Introduction
Obesity is a high risk factor for the development of
a number of human diseases, including insulin resistance, type 2
diabetes, hyperlipidemia and cancer (1). Obesity is induced by serveral
factors, such as genetic and endocrine abnormalities, certain
medicines, a low metabolic rate, nutritional and environmental
factors, as well as imbalanced energy homeostasis (2,3).
Previous studies have indicated that adipose tissue serves as an
energy reservoir and also plays a critical role in the control of
energy metabolism by secreting adipokines (3,4).
However, there is also strong evidence that the abnormal
expansion/accumulation of adipose tissue, which is largely
attributable to excessive adipocyte differentiation and an increase
in the number and size of fat cells, is closely linked to the
development of obesity (5,6).
Thus, any compound that inhibits excessive adipocyte
differentiation and adipocyte hyperplasia/hypertrophy may have
preventive and therapeutic potential against obesity.
It has previously been reported that the
differentiation of preadipocytes into mature adipocytes, also
termed adipogenesis, is controlled by numerous cellular proteins,
transcription factors, adipocyte-specific genes, lipogenic enzymes
and signaling proteins. For instance, it has previously been
demonstrated that the expression of the family of
CCAAT/enhancer-binding proteins (C/EBPs; C/EBP-α, -β and -δ) and
peroxisome proliferator-activated receptors (PPARs; PPAR-γ, -α and
-β) is critical for adipocyte differentiation (7,8).
Moreover, there is evidence indicating that Janus-activated protein
kinase-2 (JAK-2)/signal transducer and activator of transcription
(STAT)-3 and STAT-5 signaling complexes are associated with
adipocyte differentiation (9,10).
In addition, a number of signaling proteins and factors, including
protein kinase A (PKA), protein kinase C (PKC), extracellular
signal-regulated protein kinase-1/2 (ERK-1/2) and adenosine
3′,5′-cyclic monophosphate (cAMP), have been found to be of
importance for controlling adipocyte differentiation (11–13).
SGI-1776,
N-[(1-methylpiperidin-4-yl)methyl]-3-[3-(trifluoromethoxy)phenyl]imidazo[1,2-b]pyridazin-6-amine,
has been shown to inhibit three members of the Pim kinase family
(Pim-1, Pim-2 and Pim-3) (14).
Pim kinases are constitutively active serine/threonine kinases that
are known to be over-expressed in hematological malignancies, such
as acute myeloid leukemia and multiple myeloma (15). Pim kinases have multiple
substrates that are involved in transcription, protein translation,
cell proliferation and apoptosis. Due to its ability to inhibit Pim
kinase, SGI-1776 has been tested as a possible treatment for a
number of hematological malig nancies (16). Of note, it has been previously
suggested that Pim-2 is substantially expressed in adipocytes
(17) and the expression of Pim-1
in adipose tissue may be used as a marker of adipocytic
differentiation (18). There is
also compelling evidence indicating that Pim kinases are downstream
target genes of STAT-3 (19). In
a recent studies of ours, we demonstrated that a meridianin C
derivative, which inhibits protein kinases, including Pim kinases
(20), markedly inhibited
adipogenesis (21). This research
led us to further hypothesize that SGI-1776, a Pim-specific
inhibitor would suppress adipogenesis to a greater extent than a
meridianin C derivative.
In the present study, we investigated the
anti-adipogenic effects of SGI-1776 on 3T3-L1 adipocytes. To the
best of our knowledge, this is the first study which demonstrates
that SGI-1776 exerts a prominent anti-adipogenic effect without
affecting the viability of adipocytes and that the anti-adipogenic
effect of SGI-1776 is largely associated with the decreased
expression and/or phosphorylation levels of C/EBP-α, PPAR-γ, fatty
acid synthase (FAS) and STAT-3.
Materials and methods
Materials
Polyclonal C/EBP-α (sc-61), monoclonal PPAR-γ
(sc-7273), monoclonal STAT-3, monoclonal STAT-3 (sc-8019),
monoclonal phosphorylated (p-)STAT-3 (p-STAT-3; sc-8059),
polyclonal STAT-5 (sc-835) and polyclonal p-STAT-5 (sc-101806)
antibodies were all purchased from Santa Cruz Biotechnology, Inc.
(Delaware, CA, USA). Monoclonal FAS (610962) antibody was purchased
from BD Biosciences (San Jose, CA, USA). Monoclonal β-actin (A5441)
antibody, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone and
insulin were all purchased from Sigma (St. Louis, MO, USA).
SGI-1776 was purchased from Merck Millipore (Darmstadt,
Germany).
Cell culture and differentiation
As previously described (27), 3T3-L1 preadipocytes (ATCC,
Manassas, VA, USA) were grown to the contact inhibition stage and
remained in the post-confluent stage for 2 days in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS; Gibco, Grand Island, NY, USA) and
penicillin-streptomycin (Welgene, Daegu, Korea). Differentiation
was then induced by changing the medium to DMEM supplemented with
10% FBS (Welgene, Daegu, Korea) plus a cocktail of hormones (MDI),
containing 0.5 mM IBMX (M), 0.5 µM dexamethasone (D) and 5
µg/ml insulin (I) in the presence or absence of SGI-1776 or
butein (Sigma) at the indicated concentrations. Following 48 h MDI
induction, the differentiation medium was replaced with DMEM
supplemented with 10% FBS and 5 µg/ml insulin in the
presence or absence of SGI-1776 at the indicated concentrations.
The cells were then fed every other day with DMEM containing 10%
FBS in the presence or absence of SGI-1776 or butein at the
indicated concentrations until day 8. On day 8, the preadipocytes
became mature adipocytes that were rounded and filled with many oil
droplets.
Oil Red O staining
On day 8 of differentiation, the mock- (not treated
with SGI-1776 or butein) or SGI-1776- or butein-treated 3T3-L1
cells were washed twice with phosphate-buffered saline (PBS), fixed
with 10% formaldehyde for 2 h at room temperature, washed with 60%
isopropanol and dried completely. The fixed cells were then stained
with Oil Red O (Sigma, St. Louis, MO, USA) working solution for 1 h
at room temperature and were then washed twice with distilled
water. Lipid droplets were observed under a light microscope
(TS100; Nikon, Tokyo, Japan).
Cell count assay
The 3T3-L1 preadipocytes seeded in 24-well plates
were similarly grown under the above-mentioned differentiation
conditions. On day 8 of differentiation, the mock- or
SGI-1776-treated 3T3-L1 cells, which cannot be stained with trypan
blue dye, were counted under a microscope. The cell count assay was
carried out in triplicate. Data are presented as the means ±
standard error of 3 independent experiments.
Quantification of intracellular
triglyceride (TG) content by AdipoRed assay
On day 8 of differentiation, the lipid content in
the mock- or SGI-1776- or butein-treated 3T3-L1 cells was measured
using a commercially available AdipoRed Assay Reagent kit according
to the manufacturer's instructions (Lonza, Basel, Switzerland).
Following 10 min of incubation, fluorescence was measured using a
multilabel reader (Victor3; PerkinElmer, Waltham, MA, USA) with the
excitation set at 485 nm and the emission at 572 nm.
Preparation of whole cell lysates
As previously described (27), at a designated time point, the
3T3-L1 cells were washed twice with PBS and exposed to modified
RIPA buffer [50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 0.1% sodium
dodecyl sulfate, 0.25% sodium deoxycholate, 1% Triton X-100, 1%
Nonidet P-40, 1 mM EDTA, 1 mM EGTA, proteinase inhibitor cocktail
(1X)]. The cell lysates were collected and centrifuged at 12,000
rpm for 20 min at 4°C. The supernatant was saved, andthe protein
concentrations were determined using Bradford reagent (Bio-Rad,
Hercules, CA, USA).
Western blot analysis
Proteins (50 µg) were separated by SDS-PAGE
(10%) and transferred onto nitrocellulose membranes (Millipore,
Bedford, MA, USA). The membranes were washed with Tris-buffered
saline (10 mM Tris-Cl, 150 mM NaCl, pH 7.5), supplemented with
0.05% (v/v) Tween-20 (TBST), followed by blocking with TBST
containing 5% (w/v) non-fat dried milk. The membranes were
incubated overnight with antibodies specific for C/EBP-α, PPAR-γ,
STAT-3, p-STAT-3, STAT-5, p-STAT-5, FAS or β-actin at 4°C. The
membranes were then exposed to secondary antibodies conjugated to
horseradish peroxidase for 2 h at room temperature and further
washed 3 times with TBST. Immunoreactivity was detected using ECL
reagents. Equal protein loading was assessed by the expression
levels of actin.
Reverse transcription-polymerase chain
reaction (RT-PCR)
At a designated time point, total cellular RNA in
the mock- or SGI-1776-treated 3T3-L1 cells was isolated using
RNAzol-B (Tel-Test, Inc., Friendswood, TX, USA). Three micrograms
of total RNA were reverse transcribed using a random
hexadeoxy-nucleotide primer and reverse transcriptase.
Single-stranded cDNA was amplified by PCR using specific primers.
The primer sequences used for amplification were as follows:
C/EBP-α sense, 5′-TTACAACAGGCCAGGTTTCC-3′ and antisense,
5′-CTCTGGGATGGATCGATTGT-3′; PPAR-γ sense,
5′-GGTGAAACTCTGGGAGATTC-3′ and antisense,
5′-CAACCATTGGGTCAGCTCTC-3′; FAS sense, 5′-TTGCTGGCACTACAGAATGC-3′
and antisense, 5′-AACAGCCTCAGAGCGACAAT-3′; leptin sense,
5′-CCAAAACCCTCATCAAGACC-3′ and antisense,
5′-CTCAAAGCCACCACCTCTGT-3′; adiponectin sense,
5′-GGAGATGCAGGTCTTCTTGGT-3′ and antisense,
5′-TCCTGATACTGGTCGTAGGTGAA-3′; regulated on activation, normal T
cell expressed and secreted (RANTES) sense,
5′-TCCAATCTTGCAGTCGTGTTTG-3′ and antisense,
5′-TCTGGGTTGGCACACACTTG-3′; monocyte chemoattractant protein-1
(MCP-1) sense, 5′-TCCAATCTTGCAGTCGTGTTTG-3′ and antisense,
5′-TCTGGGTTGGCACACACTTG-3′; actin antisense,
5′-GGTAGGAACACGGAAGGCCA-3′. The mRNA expression levels of actin
were used as an internal control to evaluate the relative mRNA
expression of adipocyte-specific genes and adipokines.
Statistical analysis
Cell count analysis was completed in triplicate and
repeated 3 times. Data are expressed as the means ± standard error
(SE). The significant differences between groups were determined by
one-way ANOVA (Laerd Statistics, Chicago, IL, USA). All
significance testing was based upon a P-value <0.05, indicating
a statistically significant difference.
Results
SGI-1776 exerts potent anti-adipogenic
effects
Primarily, we wished to determine whether the
Pim-selective inhibitor, SGI-1776, inhibits intracellular lipid
accumulation during the differentiation of 3T3-L1 preadipocytes
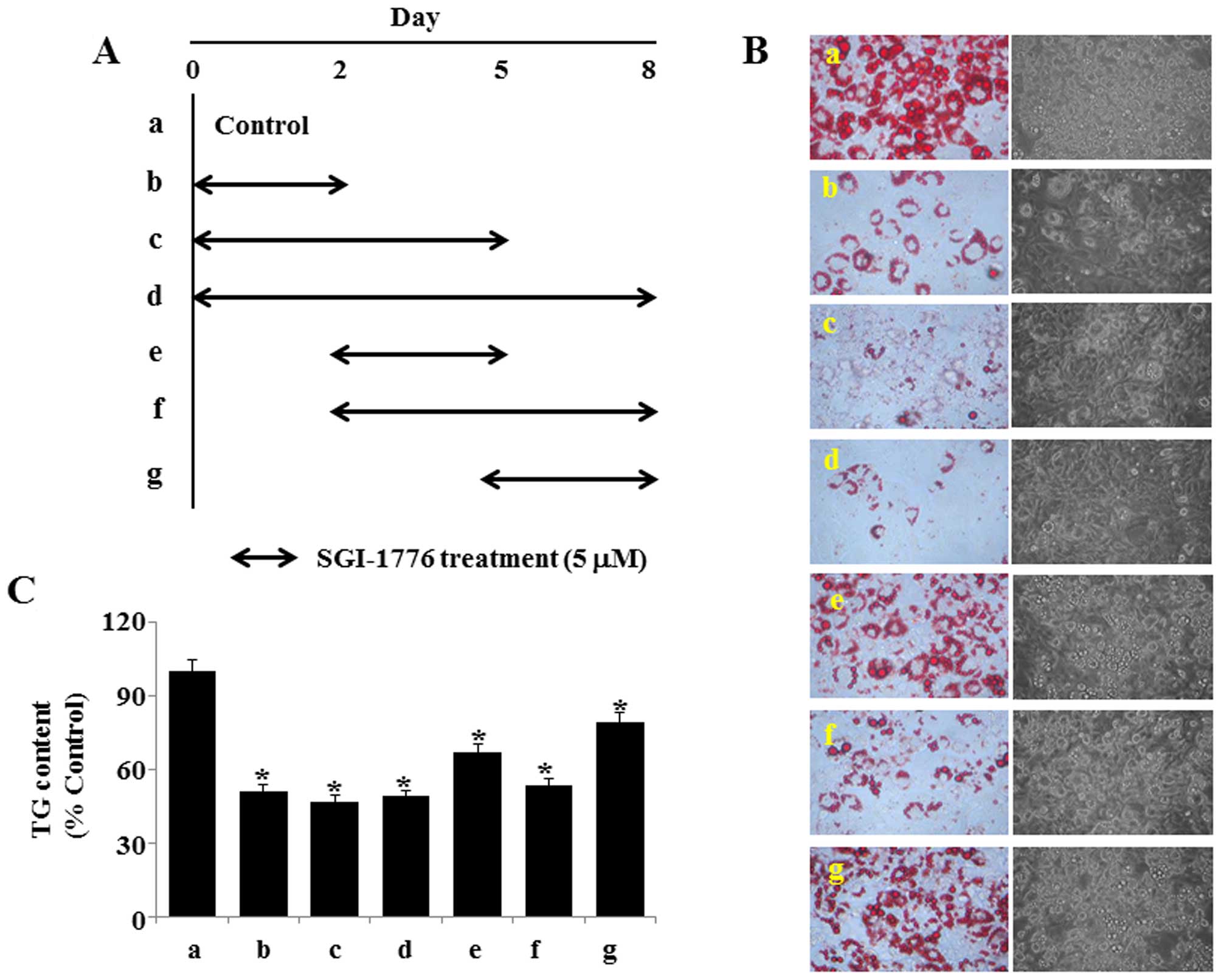
into adipocytes by Oil Red O staining. The timescale of 3T3-L1
preadipocyte differentiation is illustrated in Fig. 1A. Many lipid droplets were formed
in the differentiated 3T3-L1 preadipocytes cultured in the
induction medium on day 8 of cell differentiation, compared with
the undifferentiated cells (preadipocytes), in which no lipid
droplets had formed (Fig. 1B,
upper panels). However, treatment with SGI-1776 for 8 days markedly
reduced the amount of lipid droplets in a concentration-dependent
manner, as compared with the mock-treated cells. Evidently,
treatment with 10 µM SGI-1776 completely blocked the
accumulation of lipid droplets. The SGI-1776-mediated
lipid-reducing effect was also confirmed by light microscopy
(Fig. 1B, lower panels). In
addition, we noted that SGI-1776 decreased the levels of TG during
adipocyte differentiation in a dose-dependent manner, with maximum
reduction being achieved with an inhibitor concentration of 10
µM (Fig. 1C). However,
using a cell count assay, it was observed that SGI-1776 induced a
slight decrease in viability at 10 µM, but not at 5
µM (Fig. 1D). Certain
compounds, such as butein (40 µM), have also previously been
shown to exert anti-adipogenic effects on 3T3-L1 adipocytes
(22). In this study, we thus
compared the anti-adipogenic effect of SGI-1776 (5 µM) with
that of butein (40 µM) used as a positive control. In
agreement with the results of previous studies, treatment with
butein at 40 µM markedly inhibited both lipid droplet
formation (Fig. 1E) and TG
synthesis (Fig. 1F) during
adipocyte differentiation. Of note, treatment with SGI-1776 at 5
µM exerted more potent inhibitory effects on both lipid
droplet formation and TG synthesis than butein (40 µM).
Therefore, the concentration of 5 µM of SGI-1776 was
selected for use in further experiments due to the potent
inhibitory effects it exerted on both lipid accumulation and TG
synthesis without affecting the number of adipocytes.
Inhibition of the early adipogenic
process is critical for the SGI-1776-mediated anti-adipogenic
effects
We subsequently sought to determine which stage(s)
of adipocyte differentiation is inhibited by SGI-1776. To this end,
the 3T3-L1 preadipocytes were incubated with induction medium (MDI,
insulin and FBS) in the presence or absence of SGI-1776 (5
µM) for the indicated periods of time, as illustrated in
Fig. 2A. As expected, the
mock-treated cells incubated with MDI, insulin and FBS for 2, 5 and
8 days exhibited high lipid accumulation (treatment a). The 3T3-L1
preadipocytes treated with SGI-1776 from day 0 to 2 (treatment b),
or from day 0 to 5 (treatment c) showed a comparatively low lipid
content. The preadipocytes treated with SGI-1776 from day 0 to 8
exhibited the highest inhibition of lipid droplet accumulation
(treatment d). Of note, differentiating 3T3-L1 cells treated with
SGI-1776 from day 2 to 5, from day 2 to 8 or from day 5 to 8
(treatment e, f and g) exhibited a much weaker inhibition of
adipogenic differentiation, compared with treatment b, c and d
(Fig. 2B). The results of the
AdipoRed assay further demonstrated that treatment b, c and d
resulted in the highest reduction of TG during adipocyte
differentiation (Fig. 2C).
Treatment f also substantially reduced the TG content during
adipocyte differentiation, whereas treatment e and g led to a
slight reduction in the TG content. These results collectively
suggest that SGI-1776 inhibits adipogenesis particularly at an
early phase of differentiation.
SGI-1776 decreases the expression of
C/EBP-α and PPAR-γ and the phosphorylation levels of STAT-3 during
adipocyte differentiation
To elucidate the mechanisms underlying the
SGI-1776-mediated anti-adipogenic effect, we examined the effects
of SGI-1776 (5 µM) on the expression and/or activity
(phosphorylation) levels of adipogenic transcription factors,
including the families of C/EBPs, PPARs and STATs. Western blot
analysis revealed that SGI-1776 markedly inhibited the
adipogenesis-dependent protein expression of C/EBP-α and PPAR-γ
during adipocyte differentiation, particularly on day 5 and 8
(Fig. 3A and E). The results from
RT-PCR also revealed that SGI-1776 suppressed the mRNA expression
of C/EBP-α and PPAR-γ during adipocyte differentiation (Fig. 3B and F). These results suggest
that SGI-1776 downregulates PPAR-γ and C/EBP-α expression at the
transcriptional level. Moreover, SGI-1776 markedly decreased the
phosphorylation levels of STAT-3 on day 2 during adipocyte
differentiation (Fig. 3C and G).
By contrast, SGI-1776 did not markedly affect the phosphorylation
levels of STAT-5. Notably, SGI-1776 also had the capacity to
suppress STAT-3 phosphorylation during the early stages of
adipogenesis induced by MDI at the treatment times of 2, 4, 8 and
24 h (Fig. 3D and H). SGI-1776
did not markedly alter the total protein levels of STAT-3 and
STAT-5 during adipocyte differentiation at the designated time
points.
 | Figure 3Effect of SGI-1776 on the cellular
expression and/or phosphorylation levels of CCAAT/enhancer-binding
protein-α (C/EBP-α), peroxisome proliferator-activated receptor-γ
(PPAR-γ), signal transducer and activator of transcription (STAT)-3
and STAT-5 during 3T3-L1 adipocyte differentiation. (A–C) 3T3-L1
preadipocytes were induced to differentiate with induction medium
containing MDI, insulin and fetal bovine serum (FBS) in the
presence or absence of SGI-1776, and harvested on days 2, 5 and 8,
respectively. Cellular protein and mRNA at the indicated time
points were extracted and analyzed by (A and C) western blot
analysis and (B) RT-PCR. Each blot in (A–C) is representative of 3
independent experiments. (D) 3T3-L1 preadipocytes were treated with
induction medium containing MDI in the presence or absence of
SGI-1776 and harvested at 2, 4, 8 and 24 h, respectively. The
cellular protein at the indicated time points was extracted and
analyzed by western blot analysis. Each blot in (D) is
representative of 3 independent experiments. (E and F) The
densitometric data of (A and B), respectively, that show C/EBP-α
and PPAR-γ protein and mRNA levels normalized to actin protein and
mRNA levels as percentages of the value in the presence or absence
of SGI-1776 at the indicated days. Values represent the means ± SE
of data from 3 independent experiments with 3 replicates.
*p<0.05 compared to the value of SGI-1776-free
control on the indicated days. (G and H) The densitometric data of
(C and D), respectively, that show p-STAT-3, p-STAT-1 and p-STAT-5
protein levels normalized to total expression levels of each
protein as percentages of the value in the presence or absence of
SGI-1776 at the indicated days. *p<0.05 compared to
the value of SGI-1776-free control on the indicated days. |
SGI-1776 downregulates the protein and/or
mRNA expression of FAS, leptin and RANTES during adipocyte
differentiation
The expression and secretion of adipokines occur
during preadipocyte differentiation (28-30). Therefore, we examined whether
SGI-1776 (5 µM) regulates the expression of
adipocyte-specific genes and/or adipokines during adipocyte
differentiation. SGI-1776 markedly suppressed the protein and mRNA
expression levels of FAS on days 5 and 8 during adipocyte
differentiation (Fig. 4A–C).
Furthermore, the results from RT-PCR demonstrated that SGI-1776
markedly reduced the insulin- and FBS-induced mRNA expression of
leptin and RANTES on days 5 and 8 (Fig. 4B–D). However, SGI-1776 did not
greatly affect the mRNA levels of adiponectin or MCP-1 on days 5
and 8 (Fig. 4B and D).
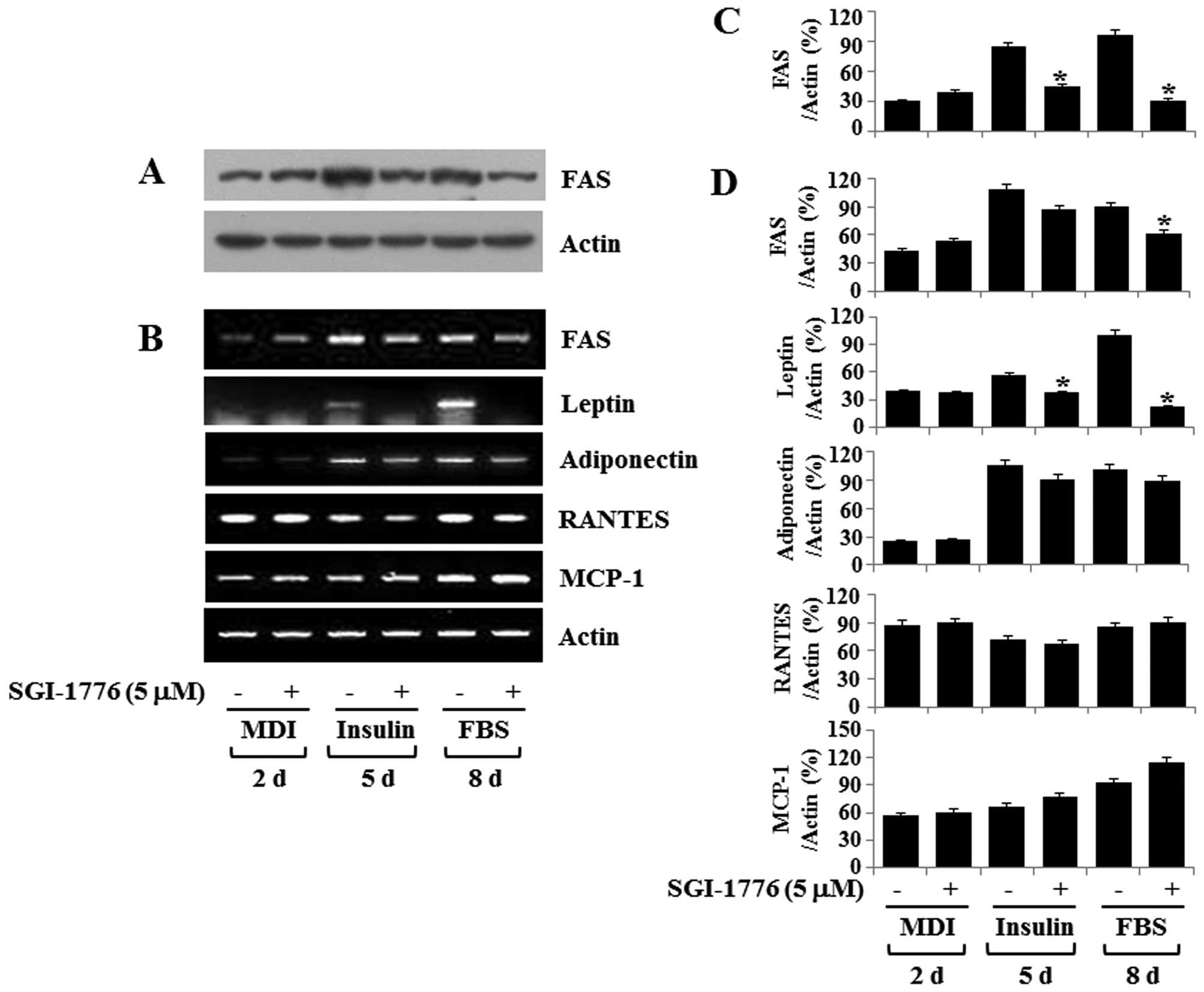 | Figure 4Effect of SGI-1776 on fatty acid
synthase (FAS) protein and mRNA expression levels of FAS and
adipokines [leptin, adiponectin, regulated on activation, normal T
cell expressed and secreted (RANTES) and monocyte chemoattractant
protein-1 (MCP-1)] during 3T3-L1 adipocyte differentiation. (A and
B) 3T3-L1 preadipocytes were induced to differentiate with
induction medium containing MDI, insulin and fetal bovine serum
(FBS) in the presence or absence of SGI-1776, and harvested at days
2, 5 and 8, respectively. Cellular protein and mRNA at the
indicated time points were extracted and analyzed by (A) western
blot analysis and (B) RT-PCR. Each blot in (A and B) is a
representative of 3 independent experiments. (C and D) The
densitometric data of (A and B), respectively, that show FAS
protein and mRNA levels and/or leptin, adiponectin, RANTES and
MCP-1 mRNA levels normalized to actin protein and/or mRNA levels as
percentages of the value in the presence or absence of SGI-1776 on
the indicated days. Values represent the means ± SE of data from 3
independent experiments with 3 replicates. *p<0.05
compared to the value of SGI-1776-free control on the indicated
days. |
Discussion
Excessive adipocyte differentiation causes the
abnormal expansion/accumulation of adipose tissue, leading to the
high secretion of adipokines, which have been implicated in
inflammation, insulin resistance and metabolic disorders (5,6).
Thus, inhibitors of adipocyte differentiation likely have
therapeutic potential as anti-obesity drugs. Recently, we
demonstrated that a meridianin C derivative, which inhibits protein
kinases including Pim kinases (20), markedly blocks adipocytic
differentiation (21). In the
present study, we focused on the role of Pim kinases in 3T3-L1
adipogenesis. We noted that when the 3T3-L1 cells differentiated in
the presence of a potent and selective Pim inhibitor, such as
SGI-1776, adipogenesis was severely inhibited.
Mature adipocytes have a spherical shape and are
filled with a high number of intracellular lipid droplets, and
these can be distinguished from fibroblast-like preadipocytes
(23). The present study
demonstrated that SGI-1776 (5 µM) markedly inhibited lipid
accumulation during the differentiation of 3T3-L1 preadipocytes
into adipocytes by 8 days of differentiation (Fig. 1B and C), suggesting that it exerts
a potent anti-adipogenic effect. Cell count analysis further
revealed that treatment with SGI-1776 (5 µM) for 8 days did
not markedly affect the viability of 3T3-L1 adipocytes (Fig. 1D).
As previously mentioned, the induction of adipocyte
differentiation is largely dependent on the expression and activity
of adipogenesis-related transcription factors, such as C/EBP-α and
PPAR-γ. It has been previously demonstrated that blocking their
expression and/or activity with either pharmacological inhibitors
or small interfering RNA leads to the inhibition of lipid
accumulation during adipocyte differentiation, and that the
knockdown of C/EBP-α or PPAR-γ decreases or impairs the
differentiation of white adipose tissue in mice (7,8,24).
The present study demonstrated that SGI-1776 inhibited the protein
and mRNA expression of C/EBP-α and PPAR-γ during adipocyte
differentiation (Fig. 3A, B, E and
F). Thus, the SGI-1776-mediated anti-adipogenic effect appears
to be closely associated with the reduced expression of C/EBP-α and
PPAR-γ. Previously, it has also been reported that the family of
STATs, including STAT-1, STAT-3, STAT-5 and STAT-6, is expressed in
both 3T3-L1 preadipocytes and adipocytes (9), and that several members of the STAT
family, including STAT-3 and STAT-5, are critical for 3T3-L1
adipocyte differentiation (10,21,25–27). Of note, previous studies have
further demonstrated that STAT-3 is rapidly (within hours)
activated during adipocyte differentiation, and that active STAT-3
plays an important role in the early stages of adipogenesis
(10,21,27). In the present study, we noted that
SGI-1776 blocked not only the early adipogenic process (Fig. 2B and C), but also MDI (but neither
insulin nor FBS) signaling to activate STAT-3 during the early
stage of adipogenesis (Fig. 3C, D and
H). It is thus likely that the STAT-3-dependent blockage of
early adipogenesis is also crucial for the SGI-1776-mediated
anti-adipogenic effect. Previous studies have indicated that STAT-3
regulates adipocyte differentiation via PPAR-γ, and that STAT-3
activity is also important for the adipogenesis-induced expression
of C/EBP-α and PPAR-γ (26,27). Hence, it is suggested in this
study that SGI-1776 inhibits adipogenesis primarily through the
inhibition of STAT-3 at the early adipogencic process, which
subsequently downregulates the expression of C/EBP-α and
PPAR-γ.
Previous research has demonstrated that the
increased expression and activity of C/EBPs and PPARs is necessary
for the expression of adipocyte-specific genes and adipokines,
including FAS, leptin, adiponectin, MCP-1 and RANTES (28–30). Of those, FAS is a lipogenic enzyme
involved in fatty acid synthesis, and its expression is highly
upregulated in cells or tissues with high rates of fatty acid
synthesis (31). In addition, it
has previously been shown that FAS mRNA and protein expression are
substantially increased during 3T3-L1 preadipocyte differentiation
(21,27,32). In this study, we noted that
SGI-1776 also had the ability to markedly inhibit the insulin- and
FBS-mediated induction of FAS at both the protein and mRNA level in
3T3-L1 adipocytes (Fig. 4). These
results suggest that SGI-1776 exerts an anti-lipogenic effect by
suppressing FAS expression, which further contributes to the
SGI-1776-mediated anti-adipogenic effect. It has been well
documented that the maturation of adipocyte differentiation is
characterized by the capacity of mature adipocytes to express and
secrete adipokines, which are involved in the endocrine control of
energy homeostasis (33,34). Therefore, considering that
SGI-1776 inhibits the mRNA expression of leptin and RANTES, but not
adiponectin, during the adipocyte differentiation process (Fig. 4B and D), we suggest that SGI-1776
further blocks the adipocyte differentiation maturation
process.
In conclusion, in this study, we demonstrate firstly
that a pan-Pim kinase inhibitor, SGI-1776, exerts potent
anti-adipogenic effects in 3T3-L1 cells that are related to the
reduced expression and/or phosphorylation levels of PPAR-γ,
C/EBP-α, FAS and STAT-3. The findings of this study thus suggest
that the Pim-specific inhibitor, SGI-1776, be considered a
potential therapeutic agent for the prevention and treatment of
obesity in which excessive adipogenesis plays pathological
roles.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea Government
(MSIP) (no. 2014R1A5A2010008) and also in part by the Korea Basic
Science Institute grants (no. D35400) and a Keimyung University
Research Grant of 2015.
References
|
1
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
2
|
Ahima RS and Flier JS: Adipose tissue as
an endocrine organ. Trends Endocrinol Metab. 11:327–332. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Havel PJ: Control of energy homeostasis
and insulin action by adipocyte hormones: leptin, acylation
stimulating protein, and adiponectin. Curr Opin Lipidol. 13:51–59.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spiegelman BM and Flier JS: Obesity and
the regulation of energy balance. Cell. 104:531–543. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bays HE: 'Sick fat', metabolic disease,
and atherosclerosis. Am J Med. 122(Suppl): S26–S37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Melnikova I and Wages D: Anti-obesity
therapies. Nat Rev Drug Discov. 5:369–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao Z, Umek RM and McKnight SL: Regulated
expression of three C/EBP isoforms during adipose conversion of
3T3-L1 cells. Genes Dev. 5:1538–1552. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stephens JM, Morrison RF and Pilch PF: The
expression and regulation of STATs during 3T3-L1 adipocyte
differentiation. J Biol Chem. 271:10441–10444. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang K, Guo W, Yang Y and Wu J:
JAK2/STAT3 pathway is involved in the early stage of adipogenesis
through regulating C/EBPβ transcription. J Cell Biochem.
112:488–497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martini CN, Plaza MV and Vila MC:
PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts
differentiation. Mol Cell Endocrinol. 298:42–47. 2009. View Article : Google Scholar
|
|
12
|
Zhou Y, Wang D, Li F, Shi J and Song J:
Different roles of protein kinase C-betaI and -δ in the regulation
of adipocyte differentiation. Int J Biochem Cell Biol.
38:2151–2163. 2006. View Article : Google Scholar
|
|
13
|
Prusty D, Park BH, Davis KE and Farmer SR:
Activation of MEK/ERK signaling promotes adipogenesis by enhancing
peroxisome proliferator-activated receptor gamma (PPARgamma) and
C/EBPalpha gene expression during the differentiation of 3T3-L1
preadipocytes. J Biol Chem. 277:46226–46232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen LS, Redkar S, Taverna P, Cortes JE
and Gandhi V: Mechanisms of cytotoxicity to Pim kinase inhibitor,
SGI-1776, in acute myeloid leukemia. Blood. 118:693–702. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nawijn MC, Alendar A and Berns A: For
better or for worse: the role of Pim oncogenes in tumorigenesis.
Nat Rev Cancer. 11:23–34. 2011. View
Article : Google Scholar
|
|
16
|
Cervantes-Gomez F, Chen LS, Orlowski RZ
and Gandhi V: Biological effects of the Pim kinase inhibitor,
SGI-1776, in multiple myeloma. Clin Lymphoma Myeloma Leuk. 13(Suppl
2): S317–S329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Behan JW, Yun JP, Proektor MP, Ehsanipour
EA, Arutyunyan A, Moses AS, Avramis VI, Louie SG, Butturini A,
Heisterkamp N and Mittelman SD: Adipocytes impair leukemia
treatment in mice. Cancer Res. 69:7867–7874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nga ME, Swe NN, Chen KT, Shen L, Lilly MB,
Chan SP, Salto-Tellez M and Das K: PIM-1 kinase expression in
adipocytic neoplasms: diagnostic and biological implications. Int J
Exp Pathol. 91:34–43. 2010. View Article : Google Scholar
|
|
19
|
Meloche J, Paulin R, Courboulin A, Lambert
C, Barrier M, Bonnet P, Bisserier M, Roy M, Sussman MA, Agharazii M
and Bonnet S: RAGE-dependent activation of the oncoprotein Pim1
plays a critical role in systemic vascular remodeling processes.
Arterioscler Thromb Vasc Biol. 31:2114–2124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
More KN, Jang HW, Hong VS and Lee J: Pim
kinase inhibitory and antiproliferative activity of a novel series
of meridianin C derivatives. Bioorg Med Chem Lett. 24:2424–2428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park YK, Lee TY, Choi JS, Hong VS, Lee J,
Park JW and Jang BC: Inhibition of adipogenesis and leptin
production in 3T3-L1 adipocytes by a derivative of meridianin C.
Biochem Biophys Res Commun. 452:1078–1083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song NJ, Yoon HJ, Kim KH, Jung SR, Jang
WS, Seo CR, Lee YM, Kweon DH, Hong JW, Lee JS, et al: Butein is a
novel anti-adipogenic compound. J Lipid Res. 54:1385–1396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosen ED and Spiegelman BM: Molecular
regulation of adipogenesis. Annu Rev Cell Dev Biol. 16:145–171.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linhart HG, Ishimura-Oka K, DeMayo F, Kibe
T, Repka D, Poindexter B, Bick RJ and Darlington GJ: C/EBPalpha is
required for differentiation of white, but not brown, adipose
tissue. Proc Natl Acad Sci USA. 98:12532–12537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shang CA and Waters MJ: Constitutively
active signal transducer and activator of transcription 5 can
replace the requirement for growth hormone in adipogenesis of
3T3-F442A preadipocytes. Mol Endocrinol. 17:2494–2508. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang D, Zhou Y, Lei W, Zhang K, Shi J, Hu
Y, Shu G and Song J: Signal transducer and activator of
transcription 3 (STAT3) regulates adipocyte differentiation via
peroxisome-proliferator-activated receptor gamma (PPARgamma). Biol
Cell. 102:1–12. 2010. View Article : Google Scholar
|
|
27
|
Park YK, Lee J, Hong VS, Choi JS, Lee TY
and Jang BC: Identification of KMU-3, a novel derivative of gallic
acid, as an inhibitor of adipogenesis. PLoS One. 9:e1093442014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Christy RJ, Yang VW, Ntambi JM, Geiman DE,
Landschulz WH, Friedman AD, Nakabeppu Y, Kelly TJ and Lane MD:
Differentiation-induced gene expression in 3T3-L1 preadipocytes:
CCAAT/enhancer binding protein interacts with and activates the
promoters of two adipocyte-specific genes. Genes Dev. 3:1323–1335.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gustafson B, Jack MM, Cushman SW and Smith
U: Adiponectin gene activation by thiazolidinediones requires PPAR
gamma 2, but not C/EBP alpha-evidence for differential regulation
of the aP2 and adiponectin genes. Biochem Biophys Res Commun.
308:933–939. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hwang CS, Loftus TM, Mandrup S and Lane
MD: Adipocyte differentiation and leptin expression. Annu Rev Cell
Dev Biol. 13:231–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lakshmanan MR, Nepokroeff CM and Porter
JW: Control of the synthesis of fatty-acid synthetase in rat liver
by insulin, glucagon, and adenosine 3′:5′ cyclic monophosphate.
Proc Natl Acad Sci USA. 69:3516–3519. 1972. View Article : Google Scholar
|
|
32
|
Rubin CS, Hirsch A, Fung C and Rosen OM:
Development of hormone receptors and hormonal responsiveness in
vitro. Insulin receptors and insulin sensitivity in the
preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem.
253:7570–7578. 1978.PubMed/NCBI
|
|
33
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998.PubMed/NCBI
|
|
34
|
Fève B: Adipogenesis: Cellular and
molecular aspects. Best Pract Res Clin Endocrinol Metab.
19:483–499. 2005. View Article : Google Scholar : PubMed/NCBI
|