cell Signaling
Technology, Inc.) and actin (1:1,000, SC-1616; goat polyclonal,
Santa Cruz Biotechnology, Inc.). Actin and lamin B were used as the
internal controls for cytosolic and nuclear fractions,
respectively. In order to examine the effects of MAPK signaling
pathway on the activation of Nrf2 and the induction of HO-1 by
garlic saponins, specific inhibitors of MAPKs such as PD98059 (an
ERK inhibitor, Cell Signaling Technology, Inc.), SP600125 (a JNK
inhibitor, Sigma-Aldrich Chemical Co.) and SB203580 (a p38 MAPK
inhibitor, Cell Signaling Technology, Inc.) were applied.
Small interfering RNA (siRNA)
transfection
siRNA targeting Nrf2 (Nrf2 siRNA) and control siRNA
were purchased from Santa Cruz Biotechnology. The siRNA was
transfected into the cells following the manufacturer's
instructions using Lipofectamine 2000 Transfection Reagent (Life
Technologies, Carlsbad, CA, USA). For transfection, the cells were
seeded in 6-well culture plates and incubated with control siRNA or
Nrf2 siRNA at 50 nM for 6 h in serum-free OPTI-MEM medium (Life
Technologies). Following transfection, the cells were treated with
garlic saponins (500 µg/ml) for 6 h or pre-treated with
garlic saponins (500 µg/ml) for 1 h and then stimulated with
or without 1 mM H2O2 (1 mM) in the presence
of garlic saponins for a further 6 h. The cells were then lysed and
equal amounts of cell lysates were subjected to western blot
analysis.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD) values. One-way analysis of variance (ANOVA) was used for
comparisons in the experiments with multiple time points and
concentrations. When ANOVA indicated statistical significance,
Duncan's multiple range test was used to determine which means were
significantly different. A probability value of P<0.05 was used
as the criterion for statistical significance.
Results
Garlic saponins protect C2C12 cells from
H2O2-induced cytotoxicity
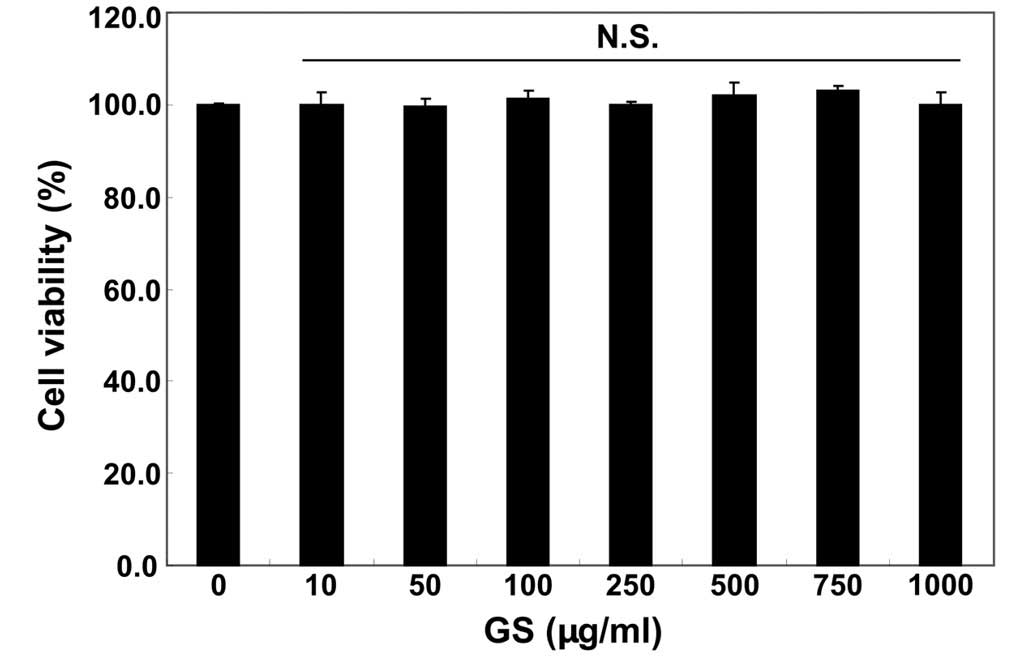
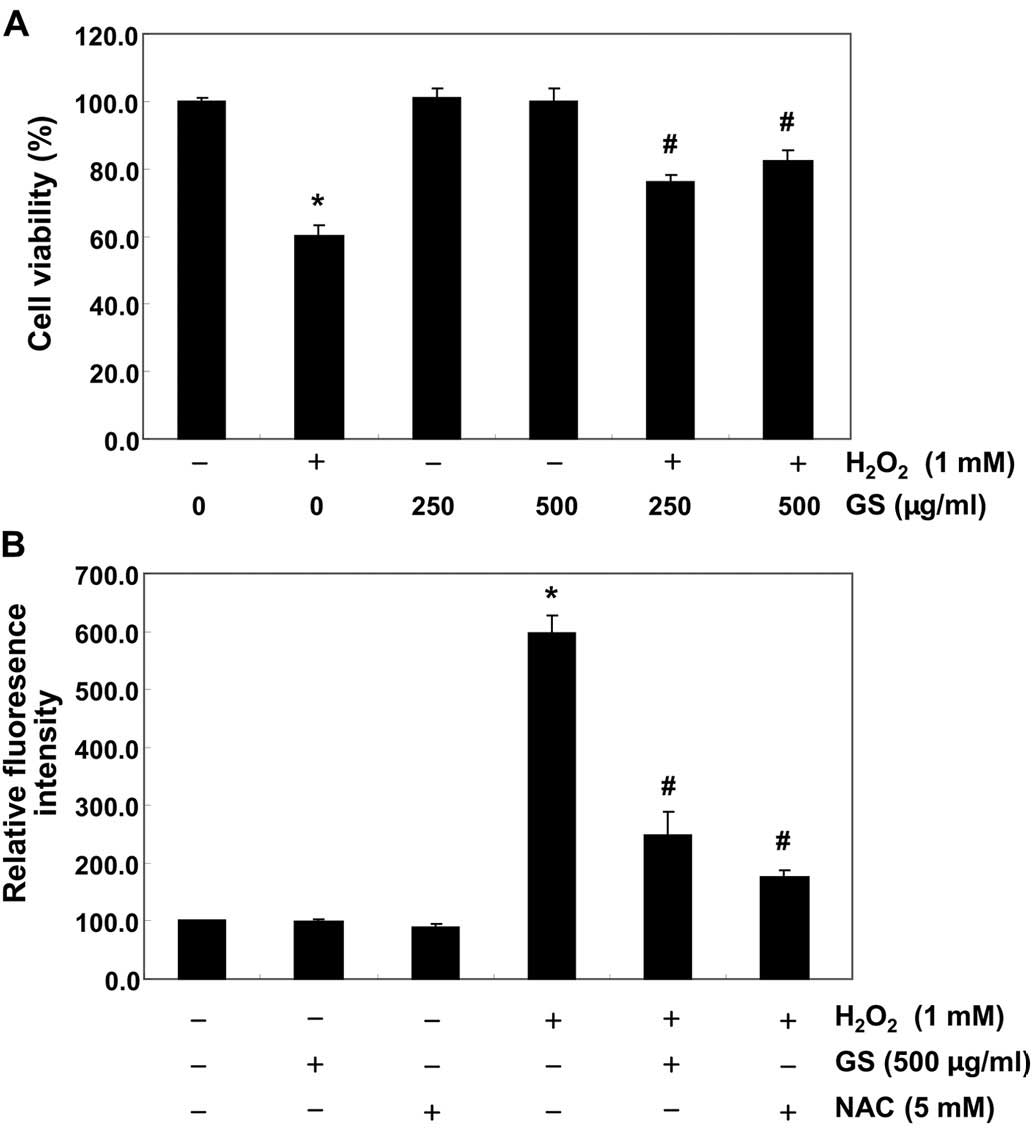
We first examined the effects of garlic saponins on
the viability of C2C12 cells by MTT assay. As shown in Fig. 1, the results revealed that
treatment with garlic saponins (10–1,000 µg/ml) alone had no
obvious effect on C2C12 cell viability. To examine the protective
effects of garlic saponins against oxidative stress-induced
cytotoxicity in C2C12 cells, the cells were pre-treated with garlic
saponins for 1 h and exposed to H2O2 for an
additional 6 h. The results revealed that treatment of the C2C12
cells with 1 mM of H2O2 for 6 h resulted in
approximately a 40% loss of cellular viability, as compared with
the control cells. However, the H2O2-induced
reduction in cell viability was significantly reversed by
pre-treatment with garlic saponins in a concentration-dependent
manner (Fig. 2A). These results
indicate that garlic saponins have properties that protect C2C12
cells against oxidative stress.
Garlic saponins modulate
H2O2-induced ROS generation in C2C12
cells
We then measured the intracellular ROS levels in
order to investigate whether garlic saponins had any effect on
intracellular ROS generation induced by stimulation with
H2O2. As expected, exposure of the C2C12
cells to H2O2 for 6 h induced an increase in
intracellular ROS levels (Fig.
2B). However, pretreatment of the cells with garlic saponins
(500 µg/ml for 1 h) significantly reduced the
H2O2-induced ROS production. As a positive
control, the ROS scavenger, NAC, was used, and we noted that this
also reduced H2O2-induced ROS generation.
Moreover, we noted that the garlic saponins themselves did not
contribute to ROS generation, suggesting that pre-treatment with
garlic saponins induced a cellular antioxidant response.
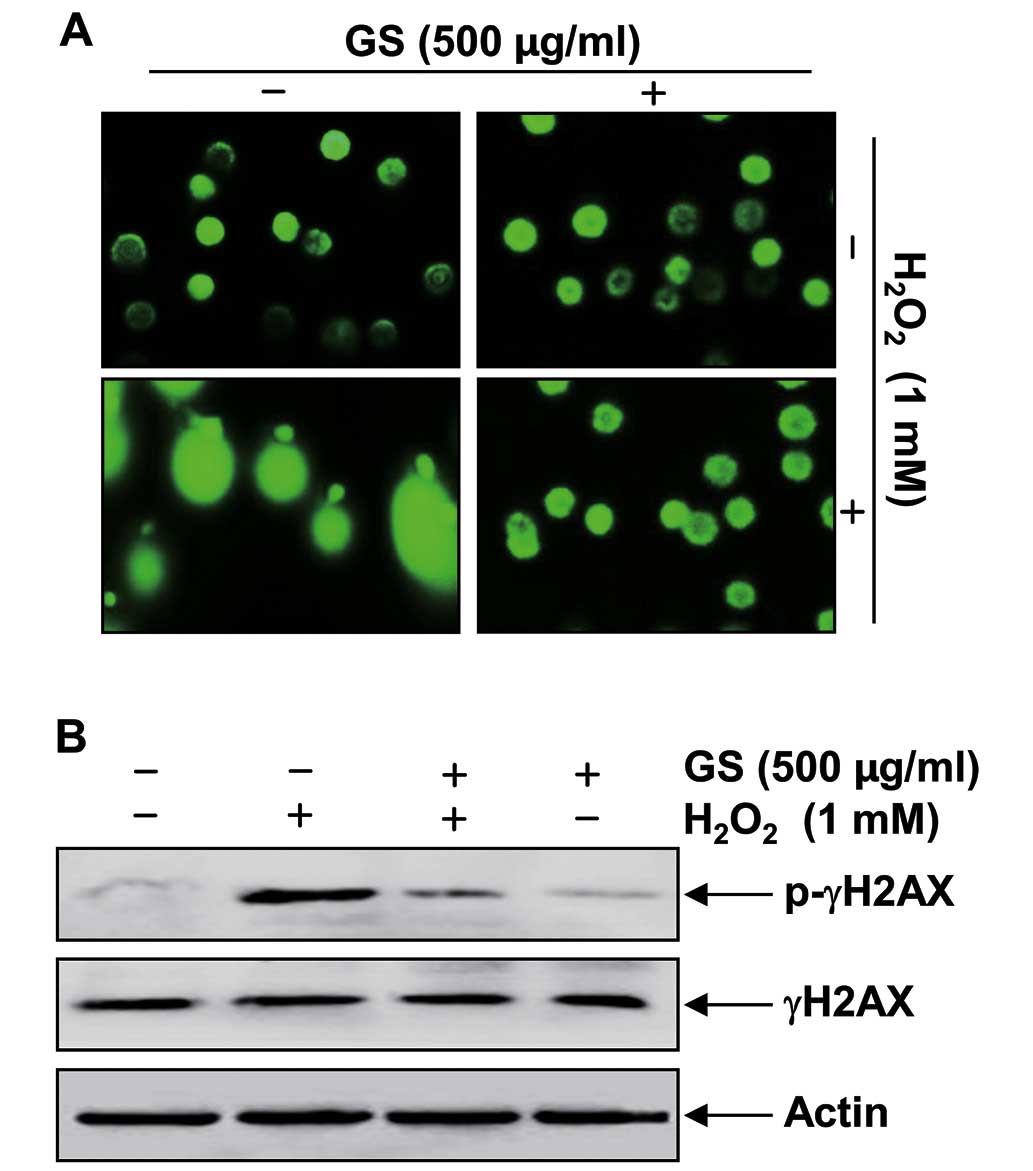
Garlic saponins attenuate
H2O2-induced DNA damage in C2C12 cells
We further examined the effects of garlic saponins
on DNA damage induced by H2O2 using
single-cell gel electrophoresis (comet assay) and western blot
analysis. As shown in Fig. 3A,
stimulation with H2O2 alone significantly
increased the number of DNA breaks, resulting in an increase in
fluorescence intensity in the tails of the comet-like structures in
C2C12 cells. These adverse effects were markedly reduced by
pre-treatment with garlic saponins. In addition, stimulation of the
C2C12 cells with H2O2 alone resulted in the
upregulation of the level of the phosphorylated histone variant
H2AX at serine 139 (p-γH2AX), a sensitive marker for DNA
double-strand breaks (25)
(Fig. 3B). By contrast,
pre-treatment with garlic saponins resulted in a decreased p-γH2AX
expression, which again indicates that garlic saponins exert a
protective effect against H2O2-induced DNA
damage.
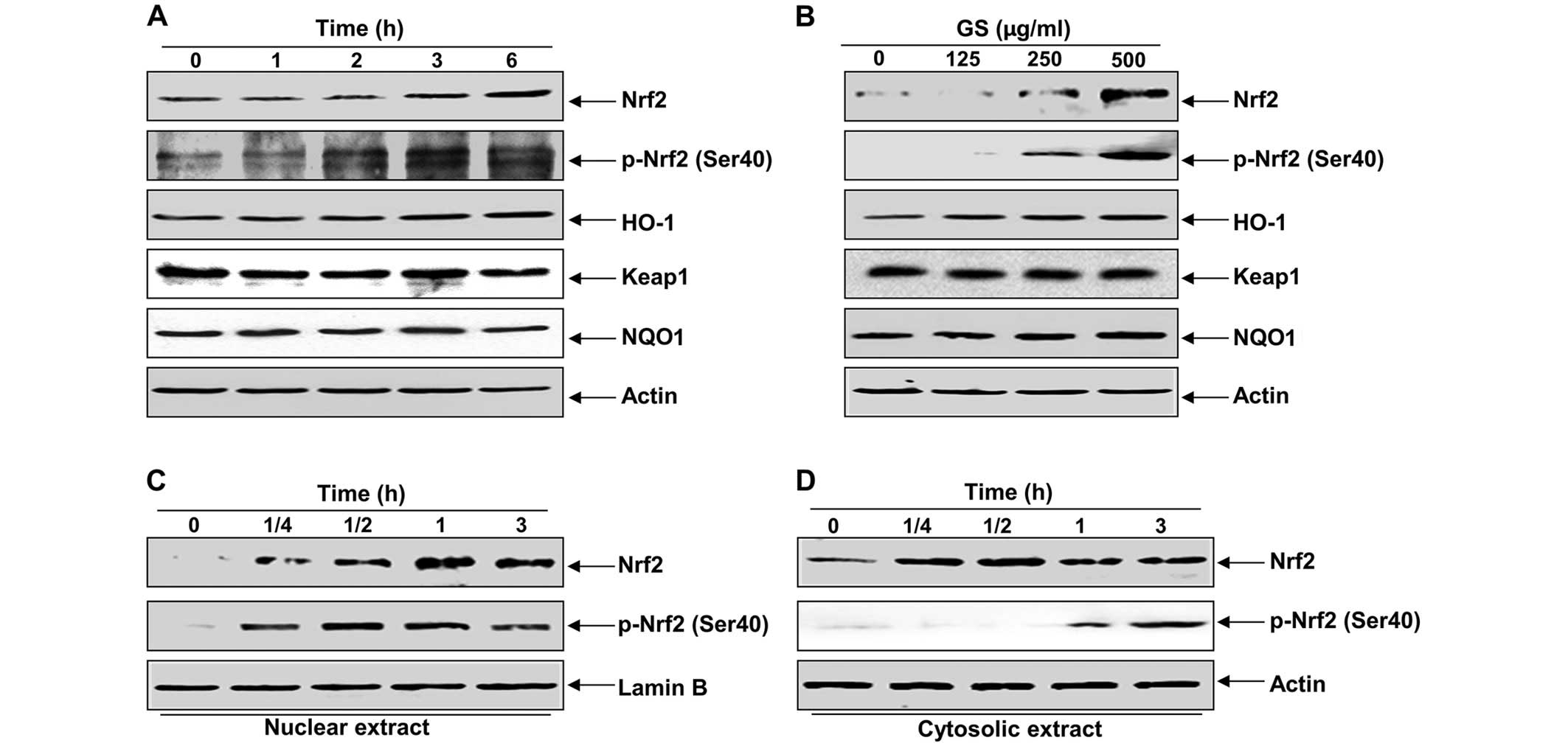
Garlic saponins enhance the expression of
Nrf2 and HO-1 in C2C12 cells
The fact that Nrf2 signaling regulates the cellular
antioxidant response by promoting ARE-dependent gene expression has
been well documented (3,7,26).
As a result, we wished to determine whether garlic saponins protect
cells from intracellular oxidative stress by activating the Nrf2
signaling pathway. As shown in Fig.
4A and B, treatment of the C2C12 cells with garlic saponins
induced Nrf2 expression and the phosphorylation of Nrf2 at Ser40 in
a duration- and dose-dependent manner and was associated with the
induction of HO-1. However, NQO1 and Keap1 were relatively
unaffected by treatment with garlic saponins. We then examined the
effect of garlic saponins on the intracellular localization of Nrf2
and found that there was an increased nuclear translocation of
phosphorylated Nrf2 proteins following treatment with garlic
saponins (Fig. 4C and D).
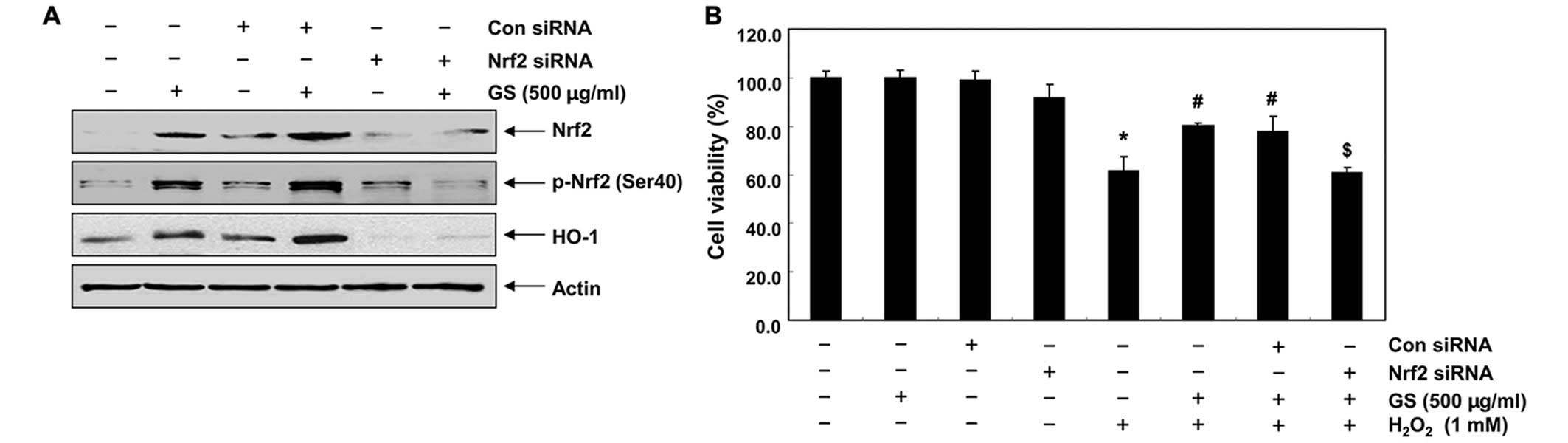
Garlic saponins upregulate HO-1
expression through the activation of Nrf2 in C2C12 cells
We then developed an Nrf2 gene knockdown model using
siRNA transfection to demonstrate the contribution of Nrf2
signaling to the counteractive effects of garlic saponins on
H2O2-induced cytotoxicity. Western blot
analysis revealed that Nrf2 siRNA reduced the expression of Nrf2
and the phosphorylation of Nrf2 induced by treatment with garlic
saponins. The expression of HO-1 which was induced by treatment
with garlic saponins was also blocked following transfection of the
cells with Nrf2 siRNA (Fig. 5A),
which is evidence that the augmentation of HO-1 expression is
mediated by Nrf2. To confirm the involvement of Nrf2, the
protective effects of garlic saponins against the
H2O2-induced reduction in cell viability were
determined in cells in which Nrf2 was knocked down. As shown in
Fig. 5B, transfection with Nrf2
siRNA cancelled out the cytoprotective effects of garlic saponins
when compared with the control siRNA-transfected cells, providing
evidence that garlic saponins initiate the cellular antioxidant
defense system through the activation of the Nrf2/HO-1 signaling
pathway.
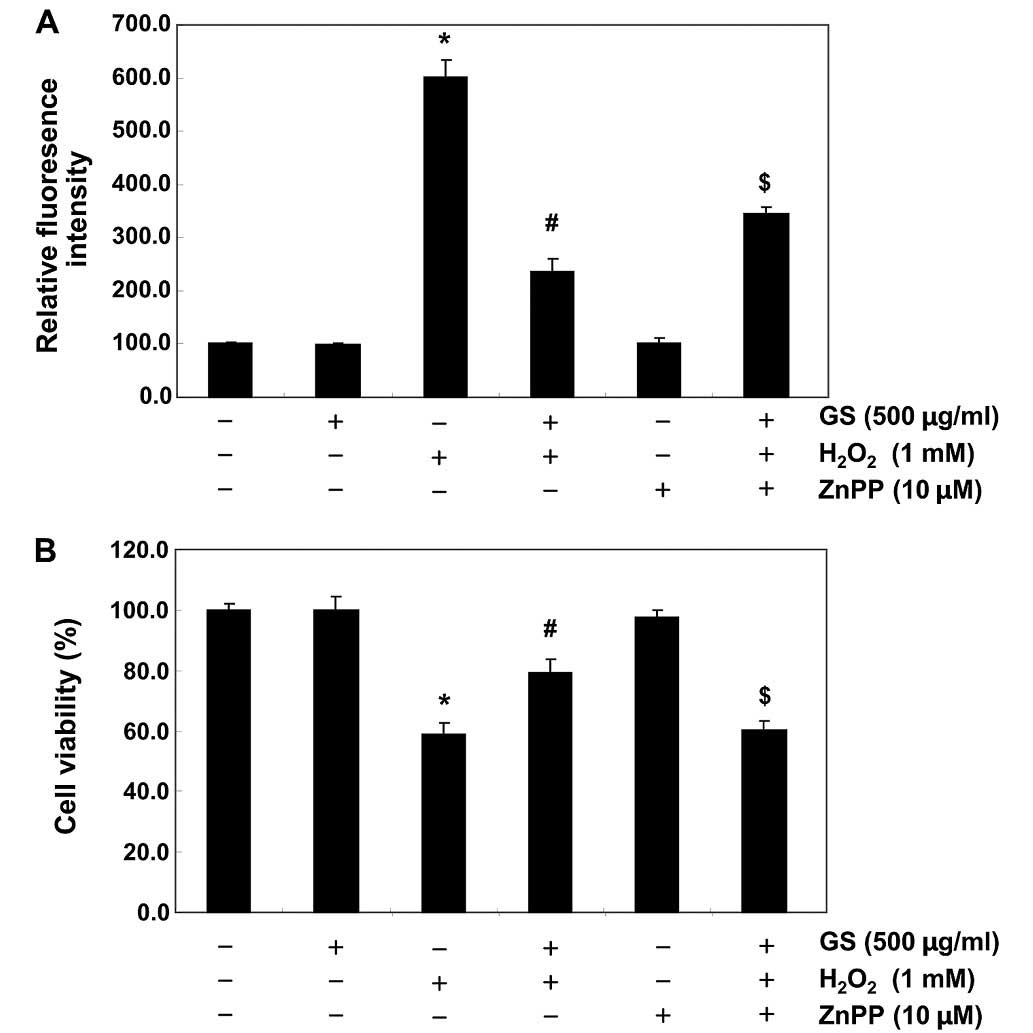
Nrf2/HO-1 pathway is involved in the
cytoprotective effects of saponins in C2C12 cells
To provide further confirmation that the antioxidant
and cytoprotective activities of garlic saponins against oxidative
stress in C2C12 cells are mediated through the activation of the
Nrf2/HO-1 signaling pathway, the C2C12 cells were pre-incubated
with or without ZnPP, a specific inhibitor of HO-1. The ROS levels
and cell viability were also assessed. As shown in Fig. 6, ZnPP nullified the protective
effect of garlic saponins on the H2O2-induced
production of ROS and the reduction in cell viability. These data
suggest that garlic saponins exert their protective effects by
activating the cellular defense mechanisms against oxidative stress
through the Nrf2-related cytoprotective pathway. The subsequent
upregulation of HO-1 thus plays a crucial role in the protective
effects of saponins in C2C12 cells.
Garlic saponins induce HO-1 expression
through the extracellular signal-regulated kinase (ERK)-Nrf2
signaling pathway
Previous studies have demonstrated that multiple
phosphorylation cascades participate in regulating the
translocation of Nrf2 and Nrf2-mediated HO-1 gene expression
(27–29). To identify the upstream signaling
events involved in the activation of Nrf2 and the induction of HO-1
by garlic saponin, the potential involvement of MAPKs was explored.
MAPKs are classified into three major subgroups, namely ERK, c-Jun
N-terminal kinase (JNK) and p38 MAPK. Although garlic saponins
induced the phosphorylation of JNK to a certain extent, it was
found that their effect was only significant on the phosphorylation
of ERK in a duration-dependent manner. There were no significant
changes observed in the levels of phosphorylated p38 MAPK compared
with the controls (Fig. 7A). To
determine whether garlic saponins induce Nrf2 expression and
phosphorylation, and HO-1 expression through the activation of ERK,
the cells were pre-treated with garlic saponins for 1 h and then
incubated with MAPK inhibitors. As shown in Fig. 7B, when the cells were incubated
with a selective inhibitor of ERK (PD98059), the induction and
phosphorylation of Nrf2 were blocked; HO-1 induction was diminished
accordingly. However, the p38 MAPK inhibitor (SB203580) and JNK
inhibitor (SP600125) were unable to reduce Nrf2 and HO-1 expression
and Nrf2 phosphorylation induced by garlic saponins. Taken
together, these observations indicate that the way in which garlic
saponins activate the Nrf2/HO-1 signaling pathway involves the ERK
pathway.
Discussion
It has been reported that oxidative stress
accompanies inflammation, aging, and neurodegenerative and
cardiovascular diseases. Oxidative stress can affect the myoblast
cytoskeleton and induce cell apoptosis. Both mechanical trauma and
prolonged ischemia have been proven to increase the permeability of
the plasma membrane for Ca2+, leading to the increased
production of ROS (30,31). Chronic inflammation in vivo
is also associated with chronic oxidative stress. It has been
demonstrated that post-ischemic reperfusion leads to oxidative
surges and thus has also been cited as a factor in the formation of
pressure ulcers (31,32). Although some studies have examined
how oxidative stress quantitatively affects the load-carrying
capacity of muscle cells (33,34), whether oxidative stress in
myoblasts is accompanied by the dysfunction of muscles has not yet
been determined. In the present study, as part of the screening
program for therapeutic antioxidant agents from traditional food
sources, we examined whether garlic saponins offer protection from
oxidative stress-induced cytotoxicity using a C2C12 myoblast cell
model. We first observed that, when the C2C12 myoblasts were
treated with garlic saponins in the presence of
H2O2, cell viability recovered significantly
due to the inhibition of H2O2-induced ROS
generation, compared to stimulation with H2O2
alone. Our data also indicated that stimulation with
H2O2 increased the tail length and expression
of p-γH2AX; however, these effects were mitigated in the C2C12
cells which had been treated with garlic saponins prior to exposure
to H2O2 (Fig.
3). As a result, these findings suggest that garlic saponins
are useful for the prevention of H2O2-induced
cytotoxicity due to their prominent antioxidant effects.
It has previoulsy been suggested that the mammalian
oxidative stress response is coordinated by the Nrf2 transcription
factor. Under normal cellular conditions, Nrf2 is inactive and
bound in the cytosol by Keap1 (3,4).
The translocation of Nrf2 into the nucleus is essential for the
transactivation of Nrf2-inducible genes, such as those encoding
HO-1, which is a key component of protection against oxidative
stress (3,7,26).
In addition, the phosphorylation of Nrf2 at Ser40 by several
kinases is also a critical process in its stabilization and nuclear
translocation (5–7). As illustrated in Fig. 4, we observed that treatment with
garlic saponins increased the levels of total and phosphorylated
Nrf2, along with the nuclear accumulation of HO-1 (Fig. 5A). In addition, the silencing of
Nrf2 halted the protective efects of the garlic saponins on
H2O2-induced growth inhibition of C2C12 cells
(Fig. 5B), and the inhibition of
HO-1 function using the HO-1 inhibitor, ZnPP, significantly
weakened the protective effects of garlic saponins on
H2O2-induced ROS generation and growth
inhibition (Fig. 6). These
results suggest that the Nrf2-dependent induction of HO-1 by garlic
saponins helps to protect cells against oxidative stress.
A number of studies have suggested that diverse
protein kinases are involved in the signals that trigger the
Nrf2-Keap1 dissociation, the phosphorylation of Nrf2 and the
antioxidant-induced activation of the Nrf2/HO-1 signaling pathway
(8–19). In certain studies, it has been
demonstrated that MAPKs play a crucial role in the cellular
response to a wide variety of signals elicited by growth factors,
hormones and cytokines, and to genotoxic and oxidative stressors
(35,36). Recent research has demonstrated
that the activation of MAPK signaling leads to the phosphorylation
and/or translocation of Nrf2 to the nucleus. For example, the
flavonoid, morin, has been shown to upregulate the activity of HO-1
through the ERK/Nrf2 signaling pathway (37). The phenolic glucoside, gastrodin,
has also been shown to stimulate HO-1 expression through the
activation of the p38 MAPK/Nrf2 signaling pathway (38). In addition, eckol, a phlorotannin
isolated from brown algae, has been shown to induce Nrf2-dependent
HO-1 expression through the JNK and PI3K/Akt signaling pathways
(39). These findings suggest
that the role of each pathway in the activation of Nrf2/HO-1
signaling, and their molecular targets, may be specific to the
stimulus and cell type. The results of the present study
demonstrate that JNK and p38 MAPK are not involved in the
activation of Nrf2/HO-1 signaling induced by garlic saponin, since
their inhibitors had no effect on garlic saponin-induced HO-1 and
Nrf2 expression or Nrf2 phosphorylation. However, the ERK
inhibitor, PD98059, suppressed the garlic saponin-induced changes
to HO-1 and Nrf2 (Fig. 7B). This
suggests that ERK plays a crucial role in the Nrf2-dependent
induction of HO-1.
In conclusion, in the present study, we demonstrate
that garlic saponins markedly induces Nrf2-mediated HO-1 expression
through the ERK/Nrf2 signaling pathway, which contributes, at least
in part, to the cellular defense mechanism against oxidative
stress-induced genotoxic events. Although such complex molecular
mechanisms require further investigation to identify the active
saponins contained in crude garlic saponins, the findings of our
study suggest that garlic saponins have potential therapeutic value
as antioxidant agents.
Acknowledgments
This study was supported by the R&D program of
MOTIE/KEIT (10040391, Development of Functional Food Materials and
Device for Prevention of Aging-associated Muscle Function Decrease)
and the National Research Foundation of Korea grant funded by the
Korean government (2013 041811, NRF-2014R1A2A1A09006983 and
2015R1A2A2A01004633).
References
|
1
|
Kregel KC and Zhang HJ: An integrated view
of oxidative stress in aging: basic mechanisms, functional effects,
and pathological considerations. Am J Physiol Regul Integr Comp
Physiol. 292:R18–R36. 2007. View Article : Google Scholar
|
|
2
|
Finkel T: Signal transduction by reactive
oxygen species. J Cell Biol. 194:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venugopal R and Jaiswal AK: Nrf1 and Nrf2
positively and c-Fos and Fra1 negatively regulate the human
antioxidant response element-mediated expression of NAD(P)H:quinone
oxido-reductase1 gene. Proc Natl Acad Sci USA. 93:14960–14965.
1996. View Article : Google Scholar
|
|
4
|
Zhang Y and Gordon GB: A strategy for
cancer prevention: stimulation of the Nrf2-ARE signaling pathway.
Mol Cancer Ther. 3:885–893. 2004.PubMed/NCBI
|
|
5
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niture SK, Khatri R and Jaiswal AK:
Regulation of Nrf2-an update. Free Radic Biol Med. 66:36–44. 2014.
View Article : Google Scholar
|
|
7
|
Surh YJ, Kundu JK and Na HK: Nrf2 as a
master redox switch in turning on the cellular signaling involved
in the induction of cytoprotective genes by some chemopreventive
phytochemicals. Planta Med. 74:1526–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qaisiya M, Coda Zabetta CD, Bellarosa C
and Tiribelli C: Bilirubin mediated oxidative stress involves
antioxidant response activation via Nrf2 pathway. Cell Signal.
26:512–520. 2014. View Article : Google Scholar
|
|
9
|
Nguyen CN, Kim HE and Lee SG:
Caffeoylserotonin protects human keratinocyte HaCaT cells against
H2O2-induced oxidative stress and apoptosis
through upregulation of HO-1 expression via activation of the
PI3K/Akt/Nrf2 pathway. Phytother Res. 27:1810–1818. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bates DJ, Smitherman PK, Townsend AJ, King
SB and Morrow CS: Nitroalkene fatty acids mediate activation of
Nrf2/ARE-dependent and PPARγ-dependent transcription by distinct
signaling pathways and with significantly different potencies.
Biochemistry. 50:7765–7773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Landete JM: Dietary intake of natural
antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr.
53:706–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwak JS, Kim JY, Paek JE, Lee YJ, Kim HR,
Park DS and Kwon O: Garlic powder intake and cardiovascular risk
factors: a meta-analysis of randomized controlled clinical trials.
Nutr Res Pract. 8:644–654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rana SV, Pal R, Vaiphei K, Sharma SK and
Ola RP: Garlic in health and disease. Nutr Res Rev. 24:60–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Capasso A: Antioxidant action and
therapeutic efficacy of Allium sativum L. Molecules. 18:690–700.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nencini C, Menchiari A, Franchi GG and
Micheli L: In vitro antioxidant activity of aged extracts of some
Italian Allium species. Plant Foods Hum Nutr. 66:11–16. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lanzotti V, Barile E, Antignani V,
Bonanomi G and Scala F: Antifungal saponins from bulbs of garlic,
Allium sativum L. var. Voghiera. Phytochemistry. 78:126–134. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khalil WK, Ahmed KA, Park MH, Kim YT, Park
HH and Abdel-Wahhab MA: The inhibitory effects of garlic and Panax
ginseng extract standardized with ginsenoside Rg3 on the
genotoxicity, biochemical, and histological changes induced by
ethylenediaminetetraacetic acid in male rats. Arch Toxicol.
82:183–195. 2008. View Article : Google Scholar
|
|
18
|
Amagase H: Clarifying the real bioactive
constituents of garlic. J Nutr. 136(Suppl 3): 716S–725S.
2006.PubMed/NCBI
|
|
19
|
Matsuura H: Saponins in garlic as
modifiers of the risk of cardiovascular disease. J Nutr. 131(3s):
1000S–1005S. 2001.PubMed/NCBI
|
|
20
|
Lacaille-Dubois MA and Wagner H: A review
of the biological and pharmacological activities of saponins.
Phytomedicine. 2:363–386. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fehresti Sani M, Montasser Kouhsari S and
Moradabadi L: Effects of three medicinal plants extracts in
experimental diabetes: antioxidant enzymes activities and plasma
lipids profiles in comparison with metformin. Iran J Pharm Res.
11:897–903. 2012.PubMed/NCBI
|
|
22
|
Luo H, Huang J, Liao WG, Huang QY and Gao
YQ: The antioxidant effects of garlic saponins protect PC12 cells
from hypoxia-induced damage. Br J Nutr. 105:1164–1172. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song JL, Choi JH, Seo JH, Kil JH and Park
KY: Antioxidative effects of fermented sesame sauce against
hydrogen peroxide-induced oxidative damage in LLC-PK1 porcine renal
tubule cells. Nutr Res Pract. 8:138–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang JS, Han MH, Kim GY, Kim CM, Kim BW,
Hwang HJ and Hyun Y: Nrf2-mediated HO-1 induction contributes to
antioxidant capacity of a Schisandrae Fructus ethanol extract in
C2C12 myoblasts. Nutrients. 6:5667–5678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SJ, Ho Hur J, Park C, Kim HJ, Oh GS,
Lee JN, Yoo SJ, Choe SK, So HS, Lim DJ, Moon SK and Park R:
Bucillamine prevents cisplatin-induced ototoxicity through
induction of glutathione and antioxidant genes. Exp Mol Med.
47:e1422015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pischke SE, Zhou Z, Song R, Ning W, Alam
J, Ryter SW and Choi AM: Phosphatidylinositol 3-kinase/Akt pathway
mediates heme oxygenase-1 regulation by lipopolysaccharide. Cell
Mol Biol (Noisy-le-grand). 51:461–470. 2005.
|
|
28
|
Paine A, Eiz-Vesper B, Blasczyk R and
Immenschuh S: Signaling to heme oxygenase-1 and its
anti-inflammatory therapeutic potential. Biochem Pharmacol.
80:1895–1903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang JJ, Tao H, Huang C and Li J: Nuclear
erythroid 2-related factor 2: a novel potential therapeutic target
for liver fibrosis. Food Chem Toxicol. 59:421–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abruzzo PM, Esposito F, Marchionni C, di
Tullio S, Belia S, Fulle S, Veicsteinas A and Marini M: Moderate
exercise training induces ROS-related adaptations to skeletal
muscles. Int J Sports Med. 34:676–687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar S, Kain V and Sitasawad SL: High
glucose-induced Ca2+ overload and oxidative stress
contribute to apoptosis of cardiac cells through mitochondrial
dependent and independent pathways. Biochim Biophys Acta.
1820:907–920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li NS, Luo XJ, Zhang YS, He L, Liu YZ and
Peng J: Phloroglucinol protects gastric mucosa against
ethanol-induced injury through regulating myeloperoxidase and
catalase activities. Fundam Clin Pharmacol. 25:462–468. 2011.
View Article : Google Scholar
|
|
33
|
Klimathianaki M, Vaporidi K and
Georgopoulos D: Respiratory muscle dysfunction in COPD: from
muscles to cell. Curr Drug Targets. 12:478–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao Y, Xiao Z, Wong S, Hsu YC, Cheng T,
Chang CC, Bian L and Mak AF: The effects of oxidative stress on the
compressive damage thresholds of C2C12 mouse myoblasts:
implications for deep tissue injury. Ann Biomed Eng. 43:287–296.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Winter-Vann AM and Johnson GL: Integrated
activation of MAP3Ks balances cell fate in response to stress. J
Cell Biochem. 102:848–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park JY, Kang KA, Kim KC, Cha JW, Kim EH
and Hyun JW: Morin induces heme oxygenase-1 via ERK-Nrf2 signaling
pathway. J Cancer Prev. 18:249–256. 2013. View Article : Google Scholar
|
|
38
|
Jiang G, Hu Y, Liu L, Cai J, Peng C and Li
Q: Gastrodin protects against MPP(+)-induced oxidative stress by up
regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway
in human dopaminergic cells. Neurochem Int. 75:79–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jun YJ, Lee M, Shin T, Yoon N, Kim JH and
Kim HR: eckol enhances heme oxygenase-1 expression through
activation of Nrf2/JNK pathway in HepG2 cells. Molecules.
19:15638–15652. 2014. View Article : Google Scholar : PubMed/NCBI
|