Introduction
Osteoporosis is a systemic disease characterized by
low bone mass, increased bone fragility and susceptibility to
fracture (1). Although the
detailed pathological mechanisms remain unknown, previous studies
have suggested that the structural abnormalities associated with
osteoporotic bones are attributable to the dysfunction of bone
marrow-derived stromal cell (BMSC) differentiation (2–6).
BMSCs are self-renewable, multipotent stem cells with the capacity
to differentiate into lineages of mesenchymal tissues, such as
chondrocytes, osteoblasts and adipocytes, when cultivated under
appropriate conditions using specific hormonal inducers or growth
factors (7,8). Bone regeneration is a complex
process mediated by the close association between the activities of
osteogenic and adipogenic progenitor cells, which both derive from
BMSCs (7,8). The balance between the osteogenesis
and adipogenesis of BMSCs can be disrupted. BMSCs have a reduced
capacity to differentiate into osteoblasts, but an increased
capacity for adipocyte differentiation. The increase in the
proportion of fat in bone marrow subsequently induces the apoptosis
of osteoblasts and the proliferation of osteoclasts, which results
in further bone resorption and overall bone loss (9,10).
Mechanical loads on bone tissue increase bone
formation and improve bone strength (11). The removal of mechanical stimuli
during immobilization or in microgravity results in a rapid loss of
bone mass, whereas the application of exogenous mechanical loading
leads to increased bone formation in the modeling skeleton
(12). The molecular mechanisms
mediating the conversion of mechanical stimuli into biochemical
signaling remain poorly understood. Previous studies have suggested
that extracellular nucleotides, such as adenosine 5′-triphosphate
(ATP) and uridine triphosphate (UTP), are soluble factors released
in response to mechanical stimulation in different cell types
(13–15). Once released, extracellular
nucleotides stimulate plasma membrane-localized nucleotide
receptors: P2 receptors play a significant role in bone remodeling
(16–18). Based on their molecular structure
and activated signaling pathways, the P2 receptor family is divided
into 2 subfamilies: the P2X and P2Y receptors (19). Currently, 7 P2X (P2X1-7) and 8 P2Y
(P2Y1, 2, 4, 6, 11, 12, 13 and 14) receptors have been identified,
each of which has been cloned, characterized and assigned distinct
tissue expression patterns and pharmacology. P2XRs are ligand-gated
ion channels, whereas P2YRs are G protein-coupled receptors
(20). The pattern of expression
of different P2R subtypes on cell membranes influences the activity
and the effects of nucleotides (20).
In particular, UTP stimulates the P2Y2 and P2Y4
receptors. In addition, UTP is hydrolyzed to uridine diphosphate
(UDP), which acts on the P2Y6 receptor (21). UTP has been implicated in the
regulation of osteogenesis in a number of cell types, including rat
osteoblasts and human BMSCs. However, these results appear to
demonstrate certain discrepancies: for example, UTP, but not ADP or
UDP, promotes alkaline phosphatase (ALP) activity and bone
mineralization, and increases the mRNA levels of ALP, bone
morphogenetic protein (BMP)-2, BMP-4, BMP-5 and bone sialoprotein
(BSP) through the P2Y2 receptor in rat primary ostoblasts (22). UTP and UDP facilitate the
osteogenic differentiation of cells which is indicated by an
increase in ALP activity through the activation of UDP-sensitive
P2Y6 receptors, but not through P2Y2 and P2Y4 receptors, in the
BMSCs of post-menopausal women (17). A component of the inhibitory
action of ATP and UTP on bone mineralization could thus be mediated
directly by PPi, independently of P2 receptors (23). UTP, signaling via the P2Y2
receptor on osteoblasts, blocks bone mineralization and bone
formation (24,25). The various physiological effects
of UTP and P2Y receptors have also been studied in adipocytes. UTP
and UDP have been shown to increase intracellular Ca2+
levels in brown adipocytes (26).
UTP has also been shown to effectively elevate the intracellular
calcium levels in white adipocytes via the P2Y2 receptor, and the
activation of the P2Y11 receptor inhibited leptin production and
stimulated lipolysis (27). Based
on the above-mentioned evidence, we hypothesized that UTP and P2Y
receptors play a critical role in the osteogenic and adipogenic
differentiation of BMSCs. Thus far, little is known about the
expression of P2Y receptor subtypes and the potential effects of
UTP on the differentiation process of rat BMSCs. Thus, in the
present study, we aimed to determine whether UTP regulates the
osteogenic and adipogenic differentiation of BMSCs and if so, to
identity which of the P2Y receptors mediate such a response, and to
elucidate the underlying mechanisms.
Materials and methods
Reagents
Unless otherwise stated, all cell culture reagents
were purchased from Gibco (Paisley, UK). TRIzol reagent was
supplied by Invitrogen (Grand Island, NY, USA). ALP kits, alizarin
red S, Oil Red O, UTP and MRS2578 (P2Y6 receptor antagonist; 1
µM was added to the cell cultures 1 h prior to UTP
treatment) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
U0126 [a mitogen-activated protein kinase inhibitor (MAPK; 5
µM were added to the cell cultures 1 h prior to UTP
treatment)] was procured from Beyotime Institute of Biotechnology
(Shanghai, China). Lipofectamine 3000 was purchased from
Invitrogen. Rat mesenchymal stem cell (MSC) adipogenic and
osteogenic differentiation medium were purchased from Cyagen
Biosciences Inc. (Santa Clara, CA, USA).
BMSC culture
Rat BMSCs were isolated from 4-week-old male
Sprague-Dawley (SD) rats and expanded in accordance with previously
published techniques (28). All
animal experiments were approved by the Animal Care and Use
Committee for Teaching and Research, of Huazhong University of
Science and Technology (Wuhan, China). The cells were maintained in
expansion medium, consisting of Dulbecco's modified Eagle's
medium/F12 (1:1) and 10% fetal bovine serum (FBS) supplemented with
100 U/ml penicillin and 100 U/ml streptomycin, in a humidified
atmosphere containing 5% CO2. Upon reaching confluence,
the cells were detached with 0.25% trypsin (Boster Inc., Wuhan,
China) and passaged at a ratio of 1:2. BMSCs of passages 3–5 were
used in the experiments. Cells maintained in expansion medium were
used as undifferentiated cells. To induce osteogenic
differentiation, the cells were cultured in osteogenic
differentiation medium (10 nM dexamethasone, 50 µg/ml
ascorbic acid and 10 mM β-glycerophosphate in expansion medium)
supplemented with UTP for 21 days. The medium was changed every 3
days.
CCK-8 proliferation assay
The cells were seeded in 96-well plates at a density
of 2×103 cells/well, and divided into 4 groups as
follows: the control (without any treatment), and the cells treated
with 5, 25 and 125 µM UTP, respectively. Each group
comprised 5 sub-wells. Cell proliferation was assessed by CCK-8
(Beyotime Institute of Biotechnology) assay, after processing for
0, 24, 48 and 72 h. Briefly, 10 µl CCK-8 solution were added
to each well followed by incubation in the dark for 1.5 h and the
absorbance was then read using a microplate reader (Sunrise RC;
Tecan, Mannedorf, Switzerland) at 450 nm.
Total RNA extraction and quantitative PCR
(qPCR)
Total RNA was extracted using TRIzol reagent. The
purity and concentration of the RNA samples were determined
spectroscopically. First-strand cDNA was synthesized from 3
µg RNA, using an EasyScript First-Strand cDNA synthesis
super mix kit (TransGen Biotech Co., Ltd., Beijing, China) and used
for qPCR. The expression of runt-related transcription factor 2
(RUNX2), ALP and osteopontin (OPN) was quantified using a Bio-Rad
MyiQ2 sequence detection system and TransStart Eco Green qPCR
SuperMix (TransGen Biotech Co., Ltd.). The primers were synthesized
by Invitrogen, and their sequences are listed in Table I. The reactions were incubated at
95°C for 30 sec, followed by 40 cycles of 94°C for 5 sec and 60°C
for 35 sec. The relative expression of gene-specific products was
analyzed using the 2−ΔΔCt method and normalized to the
corresponding GAPDH values.
 | Table IList of specific primers used in the
present study. |
Table I
List of specific primers used in the
present study.
| Gene | Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
| GAPDH |
GGCACAGTCAAGGCTGAGAATG |
ATGGTGGTGAAGACGCCAGTA |
| RUNX2 |
GCACCCAGCCCATAATAGA |
TTGGAGCAAGGAGAACCC |
| PPARγ |
CCTTTACCACGGTTGATTTCTC |
GGCTCTACTTTGATCGCACTTT |
| ALP |
CAAGGACCAACTACAACCA |
AGGGAAGGGTCAGTCAGGTT |
| OPN |
CCTGGACCTCATCAGCATTT | GGAGAC
AGGAGGCAAGG |
| Adipsin |
CACGTGTGCGGTGGCACCCTG |
CCCCTGCAAGTGTCCCTGCGGT |
| Fabp4 |
GCGTAGAAGGGGACTTGGTC |
TTCCTGTCATCTGGGGTGATT |
ALP staining and quantification
The cells were seeded at a density of 105
cells/well in 35-mm plastic dishes with or without UTP (125
µM) in osteogenic medium. After 7 days, ALP staining was
performed using ALP kits. Briefly, after discarding the medium, the
cells were gently washed with PBS 3 times and fixed with 4%
paraformaldehyde for 15 min at 4°C. The cells were washed with
deionized water and then stained with naphthol AS-MX phosphate for
30 min in the dark and washed 3 times with PBS. Images were
acquired using a light microscope (Eclipse Ti; Nikon, Tokyo,
Japan). Image-Pro Plus 5.0 was used to analyze the quantity of the
dyed areas.
Alizarin red S staining and
quantification
The cells were cultured in osteogenic medium in
35-mm plastic dishes for 21 days with or without UTP (125
µM). Briefly, the cells were washed with PBS and fixed with
4% paraformaldehyde, for 30 min at room temperature. After washing
with deionized water, the fixed cells were stained with 2% alizarin
red S (pH 4.2) in deionized water. After 20 min, the cells were
washed with deionized water and observed under a light microscope
(Eclipse Ti; Nikon). Image-Pro Plus 5.0 was used to quantify the
nodule areas.
Oil Red O staining and
quantification
To induce adipogenic differentiation, the BMSCs were
seeded at 2×104 cells/cm2 on 35-mm plastic
dishes and grown for 3 days in adipogenic induction medium (Cyagen
Biosciences, Inc.) containing additional SD rat MSC adipogenic
differentiation basal medium A, FBS, insulin, glutamine,
rosiglitazone, dexamethasone, 3-isobutyl-1-methylxanthine and
penicillin/streptomycin, followed by 1 day in adipogenic
maintenance medium containing SD rat MSC adipogenic differentiation
basal medium A, FBS, insulin, glutamine and penicillin/streptomycin
(1 cycle). Both steps were repeated up to day 21 (indicated as the
5th cycle), when the cell culture was terminated for Oil Red O
staining. UTP (125 µM) was added to the culture medium every
3 days. Briefly, the cells were washed with PBS and fixed with 4%
paraformaldehyde, for 30 min at room temperature. After washing
with PBS, the fixed cells were stained with Oil Red O in deionized
water. After 20 min, the cells were washed with PBS twice and
observed under a light microscope (Eclipse Ti; Nikon). Image-Pro
Plus 5.0 was used to quantify the nodule areas.
Western blot analysis
The cells were lysed using the protein extraction
reagent RIPA supplement, with protease and phosphatase inhibitor
and phenylmethylsulfonyl fluoride (all from Beyotime Institute of
Biotechnology). Cell homogenates were sonicated for 5 min and
protein concentrations from the lysates were determined by BCA
protein assay (Boster Inc.). Prior to loading, total protein
samples were denatured by incubation at 95°C for 5 min in the
presence of 5X reducing sample buffer (60 mM Tris-HCl pH 6.8, 25%
glycerol, 2% SDS, 10% β-mercaptoethanol and 0.1% bromophenol blue).
Thirty micrograms of protein sample were separated by
SDS-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride (PVDF) membranes. Following incubation in
5% BSA at room temperature, the membranes were incubated with
rabbit anti-total-ERK1/2 antibody (1:1,000; Cat. no. 4695P; Cell
Signaling Technology, Inc., Beverly, MA, USA), rabbit anti-p-ERK1/2
antibody (1:1,000; Cat. no. 4377S; Cell Signaling Technology,
Inc.), rabbit anti-total-JNK antibody (1:1000; Cat. no. 9252S; Cell
Signaling Technology, Inc.), rabbit anti-p-JNK antibody (1:1,000;
Cat. no. 4668T; Cell Signaling Technology, Inc.), rabbit
anti-total-p38 antibody (1:1,000; Cat. no. 8690P; Cell Signaling
Technology, Inc.), mouse anti-p-p38 antibody (1:1,000; Cat. no.
9216S; Cell Signaling Technology, Inc.), mouse anti-GAPDH antibody
(1:5,000; Cat. no. BM1623; Boster Inc.), mouse anti-RUNX2 antibody
(1:400; Cat. no. ab76596; Abcam, Cambridge, UK), rabbit anti-ALP
antibody (1:1,000; Cat. no. ab133602; Abcam), mouse anti-OPN
antibody (1:500; Cat. no. ab69498; Abcam), rabbit anti-PPARγ
antibody (1:400; Cat. no. ab133602; Abcam), rabbit anti-FABP4
antibody (1:1,000; Cat. no. ab92501; Abcam), goat anti-adipsin
antibody (1:400; Cat. no. sc12403; Santa Cruz Biotechnology, Inc.,
CA, USA) at 4°C overnight. Anti-mouse horseradish peroxidase
(HRP)-conjugated IgG (1:5,000; Cat. no. 7076P2) and anti-rabbit
HRP-conjugated IgG (1:5,000; Cat. no. 7074P2; both from Cell
Signaling Technology, Inc., Beverly, MA, USA) were used as the
secondary antibodies. The immunostained protein bands were detected
by chemiluminescence. Protein levels were determined by normalizing
to GAPDH.
Gene silencing by small interfering RNA
(siRNA)
Scrambled siRNA, P2Y2 siRNA and P2Y4 siRNA were
designed and synthesized by RiboBio (Guangzhou, China). According
to the manufacturer's instructions (RiboBio and Invitrogen), BMSCs
were seeded on 6-well plates at a density of 5×104
cells/well in normal medium and grown to 70–90% confluence prior to
transfection. A transfection mixture containing Lipofectamine 3000
(7.5 µl/well; Invitrogen) and 100 nM siRNA targeting P2Y2 or
P2Y4 receptor or scrambled siRNA (all from RiboBio) sequence was
prepared in 250 µl Opti-MEM (Invitrogen) and incubated for 5
min at room temperature. After being washed with Opti-MEM, the
cells were incubated with the transfection mixture in 1,750
µl Opti-MEM for 4 days at 37°C. The effects of gene
silencing were determined by qPCR at 24, 48, 72 and 96 h following
transfection.
Immunofluorescence staining
Rat BMSCs were seeded onto sterile 1-cm-diameter
discs in 24-well trays at 2.5×104 cells/disc for 5 days.
The discs were removed and fixed with 4% para-formaldehyde for 15
min at room temperature; after washing 3 times with PBS, the cells
were blocked for 1 h with PBS containing 5% FBS. The samples were
then incubated overnight at 4°C with anti-P2Y2 (1:200; Cat. no.
ab10270; Abcam) primary antibody, washed 3 times with PBS and
incubated for 1 h at room temperature with the goat anti-rabbit
Cy3-labelled secondary antibody solution (1:500; Cat. no. AB6939;
Abcam) diluted in blocking solution. The cells were further
counter-stained with DAPI (1:3,000; Cat. no. AR1176; Boster Inc.).
Fluorescent images were obtained with an inverted fluorescent
microscope (Eclipse Ti; Nikon). Cy3 absorbance and emission at 552
and 565 nm, and DAPI absorbance and emission at 360 and 460 nm,
respectively were assessed.
Statistical analysis
The results are expressed as the means ± SD (n=3).
Statistical comparisons were performed using one-way ANOVA,
followed by Tukey's post hoc test, which was carried out using SPSS
19.0 software. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
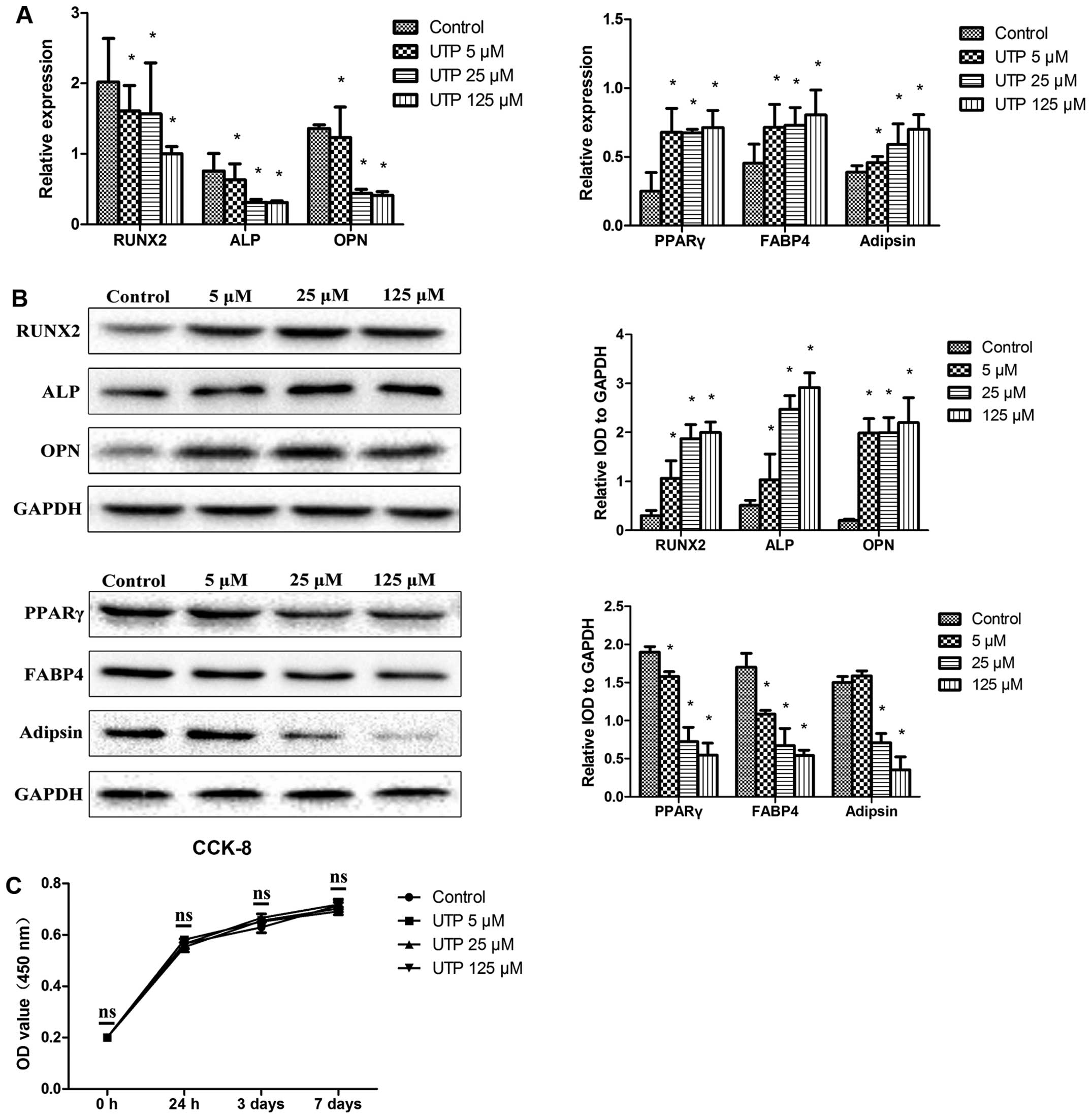
UTP decreases the expression of
osteogenic-related markers and increases the expression of
adipogenic-related markers in undifferentiated BMSCs
BMSCs were cultured in expansion medium, with
various concentrations of UTP (5–125 µM), for 7 days. The
expression levels of osteogenic- and adipogenic-related markers
were measurd by qPCR and western blot analysis. As shown in
Fig. 1A and B, UTP decreased
RUNX2, ALP and OPN mRNA and protein expression and increased
peroxisome proliferator-activated receptor γ (PPAR γ), fatty acid
binding protein 4 (FABP4) and adipsin mRNA and protein expression
in a dose-dependent manner.
The number of cells was measured using a CCK-8 kit,
in order to examine the effects of UTP on the proliferation of
BMSCs. As shown in Fig. 1B, no
significant differences in the numbers of cells were observed
(Fig. 1C), indicating that UTP
affected the differentiation potential of BMSCs and was not
cytotoxic. The concentration of 125 µM UTP did not affect
the proliferation of the BMSCs, but had the maximum effect on
differentiation, and was therefore used in the following
experiments.
UTP inhibits the osteogenic and enhances
the adipogenic differentiation of stimulated BMSCs
To determine the effects of UTP on the
differentiation of BMSCs into osteoblasts, the cells were cultured
in osteogenic medium, with or without UTP (125 µM) treatment
for 7 days. The expression levels of osteogenic- and
adipogenic-related markers were measured by qPCR and western blot
analysis. As shown in Fig. 2A and
B, the osteogenic medium-induced upregulation of RUNX2, ALP and
OPN mRNA and protein expression was significantly reduced by UTP
treatment.
 | Figure 2Uridine triphosphate (UTP) inhibits
the osteogenic and enhances the adipogenic differentiation of
stimulated bone marrow-derived stromal cells (BMSCs). Osteogenic
medium-induced upregulation of osteogenic-related (A) mRNA and (B)
protein expression was significantly reduced by UTP (125 µM)
treatment. Results are expressed as percentages with respect to
GAPDH expression. Data represent the means ± SD, n=3,
*P<0.05 vs. control. (C) Representative images and
semi-quantitative analysis of alkaline phosphatase (ALP) staining
[7 days (upper panel) and 21 days (middle panel)] and alizarin red
S staining (lower panel). Bar, 50 µm; panels a, control;
panels b, UTP 125 µM. Data represent the means ± SD, n=3,
*P<0.05 vs. control. UTP increased adipogenic-related
(D) mRNA and (E) protein expression compared to control in BMSCs
cultured in adipogenic medium. Results are expressed as percentages
with respect to GAPDH expression. Data represent the means ± SD,
n=3, *P<0.05 vs. control. (F) Representative images
and semi-quantitative analysis of Oil Red O staining. Bar, 50
µm; panel a, control; panel b, UTP 125 µM. Data
represent the means ± SD, n=3, *P<0.05 vs.
control. |
Extracellular matrix mineralization was measured
using alizarin red staining when the BMSCs were treated with UTP in
osteogenic medium for 21 days. The BMSCs formed abundant
characteristic nodules in the control cultures, and the number of
these nodules was markedly decreased in the UTP-treated cultures
(Fig. 2C). Thus, our data suggest
that ALP plays a key role in bone mineralization. The effects of
UTP on ALP expression were also examined at 7 and 21 days of
culture in osteogenic medium. UTP inhibited ALP expression compared
to the controls both at 7 and 21 days of culture (Fig. 2C).
We then examined the effects of UTP on the
adipogenic differentiation of BMSCs. The BMSCs were cultured in
adipogenic medium with or without UTP. As shown in Fig. 2D and E, UTP increased the mRNA and
protein expression levels of PPAR, FABP4 and adipsin compared to
the control on day 7. After 3 weeks of adipogenic differentiation,
numerous lipid drops were observed in the intracellular spaces of
the differentiated cells. The lipid content of the cells was
demonstrated by Oil Red O staining; lipid accumulation was more
evident in the UTP-treated cells compared to the untreated cell
cultures (Fig. 2F).
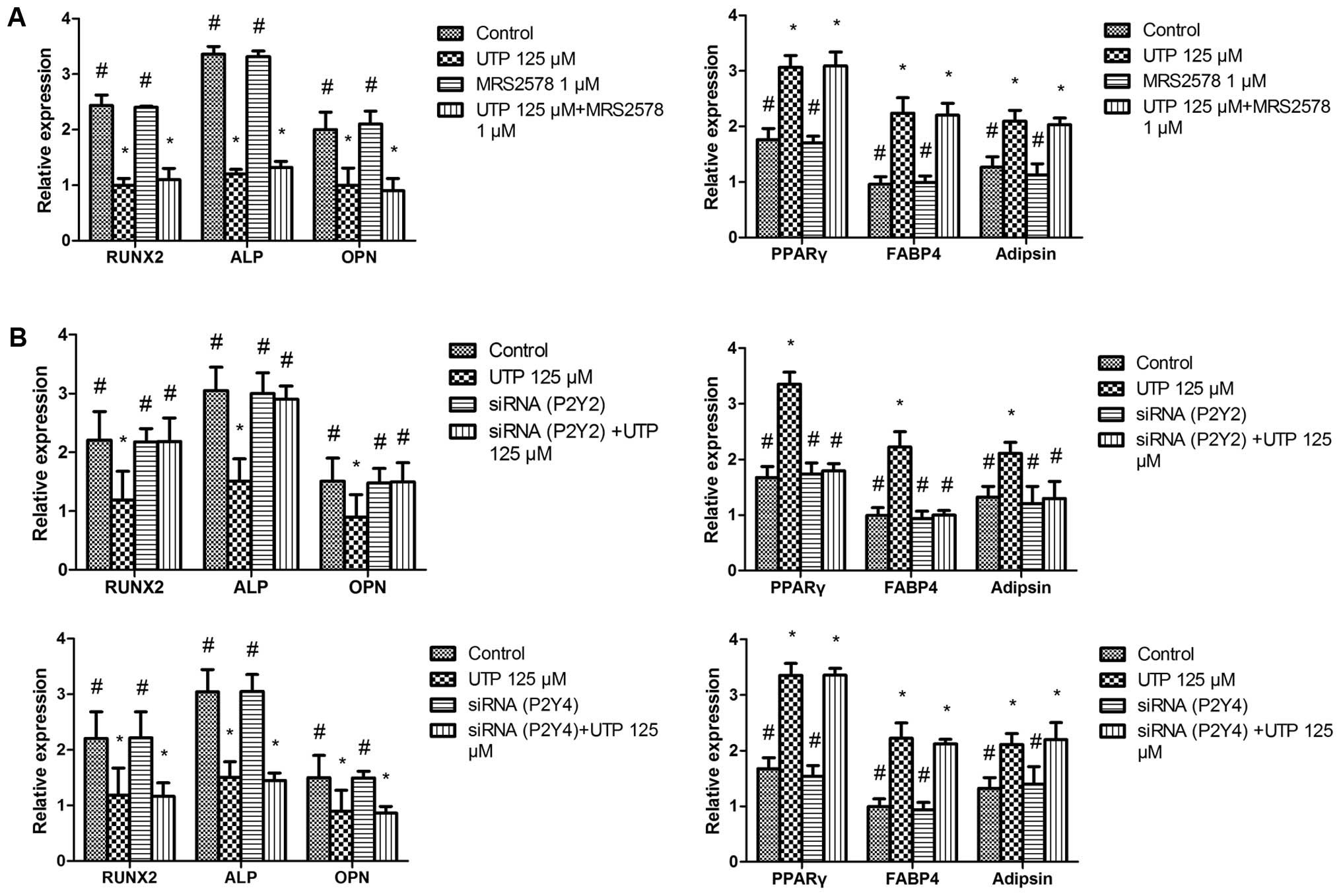
UTP regulates the osteogenic and
adipogenic differentiation of BMSCs via the P2Y2 receptor
UTP stimulates the P2Y2 and P2Y4 receptors. In
addition, UTP is hydrolyzed to UDP, which acts on the P2Y6 receptor
(28). Thus, to identify which of
the P2Y receptor subtypes is associated with the effects of UTP on
BMSCs, we added the selective P2Y6 receptor antagonist, MRS2578 (1
µM) (29,30), to the cell cultures 1 h prior to
UTP treatment. We observed that the effects of UTP + MRS2578 on
osteogenic- and adipogenic-related gene expression were similar to
those observed wtih UTP treatment (Fig. 3A), suggesting that the effects of
UTP on BMSCs are mediated via P2Y2 or P2Y4 receptors rather than
the P2Y6 receptor.
A number of previous studies have reported that UTP
inhibits bone mineralization in vitro via the P2Y2 receptor
in rat primary osteoblasts (23–25,31). To determine whether the effects
induced by UTP are mediated through the P2Y2 receptor, we employed
siRNAs targeting the P2Y2 and P2Y4 receptor genes. The P2Y2 and
P2Y4 siRNA silencing efficiency were both 85% at 2 days following
transfection (data not shown). To examine the effects of P2Y2 and
P2Y4 siRNA on BMSC differentiation, the cells were incubated with
the transfection mixture for 2 days. Subsequently, the transfection
mixture was replaced with osteogenic or adipogenic medium with UTP
(125 µM) and the cells were cultured for 5 days. Total RNA
extraction and qPCR were then performed to determine the expression
of osteogenic- and adipogenic-related genes. We found that P2Y2
siRNA prevented the downregulation of osteogenic-related gene
expression and the upregulation of adipogenic-related genes induced
by UTP, whereas P2Y4 receptor siRNA did not have a marked effect on
the expression of these genes (Fig.
3B).
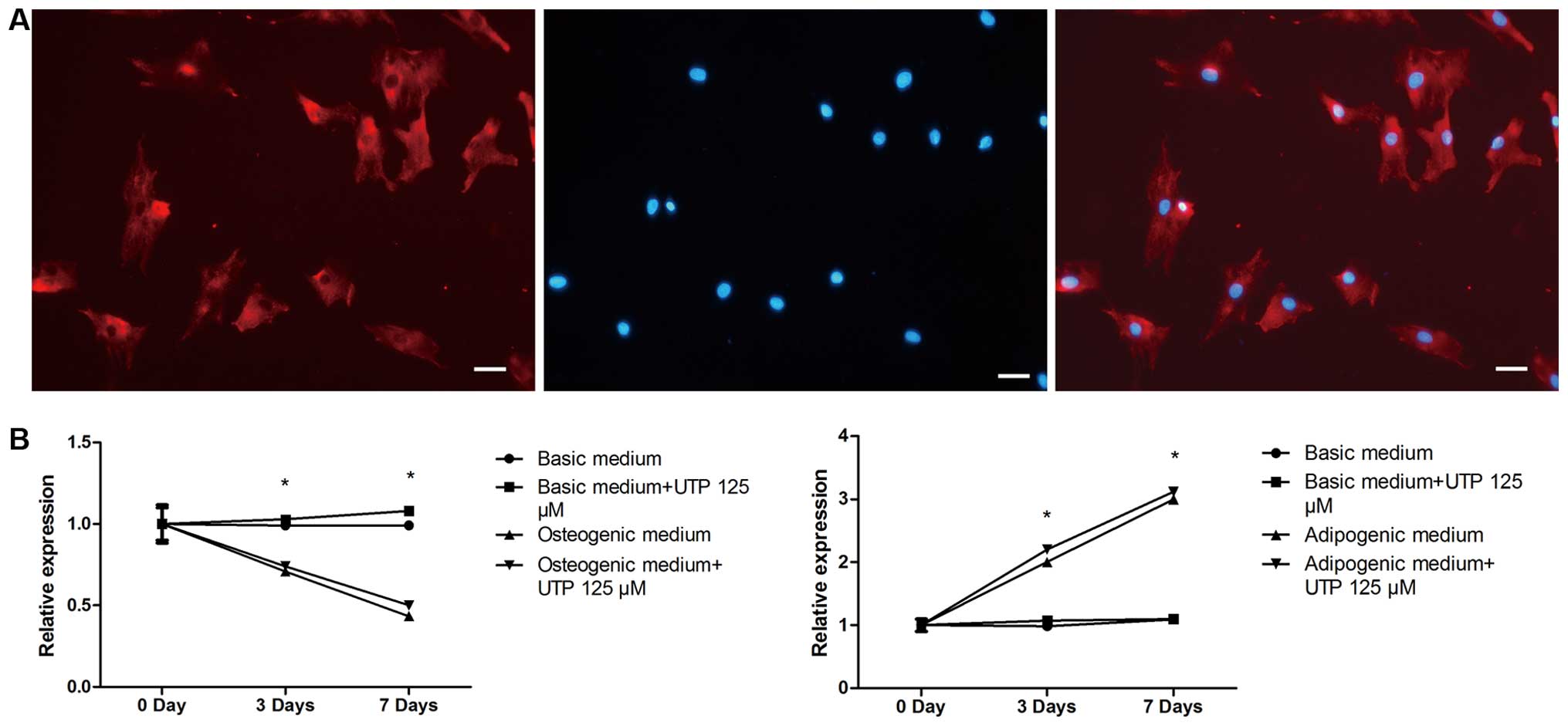
Effect of BMSC differentiation on the
expression of P2Y2 receptor
We first confirmed that the P2Y2 receptor was
expressed by rat BMSCs using immunofluorescence staining (Fig. 4A). P2Y2 receptor expression was
evaluated by qPCR, in both stimulated (osteogenic and adipogenic
medium) and unstimulated (expansion medium) BMSCs, with or without
UTP treatment, on days 0, 3 and 7. As shown in Fig. 4B, in the cells cultured in
adipogenic medium, the mRNA expression of the P2Y2 receptor
increased on days 3 and 7 compared to day 0. However, the mRNA
expression of the P2Y2 receptor in the cells cultured in osteogenic
medium decreased on days 3 and 7 compared to day 0. Furthermore,
UTP treatment failed to affect the expression of the P2Y2 receptor
in both the osteogenic and adipogenic media. P2Y2 receptor
expression remained relatively unaltered in the unstimulated
cells.
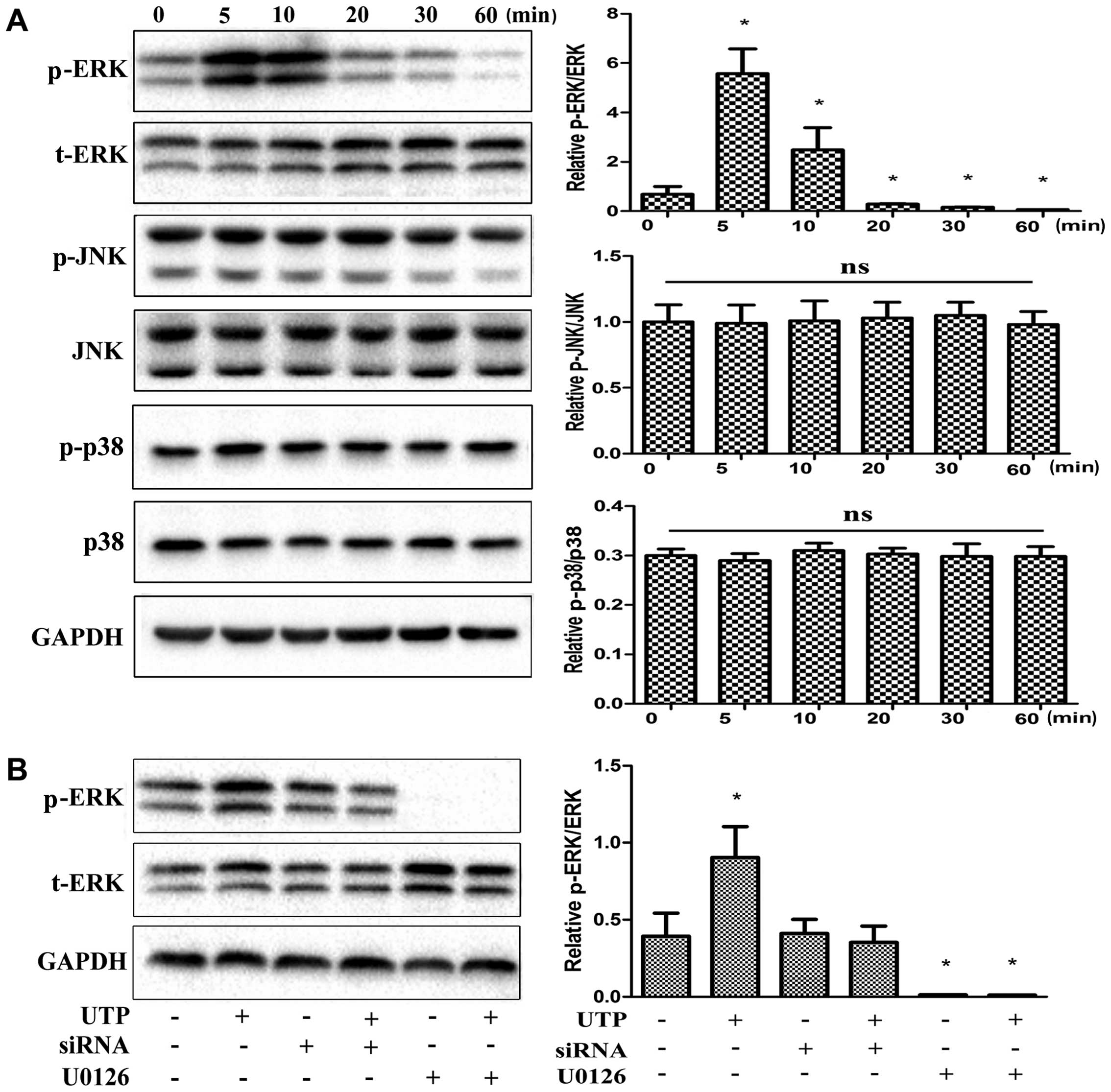
UTP activates the ERK1/2 signaling
pathway in BMSCs
MAPKs have been shown to be important in the
differentiation of a number of cell types, including BMSCs
(32–38). Thus, in this study, we examined
the effects of treatment with UTP on the phosphorylation of 3
members of the MAPK family in rat BMSCs. As shown in Fig. 5A, of the 3 MAPK isoforms, only
ERK1/2 was significantly phosphorylated following treatment with
UTP. Maximal ERK1/2 activation was observed at 5 min and remained
activated for up to 60 min. Furthermore, U0126 (5 µM), a
selective MAPK inhibitor, completely abolished the phosphorylation
of ERK1/2 induced by UTP (Fig.
5B). P2Y2 receptor siRNA was also used to suppress receptor
expression in order to examine its role in ERK1/2 phosphorylation.
UTP-induced ERK1/2 phosphorylation was attenuated by approximately
90% when the cells were treated with P2Y2 siRNA (Fig. 5B).
Role of the ERK1/2 signaling pathway in
the osteogenic and adipogenic differentiation of BMSCs induced by
UTP
To determine the role of ERK1/2 in the
differentiation of BMSCs, we assessed the effects of UTP on
osteogenic- and adipogenic-related gene expression in BMSCs in the
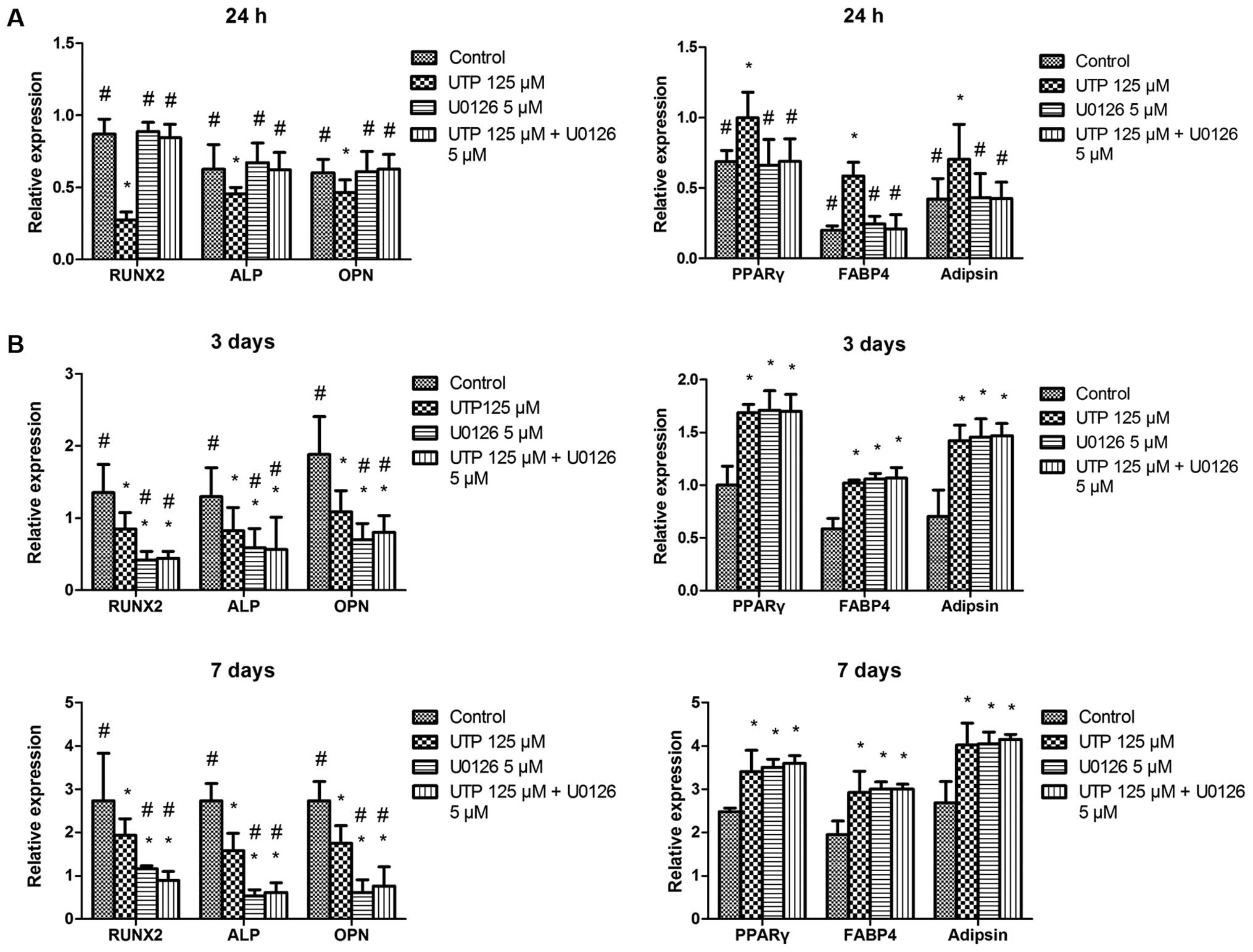
presence or absence of U0126 (5 µM). UTP significantly
inhibited osteogenic-related gene expression and increased
adipogenic-related gene expression. These effects were
significantly attenuated by U0126 in the cells cultured for 24 h
(Fig. 6A). However, U0126 failed
to prevent the effects induced by UTP on 3- and 7-day-old cell
cultures (Fig. 6B). In addition,
U0126 and U0126 + UTP induced the downregulation of
osteogenic-related genes and the upregulation of adipogenic-related
genes compared with the controls on days 3 and 7.
Discussion
This study demonstrated that, in relation to BMSCs,
UTP inhibited osteogenesis and enhanced adipogenesis, without
affecting cell growth. Furthermore, we confirmed that these effects
induced by UTP acted via P2Y2 receptors. We also demonstrated that
ERK1/2 signaling played differential roles in the differentiation
of BMSCs. These findings help to broaden our understanding of the
role of purinergic receptors, particularly the P2Y2 receptor, in
the functional differentiation of BMSCs.
It has previously been demonstrated that
extracellular UTP exerts profound inhibitory effects on the bone
mineralization mediated by P2Y receptors in primary osteoblasts
(23–25). In the present study, we analyzed
the molecular pathway activated by extracellular UTP during the
BMSC differentiation process. Our findings revealed that the
ability of UTP to modulate the differentiation of BMSCs into the
osteoblastic lineage is relevant at a physiological level, as the
number of mineralized nodules and ALP expression induced by culture
in osteogenic medium were markedly reduced in UTP-treated cell
cultures. Furthermore, UTP also decreased osteogenic-related mRNA
and protein expression in unstimulated BMSCs, indicating that UTP
may prevent precursor cells from differentiating into osteoblasts,
and also inhibited extracellular matrix mineralization in
osteoblasts differentiated from BMSCs. Few studies have, however,
reported different results. It has previously been noted that UTP
stimulated BMP gene expression and mineralization in rat primary
osteoblasts (22), and enhanced
the osteogenic differentiation of BMSCs obtained from
post-menopausal women (17). We
suggest that differences between cell types, osteogenic culture
systems and variable methods for detecting osteogenic
differentiation contribute to explaining the discrepancies. For
example, first, osteoblasts from Wistar rats and BMSCs from
post-menopausal women were used in these studies, whereas in the
present study, we used BMSCs from SD rats. Second, in this previous
study, dexamethasone was not added to the osteogenic medium during
osteoblast differentiation (22).
It has been noted that dexamethasone downregulates Runx2, a
transcription factor required for osteoblast differentiation
(39), and inhibits
Wnt/β-catenin, a signaling pathway involved in the osteoblast
differentiation of mesenchymal progenitor cells (40). Third, the detection indices and
methods used in these studies differed from those used in our
research. Any one or a combination of these factors explains the
differences in these studies.
The differentiation of BMSCs into the osteoblastic
or adipogenic lineages is not an independent process: molecular
components promoting one cell fate inhibit the mechanisms governing
the differentiation of the alternative lineage (5,41).
This is also the case for UTP. In the present study, UTP increased
the expression of adipogenic-related markers in a dose-dependent
manner. Furthermore, the formation of lipid droplets, another
specific characteristic of adipogenic differentiation, was also
increased by UTP treatment. Consistent with our study, Ciciarello
et al reported that extracellular UTP increased the mRNA
expression of PPAR in human BMSCs (42). However, a previous study reported
that ATP and adenine compounds, but not other nucleotides (UTP,
UDP, CTP, GTP, ITP and diadenosine tetraphosphate), stimulated
lipogenesis in adipocytes (43).
In this previous study, the lipogenesis of adipocytes derived from
the epididymal fat pads of male Wistar rats was detected by
measuring the incorporation of D-[3-3H]glucose in
toluene extractable lipids, but without mRNA or protein data on any
key adipogenic related genes, such as PPARγ. Adipogenesis consists
of integrated cascades that involve several transcription factors.
The initial step of adipogenesis is the lineage commitment of MSC
followed by the expansion of preadipocytes. PPARγ is a critical
component in adipogenesis, as indicated by the fact that loss of
PPARγ expression in murine embryonic fibroblasts leads to a
complete absence of adipogenic capacity (44). MSCs differentiate into adipocytes
when they express PPARγ, which enhances the expression of
adipogenic genes (45). Although
it is not clear whether the apparent discrepancy is a matter of the
detection method or of the different cell types used in
experiments, we consider that the results of qPCR and
immunohistochemical staining in our study strongly indicate that
UTP is a positive stimulus of adipogenesis in rat BMSCs.
UTP stimulates the P2Y2 receptor and, following
degradation to UDP, also acts on the P2Y6 receptor (21). In this study, we demonstrated that
UTP regulated the differentiation of BMSCs into adipogenic cells,
but not osteoblasts by activating the P2Y2 receptor rather than
P2Y4 or P2Y6 receptors. Studies have previously reported the
effects of P2Y receptors on the osteogenic and adipogenic
differentiation of BMSCs, with varying results. For example,
Ciciarello et al reported that ATP stimulated adipogenic
differentiation of human BMSCs, mainly acting through P2Y1 and P2Y4
subtypes (42). Conversely,
adenosine resulting from ATP degradation increased BMSC osteogenic
differentiation, by activating the A2B adenosine-specific receptor
subtype (42). Zippel et
al reported that ATP, but not UTP partially compensated for the
potent inhibitory effects on matrix mineralization induced by
suramin and PPADS (P2 receptor antagonists) in human BMSCs,
indicating that P2Y2 and P2Y4 receptors had no effect on
osteogenesis (18). UTP, but not
ATP, partly compensated for the decrease in formation of lipid
droplets induced by PPADS, thus suggesting the involvement of P2Y4
receptor (18). In these two
studies, the authors used several agonists and antagonists of P2
receptors to identify which receptors were activated in the
processes of osteogenic and adipogenic differentiation. Indeed,
many of the P2 receptor subtypes are still lacking potent and
selective synthetic agonists and antagonists. These reagents are
considered effective stimulators and inhibitors of P2 receptors.
Thus, to delineate the role of each P2 receptor in osteogenesis and
adipogensis of BMSCs, several issues need to be addressed,
including specific agonists and antagonists of P2 receptors, and
gene knockout models.
In the present study, pharmacological approaches
revealed that UTP enhanced the differentiation of BMSCs into
adipocytes, but not osteoblasts by stimulating the ERK1/2 signaling
pathway in a P2Y2R-dependent manner. In agreement with our data, it
has been previously reported that the activation of P2Y2 receptors
by extracellular nucleotides is responsible for the phosphorylation
of ERK1/2 in osteoblasts. For example, Costessi et al
reported that extracellular ATP and UTP stimulate the
ERK1/2-dependent activation of the transcription factor Runx2 via
the P2Y2 receptor in the osteoblast-like HOBIT cell line (46). Katz et al reported that
P2Y2 receptor stimulation by ATP in osteoblasts sensitizes
mechanical stress-activated calcium channels, leading to calcium
influx and fast activation of the ERK1/2 and p38 MAPK pathways
(47). However, in studies on
MAPK signaling and its role in the differentiation of BMSCs, the
ERK1/2 pathway is widely reported as a positive regulator of
osteogenesis (32,33,35,36) and a negative regulator of
adipogenesis (37,38). To investigate the differences
between our observations and previous studies, in the present study
we employed U0126, a MAPK inhibitor. Consistent with these studies,
we observed that the inhibition of ERK1/2 activation by U0126
inhibited osteogenic-related gene expression, and enhanced
adipogenic-related gene expression in 3- and 7-day-old cultures.
However, U0126 significantly attenuated the effects of UTP on these
genes at 24 h of incubation. Our study on ERK1/2 modulation by UTP
showed that purinergic activation rapidly stimulated MAPK
phosphorylation in BMSCs. UTP-induced ERK1/2 activation reached a
peak at 5 min and was weaker at 60 min. In view of this finding, we
speculate that ERK1/2 is a branch or a feedback loop of the complex
signaling pathways involved in the onset of differentiation of
BMSCs. In other words, we suggest that UTP mediates the early stage
of osteogenic and adipogenic differentiation of BMSCs via
activation of the ERK1/2 signaling pathway. We believe that the
reverse effect of ERK1/2 signaling may be a possible interpretation
for contrary views widely reported in the literature and may
provide new insight into the molecular regulation of the osteogenic
differentiation of rat BMSCs. The exact downstream components of
the signaling pathway remain unknown. Thus, we have great interest
in researching them in future studies.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301552).
Abbreviations:
|
UTP
|
uridine triphosphate
|
|
BMSCs
|
bone marrow-derived stromal cells
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
References
|
1
|
Raisz LG: Pathogenesis of osteoporosis:
concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hess R, Pino AM, Ríos S, Fernández M and
Rodríguez JP: High affinity leptin receptors are present in human
mesenchymal stem cells (MSCs) derived from control and osteoporotic
donors. J Cell Biochem. 94:50–57. 2005. View Article : Google Scholar
|
|
3
|
Dalle Carbonare L, Valenti MT, Zanatta M,
Donatelli L and Lo Cascio V: Circulating mesenchymal stem cells
with abnormal osteogenic differentiation in patients with
osteoporosis. Arthritis Rheum. 60:3356–3365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egermann M, Heil P, Tami A, Ito K, Janicki
P, Von Rechenberg B, Hofstetter W and Richards PJ: Influence of
defective bone marrow osteogenesis on fracture repair in an
experimental model of senile osteoporosis. J Orthop Res.
28:798–804. 2010.
|
|
5
|
Nuttall ME and Gimble JM: Controlling the
balance between osteoblastogenesis and adipogenesis and the
consequent therapeutic implications. Curr Opin Pharmacol.
4:290–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Post S, Abdallah BM, Bentzon JF and Kassem
M: Demonstration of the presence of independent pre-osteoblastic
and pre-adipocytic cell populations in bone marrow-derived
mesenchymal stem cells. Bone. 43:32–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barry FP and Murphy JM: Mesenchymal stem
cells: clinical applications and biological characterization. Int J
Biochem Cell Biol. 36:568–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maurin AC, Chavassieux PM, Frappart L,
Delmas PD, Serre CM and Meunier PJ: Influence of mature adipocytes
on osteoblast proliferation in human primary cocultures. Bone.
26:485–489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan Y, Chong LW and Evans RM: PPAR-gamma
regulates osteoclastogenesis in mice. Nat Med. 13:1496–1503. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robling AG, Hinant FM, Burr DB and Turner
CH: Improved bone structure and strength after long-term mechanical
loading is greatest if loading is separated into short bouts. J
Bone Miner Res. 17:1545–1554. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duncan RL and Turner CH:
Mechanotransduction and the functional response of bone to
mechanical strain. Calcif Tissue Int. 57:344–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoebertz A, Arnett TR and Burnstock G:
Regulation of bone resorption and formation by purines and
pyrimidines. Trends Pharmacol Sci. 24:290–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riddle RC, Taylor AF, Rogers JR and
Donahue HJ: ATP release mediates fluid flow-induced proliferation
of human bone marrow stromal cells. J Bone Miner Res. 22:589–600.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rumney RM, Sunters A, Reilly GC and
Gartland A: Application of multiple forms of mechanical loading to
human osteoblasts reveals increased ATP release in response to
fluid flow in 3D cultures and differential regulation of immediate
early genes. J Biomech. 45:549–554. 2012. View Article : Google Scholar :
|
|
16
|
Ferrari D, Gulinelli S, Salvestrini V,
Lucchetti G, Zini R, Manfredini R, Caione L, Piacibello W,
Ciciarello M, et al: Purinergic stimulation of human mesenchymal
stem cells poten-tiates their chemotactic response to CXCL12 and
increases the homing capacity and production of proinflammatory
cytokines. Exp Hematol. 39:360–374. 2011. View Article : Google Scholar
|
|
17
|
Noronha-Matos JB, Costa MA,
Magalhães-Cardoso MT, Ferreirinha F, Pelletier J, Freitas R, Neves
JM, Sévigny J and Correia-de-Sá P: Role of ecto-NTPDases on
UDP-sensitive P2Y(6) receptor activation during osteogenic
differentiation of primary bone marrow stromal cells from
postmenopausal women. J Cell Physiol. 227:2694–2709. 2012.
View Article : Google Scholar
|
|
18
|
Zippel N, Limbach CA, Ratajski N, Urban C,
Luparello C, Pansky A, Kassack MU and Tobiasch E: Purinergic
receptors influence the differentiation of human mesenchymal stem
cells. Stem Cells Dev. 21:884–900. 2012. View Article : Google Scholar
|
|
19
|
Erlinge D and Burnstock G: P2 receptors in
cardiovascular regulation and disease. Purinergic Signal. 4:1–20.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burnstock G: Purinergic signalling: past,
present and future. Braz J Med Biol Res. 42:3–8. 2009. View Article : Google Scholar
|
|
21
|
Abbracchio MP, Burnstock G, Boeynaems JM,
Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C,
Jacobson KA and Weisman GA: International Union of Pharmacology
LVIII: update on the P2Y G protein-coupled nucleotide receptors:
from molecular mechanisms and patho-physiology to therapy.
Pharmacol Rev. 58:281–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ayala-Peña VB, Scolaro LA and Santillán
GE: ATP and UTP stimulate bone morphogenetic protein-2,-4 and -5
gene expression and mineralization by rat primary osteoblasts
involving PI3K/AKT pathway. Exp Cell Res. 319:2028–2036. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orriss IR, Utting JC, Brandao-Burch A,
Colston K, Grubb BR, Burnstock G and Arnett TR: Extracellular
nucleotides block bone mineralization in vitro: evidence for dual
inhibitory mechanisms involving both P2Y2 receptors and
pyrophosphate. Endocrinology. 148:4208–4216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orriss IR, Knight GE, Ranasinghe S,
Burnstock G and Arnett TR: Osteoblast responses to nucleotides
increase during differentiation. Bone. 39:300–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoebertz A, Mahendran S, Burnstock G and
Arnett TR: ATP and UTP at low concentrations strongly inhibit bone
formation by osteoblasts: a novel role for the P2Y2 receptor in
bone remodeling. J Cell Biochem. 86:413–419. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SC, Vielhauer NS, Leaver EV and
Pappone PA: Differential regulation of ca(2+) signaling and
membrane trafficking by multiple p2 receptors in brown adipocytes.
J Membr Biol. 207:131–142. 2005. View Article : Google Scholar
|
|
27
|
Lee H, Jun DJ, Suh BC, Choi BH, Lee JH, Do
MS, Suh BS, Ha H and Kim KT: Dual roles of P2 purinergic receptors
in insulin-stimulated leptin production and lipolysis in
differentiated rat white adipocytes. J Biol Chem. 280:28556–28563.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sreejit P, Dilip KB and Verma RS:
Generation of mesenchymal stem cell lines from murine bone marrow.
Cell Tissue Res. 350:55–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barragán-Iglesias P, Mendoza-Garcés L,
Pineda-Farias JB, Solano-Olivares V, Rodríguez-Silverio J,
Flores-Murrieta FJ, Granados-Soto V and Rocha-González HI:
Participation of peripheral P2Y1, P2Y6 and P2Y11 receptors in
formalin-induced inflammatory pain in rats. Pharmacol Biochem
Behav. 128:23–32. 2015. View Article : Google Scholar
|
|
30
|
Rodrigues-Ribeiro R, Alvarenga EC, Calio
ML, Paredes-Gamero EJ and Ferreira AT: Dual role of P2 receptors
during osteoblast differentiation. Cell Biochem Biophys.
71:1225–1233. 2015. View Article : Google Scholar
|
|
31
|
Orriss IR, Knight GE, Utting JC, Taylor
SE, Burnstock G and Arnett TR: Hypoxia stimulates vesicular ATP
release from rat osteoblasts. J Cell Physiol. 220:155–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yong Y, Ming ZD, Feng L, Chun ZW and Hua
W: Electromagnetic fields promote osteogenesis of rat mesenchymal
stem cells through the PKA and ERK1/2 pathways. J Tissue Eng Regen
Med. Mar 16–2014.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Yan M, Wang Z, Zheng Y, Li J, Ma S,
Liu G and Yu J: 17beta-estradiol promotes the odonto/osteogenic
differentiation of stem cells from apical papilla via
mitogen-activated protein kinase pathway. Stem Cell Res Ther.
5:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu FF, Zhu H, Li XM, Yang F, Chen JD, Tang
B, Sun HG, Chu YN, Zheng RX, Liu YL, et al: Intercellular adhesion
molecule-1 inhibits osteogenic differentiation of mesenchymal stem
cells and impairs bio-scaffold-mediated bone regeneration in vivo.
Tissue Eng Part A. 20:2768–2782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu D, Yi C, Fong CC, Jin Q, Wang Z, Yu
WK, Sun D, Zhao J and Yang M: Activation of multiple signaling
pathways during the differentiation of mesenchymal stem cells
cultured in a silicon nanowire microenvironment. Nanomedicine
(Lond). 10:1153–1163. 2014.
|
|
36
|
Yu Y, Wang L, Yu J, Lei G, Yan M, Smith G,
Cooper PR, Tang C, Zhang G and Smith AJ: Dentin matrix proteins
(DMPs) enhance differentiation of BMMSCs via ERK and P38 MAPK
pathways. Cell Tissue Res. 356:171–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashimoto R, Katoh Y, Miyamoto Y, Itoh S,
Daida H, Nakazato Y and Okada T: Increased extracellular and
intracellular Ca2+ lead to adipocyte accumulation in
bone marrow stromal cells by different mechanisms. Biochem Biophys
Res Commun. 457:647–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kusuyama J, Bandow K, Shamoto M, Kakimoto
K, Ohnishi T and Matsuguchi T: Low intensity pulsed ultrasound
(LIPUS) influences the multilineage differentiation of mesenchymal
stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK
signaling pathway. J Biol Chem. 289:10330–10344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang YY, Li X, Qian SW, Guo L, Huang HY,
He Q, Liu Y, Ma CG and Tang QQ: Down-regulation of type I Runx2
mediated by dexamethasone is required for 3T3-L1 adipogenesis. Mol
Endocrinol. 26:798–808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Naito M, Omoteyama K, Mikami Y, Takahashi
T and Takagi M: Inhibition of Wnt/β-catenin signaling by
dexamethasone promotes adipocyte differentiation in mesenchymal
progenitor cells, ROB-C26. Histochem Cell Biol. 138:833–845. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Menuki K, Mori T, Sakai A, Sakuma M,
Okimoto N, Shimizu Y, Kunugita N and Nakamura T: Climbing exercise
enhances osteoblast differentiation and inhibits adipogenic
differentiation with high expression of PTH/PTHrP receptor in bone
marrow cells. Bone. 43:613–620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ciciarello M, Zini R, Rossi L, Salvestrini
V, Ferrari D, Manfredini R and Lemoli RM: Extracellular purines
promote the differentiation of human bone marrow-derived
mesenchymal stem cells to the osteogenic and adipogenic lineages.
Stem Cells Dev. 22:1097–1111. 2013. View Article : Google Scholar :
|
|
43
|
Schödel J, Weise I, Klinger R and Schmidt
M: Stimulation of lipogenesis in rat adipocytes by ATP, a ligand
for P2-receptors. Biochem Biophys Res Commun. 321:767–773. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kubota N, Terauchi Y, Miki H, Tamemoto H,
Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, et
al: PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy
and insulin resistance. Mol Cell. 4:597–609. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kawai M and Rosen CJ: PPARγ: a circadian
transcription factor in adipogenesis and osteogenesis. Nat Rev
Endocrinol. 6:629–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Costessi A, Pines A, D'Andrea P, Romanello
M, Damante G, Cesaratto L, Quadrifoglio F, Moro L and Tell G:
Extracellular nucleotides activate Runx2 in the osteoblast-like
HOBIT cell line: a possible molecular link between mechanical
stress and osteoblasts' response. Bone. 36:418–432. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Katz S, Boland R and Santillán G:
Modulation of ERK 1/2 and p38 MAPK signaling pathways by ATP in
osteoblasts: involvement of mechanical stress-activated calcium
influx, PKC and Src activation. Int J Biochem Cell Biol.
38:2082–2091. 2006. View Article : Google Scholar : PubMed/NCBI
|