Introduction
Drowning is a cause of accidental mortality.
However, survival of the initial apnoea may result in acute lung
injury (ALI) (1). The symptoms of
near drowning depend on the composition (2) and quantity of water aspirated
(3) and it is well known that ALI
induced by seawater (SW-ALI) is more severe than that of fresh
water. Additionally, SW-ALI is more complicated than ordinary ALI
because hypertonic stimulation is unique for seawater-induced lung
injury compared with other types of ALI (4). Seawater inspiration may result in
direct and indirect disorders, such as histological changes,
alveolar epithelial cell apoptosis and injuries associated with its
physical properties (5,6). Following direct injury, the
infiltration of inflammatory cells may aggravate lung damage and
the stimulated inflammatory cells release various molecules such as
pro-inflammatory cytokines, reactive oxygen species and proteases,
exacerbating the pulmonary inflammatory process (7). Therefore, it may be beneficial for
SW-ALI to inhibit inflammation following seawater inspiration.
Mounting evidence shown that nuclear factor-κB
(NF-κB) is a crucial transcriptional factor in regulating
inflammatory factors and plays a key role in the initiation and
amplification of immune and inflammatory responses (8). In most cells, the primary form of
NF-κB, consisting of a heterodimer of p50 and p65, localizes in the
cytoplasm and binds to inhibitory proteins known as IκB families.
Once stimulated by activators, such as LPS, tumor necrosis factor α
(TNF-α), IL-1 and IκBs, particularly IκBα may be completely
depredated in minutes (9).
Released NF-κB translocates into the nucleus and binds to a variety
of associated target DNA sequences known as κB sites to modulate
the associated gene expression.
Nitric oxide synthases (NOS), first reported by
Bredt and Snyder (10) comprise
the neuronal NOS (n-NOS), endothelial NOS (e-NOS) and inducible NOS
(i-NOS) isoforms. The n-NOS and e-NOS are continuously expressed,
whereas i-NOS expression is induced in organs (11). Once induced, i-NOS may lead to the
upregulation of nitric oxide (NO) at a relatively constant rate for
a long period of time. Excessive production of NO plays a crucial
role in the process of inflammatory disease. Furthermore, several
transcription factors were involved in i-NOS gene expression and
NF-κB is considered an essential factor (12,13).
Resveratrol (3,5,4′-trihydroxy-trans-stilbene, Res)
is a poly phenolic compound, which is a phytoalexin synthesized by
a wide variety of plant species. Res has broad biological and
pharmacological functions, such as cardioprotection (14), liver protection (15), radiation protection (16), anti-cancer (17), anti-aging (18), anti-oxidation (19) and neuro-protection (20). Therefore, interest of the
scientific community in resveratrol has substantially increased.
However, owing to its poor pharmacokinetic, bio-availability
properties and short half-life of 8-14 min, resveratrol cannot be
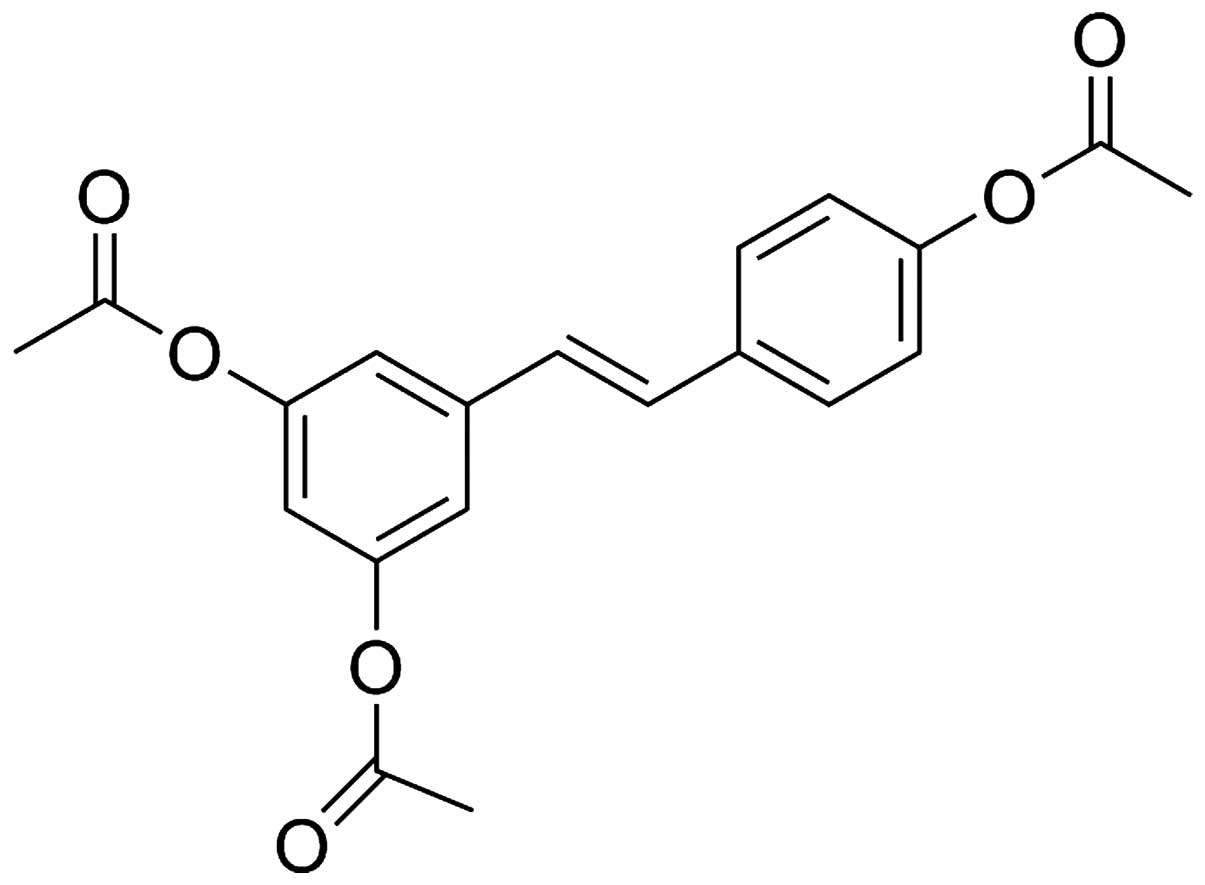
used as an injury protective drug (21). 3,5,4′-tri-O-acetylresveratrol
(AC-Res) (Fig. 1), obtained by
the complete acetylation of resveratrol, is a prodrug of
resveratrol. Previous findings confirmed that AC-Res triggers the
accumulation and concentration of resveratrol in lung (22).
Based on the abovementioned findings, we
hypothesized and proved that the abnormal expression of NF-κB and
i-NOS, followed by enhanced inflammatory response, played a
critical role in SW-ALI. Additionally, the pretreatment of AC-Res
may improve the outcome of SW-ALI through interference of the NF-κB
and i-NOS pathways.
Materials and methods
Reagents
AC-Res with an HPLC purity of >99%, was obtained
from Department of Medicinal Chemistry, School of Pharmacy, Fourth
Military Medical University (Xi'an, Shaanxi, China). Seawater
(osmolality 1,300 mmol/l, pH 8.2, SW 1.05, NaCl2 6.518
g/l, MgSO4 3.305 g/l, MgCl2 2.447 g/l,
CaCl2 1.141 g/l, KCl 0.725 g/l, NaHCO3 0.202
g/l, NaBr 0.083 g/l) was prepared as per the composition of the
East China Sea provided by the Chinese Ocean Bureau. Additionally,
seawater was filtered prior to inspiration by the animals.
Enzyme-linked immune adsorbent assay (ELISA) kits of TNF-α and
interleukin-1 β (IL-1β) were purchased from R&D Systems Inc.
(Minneapolis, MN, USA). The NO assay kit was obtained from
Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China).
Anti-pNF-κB p65, anti-i-NOS and anti-β-actin monoclonal antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Other chemicals were of high purity and were used without
purification.
Animals
Male adult Sprague-Dawley (SD) rats, weighing
180–220 g each, were obtained from the Animal Center of the Fourth
Military Medical University. The rats were acclimatized to their
environment for 1 week and housed in air-filtered,
temperature-controlled units with free access to standard rodent
chow and water. The experimental protocols were approved by the
Animal Care and Use Committee of the Fourth Military Medical
University and all experiments complied with the Declaration of the
National Institutes of Health Guide for Care and Use of Laboratory
Animals (publication no. 85–23, revised 1985).
Experimental group
Thirty-two SD rats were randomly divided into four
groups. Rats in the control (Ct) group were treated without any
intervention. In the seawater inhalation (SW) group, seawater
inhalation-induced ALI models were established in rats. Briefly,
the rats were anesthetized with pentobarbital sodium [100 mg/kg of
body weight, administered intraperitoneally (i.p.)] and maintained
in the supine position during the experiment with the head elevated
at 30°. A syringe (1 ml) was inserted into the trachea and seawater
(4 ml/kg) was instilled at a steady speed within 4 min into the
lungs.
Rats from the seawater inhalation plus
3,5,4′-tri-O-acetyl-resveratrol pretreatment (SW + AC-Res) group
were pretreated with AC-Res (50 mg/kg body weight) for 7 days prior
to modeling. SW-ALI models were established 90 min after the
previous administration of AC-Res and the modeling process was the
same as that of the SW group.
In the fourth group, the rats were treated with 50
mg/kg AC-Res for 7 days. These rats were euthanized 90 min after
the final administration of AC-Res.
The rats that suffered from seawater stimulation
were anesthetized and exsanguinated through aortic transection 4 h
after modeling. The lungs were removed rapidly from the thoraxes
and processed as described below.
Histologic examination
The rats were sacrificed 4 h after seawater exposure
(n=8 in each group). The same right lower lung lobes from all the
animals were preserved in 4% formalin for 24 h and then embedded in
paraffin. Lung tissues were cut into 5 µm sections with a
microtome and stained with hematoxylin and eosin after
deparaffinisation and dehydration. Microscopic evaluation was
performed to characterize the lung injury.
Wet to dry weight ratio of lung
samples
In order to quantify the magnitude of pulmonary
edema, we evaluated the wet to dry weight ratios of the lung
samples from each group. Samples were obtained 4 h after seawater
instillation and weighed immediately after removal prior to being
subjected to desiccation in an oven at 70°C until a stable dry
weight was achieved after 72 h. Wet to dry weight ratios were
calculated to evaluate the level of pulmonary edema.
Bronchoalveolar lavage fluid (BALF)
analysis
Lungs were removed intact and lavaged with 7 ml
ice-cold phosphate-buffered saline (PBS) 5 times 4 h after seawater
inhalation. The recover ratio of PBS was >90%. BALF from each
group was centrifuged at 500 × g for 10 min at 4°C.
The protein content in the supernatant was
determined by measuring the absorbance at 630 nm following the
addition of bromocresol green. The values were calculated according
to a standard curve and data were presented as µg protein/ml
(µg/ml).
The cell pellet was resuspended in 1 ml of red blood
cell-lysis buffer to eliminate red cells. White cells were then
re-pelleted by centrifugation at 520 × g for 20 min at 4°C. The
cell pellet was again resuspended in PBS and cells were counted
using a hemocytometer (XFA6100; Perlong Medical Equipment Co.,
Ltd., Nanjing, China).
Evaluation of vascular permeability
Vascular permeability was evaluated by extravasation
of the Evans blue dye into lung tissues. Briefly, Evans blue (20
mg/kg) was administered intravenously (1 ml/kg) via a tail vein
prior to modeling. Aliquots of lung tissues from each animal were
dried in an oven for 24 h at 37°C. Evans blue was then extracted in
1 ml formamide for 24 h at room temperature. Evans blue contents
were quantified by measuring absorbance at 620 nm and the
concentration of Evans blue was calculated according to a standard
curve (1–100 mg/ml). The results were presented as the amount of
Evans blue µg/g tissue.
NO content in lung
NO content, assessed by determining NO2−
concentration in the plams of the lung tissues, reflected the
degree of lung damage and expression of i-NOS. Briefly, the lung
tissue samples were homogenized in cool normal saline (lung tissue
to normal saline, 1:10). The homogenate was assessed according to
the manufacturer's instructions. NO content was measured by
absorbance at 550 nm and expressed as mmol/mg protein.
Measurement of cytokines
TNF-α and IL-1β content were evaluated in the lung
tissues collected at 4 h after seawater instillation. Briefly,
portions of lung tissues were homogenized in cool
phosphate-buffered saline (lung tissue to normal saline, 1:5). The
assay was carried out using a commercially available ELISA kit,
according to the manufacturer's instructions.
Cell culture and treatment
The NR8383 alveolar macrophage cell line, obtained
from the American Type Culture Collection (ATCC, Rockville, MD,
USA), was maintained in Ham's F12 medium supplemented with 10%
fetal calf serum, 100 U/ml of penicillin and 100 µg/ml of
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2 and 95% air. The cells were stimulated with seawater
(25%) for 4 h with or without resveratrol (40 µg/ml)
pretreatment. The total protein was extracted from the cells to
evaluate the expression of p-65NF-κB and i-NOS on a protein level.
Additionally, cytokines and NO content in medium were tested by
ELISA and nitrate reductive enzymatic method, and the results were
expressed as pg/ml and µmol/l, respectively.
Western blot analysis
Lungs were perfused to remove the blood cells from
pulmonary circulation. Tissue samples from each group were
collected and the total proteins were extracted. Proteins from the
lung samples and cells were quantified using a BCA protein assay
kit. Subsequently, 100 µg protein from each group was boiled
in loading buffer. The proteins from each group were loaded and
separated on a 12% SDS-PAGE gel, transferred to nitrocellulose
membranes, blocked with 5% non-fat dry milk in Tris-buffered saline
with Tween-20 and probed overnight at 4°C with antibodies against
p65NF-κB (1:1,000), i-NOS (1:500) and β-actin (1:5,000). The
secondary antibody (anti-rabbit IgG peroxidase conjugated,
1:10,000) was incubated and the relative content of the target
proteins was detected by the enhanced chemiluminescent detection
system according to the manufacturer's protocol.
Statistical analysis
Data were presented as means ± SD. Statistical
analysis was performed with analysis of variance. Statistically
significant differences between groups were identified using
one-way ANOVA followed by the Dunnett's test. A statistical
difference was accepted as significant if P<0.05.
Results
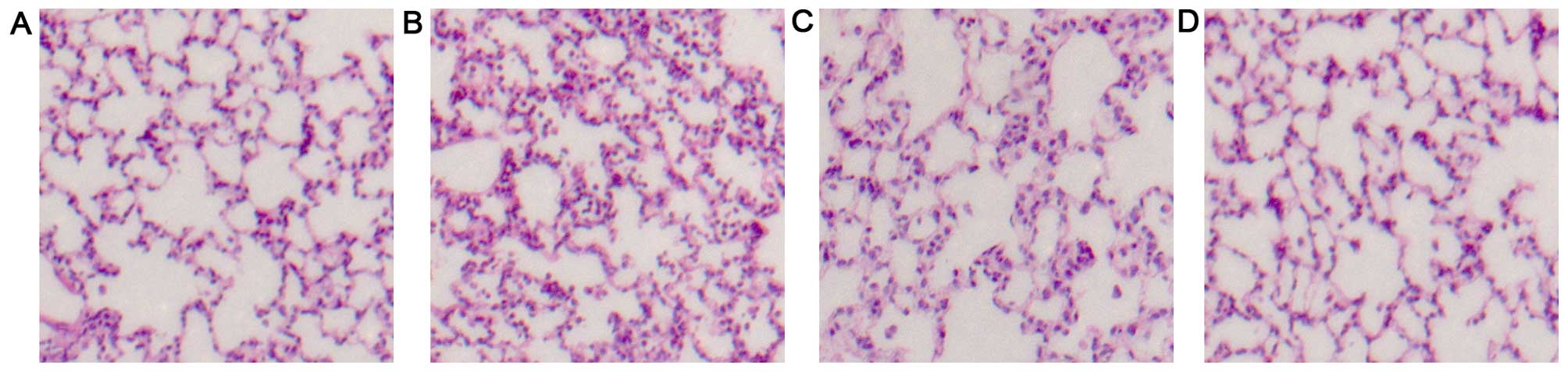
Effects of AC-Res on histopathological
changes
No histological alteration was observed in the lung
tissues in the Ct group (Fig.
2A). Alveolar damage, pulmonary edema and infiltration of
inflammatory cells in the lung tissues and alveoli were evident 4 h
after seawater instillation (Fig.
2B). AC-Res significantly reduced pulmonary edema and alveolar
damage and inhibited inflammatory cells and blood cell infiltration
(Fig. 2C).
Effects of AC-Res on lung edema
To observe lung edema resulting from seawater
inhalation and the protective effects of AC-Res, we measured the
lung wet to dry weight ratios of the tissues from each group
(Fig. 3). The ratios were
significantly increased in the seawater exposure group compared
with the control group (n=8, P<0.05). Compared with SW, the wet
to dry ratio of the AC-Res group was significantly decreased
(P<0.05).
Effects of AC-Res on vascular
permeability
Vascular permeability was evaluated to determine the
reason for lung edema and to assess the effects of AC-Res (Fig. 4). The results showed that seawater
stimulation increased the protein content in BALF (Fig. 4A), enhanced Evans blue dye and
cell infiltration from blood vessels to lung tissues (Fig. 4B and C). By contrast, AC-Res
pretreatment reduced the protein content in BALF and inhibited
Evans blue and cell infiltration (n=8, P<0.05).
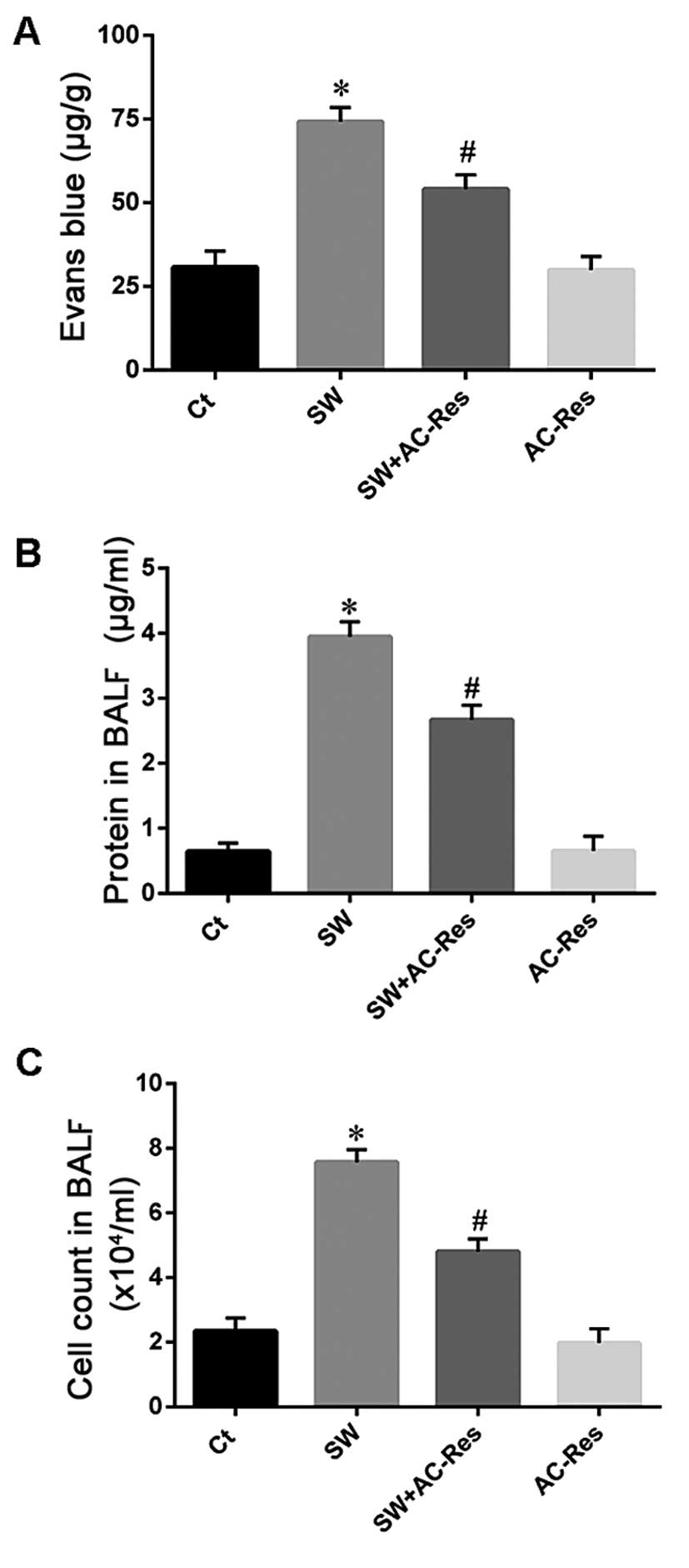 | Figure 4Effects of AC-Res on (A) Evans blue,
(B) protein and (C) cell infiltration from blood vessels to lung
tissues. *P<0.01 vs. Ct; #P<0.01 vs.
*P. Ct, control group; SW, seawater inhalation group; SW
+ AC-Res, seawater inhalation plus 3,5,4′-tri-O-acetylresveratrol
pretreatment group; AC-Res, 3,5,4′-tri-O-acetylresveratrol group.
Data are presented as means ± SD, n=8. |
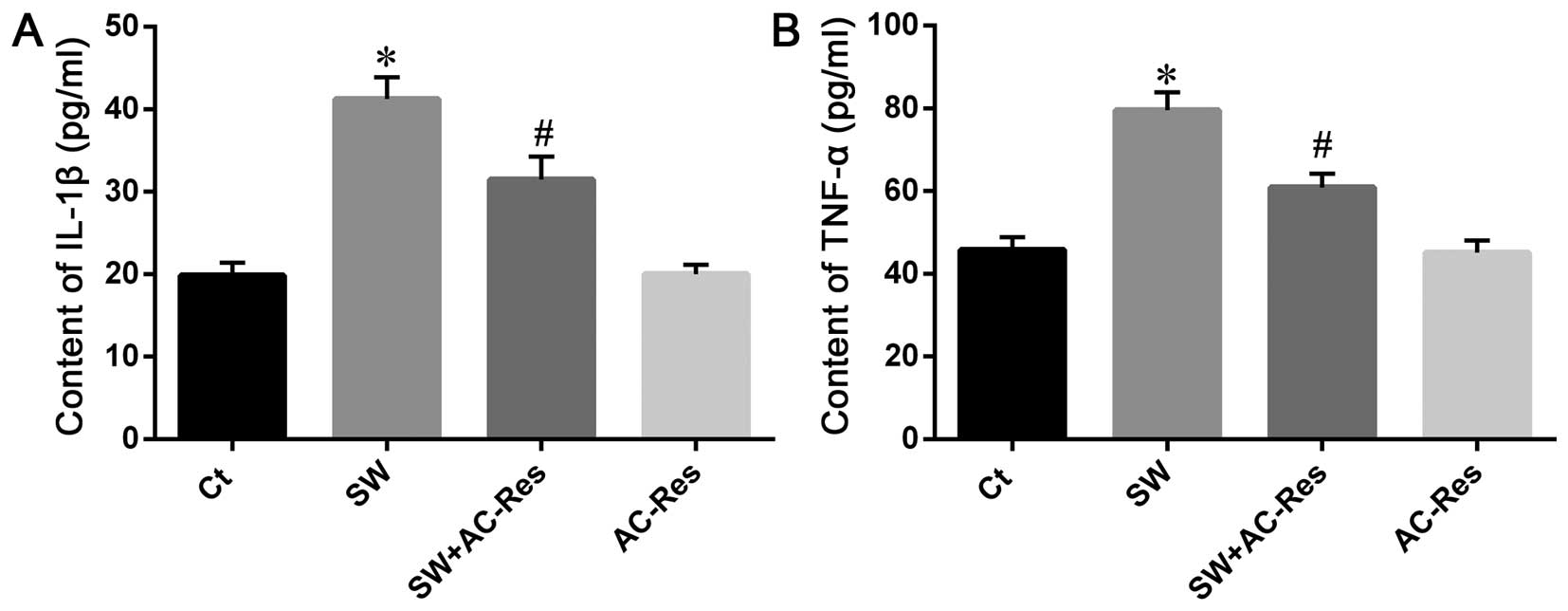
AC-Res attenuates seawater resulted in
inflammation
To determine the reason for the increase in vascular
permeability, inflammation in lung samples was examined. The
contents of TNF-α and IL-1β in lung tissues were tested using ELISA
(Fig. 5). The results showed that
seawater inspiration increased inflammatory TNF-α and IL-1β in lung
tissues (n=8, P<0.05). By contrast, AC-Res treatment reduced the
seawater stimulation, resulting in cell infiltration and TNF-α and
IL-1β in lungs stimulated by seawater.
The results from the NR8383 cells showed that
seawater stimulation increased TNF-α and IL-1β (Fig. 6) secretion. AC-Res treatment
inhibited the release of those cytokines (P<0.05).
AC-Res reduced NO content
Since NO is associated with inflammation and plays
an important role in the body, we investigated the effects of
seawater on NO content and whether AC-Res attenuated that effect
(Fig. 7A). The results showed
that seawater inhalation increased the NO production (n=8,
P<0.05), while AC-Res pre-treatment decreased NO content
compared with that of SW (n=8, P<0.05).
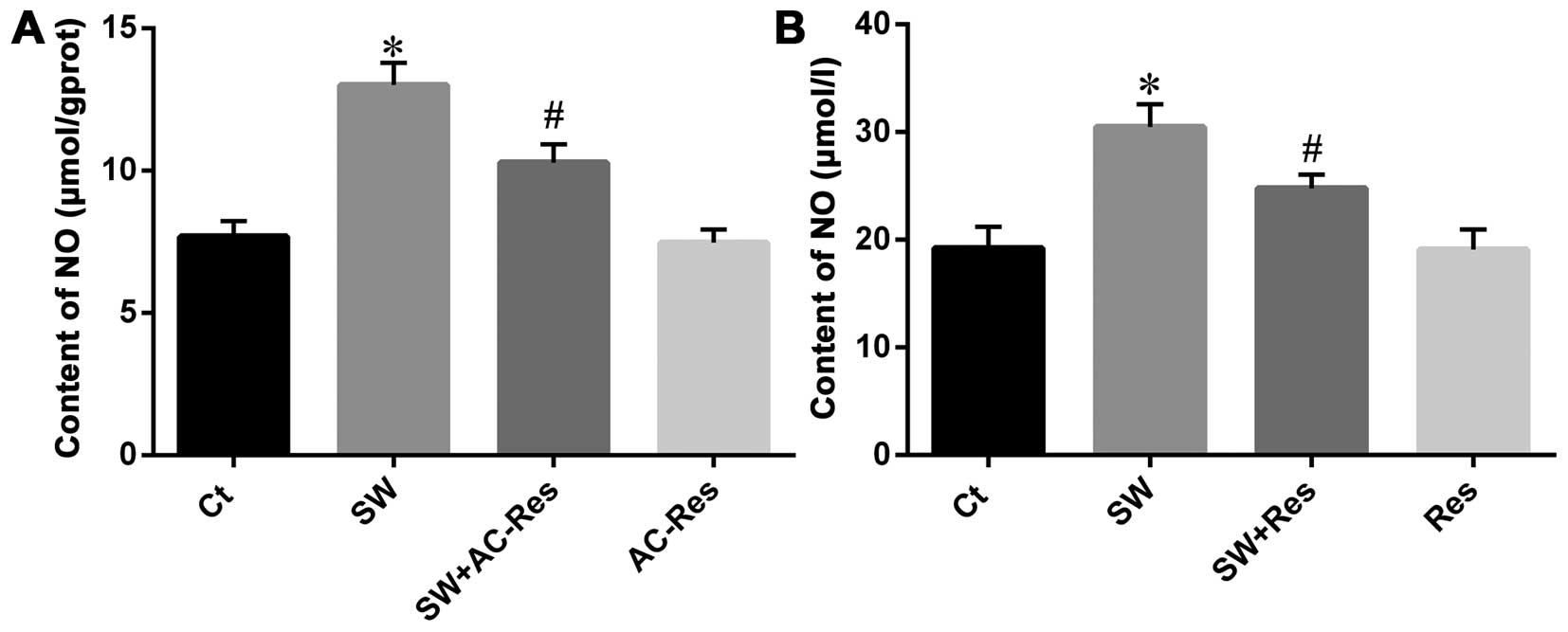 | Figure 7Effects of AC-Res and resveratrol on
NO content in (A) lung tissues and (B) NR8383 cells. Data are
presented as means ± SD, n=8. *P<0.01 vs. Ct;
#P<0.01 vs. *P. Ct, control group; SW,
seawater inhalation group; SW + AC-Res, seawater inhalation plus
AC-Res pretreatment group; AC-Res, 3,5,4′-tri-O-acetylresveratrol
group, SW + Res, resveratrol pretreatment plus seawater inhalation
group; Res, resveratrol group. |
On the other hand, NO content in medium
significantly increased when NR8383 cells were challenged with
seawater (Fig. 7B). In addition,
AC-Res pre-treatment reduced NO production (P<0.05).
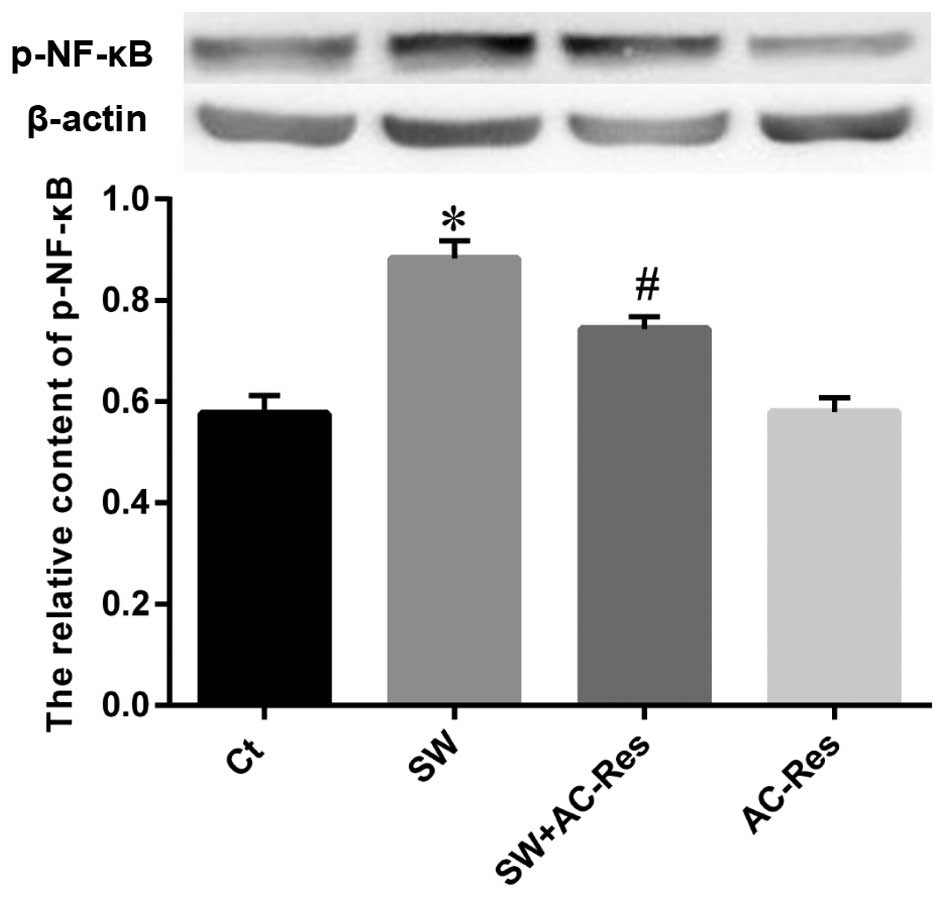
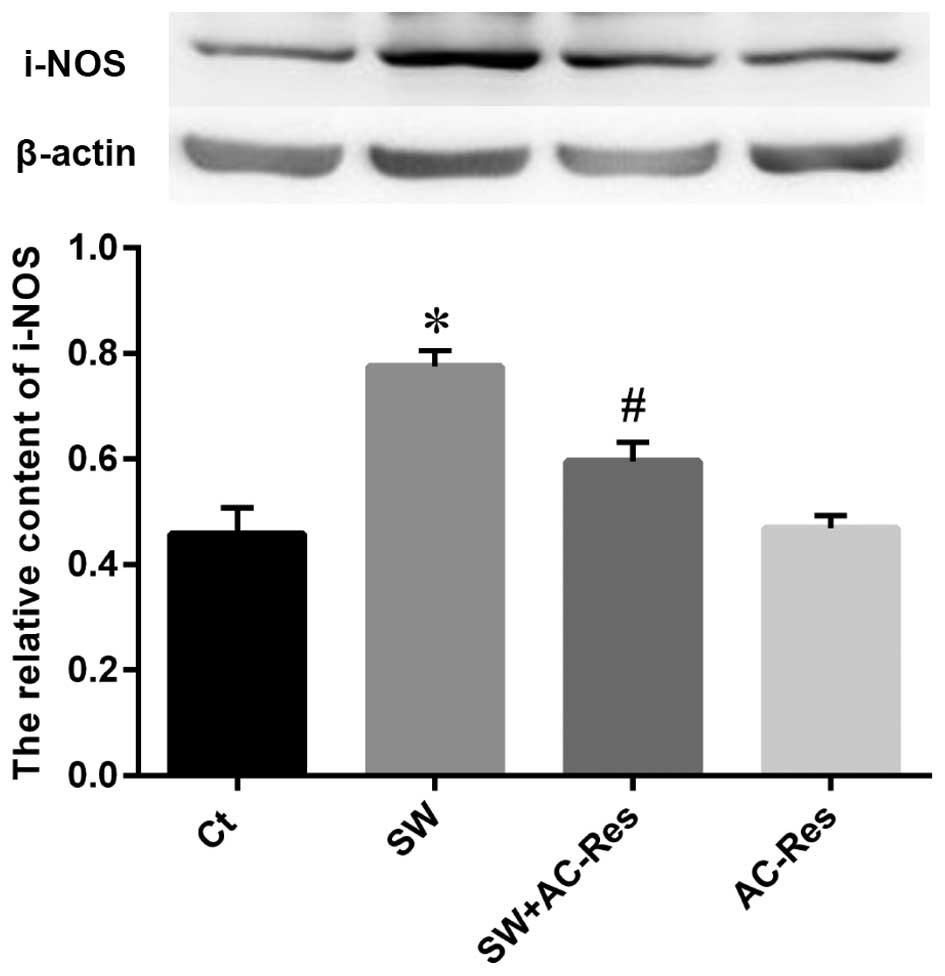
Effects of AC-Res on p-65 NF-κB and i-NOS
expression in lung
Western blot analysis was performed to examine the
status of p-65 NF-κB and i-NOS expression as well as the effects of
AC-Res. The results (Figs. 8 and
9) showed that seawater
instillation significantly upregulated the expression of p-65 NF-κB
and i-NOS (P<0.05), while the pretreatment of AC-Res reversed
the trend and decreased p-65 NF-κB and i-NOS at the protein level
(P<0.05).
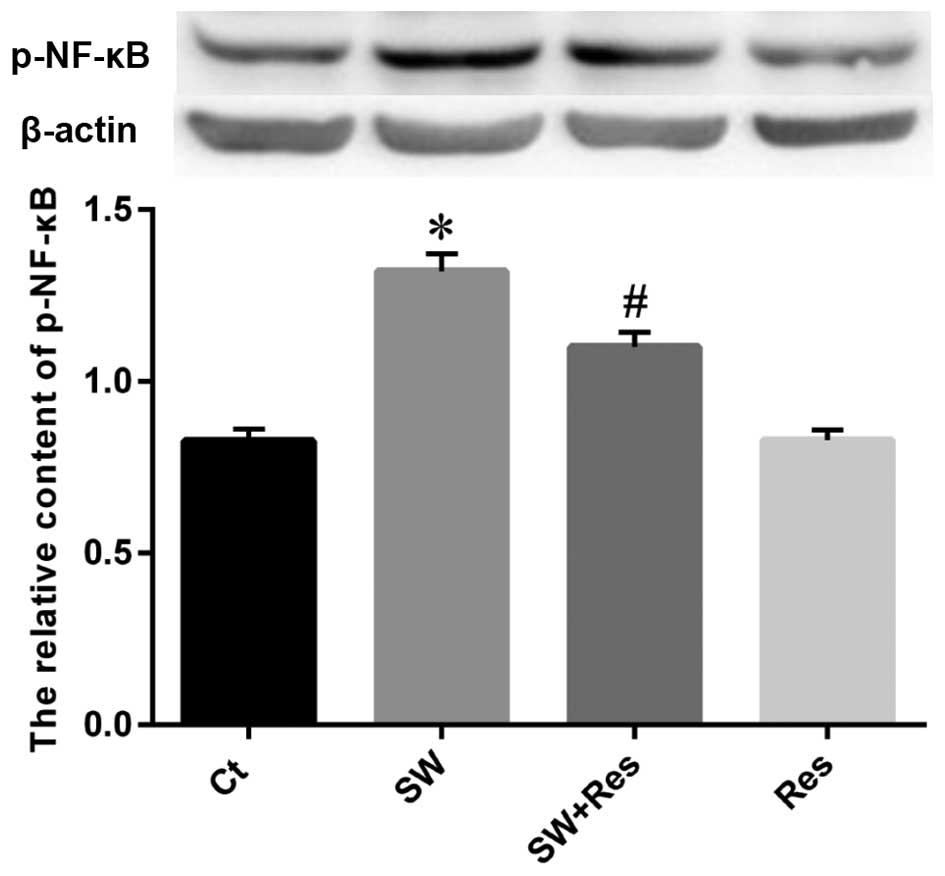
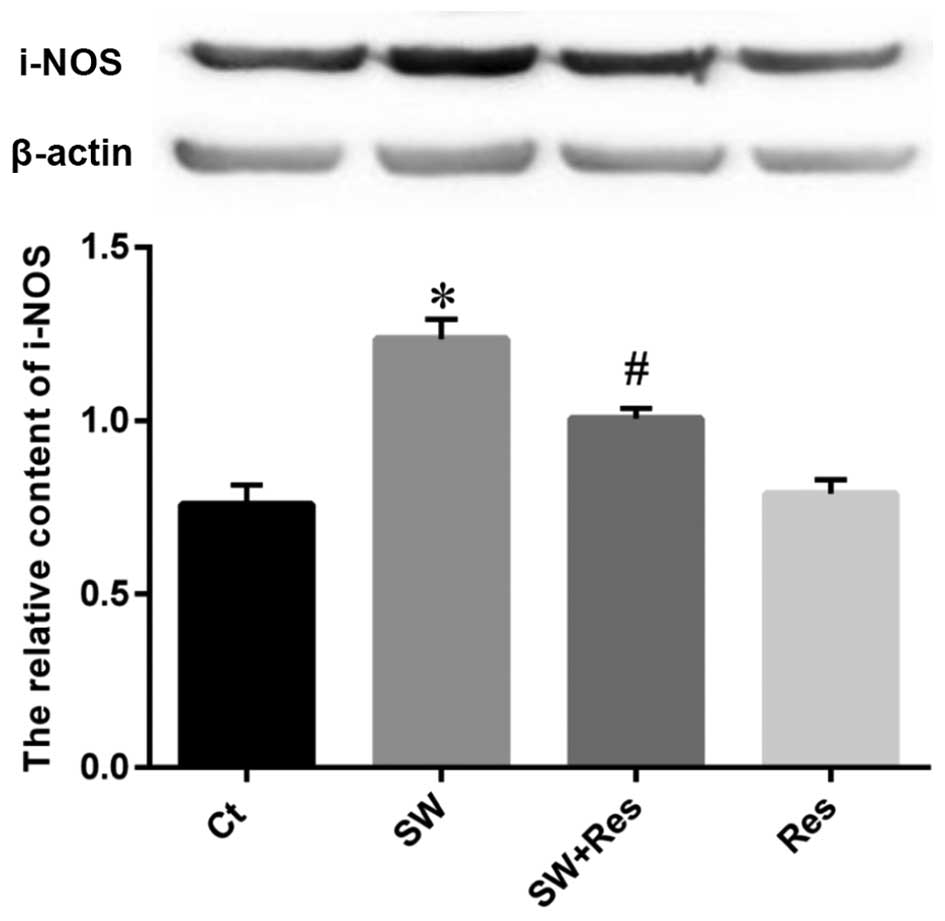
Similarly, the p-65 NF-κB and i-NOS expression
levels were enhanced in the NR8383 cells following stimulation with
seawater (Figs. 10 and 11). AC-Res inhibited the expression of
p-65 NF-κB and i-NOS in cells challenged with seawater
(P<0.05).
Discussion
Drowning is one of the most frequent causes of
accidental mortality, while the mortality of near-drowning is 9–12%
according to a large series of studies on near-drowning (23). Victims surviving the near-drowning
process, may suffer ALI characterized by alveolar collapse,
atelectasis and intrapulmonary shunting. Seawater
instillation-related lung damage is much more adverse than that of
fresh water (24). Therefore, in
the present study, we selected rats as experimental models, which
survived seawater intratracheal instillation, to examine the
potential mechanism of SW-ALI and the effects of AC-Res on it.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a
polyphenolic compound, which is a phytoalexin synthesized by a wide
variety of plant species. Resveratrol has a number of
pharmacological activities, including anti-oxidation (19) and anti-inflammation (25). However, resveratrol has
limitations. For example, it is not stable with a short half-life
and low bioavailability (21).
Resveratrol's analog, AC-Res, with three hydroxyls replaced by
acetyls, may overcome that disadvantage to some extent. Evidence
has demonstrated that it exerted anti-γ-irradiation effects by
inhibiting the expression of reactive oxygen species (ROS)
(26). Additionally, in a
previous study it was demonstrated that acetylated resveratrol
attenuated SW-ALI by interfering with intracellular communication
(27) and the HIF-1α pathway
(28). The aim of the present
study was to examine the values of the NF-κB and i-NOS pathways in
SW-ALI and the effects of AC-Res in seawater-stimulated lungs.
Following seawater inspiration into lungs, the high
osmolality caused osmotic fluid transport, driving fluid from the
vascular to alveolar space, which led to lung edema. Our previous
results proved that seawater inhalation led to an increased wet to
dry weight ratio of lung and an abnormal expression of
water-transporting proteins (29). Notably, the fluid accumulation in
alveolar space also impaired oxygen diffusion followed by severe
hypoxia, manifested by decreased PaO2, increased
PaCO2 (30,31) and upregulation of HIF-1α
expression (32). Pulmonary
inflammation may develop based on those changes and previous
evidence from our laboratory and other investigators have shown
that seawater increased cytokines and genes regulating
inflammation.
Evidence has suggested that NF-κB activation is a
crucial part of the pathological response that leads to the
development of organ dysfunction/injury. It has also been
consistently demonstrated, using an LPS model of septic shock, that
septic shock may be improved by blocking the NF-κB pathway, from
0–20% (LPS alone) to 80–100% (LPS plus NF-κB inhibitors) (33). On the other hand, as
NF-κB-dependent pro-inflammatory cytokines, TNF-α and IL-1β are
involved in lung injury by promoting endothelial cell permeability,
enhancing neutrophil recruitment and inducing further cytokine
secretion. Positive feedback between NF-κB and TNF-α and IL-1β may
also enhance the inflammatory response. In the present study, we
have demonstrated that seawater inspiration upregulated NF-κB
expression followed by increased TNF-α and IL-1β secretion, while
pretreatment with AC-Res inhibited the expression of NF-κB and
associated proinflammatory cytokines. By contrast,
seawater-stimulated NR8383 cells also increased NF-κB expression
and the secretion of TNF-α and IL-1β. Since AC-Res was altered
prior to exhibiting its pharmacological effects, resveratrol was
employed to determine the protective role of cells. The results
showed that NF-κB expression was upregulated followed by
enhancement of cytokine secretion, while resveratrol inhibited
NF-κB expression and reduced TNF-α and IL-1β secretion.
Evidence suggests that NF-κB regulated i-NOS
activation was responsible for generating NO (11,13). Furthermore, NO was closely
associated with inflammation (13,34). Previous findings have demonstrated
smoke inhalation increased i-NOS expression (35) while the de-regulation of i-NOS was
beneficial to Escherichia coli sepsis-induced lung
inflammation and injury (36). We
measured i-NOS expression, in the present study, to determine the
downstream signals of NF-κB and results from the SW-ALI models and
NR8383 cells showed that seawater inspiration significantly
increased i-NOS expression and NO generation followed by enhanced
inflammatory factor secretion. The pre-treatment of AC-Res was able
to reduce i-NOS expression and NO generation through the NF-κB
pathway. Similar results were obtained in seawater-stimulated
NR8383 cells with or without resveratrol pretreatment.
The NF-κB and i-NOS pathways were responsible for
SW-ALI. Seawater inhalation increased NF-κB expression followed by
the upregulation of i-NOS expression, NO formation and cytokine
secretion. However, the pretreatment of AC-Res inhibited activation
of the NF-κB and i-NOS pathways and attenuated lung edema and
pulmonary inflammation in lungs challenged with seawater.
Acknowledgments
This study was supported by grants from the Military
Key Projects in the 12th Five-year Plan of China (Project No.
WS13J043).
References
|
1
|
Xinmin D, Yunyou D, Chaosheng P, Huasong
F, Pingkun Z, Jiguang M, Zhiqian X and Qinzhi X: Dexamethasone
treatment attenuates early seawater instillation-induced acute lung
injury in rabbits. Pharmacol Res. 53:372–379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simcock AD: Treatment of near drowning - a
review of 130 cases. Anaesthesia. 41:643–648. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erickson SE, Martin GS, Davis JL and
Matthay MA: Recent trends in acute lung injury mortality:
1996–2005. Crit Care Med. 37:1574–1579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grünig G, Corry DB, Leach MW, Seymour BW,
Kurup VP and Rennick DM: Interleukin-10 is a natural suppressor of
cytokine production and inflammation in a murine model of allergic
bron-chopulmonary aspergillosis. J Exp Med. 185:1089–1099. 1997.
View Article : Google Scholar
|
|
5
|
Zhang M, Wang L, Dong M, Li Z and Jin F:
Endothelial Semaphorin 7A promotes inflammation in seawater
aspiration-induced acute lung injury. Int J Mol Sci.
15:19650–19661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gregorakos L, Markou N, Psalida V,
Kanakaki M, Alexopoulou A, Sotiriou E, Damianos A and Myrianthefs
P: Near-drowning: clinical course of lung injury in adults. Lung.
187:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakatani K, Takeshita S, Tsujimoto H,
Kawamura Y and Sekine I: Inhibitory effect of serine protease
inhibitors on neutrophil-mediated endothelial cell injury. J Leukoc
Biol. 69:241–247. 2001.PubMed/NCBI
|
|
8
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li DY, Xue MY, Geng ZR and Chen PY: The
suppressive effects of Bursopentine (BP5) on oxidative stress and
NF-κB activation in lipopolysaccharide-activated murine peritoneal
macrophages. Cell Physiol Biochem. 29:9–20. 2012. View Article : Google Scholar
|
|
10
|
Bredt DS and Snyder SH: Isolation of
nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl
Acad Sci USA. 87:682–685. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vaporidi K, Francis RC, Bloch KD and Zapol
WM: Nitric oxide synthase 3 contributes to ventilator-induced lung
injury. Am J Physiol Lung Cell Mol Physiol. 299:L150–L159. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kinaci MK, Erkasap N, Kucuk A, Koken T and
Tosun M: Effects of quercetin on apoptosis, NF-κB and NOS gene
expression in renal ischemia/reperfusion injury. Exp Ther Med.
3:249–254. 2012.PubMed/NCBI
|
|
13
|
Tabassum R, Vaibhav K, Shrivastava P, Khan
A, Ahmed ME, Ashafaq M, Khan MB and Islam F, Safhi MM and Islam F:
Perillyl alcohol improves functional and histological outcomes
against ischemia-reperfusion injury by attenuation of oxidative
stress and repression of COX-2, NOS-2 and NF-κB in middle cerebral
artery occlusion rats. Eur J Pharmacol. 747:190–199. 2015.
View Article : Google Scholar
|
|
14
|
Rivera L, Morón R, Zarzuelo A and Galisteo
M: Long-term resveratrol administration reduces metabolic
disturbances and lowers blood pressure in obese Zucker rats.
Biochem Pharmacol. 77:1053–1063. 2009. View Article : Google Scholar
|
|
15
|
Wang T, Zhou ZX, Sun LX, Li X, Xu ZM, Chen
M, Zhao GL, Jiang ZZ and Zhang LY: Resveratrol effectively
attenuates α-naphthyl-isothiocyanate-induced acute cholestasis and
liver injury through choleretic and anti-inflammatory mechanisms.
Acta Pharmacol Sin. 35:1527–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KO, Park H, Chun M and Kim HS:
Immunomodulatory effects of high-protein diet with resveratrol
supplementation on radiation-induced acute-phase inflammation in
rats. J Med Food. 17:963–971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seino M, Okada M, Shibuya K, Seino S,
Suzuki S, Takeda H, Ohta T, Kurachi H and Kitanaka C: Differential
contribution of ROS to resveratrol-induced cell death and loss of
self-renewal capacity of ovarian cancer stem cells. Anticancer Res.
35:85–96. 2015.PubMed/NCBI
|
|
18
|
Harikumar KB and Aggarwal BB: Resveratrol:
A multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de la Lastra CA and Villegas I:
Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and
clinical implications. Biochem Soc Trans. 35:1156–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parker AJ, Arango M, Abderrahmane S,
Lambert E, Tourette C, Catoire H and Néri C: Resveratrol rescues
mutant polyglutamine cytotoxicity in nematode and mammalian
neurons. Med Sci (Paris). 21:556–557. 2005.In French.
|
|
21
|
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr
and Walle UK: High absorption but very low bioavailability of oral
resveratrol in humans. Drug Metab Dispos. 32:1377–1382. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang L, Liu X, Wang Q, Cheng S, Zhang S
and Zhang M: Pharmacokinetics, tissue distribution and excretion
study of resveratrol and its prodrug 3,5,4′-tri-O-acetylresveratrol
in rats. Phytomedicine. 20:558–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ellis AA and Trent RB: Hospitalizations
for near drowning in California: Incidence and costs. Am J Public
Health. 85:1115–1118. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kakizaki E, Kozawa S, Sakai M and Yukawa
N: Bioluminescent bacteria have potential as a marker of drowning
in seawater: Two immersed cadavers retrieved near estuaries. Leg
Med Tokyo. 11:91–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poulsen MM, Fjeldborg K, Ornstrup MJ, Kjær
TN, Nøhr MK and Pedersen SB: Resveratrol and inflammation:
Challenges in translating pre-clinical findings to improved patient
outcomes. Biochim Biophys Acta. 1852:1124–1136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koide K, Osman S, Garner AL, Song F, Dixon
T, Greenberger JS and Epperly MW: The use of
3,5,4′-Tri-O-acetylresveratrol as a potential pro-drug for
resveratrol protects mice from γ-irradiation-induced death. ACS Med
Chem Lett. 2:270–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma L, Li Y, Zhao Y, Wang Q, Nan Y, Mu D,
Li W, Sun R, Jin F and Liu X: 3,5,4′-tri-O-acetylresveratrol
ameliorates seawater exposure-induced lung injury by upregulating
connexin 43 expression in lung. Mediators Inflamm. 2013:1821322013.
View Article : Google Scholar
|
|
28
|
Ma L, Zhao Y, Li B, Wang Q, Liu X, Chen X,
Nan Y, Liang L, Chang R, Liang L, et al:
3,5,4′-Tri-O-acetylresveratrol attenuates seawater
aspiration-induced lung injury by inhibiting activation of nuclear
factor-κB and hypoxia-inducible factor-1α. Respir Physiol
Neurobiol. 185:608–614. 2013. View Article : Google Scholar
|
|
29
|
Li J, Xu M, Fan Q, Xie X, Zhang Y, Mu D,
Zhao P, Zhang B, Cao F, Wang Y, et al: Tanshinone IIA ameliorates
seawater exposure-induced lung injury by inhibiting aquaporins
(AQP) 1 and AQP5 expression in lung. Respir Physiol Neurobiol.
176:39–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Zhang B, Xu DQ, Li WP, Xu M, Li
JH, Xie XY, Fan QX, Liu W, Mu DG, et al: Tanshinone IIA attenuates
seawater aspiration-induced lung injury by inhibiting macrophage
migration inhibitory factor. Biol Pharm Bull. 34:1052–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li JH, Xu M, Xie XY, Fan QX, Mu DG, Zhang
Y, Cao FL, Wang YX, Zhao PT, Zhang B, et al: Tanshinone IIA
suppresses lung injury and apoptosis, and modulates protein kinase
B and extracellular signal-regulated protein kinase pathways in
rats challenged with seawater exposure. Clin Exp Pharmacol Physiol.
38:269–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang M, Dong M, Liu W, Wang L, Luo Y, Li
Z and Jin F: 1α,25-dihydroxyvitamin D3 ameliorates seawater
aspiration-induced acute lung injury via NF-κB and RhoA/Rho kinase
pathways. PLoS One. 9:e1045072014. View Article : Google Scholar
|
|
33
|
Zheng C, Yin Q and Wu H: Structural
studies of NF-κB signaling. Cell Res. 21:183–195. 2011. View Article : Google Scholar :
|
|
34
|
Vaibhav K, Shrivastava P, Javed H, Khan A,
Ahmed ME, Tabassum R, Khan MM, Khuwaja G, Islam F, Siddiqui MS, et
al: Piperine suppresses cerebral ischemia-reperfusion-induced
inflammation through the repression of COX-2, NOS-2, and NF-κB in
middle cerebral artery occlusion rat model. Mol Cell Biochem.
367:73–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cox RA, Jacob S, Oliveras G, Murakami K,
Enkhbaatar P, Traber L, Schmalstieg FC, Herndon DN, Traber DL and
Hawkins HK: Pulmonary expression of nitric oxide synthase isoforms
in sheep with smoke inhalation and burn injury. Exp Lung Res.
35:104–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brovkovych V, Gao XP, Ong E, Brovkovych S,
Brennan ML, Su X, Hazen SL, Malik AB and Skidgel RA: Augmented
inducible nitric oxide synthase expression and increased NO
production reduce sepsis-induced lung injury and mortality in
myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol.
295:L96–L103. 2008. View Article : Google Scholar : PubMed/NCBI
|