Introduction
Shellfish, such as mussels, clams and abalones are a
commercially important bioresource in the fishery and food
industries. Abalone is a marine gastropod, as well as an important
shellfish and industrial resource in Asia, Africa, Australia and
America, and approximately 100 species of abalones are to be found
worldwide (1,2). Of the abalone species, the Pacific
abalone, Haliotis discus hannai (H. discus hannai), is the
most commercially important species in Korea. H. discus
hannai abalone mariculture has expanded in land- and sea-based
systems, and the total yield from Korea was estimated at 7,580
metric tons in 2009. Korea is one of the major suppliers of
abalone, and the majority of the Korean production is in the remote
Wando region (3). In addition,
the production of various types of abalone (e.g., dried, steamed,
seasoned and spiced) has also significantly increased (14).
Marine organism-derived proteins and peptides
possess various biological activities, such as, anticoagulant
(4), antimicrobial (5) and antihypertensive (6) activities, and they have also been
shown to reduce the risk of developing cardiovascular disease
(7). Depending on the composition
and the molecular size of the amino acid, bioactive peptides can be
involved in diverse biological functions (8). During gastrointestinal (GI)
digestion, proteolytic digestion can generate absorbable and
bioactive peptides in the stomach and small intestinal tract
(9,10) that may have certain physiological
benefits. Certain recent studies have reported that in vitro
GI digests of marine organisms possess biological activities that
are as potent as those of other natural antioxidants (11,12). In our recent studies, we
demonstrated that the intestinal digests of abalone, H. discus
hannai, possess potent antioxidant and anti-inflammatory
activities, and inhibit the effects of matrix metalloproteinases
(MMPs) (13,14).
Allergic diseases such as asthma, allergic rhinitis
and atopic dermatitis are typified by an undesirable reaction to a
normally harmless allergen in the environment (15). Allergens can enter the body
through various routes, such as inhalation, ingestion or external
skin contact (16). An allergy is
a condition characterized by the excessive recruitment of
lymphocytes, basophils, eosinophils and mast cells to the inflamed
site of lesions (17). Of these
cells, mast cells are central effector cells involved in the
pathogenesis of allergic diseases (18). Mast cells are commonly found at
sites exposed to the external environment, namely the skin and
mucosal membranes (19,20). Mast cells constitutively express
the high-affinity receptor for immunoglobulin (Ig) E (FcεRI) on
their surface, and the number of surface FcεRI is positively
regulated by ambient concentrations of IgE (21). The IgE-dependent activation of
mast cells, through the aggregation of FcεRI by allergen-specific
IgE, initiates a complex secretory response. Once activated, mast
cells release and generate biologically active preformed and newly
synthesized mediators, such as granule-associated mediators,
cytokines and inflammatory lipids, which can initiate the immediate
hypersensitivity responses associated with allergies (17).
In the present study, abalone intestines were
digested using an in vitro GI digestion system containing
pepsin, trypsins and α-chymotrypsin. The abalone intestine GI
digests (AIGIDs) produced by the GI digestion system were
fractionated into AIGID I (>10 kDa), II (5–10 kDa) and III
(<5 kDa) using an ultrafiltration (UF) membrane system. We
evaluated the anti-allergic effects of AIGIDs on IgE-dependent
passive cutaneous anaphylaxis (PCA) reactions in vivo, and
we investigated the regulatory mechanisms underlying the
pharmacological effects of abalone intestine GI digest peptide
(AIGIDP) on the release of phorbol-12-myristate 13-acetate (PMA)
plus calcium ionophore A23187 (PMACI)-induced inflammatory
mediators in human mast cells (HMC-1).
Materials and methods
Animals
Male (6 to 8-week-old) ICR mice were purchased from
Orient Bio Inc. (Seoul, Korea) and were allowed to acclimatize to
our animal facility for at least 1 week. All experimental animals
used in this study were maintained under a protocol approved by the
Institutional Animal Care and Use Committee of the Inje University
Medical School.
Materials
Live adult abalones (H. discus hannai) were
collected from Wando island, Wando-gun, Korea. PMA, calcium
ionophore A23187 (calcimycin; C29H37N3O6), anti-dinitrophenol (DNP)
IgE, DNP-human serum albumin (HSA) and Iscove's modified Dulbecco's
medium (IMDM) were all purchased from Sigma Chemical Co. (St.
Louis, MO, USA). Nuclear factor-κB (NF-κB) antibody was obtained
from eBioscience (San Diego, CA, USA) (Cat. no. 14–6731).
Antibodies against JNK (Cat. no. 9252), phosphorylated (p-)JNK
(Cat. no. 9251), p-extracellular signal-regulated kinase (ERK)1/2
(Cat. no. 9106), p-p38 mitogen-activated protein kinase (MAPK)
(Cat. no. 9211) and p-IκBα (Cat. no. 9246) were all purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies
against ERK1/2 (Cat. no. sc-94), p38 MAPK (Cat. no. sc-535), and
IκBα (cat. no. sc-371) were all purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Preparation of in vitro GI digestion and
fractionation on a UF membrane bioreactor system
For the digestion process, we used the method
previously described by Kapsokefalou and Miller (22). One hundred milliliters of 4% (w/v)
abalone intestine solution were brought to pH 2.2 in gastric
digestion (phase I) using 1 M HCl and 1 M NaOH while being
vigorously mixed. Pepsin was added at an enzyme-to-substrate ratio
of 1/100 (w/w) and then incubated at 37°C in a shaker. After 2 h,
the pH was set to 6.5 to mimic the conditions of intestinal
digestion (phase II). Similarly, trypsin and α-chymotrypsin were
both supplemented at an enzyme-to-substrate ratio of 1/100 (w/w).
The solution was further incubated at 37°C for 2.5 h. When the
samples were taken at the beginning and end of digestion, the pH
was adjusted to 8.0. The samples were centrifuged at 10,000 × g for
15 min at 4°C, and the supernatant was lyophilized to obtain an
AIGID dry powder. The resultant AIGID was fractionated using a UF
membrane bioreactor system with molecular weight (MW) cut-offs
(MWCOs) of 1, 5 and 10 kDa. Fractionates were designed as follows:
AIGID I with MW distribution of >10 kDa, AIGID II with a MW
distribution of 5–10 kDa and AIGID III with a MW distribution of
<5 kDa. All the AIGIDs recovered from the fractionation were
lyophilized in a freeze drier for 5 days.
Cell culture
HMC-1 cells, a human mast cell line, were provided
by Professor D. K. Kim (Chonbuk National University, Medical
School, Jeonju, Korea). The HMC-1 cells were grown in IMDM and
supplemented with 100 U/ml of penicillin, 100 µg/ml of
streptomycin and 10% fetal bovine serum (FBS) at 37°C in an
atmosphere with 5% CO2 with 95% humidity. The HMC-1
cells were treated with AIGIDP for 30 min. The cells were then
stimulated with 50 nM of PMA plus 1 µM of A23187 and
incubated at 37°C for the indicated periods of time.
Determination of cell viability
Cell viability was assessed using the Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) assay method.
Briefly, wells containing 2xl04 cells/well were treated
with AIGIDs. Following incubation for 24 h, the cells were washed
twice with phosphate-buffered saline (PBS), and CCK-8 was added to
each well and incubated at 37°C for 1 h, followed by an analysis at
450 nm using a microplate reader (model EL800; BioTek, Winooski,
VT, USA).
PCA reaction
The mice were injected intradermally with 500 ng of
anti-DNP IgE into each of 3 dorsal skin sites that had been shaved
48 h earlier. The sites were outlined with a waterproof red marker.
Forty-eight hours later, each mouse received an injection of 100
µg of DNP-HSA in PBS containing 4% Evans Blue via the tail
vein. One hour prior to this injection, the AIGIDs (50 mg/kg, each)
were administered intraperitoneally. Thirty minutes after the
antigenic challenge, the mice (n=3) were sacrificed by asphyxiation
with CO2 and the dorsal skin was removed in order to
measure the amount of pigment. The amount of dye was then
determined colormetrically following extraction with 1 ml of 0.1 N
KOH and 9 ml of a mixture of acetone and phosphoric acid (5:13).
The absorption intensity of the extraction was measured at 620 nm
using a spectrometer (model ELx800; BioTek).
Histamine assay
The HMC-1 cells were treated with various
concentrations of the AIGIDs (100–300 µg/ml) for 30 min
prior to stimulation with PMACI. The amount of histamine was
assayed using an enzyme-linked immunosorbent assay (ELISA) kit
(Oxford Biomedical Research, Rochester Hills, MI, USA) in
accordance with the manufacturer's instructions.
Preparation and identification of the
peptide (AIGIDP)
AIGID III was loaded onto a HiPrep 16/10 CM FF
ion-exchange column (16×100 mm) (from GE Healthcare Life Sciences,
Uppsala, Sweden) equilibrated with 20 mM sodium acetate buffer (pH
4.0) and eluted with a linear gradient of NaCl (0–2 M) using fast
protein liquid chromatography (FPLC). Pooled and lyophilized
fractions were then further purified on a Prime Sphere 10 C18
column (20×250 mm) (Phenomenex, Inc., Torrance, CA, USA) using
permeation reverse-phase high-performance liquid chromatography
(RP-HPLC) with a linear gradient of acetonitrile (0–35% in 30 min)
containing 0.1% trifluoroacetic acid (TFA). Finally, the accurate
molecular mass and amino acid sequence of AIGIDP was ascertained by
quadruple time-of-flight mass spectroscopy (Micromass UK Ltd.,
Altrincham, UK) coupled to an electrospray ionization source.
Cytokine assay
The HMC-1 cells were treated with various
concentrations of AIGIDP (100–300 µg/ml) for 30 min prior to
stimulation with PMACI. The levels of interleukin (IL)-1β, IL-6,
and tumor necrosis factor-α (TNF-α) were measured using ELISA kits
(BioLegend, Inc., San Diego, CA, USA). Quantification of the ELISA
results was performed using an ELISA plate reader (Dynatech
MR-7000; Dynatech Laboratories Inc., Chantilly, VA, USA) set to a
wavelength of 450 nm, according to the manufacturer's
instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). Total RNA (1.0 µg) from the
cells was reverse transcribed using M-MLV reverse transcriptase
(Promega, Madison, WI, USA) to produce cDNA. Reverse
transcription-generated cDNAs encoding IL-1β, IL-6, IL-8 and TNF-α
were amplified by PCR using selected primers (Table I). Following amplification,
portions of the PCR reactions were electrophoresed on an agarose
gel.
 | Table IInformation on primers used for
RT-PCR. |
Table I
Information on primers used for
RT-PCR.
| Genes | NCBI accesion
no. | 5′→3′ | Size (bp) |
|---|
| IL-1β | NT_022135 | F:
TGTCCTGCGTGTTGAAAGATGA | 391 |
| | R:
CAGGCAGTTGGGCATTGGTG | |
| IL-6 | NT_007819 | F:
GATGGCTGAAAAAGATGGATGC | 229 |
| | R:
TGGTTGGGTCAGGGGTGGTT | |
| TNF-α | NT_113891 | F:
CCCCAGGGACCTCTCTCTAATC | 241 |
| | R:
GGTTTGCTACAACATGGGCTACA | |
| GAPDH | NT_009759 | F:
CGTCTAGAAAAACCTGCCAA | 117 |
| | R:
TGAAGTCAAAGGAGACCACC | |
Western blot analysis
Western blot analysis was performed according to the
method previously described by Yu et al (23). Briefly, the cells were washed 3
times with PBS and lysed with lysis buffer (Mammalian Cell-PE LB;
G-Biosciences, St. Louis, MO, USA). Equal amounts of protein were
separated on 10% SDS-polyacrylamide minigels and transferred onto
nitrocellulose membranes (Amersham plc., Amersham, UK). Following
incubation with the appropriate primary antibody (ERK, p-ERK, p38,
p-38, JNK, p-JNK, NF-κB, IκBα, and p-IκBα), the membranes were
incubated for 1 h at room temperature with a secondary antibody
conjugated to horseradish peroxidase [goat anti-rabbit IgG (Cat.
no. 31460; Pierce Biotechnology, Inc., Rockford, IL, USA), goat
anti-mouse IgG (Cat. no. sc-2031; Santa Cruz Biotechnology, Inc.)].
Following 3 washes in Tris-buffered saline Tween-20 (TBST),
immunoreactive bands were visualized using the ECL detection system
(Pierce Biotechnology, Inc.).
Electrophoretic mobility shift assay
Nuclear extracts were prepared using the NE-PER
nuclear extraction reagent (Pierce Biotechnology, Inc.). As a probe
for the gel retardation assay, an oligonucleotide harboring the
Ig-κ-chain binding site (κB, 5′-GATCTCAGAGGGGACTTTCCGAGAGA-3′) was
synthesized. A non-radioactive method, whereby the 3′ end of the
probe was labeled with biotin, was used in these experiments
(Pierce Biotechnology, Inc.). The binding reactions contained 5
µg of nuclear extract protein, buffer (10 mM Tris, pH 7.5,
50 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol, 0.05%
Nonidet P-40 and 2.5% glycerol), 50 ng of poly (dI-dC) and 20
µM of biotin-labeled DNA. The reactions were incubated for
20 min at room temperature in a final volume of 20 µl. The
competition reactions were conducted by adding a 100-fold excess of
cold κB to the reaction mixture. The mixture was then separated by
electrophoresis on a 5% polyacrylamide gel in 0.5X Tris-borate
buffer and transferred onto nylon membranes. The biotin-labeled DNA
was detected using a LightShift Chemiluminescent electrophoretic
mobility shift assay (EMSA) kit (Pierce Biotechnology, Inc.).
Statistical analysis
Statistical analyses were conducted using the
Student's t-test. The results are presented as the means ± standard
error of the mean (SEM) of at least 3 separate experiments. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Preparation of in vitro GI digestion and
fractionation on the UF membrane bioreactor system
In previous studies (13,14), for the formation of AIGIDs, 2
infant formulas, gastric digests (phase 1) and intestinal digests
(phase 2) with different biological behaviors were subjected to
hydrolysis, a process which simulates physiological digestion. The
gastric digests (phase 1) corresponded to a pepsin-hydrolyzed
abalone protein-based formula and the intestinal digests (phase 2)
to pepsin-hydrolyzed abalone protein by 2 enzymes (trypsin and
α-chymotrypsin). The abalone intestinal digests (phase 2) were
further separated into 3 MW groups, AIGID I (>10 kDa), II (5–10
kDa) and III (<5 kDa), using UF membranes (MWCO = 5 and 10).
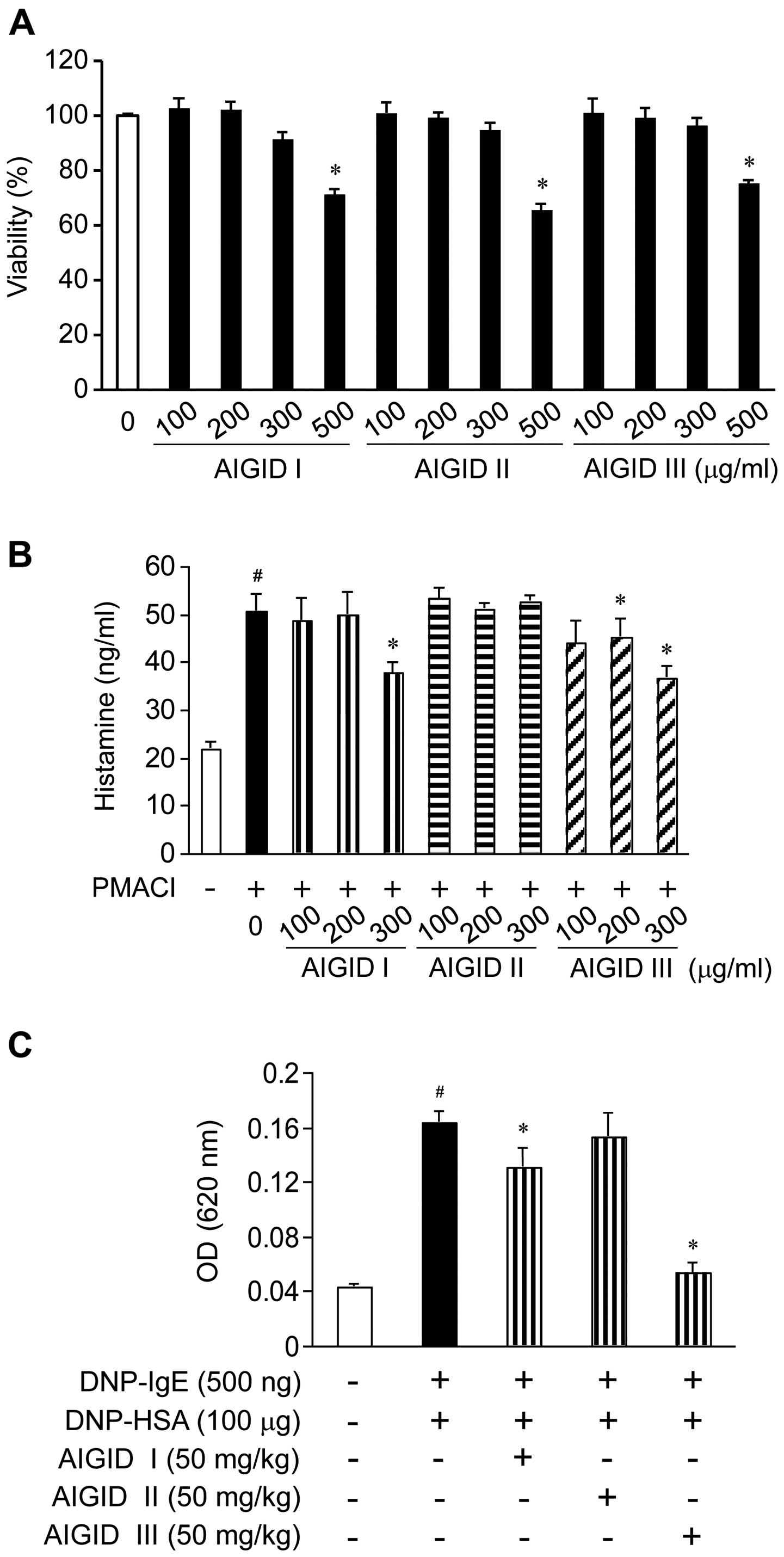
Effects of AIGIDs on the viability of
HMC-1 cells
We examined the viability of the HMC-1 cells
following treatment with 3 types of AIGIDs by CCK-8 assay. No
significant cytotoxicity was observed in the HMC-1 cells treated
with the AIGIDs at a concentration of up to 300 µg/ml;
however, cell viability was significantly reduced by 35% in the
cells treated with 500 µg/ml of the AIGIDs (Fig. 1A). Based on these results, a
concentration range of 100–300 µg/ml was selected for
treatment in the follow-up experiments.
Effect of AIGIDs on the release of
histamine from HMC-1 cells
To determine whether AIGIDs inhibit the release of
histamine from mast cells, we measured the PMACI-induced histamine
release of histamine from HMC-1 cells. The cells were treated with
the AIGIDs at concentrations ranging from 100–300 µg/ml for
1 h prior to stimulation with PMACI. As shown in Fig. 1B, the release of histamine from
the PMACI-treated HMC-1 cells was markedly increased when compared
with that of the control group. By contrast, treatment with 300
µg/ml of AIGID I and AIGID III decreased the release of
histamine from the cells. However, AIGID II had not significant
effect on the release of histamine.
Effects of AIGIDs on the IgE-mediated PCA
reaction in mice
To assess the anti-allergic effects of AIGIDs in
vivo, we used a mouse model of PCA. Localized extravasation was
induced by an injection of DNP-IgE, followed by an antigenic
challenge (DNP-HSA). As shown in Fig.
1C, of the AIGIDs, the administration of AIGID III (50 mg/kg)
markedly inhibited in the PCA reaction. These results suggest that
AIGID III has more potential than AIGID I or II as an allergy
therapeutic. Thus, AIGID III was selected for treatment in the
follow-up in vitro experiments.
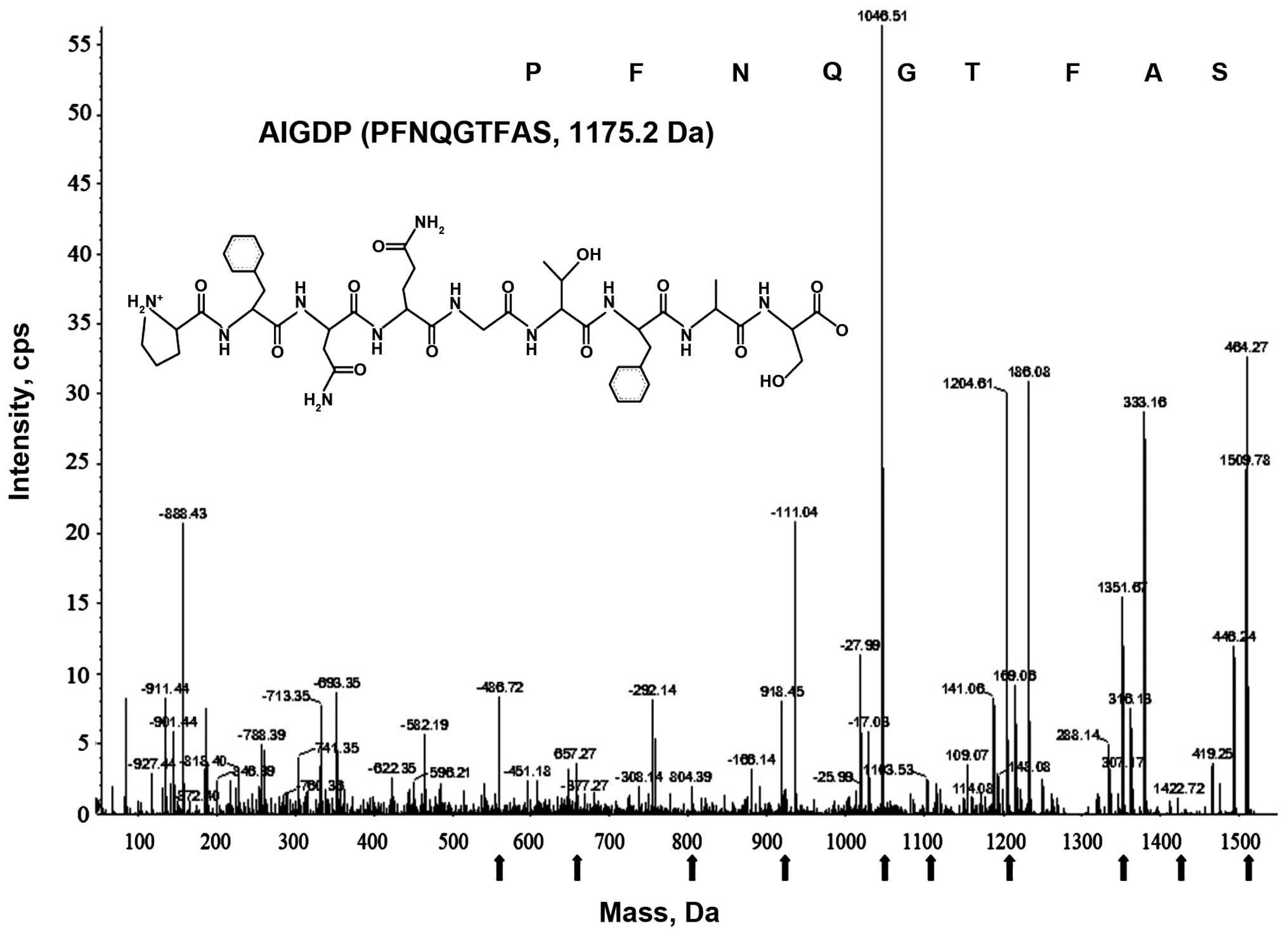
Purification and identification of the
peptide (AIGIDP)
AIGID III was purified using chromatographic
methods, combining FPLC on a HiPrep 16/10 CM FF ion-exchange column
(16×100 mm) and repeated RP-HPLC on a Prime Sphere 10 C18 column
(data not shown), as previously described (14). AIGIDP was over 99% pure according
to RP-HPLC and N-terminal sequence analyses. The molecular mass of
the peptide (AIGIDP) isolated from AIGID III was determined to be
1175.2 Da by analyzing the ESI/MS spectroscopic data, and its full
amino acid sequence was found to be PFNQGTFAS (Fig. 2).
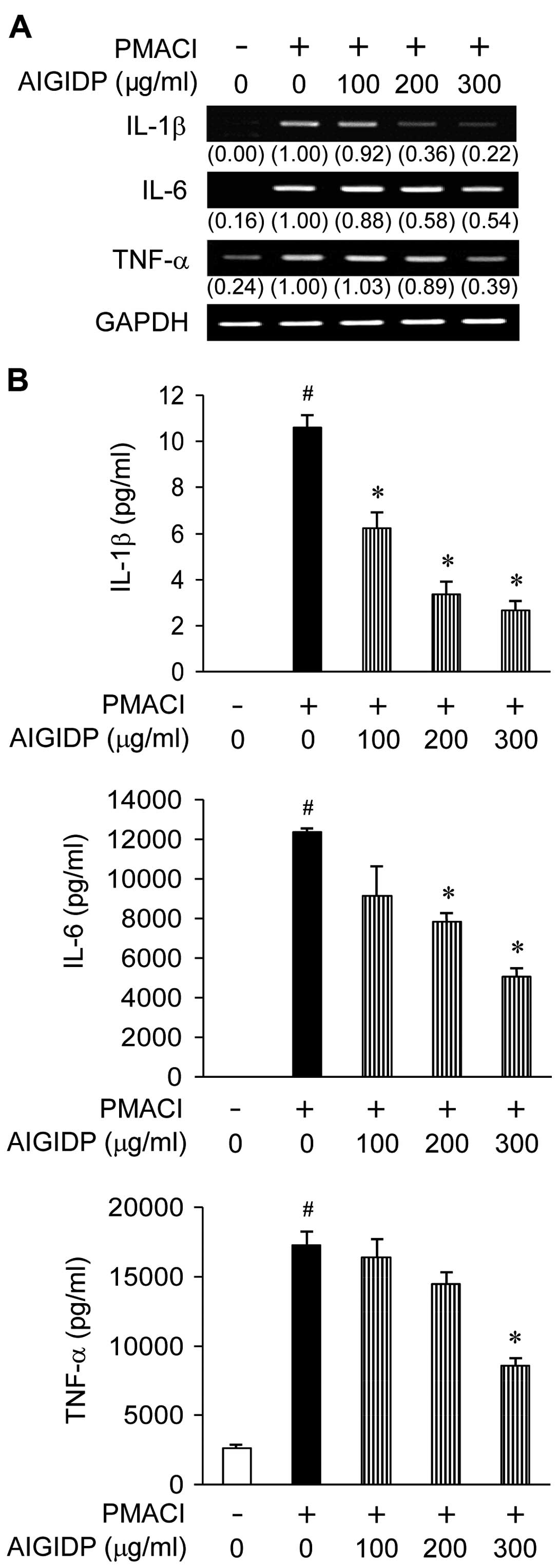
Effect of AIGIDP on the gene expression
and secretion of pro-inflammatory cytokines in HMC-1 cells
To examine the effects of AIGIDP on the production
of pro-inflammatory cytokines, we treated the cells with AIGIDP
(100–300 µg/ml) prior to stimulation with PMACI for 8 h.
IL-1β, IL-6 and TNF-α are pro-inflammatory cytokines which play an
important role in the immediate hypersensitivity responses
associated with allergies (24).
Thus, we examined the effects of AIGIDP on the secretion and gene
expression of cytokines induced by PMACI in HMC-1 cells by ELISA
and RT-PCR. Treatment with AIGIDP suppressed the PMACI-induced mRNA
expression of IL-1β, IL-6 and TNF-α (Fig. 3A). In addition, the PMACI-induced
production of pro-inflammatory cytokines from the mast cells was
decreased by treatment with AIGIDP in a dose-dependent manner
(Fig. 3B).
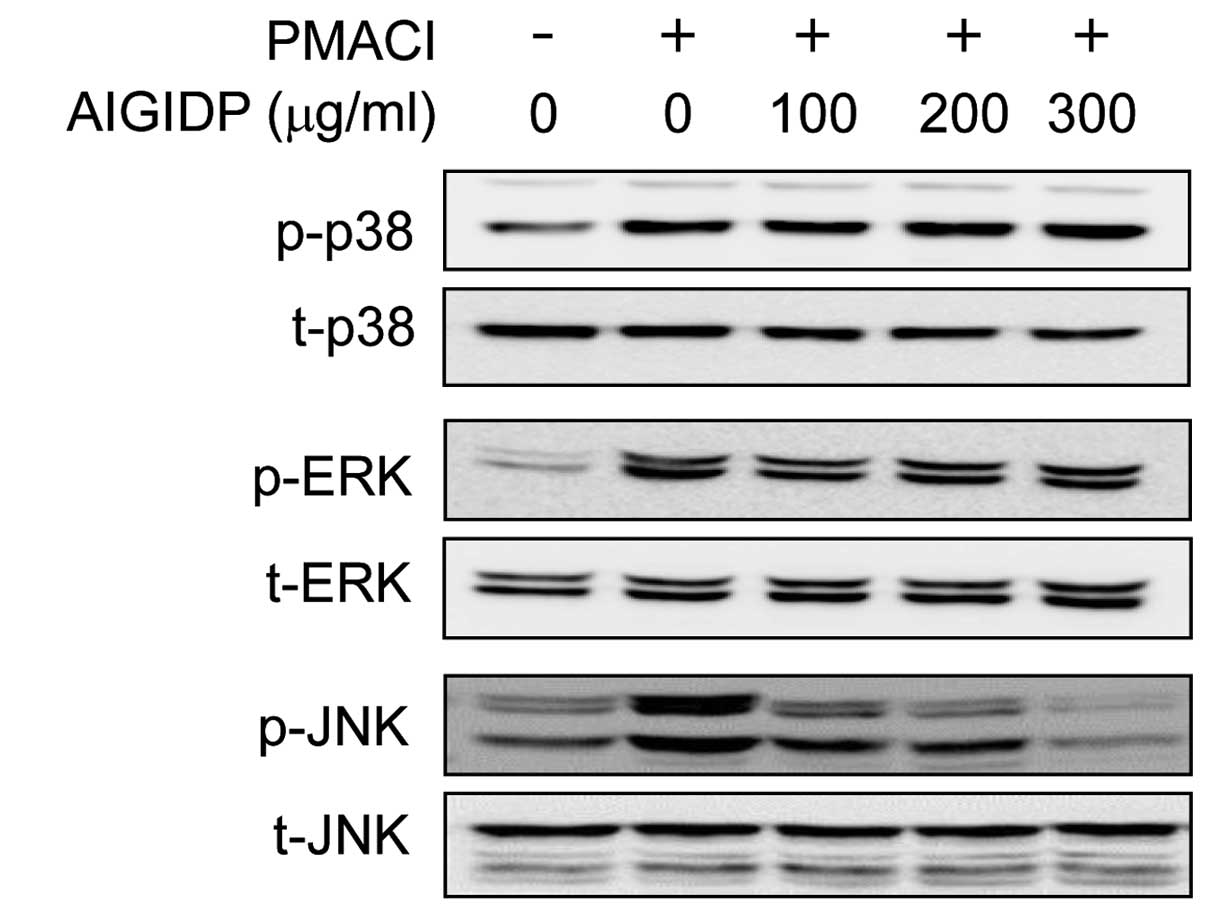
Effects of AIGIDP on the activation of
MAPKs in PMACI-stimulated HMC-1 cells
In order to elucidate the mechanisms underlying the
anti-inflammatory effects of AIGIDP, we examined the activation of
MAPKs using western blot analysis. The activation of MAPKs has
previously been shown to induce the production of pro-inflammatory
cytokines (25). In the present
study, we noted that the stimulation of HMC-1 cells with PMACI
resulted in the increased phosphorylation of all 3 types of MAPKs:
JNK, p38 and ERK1/2. The cells were treated for 30 min with AIGIDP
and then stimulated for 30 min with PMACI. As shown in Fig. 4, treatment with AIGIDP attenuated
the PMACI-induced phosphorylation of JNK; however, it did not
affect the phosphorylation of ERK1/2 and p38 MAPK.
Effects of AIGIDP on the activation of
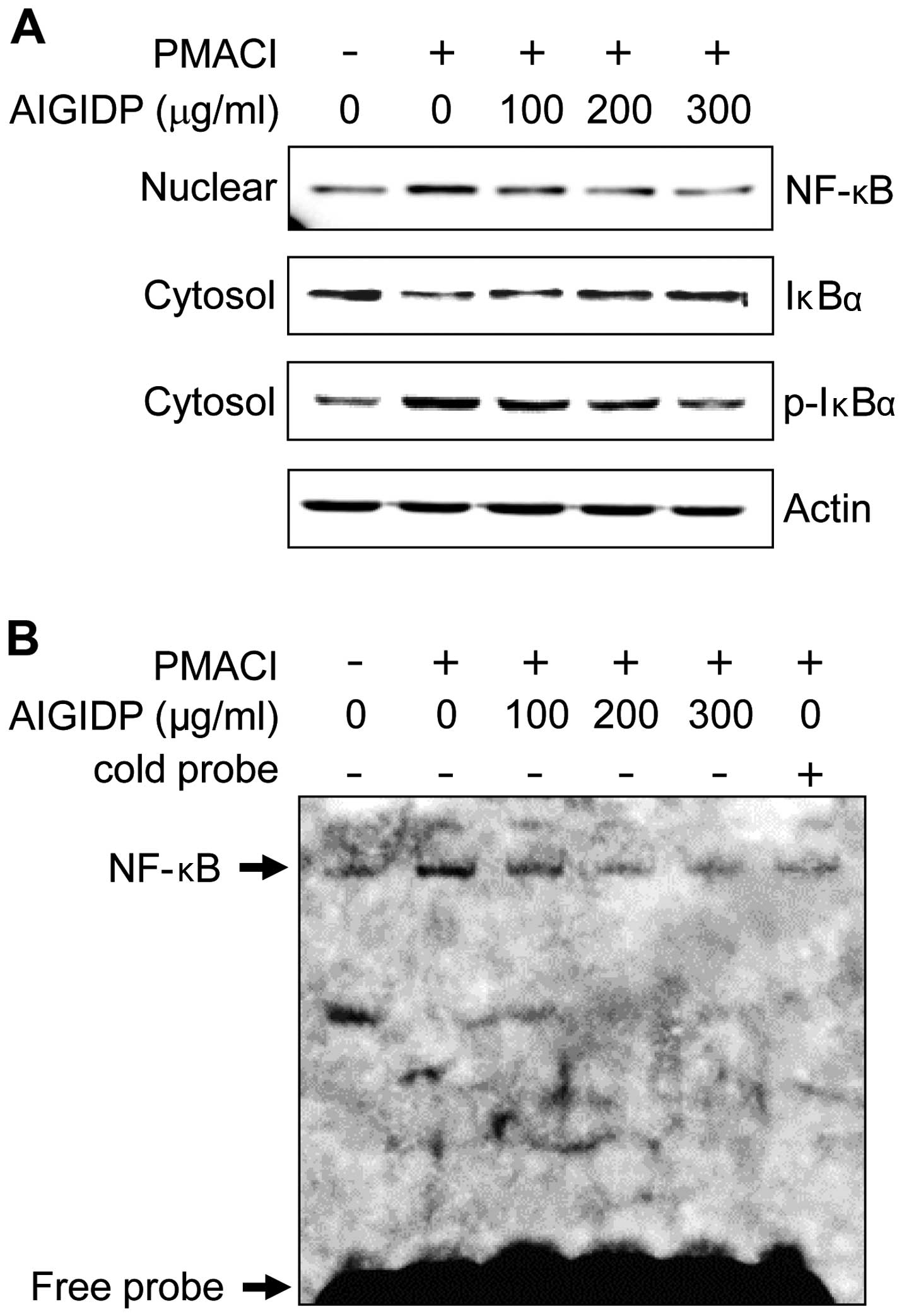
NF-κB in PMACI-stimulated HMC-1 cells
The expression of pro-inflammatory cytokines is
regulated by the transcription factor, NF-κB (26). Thus, in order to elucidate the
mechanisms through which AIGIDP affects the expression of
pro-inflammatory cytokines, we examined the effects of AIGIDP on
the activation of NF-κB. The majority of the inhibitors of NF-κB
activation exert their effects through the suppression of IκBα
phosphorylation and degradation (27). In this study, we found that AIGIDP
inhibited the PMACI-induced phosphorylation and degradation of
IκBα, as well as the nuclear translocation of p65 NF-κB (Fig. 5A). Subsequently, we examined the
effect of AIGIDP on the DNA-binding activity of NF-κB, using an
EMSA kit (Fig. 5B). Treatment
with PMACI treatment a significant increase in the DNA-binding
activity of NF-κB, whereas treatment with AIGIDP markedly reduced
the PMACI-induced DNA-binding activity of NF-κB.
Discussion
The human GI tract is composed of the stomach and
intestines, and includes all of the organs from the mouth to the
anus. The process of digestion converts food into substances that
can be easily absorbed and assimilated by the body through the
action of digestive enzymes. These enzymes break down proteins into
peptides in the GI tract (28,29). Abalone is a marine gastropod and
an important resource in the fishery and food industries and is
widely cultivated in Asia, Africa, Australia and America. To meet
the increasing demand of the Asian market, abalone mari-culture has
been expanding in land- and sea-based systems in Korea, and the
total yield was estimated at 7,580 tons of abalone in 2009 (Marine
Institute of Korea) (14). In
addition, the manufacture of different types of abalone products
(dried, steamed and spiced abalone) has also significantly
increased (13,14). It is currently accepted that
marine organisms possess various bioactive natural components with
a number of physiological functions related to their nutraceutical
and pharmaceutical activities such as antioxidant,
anti-inflammatory, anti-bacterial, anticoagulant, antifungal,
anti-inflammatory, anti-malarial, anti-protozoal, anti-tuberculosis
and anti-viral activities (30).
In the present study, we digested the abalone intestine using the
digestive enzymes, pepsin, trypsin and α-chymotrypsin and prepared
in vitro GI digests of abalone intestines, a byproduct
commonly discarded in the manufacturing process. The AIGIDs were
fractionated into 3 MW groups: AIGID I (>10 kDa), AIGID II (5–10
kDa), and III (<5 kDa) using a UF membrane system (MWCO = 5 and
10 kDa).
Mast cells clearly play a central role in the
pathogenesis of allergic diseases and participate in both the
initiation of the innate immune response and the coordination of
the adaptive immune response. Once activated, mast cells release
biologically active, preformed mediators. The release of preformed
granular mediators, such as histamine, serotonin and
β-hexosaminidase from mast cells is a consequence of complex
biochemical events during the process of degranulation (31). Of these granular mediators,
histamine has long been known to be a major promoter of allergic
inflammatory conditions. Therefore, approaches to controlling the
release of histamine may be utilized for the management of allergic
disorders. In the present study, we investigated the inhibitory
effects of fractionated AIGIDs on the PMACI-induced release of
histamine from mast cells. Of the separated peptides, AIGID I and
AIGID III, but not AIGID II, attenuated the release of histamine in
the PMACI-stimulated HMC-1 cells. Subsequently, in order to
elucidate the anti-allergic properties of AIGIDs in vivo, we
designed a PCA reaction test in mice. PCA can be used in animal
models to mimic the IgE-mediated immediate allergic reaction, which
is known to be induced by mediators, such as histamine that are
secreted from mast cells (32).
As shown in Fig. 1C, when the
mice were administered 3 types of AIGID peptides, AIGID III
exhibited the most promiment suppressive effects on local allergic
reactions compared to the other fractions. However, AIGID II did
not suppress the allergic reaction activity. These results suggest
that AIGID III may be more useful than the other fractions in
treating allergic disorders. Additionally, we purified and
characterized a peptide (AIGIDP) from AIGID III (Fig. 2). Recently, bioactive peptides
from protein hydrolysates have received much attention due to the
unraveling of their structural, compositional and sequential
properties, as well as their biological activities. They can be
used as versatile raw materials for producing nutraceuticals and
pharmaceuticals for humans (33,34). The sequence (AIGIDP: PFNQGTFAS,
1175.2 Da) (Fig. 2) is composed
of a mixture of essential and non-essential amino acids, with a
high concentration of branched chain amino acids (proline) and a
low concentration of methionine. This amino acid composition has
been specifically formulated to build up tolerance to inflammatory
disease as a nutritional supplement. Notably, it has been suggested
that bioactive peptides with low molecular weight are able to cross
the intestinal barrier (9).
Previous research has confirmed that low molecular-weight peptides
are involved in potent bioactivities (35). Based on these results, AIGIDP was
selected during the screening of anti-allergic activity for our
follow-up experiments.
Mast cell-derived pro-inflammatory cytokines, such
as IL-1β, IL-6 and TNF-α are key indicators of inflammatory
allergic disease (36). IL-1β
receptor antagonists have been shown to alleviate the late
asthmatic reaction in animal models (37). IL-6 is produced from mast cells
and can influence B-cell and dendritic cell biology (38). TNF-α has an important amplifying
effect in asthmatic inflammation and stimulates airway epithelial
cells to produce cytokines (39).
Therefore, a reduction in the levels of these pro-inflammatory
cytokines is one of the key indicators of an attenuation in
allergic inflammatory symptoms.
In the present study, to evaluate the mechanisms
responsible for the inhibitory effects of AIGIDP on the production
of pro-inflammatory cytokines, we examined the activation of the
transcription factor, NF-κB, and MAPKs. The MAPK (JNK, ERK1/2 and
p38 MAPK) cascade is one of the important signaling pathways in
immune responses, and these pathways play critical roles in the
activation, survival and differentiation of, as well as cytokine
production in mast cells (40).
Therefore, MAPK pathways are appropriate targets for the
pharmacological treatment of allergic diseases. In this respect, we
examined the inhibitory effects of AIGIDP on the activation of
MAPKs in PMACI-stimulated HMC-1 cells. As shown in Fig. 4, AIGIDP inhibited the
phosphorylation of JNK, but not that of p38 MAPK and ERK1/2. Many
transcription factors have been implicated in the pathophysiology
of allergic disease. NF-κB can be activated by multiple stimuli,
such as allergens (41). NF-κB
dimers are usually present in the cytoplasm of most cells in an
inactive form, as they bind to an inhibitor protein referred to as
IκBα (42). After an inflammatory
stimulus, the phosphorylation of IκBα triggers their degradation
and the translocation of NF-κB to the nucleus, where it induces the
expression of a broad variety of inflammatory genes, including
cytokines, enzymes, adhesion molecules, and acute-phase proteins
(43). In the present study, we
noted that AIGIDP inhibited PMACI-induced NF-κB activation by
suppressing IκBα phosphorylation and its degradation.
In conclusion, of the AIGIDs, AIGID III was clearly
more potently anti-allergic than the other fractions. Thus, mice
treated with AIGID III were protected from the IgE-mediated PCA.
The molecular mass of the novel peptide (AIGIDP) isolated from
AIGID III was determined to be 1175.2 Da according to ESI/MS
spectroscopy data, and the amino acid sequence was found to be
PFNQGTFAS. It was demonstrated that AIGIDP regulated the production
of IL-1β, IL-6 and TNF-α in PMA plus A23187-stimulated HMC-1 cells
and decreased the release of histamine. In addition, AIGIDP
inhibited the activation of the JNK and NF-κB pathways. Therefore,
we suggest that the regulation of the JNK and NF-κB signalling
pathways by AIGIDP in HMC-1 cells has the potential to be used in
the prevention or treatment of mast cell-mediated allergic
diseases.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), funded by the Ministry of Education, Science and Technology
(no. 2013R1A1A1A05013577), and was also supported by the Study for
Establishment of Marine Natural Products Library, funded by the
National Marine Biodiversity Institute of Korea (MABIK) and Marine
Biotechnology Program (no. 20150220) funded by the Ministry of
Oceans and Fisheries of Korea.
References
|
1
|
Ekanayake PM, De Zoysa M, Kang HS, Wan Q,
Jee Y, Lee YH, Kim SJ and Lee J: Cloning, characterization and
tissue expression of disk abalone (Haliotis discus discus)
catalase. Fish Shellfish Immunol. 24:267–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu BW, Wang LS, Zhou DY, Li DM, Sun LM,
Yang JF, Wu HT, Zhou XQ and Tada M: Antioxidant activity of
sulphated polysaccharide conjugates from abalone (Haliotis discus
hannai Ino). Eur Food Res Technol. 227:1663–1668. 2008. View Article : Google Scholar
|
|
3
|
Cook PA and Gordon HR: World abalone
supply, markets, and pricing. J Shellfish Res. 29:569–571. 2010.
View Article : Google Scholar
|
|
4
|
Jo HY, Jung WK and Kim SK: Purification
and characterization of a novel anticoagulant peptide from marine
echiuroid worm, Urechis unicinctus. Process Biochem. 43:179–184.
2008. View Article : Google Scholar
|
|
5
|
Liu Z, Liu H, Liu X and Wu X: Purification
and cloning of a novel antimicrobial peptide from salivary glands
of the hard tick, Ixodes sinensis. Comp Biochem Physiol B Biochem
Mol Biol. 149:557–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Byun HG and Kim SK: Purification and
characterization of angiotensin I converting enzyme (ACE)
inhibitory peptides from Alaska Pollack (Theragra chalcogramma)
skin. Process Biochem. 36:1155–1162. 2001. View Article : Google Scholar
|
|
7
|
Erdmann K, Cheung BWY and Schröder H: The
possible roles of food-derived bioactive peptides in reducing the
risk of cardiovascular disease. J Nutr Biochem. 19:643–654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elias RJ, Kellerby SS and Decker EA:
Antioxidant activity of proteins and peptides. Crit Rev Food Sci
Nutr. 48:430–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roberts PR, Burney JD, Black KW and Zaloga
GP: Effect of chain length on absorption of biologically active
peptides from the gastrointestinal tract. Digestion. 60:332–337.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vermeirssen V, van der Bent A, Van Camp J,
van Amerongen A and Verstraete W: A quantitative in silico analysis
calculates the angiotensin I converting enzyme (ACE) inhibitory
activity in pea and whey protein digests. Biochimie. 86:231–239.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung WK, Qian ZJ, Lee SH, Choi SY, Sung
NJ, Byun HG and Kim SK: Free radical scavenging activity of a novel
antioxidative peptide isolated from in vitro gastrointestinal
digests of Mytilus coruscus. J Med Food. 10:197–202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian ZJ, Jung WK, Byun HG and Kim SK:
Protective effect of an antioxidative peptide purified from
gastrointestinal digests of oyster, Crassostrea gigas against free
radical induced DNA damage. Bioresour Technol. 99:3365–3371. 2008.
View Article : Google Scholar
|
|
13
|
Qian ZJ, Kim SA, Lee JS, Kim HJ, Choi IH
and Jung WK: The antioxidant and anti-inflammatory effects of
abalone intestine digest, Haliotis discus hannai in RAW 264.7
macrophages. Biotechnol Bioprocess Eng; BBE. 17:475–484. 2012.
View Article : Google Scholar
|
|
14
|
Nguyen VT, Qian ZJ, Ryu B, Kim KN, Kim D,
Kim YM, Jeon YJ, Park WS, Choi IW, Kim GH, et al: Matrix
metalloproteinases (MMPs) inhibitory effects of an octameric
oligopeptide isolated from abalone Haliotis discus hannai. Food
Chem. 141:503–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conrad ML, Renz H and Blaser K:
Immunological approaches for tolerance induction in allergy. Curr
Top Microbiol Immunol. 352:1–26. 2011.PubMed/NCBI
|
|
16
|
Marsella R, Nicklin C and Lopez J: Studies
on the role of routes of allergen exposure in high IgE-producing
beagle dogs sensitized to house dust mites. 17:306–312. 2006.
|
|
17
|
Stone KD, Prussin C and Metcalfe DD: IgE,
mast cells, basophils, and eosinophils. J Allergy Clin Immunol.
125:S73–S80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Metz M and Maurer M: Mast cells - key
effector cells in immune responses. Trends Immunol. 28:234–241.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galli SJ, Maurer M and Lantz CS: Mast
cells as sentinels of innate immunity. Curr Opin Immunol. 11:53–59.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marshall JS: Mast-cell responses to
pathogens. Nat Rev Immunol. 4:787–799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawakami T and Galli SJ: Regulation of
mast-cell and basophil function and survival by IgE. Nat Rev
Immunol. 2:773–786. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kapsokefalou M and Miller DD: Effects of
meat and selected food components on the valence of nonheme iron
during in vitro digestion. J Food Sci. 56:352–355. 1991. View Article : Google Scholar
|
|
23
|
Yu BC, Lee DS, Bae SM, Jung WK, Chun JH,
Urm SH, Lee DY, Heo SJ, Park SG, Seo SK, et al: The effect of
cilostazol on the expression of matrix metalloproteinase-1 and type
I procollagen in ultraviolet-irradiated human dermal fibroblasts.
Life Sci. 92:282–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shakoory B, Fitzgerald SM, Lee SA, Chi DS
and Krishnaswamy G: The role of human mast cell-derived cytokines
in eosinophil biology. J Interferon Cytokine Res. 24:271–281. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
26
|
Paeng SH, Park WS, Jung WK, Lee DS, Kim
GY, Choi YH, Seo SK, Jang WH, Choi JS, Lee YM, et al: YCG063
inhibits Pseudomonas aeruginosa LPS-induced inflammation in human
retinal pigment epithelial cells through the TLR2-mediated
AKT/NF-κB pathway and ROS-independent pathways. Int J Mol Med.
36:808–816. 2015.PubMed/NCBI
|
|
27
|
Yu GJ, Choi IW, Kim GY, Kim BW, Park C,
Hong SH, Moon SK, Cha HJ, Chang YC, Paek KY, et al:
Anti-inflammatory potential of saponins derived from cultured wild
ginseng roots in lipopolysaccharide-stimulated RAW 264.7
macrophages. Int J Mol Med. 35:1690–1698. 2015.PubMed/NCBI
|
|
28
|
Guerra A, Etienne-Mesmin L, Livrelli V,
Denis S, Blanquet-Diot S and Alric M: Relevance and challenges in
modeling human gastric and small intestinal digestion. Trends
Biotechnol. 30:591–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borgström B, Dahlqvist A, Lundh G and
Sjovall J: Studies of intestinal digestion and absorption in the
human. J Clin Invest. 36:1521–1536. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mayer AMD, Rodríguez AD, Berlinck RG and
Fusetani N: Marine pharmacology in 2007–8: Marine compounds with
antibacterial, anticoagulant, antifungal, anti-inflammatory,
antimalarial, antiprotozoal, antituberculosis, and antiviral
activities; affecting the immune and nervous system, and other
miscellaneous mechanisms of action. Comp Biochem Physiol C Toxicol
Pharmacol. 153:191–222. 2011. View Article : Google Scholar
|
|
31
|
Ma HT and Beaven MA: Regulation of
Ca2+ signaling with particular focus on mast cells. Crit
Rev Immunol. 29:155–186. 2009. View Article : Google Scholar
|
|
32
|
Kemp SF and Lockey RF: Anaphylaxis: a
review of causes and mechanisms. J Allergy Clin Immunol.
110:341–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li-Chan ECY: Bioactive peptides and
protein hydrolysates: research trends and challenges for
application as nutraceuticals and functional food ingredients. Curr
Opin Food Sci. 1:28–37. 2015. View Article : Google Scholar
|
|
34
|
Lordan S, Ross RP and Stanton C: Marine
bioactives as functional food ingredients: potential to reduce the
incidence of chronic diseases. Mar Drugs. 9:1056–1100. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Je JY, Park PJ, Kwon JY and Kim SK: A
novel angio-tensin I converting enzyme inhibitory peptide from
Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. J
Agric Food Chem. 52:7842–7845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Min YD, Choi CH, Bark H, Son HY, Park HH,
Lee S, Park JW, Park EK, Shin HI and Kim SH: Quercetin inhibits
expression of inflammatory cytokines through attenuation of
NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res.
56:210–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okada S, Inoue H, Yamauchi K, Iijima H,
Ohkawara Y, Takishima T and Shirato K: Potential role of
interleukin-1 in allergen-induced late asthmatic reactions in
guinea pigs: suppressive effect of interleukin-1 receptor
antagonist on late asthmatic reaction. J Allergy Clin Immunol.
95:1236–1245. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galli SJ, Nakae S and Tsai M: Mast cells
in the development of adaptive immune responses. Nat Immunol.
6:135–142. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakae S, Lunderius C, Ho LH, Schäfer B,
Tsai M and Galli SJ: TNF can contribute to multiple features of
ovalbumin-induced allergic inflammation of the airways in mice. J
Allergy Clin Immunol. 119:680–686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sundström M, Alfredsson J, Olsson N and
Nilsson G: Stem cell factor-induced migration of mast cells
requires p38 mitogen-activated protein kinase activity. Exp Cell
Res. 267:144–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barnes PJ: Pathophysiology of allergic
inflammation. Immunol Rev. 242:31–50. 10112011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: a pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|