Introduction
Atherosclerosis is a multifactor cardiovascular
disease, which is associated with physiological and behavioral risk
factors, such as age, gender, hypertension, hypercholesterolemia,
obesity, diabetes, smoking and a sedentary lifestyle (1). Studies have indicated that chronic
inflammatory responses and oxidative stress conditions in vascular
tissue are associated with the pathogenesis of atherosclerosis
(2). Oxidized low-density
lipoprotein (ox-LDL) is a particularly important risk factor for
the pathogenesis of atherosclerosis. It is well known that ox-LDL
promotes the occurrence and development of atherosclerosis through
various mechanisms, including the induction of endothelial cell
damage. In addition, ox-LDL causes endothelial cell activation,
dysfunction and death, as well as impaired vasorelaxation, which
contribute causally to the development and progression of
atherosclerosis (3–5). Accumulating has indicated that
ox-LDL-mediated biological processes may be related to the
increased activity of NADPH oxidase (6–8).
NADPH oxidase, a multisubunit enzymatic complex comprised of two
membrane-bound subunits, gp91 and p22phox, is the major
source of intracellular reactive oxygen species (ROS) in vascular
cells. Moreover, cytoplasmic subunits, such as p47phox
and p67phox are critical components of endothelial NADPH
oxidase. For example, it has been demonstrated that the activation
of Rac-1 and p47phox is involved in the generation of
superoxide, a molecule that stimulates inflammatory gene expression
through a redox-sensitive signaling pathway in vascular endothelial
cells (9).
Green tea is a one of the most ancient and popular
beverages consumed worldwide, and it has been suggested to prevent
the development of a variety of diseases, including diabetes,
hypertension, cancer and cardiovascular diseases (10). The effects of green tea are
attributed to its abundant and biologically active catechin,
epigallocatechin-3-gallate (EGCG), which has antioxidant (11), anti-inflammatory (12), anti-tumorigenic (13) and anti-angiogenic (14) effects. Accumulating evidence has
indicated that EGCG plays an important role in the protection
against the initiation and/or development of atherosclerosis
(15). Previous studies have
demonstrated that EGCG possesses potent antioxidant properties,
which attenuate oxidative injury induced by ox-LDL in endothelial
cells (16–19). In a recent study, Cai et al
demonstrated that green tea EGCG attenuated Porphyromonas
gingivalis-induced atherosclerosis (15). Based on the findings of previous
studies, we hypothesized that EGCG may protect endothelial cells
against ox-LDL-induced damage by suppressing the ox-LDL-induced
activity of NADPH oxidase.
The Notch pathway is an evolutionary highly
conserved signaling system. Thus far, 4 Notch receptors (Notch1–4)
and 5 ligands [Delta-like (Dll)-1, -3, -4 and Jagged (JAG)-1 and
-2] have been identified in vertebrates. The Notch signaling
pathway shows functional significance in neural development
(20,21), multiple cellular processes,
embryonic development and self-renewing adult tissues (22,23). Recently, Notch signaling was
proven to be critical for arterial specification, sprouting
angiogenesis and vessel maturation (24–27). However, little is known about the
function of the Jagged-1/Notch pathway in the protective effects
exerted by EGCG against ox-LDL-induced endothelial cell damage.
In this study, ox-LDL-exposed endothelial cells were
treated with various concentrations of EGCG, to examine the
hypothesis that 50 µM EGCG may hamper ox-LDL-induced
endothelial cell damage by modulating the Jagged-1/Notch
pathway.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from the American Type Culture Collection (ATCC;
Rockville, MD, USA) and cultured in RPMI-1640 (Invitrogen,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS)
and 100 µg/ml penicillin/streptomycin (both from
Invitrogen).
Isolation of endothelial progenitor cells
(EPCs)
The isolation and culture of EPCs was performed as
previously described (28).
Mononuclear cells were isolated from the bone marrow of
apolipoprotein E (ApoE) knockout (ApoE-KO or ApoE−/−)
mice by flushing the femurs and tibias of the mice. Briefly, the
mice (n=5) were anesthetized by an intraperitoneal injection of 10%
chloral hydrate (0.3 ml/100 g) and then sacrificed by cervical
dislocation. After disinfecting the area with alcohol and
performing muscle incision, the whirlbone of the thigh was clipped
and a needle was inserted into the bone tube to flush out the bone
marrow using sterilized PBS. The bone marrow was then cultured in
selective medium (EGM-2; CC-3162; Lonza, Walkersville, MD, USA) for
14 days. The Institutional Animal Care and Use Committee of Central
South University approved all the animal protocols. Following
isolation, the cells were plated immediately onto 6-well plates
pre-coated with fibronectin (Sigma-Aldrich, St. Louis, MO USA) at a
density of 5×106 cells/well and cultured in RPMI-1640
(Gibco Life Technologies, Grand Island, NY, USA) with 10% FBS
(Gibco Life Technologies). The cells were incubated at 37°C in 5%
CO2. The culture medium was changed every 3 days. After
4 days in culture, the non-adherent cells were washed away with
0.01 mol/l phosphate-buffered saline (PBS; pH 7.4) and fresh medium
was then added. The EPCs after 7 days of culture were used in the
experiments. To confim the identity of the EPCs prior to use,
western blot analysis was performed to detect EPC-specific surface
markers [CD34, CD133 and vascular endothelial growth factor
receptor (VEGFR)-2] (data not shown).
Preparation of ox-LDL
Human ox-LDL was obtained from Shanghai Luwen
Biotech Inc. (LW-6002; Shanghai, China). LW Human LDL had been
purified to homogeneity via ultra-centrifugation (1.019–1.063 g/cc)
and had been oxidized using 5 µM
Cu2SO4 (oxidant) ihn PBS at 37°C for 20 h.
The reaction was terminated by the addition of EDTA-Na2.
The concentration of ox-LDL used in the present study was 100
mg/l.
Mice and treatments
Six-week-old male ApoE-KO mice were obtained from
Xiangya Hospital of Central South University, Changsha, China. The
mice were randomly divided into 3 groups (n=5) and administered for
7 weeks (via their drinking water) with the treatments: 2 groups
were administered distilled water, and the other group was
administered 0.8 g/l EGCG (Sigma-Aldrich). As previously described
(15), the mice in the EGCG group
and those in the distilled water groups were fed a high-fat diet
(HFD). All the mice were monitored until sacrifce (by cervical
dislocation) at the age of 15 weeks and the tissue samples of the
ApoE−/− mice were then collected. All the animal
protocols were approved by the Institutional Animal Care and Use
Committee of Central South University.
Manipulation of Jagged-1 expression
levels
pCDNA3.1 vectors containing Jagged-1
(pCDNA3.1-Jagged-1) and pRNAT-U6.1/Neo vectors containing Jagged-1
shRNA (pRNAT-U6.1/Neo-Jagged-1-sh) were constructed and transfected
into the HUVECs. To confirm the effects of the vectors on the
expression of Jagged-1, western blot analysis was performed to
measure the protein expression levels of Jagged-1 in the HUVECs.
The transfected cells were expanded and harvested for further
analysis. Untransfected cells were used as controls (Con) and cells
transfected with empty carrier vectors (pCDNA3.1 or pRNAT-U6.1/Neo)
served as the negative control (NC).
Isolation of mRNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the HUVECs or the EPCs
using TRIzol reagent (Invitrogen) and then reverse transcribed into
cDNA using the RevertAid™ First Stand cDNA Synthesisi kit (Thermo
Fisher Scientific, Waltham, MA, USA). The relative expression
levels were detected using Real-Time PCR SYBR-Green Reagents
(Dongsheng, Xian, China) in accordance with the manufacturer's
instructions. Target RNA levels were normalized to β-actin. The
primer sequences used in this study are listed in Table I.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Gene | | Primer sequence
(5′→3′) |
|---|
|
p22phox | Sense |
ATTGTGGCGGGCGTGTT |
| Antisense |
GCACCGAGAGCAGGAGAT |
|
p47phox | Sense |
CCTGACGAGACGGAAGACC |
| Antisense |
CTTTCCTGATGACCCACCA |
|
p67phox | Sense |
CAGACAGAGAAATATGATTTGGC |
| Antisense |
GGATCACCACTGGCTCATATAG |
| HES1 | Sense |
GAAGGAAGTGGTCGAAGCTC |
| Antisense |
ATGCGCGTCACTTTCCAG |
| Jagged-1 | Sense |
ACCTGCCAGTGCCTGAATG |
| Antisense |
AGGCAAGGTCGAGGGCC |
| β-actin | Sense |
AGGGGCCGGACTCGTCATACT |
| Antisense |
GGCGGCACCACCATGTACCCT |
Cell survival assay
The
3-(4,5-dimethylthiazal-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay was used to estimate cell viability, as prevoiusly described
(29). Briefly, the cells were
plated at a density of 1×104 cells/well in 96-well
plates. After being subjected to the specific treatments, the cells
were incubated with MTT solution at a final concentration of 0.5
mg/ml for 4 h at 37°C. After the removal of the medium, 150 mM DMSO
were added to dissolve the formazan crystals. The absorbance was
read at 570 nm using a multi-well scanning microplate reader
(Thermo Fisher Scientific). The cells in the control group were
considered 100% viable.
Western blot analysis
Protein lysates were separated by 10%
SDS-polyacrylamide gel electrophoresis and then electroblotted onto
PVDF membranes. Primary antibodies against inducible nitric oxide
synthase (iNOS), endothelial nitric oxide synthase (eNOS), NADPH
(p47phox), NADPH (p67phox), NADPH
(p22phox), Jagged-1, hairy and enhancer of split (HES)1,
HES5 and Math1 were used, with β-actin antibody as an internal
control. Densitometric analysis was performed using LabWorks Image
Acquisition and Analysis software (UVP, Inc., Upland, CA, USA).
Annexin V and propidium iodide (PI)
binding assay
The HUVECs were cultured in 6-well plates and
exposed to ox-LDL (50 µg/ml) for 0, 12, 24 and 48 h. The
cells were harvested and stained using the Annexin V-FITC Apoptosis
Detection kit (Beyotime Biotech, Jiangsu, China) according to the
manufacturer's instructions. Briefly, 5×105 cells were
suspended in 500 µl 1X binding buffer (10 mM HEPES pH 7.4,
140 mM NaCl, 2.5 mM CaCl2). The cells were then
incubated with Annexin V (1:20) for 5 min followed by incubation
with PI (1 mg/ml) for 15 min. The apoptotic rate was evaluated by
flow cytometry.
Adhesion assay
The HUVECs (1×105 cells/ml) were cultured
in 96-well flat-bottom plates (0.1 ml/well) for 1–2 days. The cells
were then pre-treated with the indicated concentrations of EGCG and
incubated with ox-LDL. The wells were incubated at 37°C for 50 min
in a 5% CO2 incubator and washed 3 times with PBS to
remove the non-adherent cells.
Morphological and immunohistochemistry
analysis
The root of the aorta was obtained from the
ApoE−/− mice and fixed in 4% paraformaldehyde overnight.
Tissue specimens were then cut at 5 µm thickness for
subsequent hematoxylin and eosin (H&E) staining or
immunohistochemical analysis. The method for H&E staining of
the aortic tissues was conducted as previously described (30). Immunohistochemical analysis was
performed according to the manufacturer's instructions. The
staining results were observed and captured using an AE31 light
microscope (Motic, Xiamen, China).
Statistical analysis
Each experiment was repeated at least 3 times. Data
are presented as the means ± SE and analyzed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Statistical comparisons
between the groups were analyzed using a Student's t-test and a
two-tailed value of P<0.05 was considered to indicate a
statistically significant difference.
Results
ox-LDL induces the apoptosis of
endothelial cells
It has been previously demonstrated that ox-LDL
induces endothelial cell apoptosis (31). As shown in Fig. 1A, MTT assay revealed that the
incubation of HUVECs with ox-LDL (100 mg/l) enhanced endothelial
cell apoptosis in a time-dependent manner. This result was also
confirmed by flow cytometry (Fig. 1B
and C). The HUVECs that were incubated with ox-LDL for 72 h
were used in the subsequent experiments.
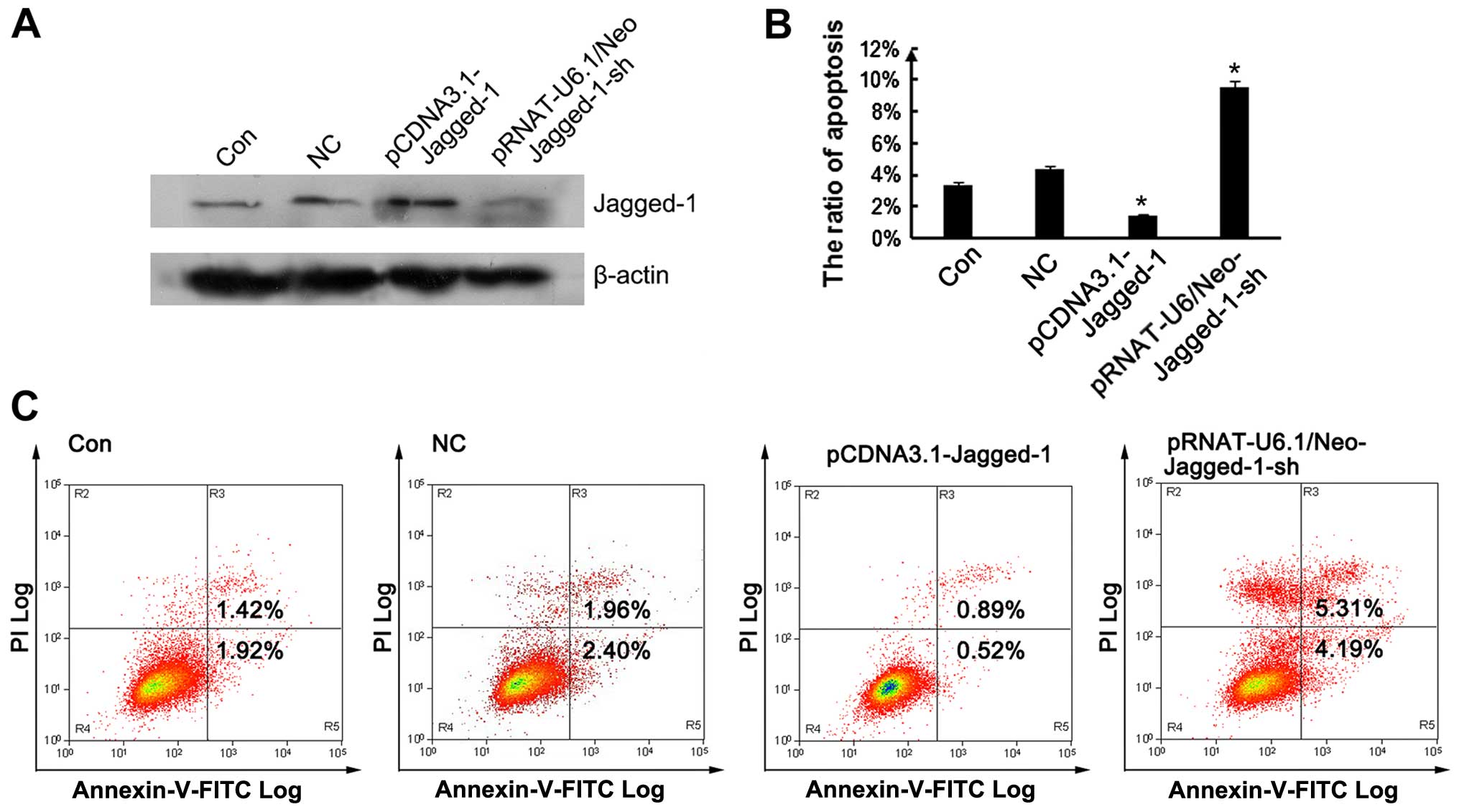
Jagged-1 affects the apoptosis of
endothelial cells
To determine the potential effect of Jagged-1 on
endothelial cell apoptosis, we transfected pCDNA3.1 vectors
containing Jagged-1 (pCDNA3.1-Jagged-1) and transfected
pRNAT-U6.1/Neo vectors containing Jagged-1 shRNA
(pRNAT-U6.1/Neo-Jagged-1-sh) into the HUVECs in order to induce the
overexpression or to silence Jagged-1, respectively. Western blot
analysis revealed that the Jagged-1 levels were effectively
downregulated following transfection of the cells with Jagged-1
shRNA, and significantly enhanced following transfection with
pCDNA3.1-Jagged-1 (Fig. 2A). As
shown in Fig. 2B and C, the
silencing of Jagged-1 significantly enhanced the apoptosis of the
HUVECs compared with the control group, and this effect was
reversed by the overexpression of Jagged-1.
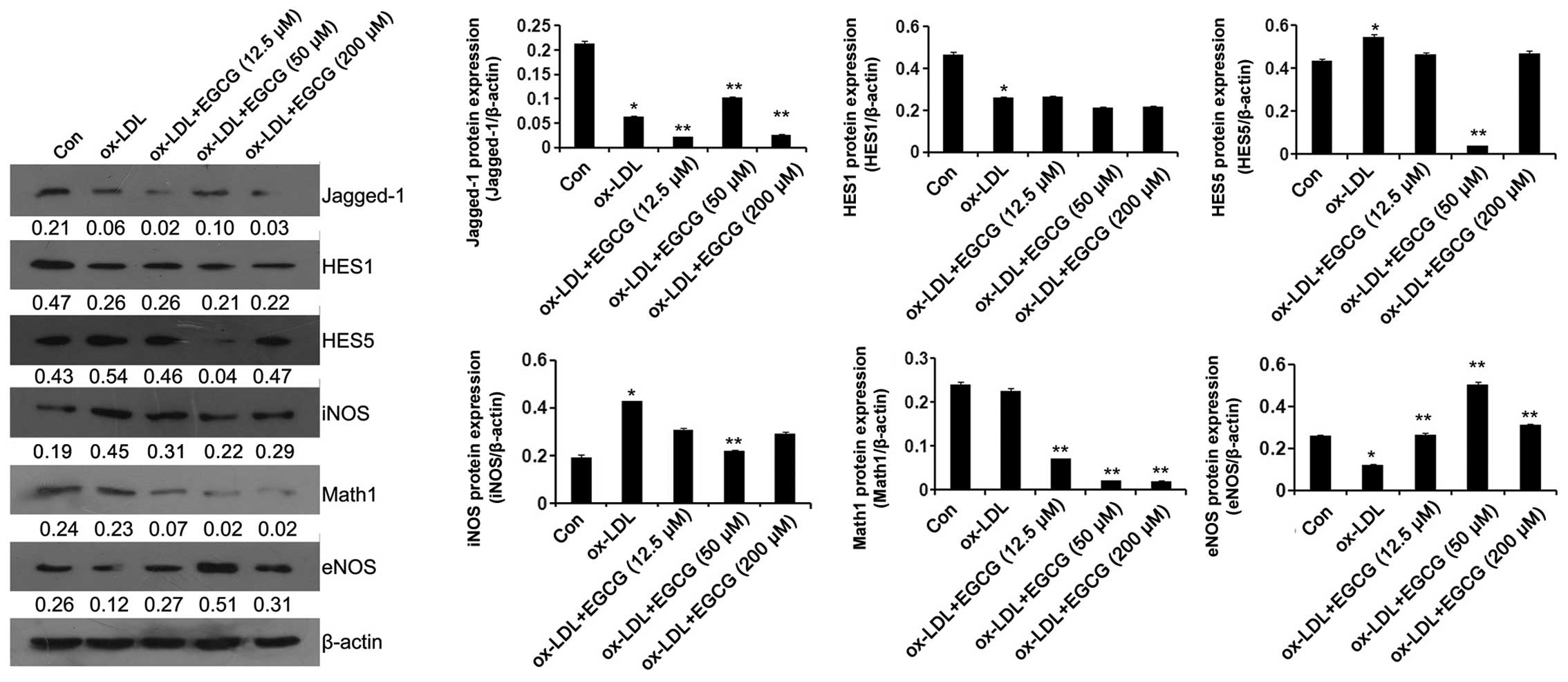
EGCG protects against ox-LDL-induced
endothelial dysfunction
It has been previously demonstrated that the
activation of NADPH oxidase is associated with ox-LDL-induced
endothelial dysfunction (32,33). As active NADPH oxidase is
assembled on the membrane, the effects of EGCG on the membrane
translocation of p22phox, p47phox and
p67phox were examined by RT-qPCR. As shown in Fig. 3, the levels of membrane-bound
p22phox and p47phox were markedly decreased
in the cells treated with ox-LDL for 72 h compared with the
untreated cells (Fig 3). Of note,
this decreasing effect on p47phox was enhanced by
treatment with EGCG in a dose-dependent manner. In addition,
treatment with EGCG enhanced p22phox expression, with a
significant increase in expression being observed following
treatment with 50 µM EGCG (P<0.01). As regards
p67phox, the expression levels were slightly increased
by ox-LDL and then decreased following treatment with 12.5
µM EGCG. However, treatment with 50 and 200 µM EGCG
increased the levels of p67phox even further than ox-LDL
did. The most significant effect on p67phox expression
was observed following treatment with 200 µM EGCG (Fig. 3A–C). Moreover, ox-LDL decreased
the expression of Jagged-1, and this effect was attenuated by
treatment with 50 µM EGCG (Fig. 3D). Western blot analysis revealed
that treatment with 12.5 µM EGCG further decreased Jagged-1
protein expression. However, treatment with 50 µM EGCG
attenuated this effect (Fig.
4).
Notch signaling has been proven to be critical for
arterial specification, sprouting angiogenesis and vessel
maturation (24–27). Thus, in this study, we examined
the effects of Notch signaling on the EGCG-mediated protection
against ox-LDL-induced endothelial cell dysfunction. As shown in
Fig. 4, the expression level of
HES1 was significantly decreased following the exposure of the
cells to ox-LDL. However, EGCG had no effect on HES1 expression in
the ox-LDL + EGCG (12.5, 50 and 200 µM) groups compared with
the ox-LDL group. The expression level of HES5 was increased
following exposure of the cells to ox-LDL, and this effect was
attenuated by treatment with 50 µM EGCG. EGCG (12.5 and 200
µM) did not affect HES5 expression in the ox-LDL + EGCG
(12.5 and 200 µM) groups compared with the ox-LDL group.
Exposure of the cells to ox-LDL did not affect Math1 expression;
however, treatment with EGCG decreased Math1 expression in a
dose-dependent manner (Fig.
4).
It has been previously demonstrated that ox-LDL
reduces the expression of eNOS, thereby altering endothelial
biology (34). In the present
study, we examined the effects of EGCG on the protein expression
levels of eNOS and iNOS in endothelial cells exposed to ox-LDL. Our
results revealed that ox-LDL significantly reduced eNOS protein
expression and increased iNOS protein expression compared with the
control group. This effect was attenuated significantly in the
cells treated with 50 µM EGCG. Treatment with 50 µM
EGCG signficantly increased eNOS protein expression compared with
the cells in the ox-LDL group. Treatment with EGCG at 12.5
µM promoted ox-LDL-induced endothelial dysfunction, whereas
EGCG at 50 µM protected the cells against ox-LDL-induced
endothelial cell dysfunction through the Notch signaling pathway.
Thus, the concentration of 50 µM EGCG was used in the
subsequent experiments.
Jagged-1 is the key effector protein
through which EGCG exerts its protective effects against
ox-LDL-induced endothelial cell dysfunction
The overexpression and silencing of Jagged-1 was
induced in order to determine the role of Jagged-1 in the
EGCG-mediated protection against ox-LDL-induced endothelial
dysfunction. EPCs were obtained from ApoE−/− mice and
treated with EGCG (50 µM) for 24 h followed by exposure to
100 mg/l ox-LDL for 72 h. As shown in Fig. 5, the overexpression of Jagged-1
markedly decreased the expression levels of HES5, iNOS and Math1,
and increased the expression levels of eNOS in the Jagged-1 +
ox-LDL + EGCG group compared with the ox-LDL + EGCG group. By
contrast, the silencing of Jagged-1 markedly increased the
expression levels of HES5, iNOS and Math1, and decreased the
expression levels of eNOS in the Jagged-1-sh + ox-LDL + EGCG group
compared with the ox-LDL + EGCG group.
Furthermore, we examined the effects of Jagged-1 on
apoptosis, as well as on the adhesiveness of EPCs. As shown in
Fig. 6A and B, MTT assay and flow
cytometric analysis revealed that EGCG suppressed ox-LDL-induced
cell apoptosis. This effect was enhanced by the overexpression of
Jagged-1, but inhibited by the silencing of Jagged-1. In addition,
EGCG attenuated the decrease in cell adhesion induced by ox-LDL,
and this effect was inhibited by the silencing of Jagged-1
(Fig. 6C). Thus, our results
indicate that Jagged-1 is the key effector protein through which
EGCG exerts its protective effects against ox-LDL-induced
endothelial dysfunction.
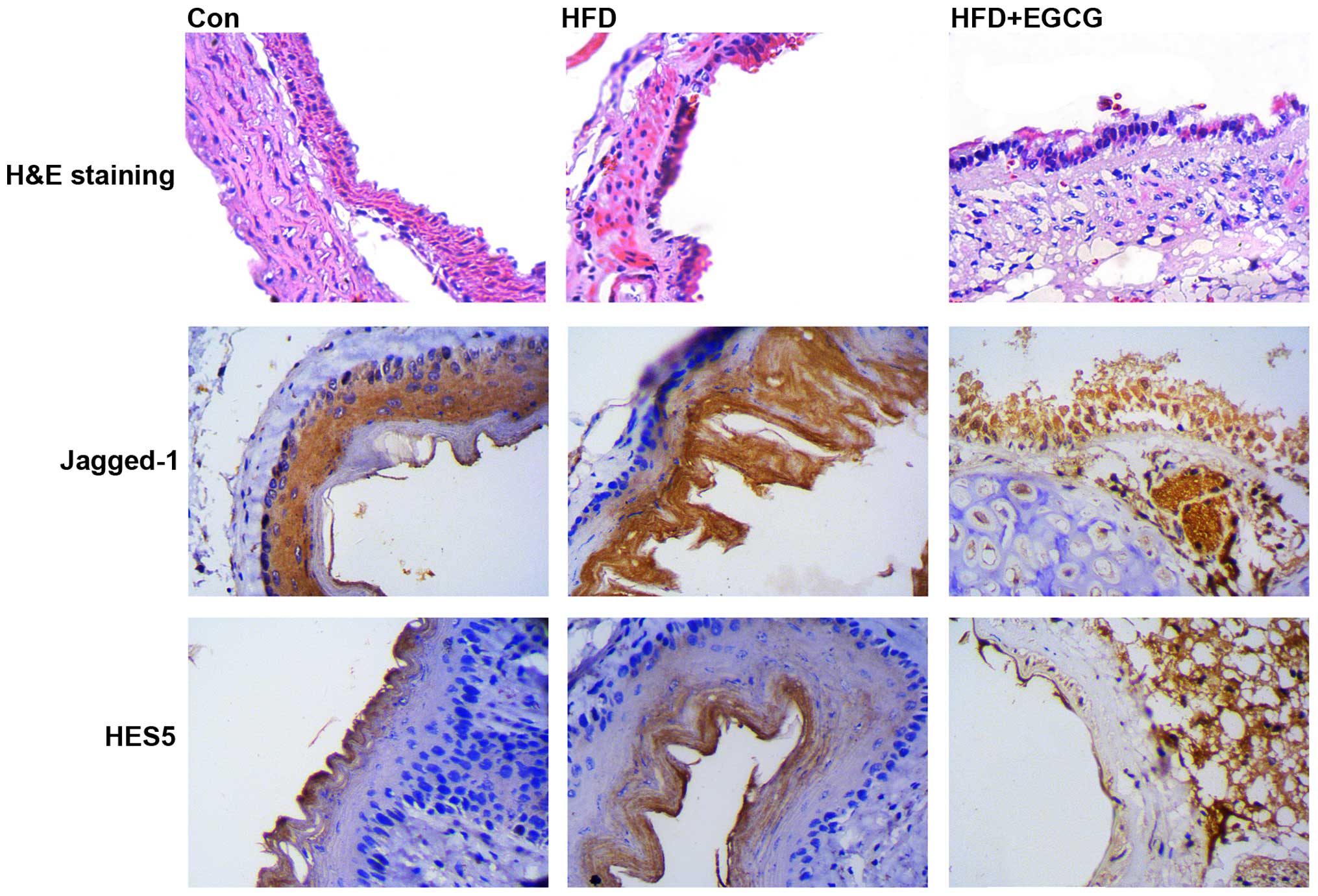
Histomorphometric analysis of the aortic
sinus
To directly determine the protective effects of EGCG
against the development of atherosclerosis, the characteristics of
arterial lesions were examined by pathological section H&E
staining using light microscopy (Fig.
7). In the control group, the vessel walls were round with even
thicknesses. The endothelial cell core was stained and evenly
arranged. In the HFD group, the vessel walls were uneven, and
significant intimal hyperplasia was present. The inner elastic
plates were broken. Treatment with EGCG resulted in more even blood
vessels and smoother intima. Histomorphological analysis revealed
that EGCG attenuated the HFD-induced accumulation of
atherosclerotic plaque. Furthermore, we found that the expression
of Jagged-1 and HES5 was upregulated in the HFD group (shown by
increased dark brown staining), and this effect was attenuated in
the HFD + EGCG group. These results indicated that impairment of
the vascular endothelium induced the activation of the
Jagged-1/Notch pathway, which was associated with significant
intimal hyperplasia.
Discussion
Atherosclerosis is a multifactor cardiovascular
disease, and ox-LDL is a particularly important factor in the
pathogenesis of atherosclerosis and it contributes to endothelial
damage. In the present study, we demonstrate that EGCG at a
concentration of 50 µM protected against ox-LDL-induced
endothelial cell apoptosis and inhibited the development and
progression of atherosclerosis. We also investigated the possible
mechanisms responsible for these effects.
EGCG is found in green tea, and it has potent
antioxidant, anti-mitotic and anti-angiogenic properties (35). The antioxidant activity of EGCG
has been widely demonstrated in vitro and in vivo
(15,36). The antioxidant activity of EGCG is
repsonsibel for its protective effects against atherosclerosis
(37). Consistent with these
results, the present study demonstrated that treatment with 50
µM EGCG evidently reduced ox-LDL-induced cell apoptosis and
the ox-LDL-induced decrease in the adhesion of endothelial cells.
However, treatment with a lower concentration of EGCG (12.5
µM) for 24 h may not be sufficient to exert antioxidant
effects, and the higher concentration of EGCG (200 µM) may
promote the apoptosis of damaged endothelial cells. Taken together,
these findings suggest that treatment with EGCG at the
concentration of 50 µM exerts the optimal antioxidant
effects.
iNOS is known to play a role in producing NO during
inflammation, and thus it contributes to the initiation and
development of inflammatory cardiovascular diseases, such as
atherosclerosis. As previously demonstrated, mice lacking the
endothelial isoform are generally hypertensive and have a more
severe outcome to diet-induced atherosclerosis. Mice lacking the
neuronal isoform have increased diet-induced atherosclerosis. Mice
lacking the inducible isoform show reduced hypotension to septic
shock (38). ox-LDL has been
shown to reduce the expression of eNOS, thereby altering
endothelial biology (34). It has
also been demonstrated that EGCG prevents the ox-LDL-decrease in
eNOS protein expression HUVECs (39). In this study, we found that
treatment with 50 µM EGCG prevented the ox-LDL-induced
decrease in eNOS expression and the ox-LDL-induced increase in iNOS
protein expression in HUVECs. Accumulating evidence has indicated
that the ox-LDL-mediated biological processes may be related to the
increased activity of NADPH oxidase (6–8).
It has been demonstrated that ox-LDL-induced endothelial
dysfunction is caused by an increase in NADPH oxidase-generated
superoxide concentrations and a decrease in antioxidant enzyme
activity (40). This indicates
that ox-LDL mediates endothelial cell damage by suppressing the
activity of NADPH oxidase. In this study, we demonstrated that EGCG
attenuated the ox-LDL-induced decrease in NADPH oxidase activity by
significantly increasing the expression of p22phox in
endothelial cells, indicating that EGCG protects against
ox-LDL-mediated endothelial cell (HUVEC) damage by increasing the
expression of NADPH oxidase p22phox in endothelial
cells.
Notch signaling within endothelial cells plays a
critical role during developmental angiogenesis, providing
instructive cues to neighboring endothelial cells through Notch
ligand-receptor interactions typical of the canonical Notch
signaling pathway. The Notch pathway is a highly conserved
signaling system that is essential for vascular development,
homeostasis and angiogenesis. In growing blood vessels, the
sprouting of endothelial tip cells is inhibited by Notch signaling
and the precise equilibrium between two Notch ligands with distinct
spatial expression patterns and opposing functional roles regulates
angiogenesis (41). In the
present study, ox-LDL suppressed Notch ligand Jagged-1 expression
and induced Notch target gene HES5 expression. These effects were
reversed by treatment with EGCG. Moreover, Jagged-1 suppressed
apoptosis and promoted adhesion of EPCs. In conclusion, Jagged-1 is
the key effector protein in the protective effects of EGCG against
ox-LDL-induced endothelial dysfunction through the Notch
pathway.
To directly determine the effects of EGCG on the
development of atherosclerosis and the mechanisms involved, the
characteristics of arterial lesions were examined by pathological
section H&E staining using light microscopy. The results
demonstrated that EGCG evidently inhibited HFD-induced
atherosclerosis in ApoE-KO mice, which was associated with the
expression of Jagged-1 and HES5, indicating that EGCG protects
ApoE-KO mice from atherosclerosis through the Jagged-1/Notch
pathway. These findings are consistent with those of a previous
study indicating that EGCG prevented the development of
atherosclerosis in ApoE-KO mice by reducing LDL-induced
susceptibility to oxidation (42).
In conclusion, the findings of our study demonstrate
that EGCG protects against ox-LDL-induced endothelial cell damage
through the Jagged-1-mediated Notch pathway, both in vitro
and in vivo. The manipulation of the components of this
mechanism may prove to be a potential therapeutic strategy for
preventing atherosclerosis.
References
|
1
|
Stocker R and Keaney JF Jr: Role of
oxidative modifications in atherosclerosis. Physiol Rev.
84:1381–1478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galkina E and Ley K: Immune and
inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol.
27:165–197. 2009. View Article : Google Scholar :
|
|
3
|
Parthasarathy S, Raghavamenon A, Garelnabi
MO and Santanam N: Oxidized low-density lipoprotein. Methods Mol
Biol. 610:403–417. 2010. View Article : Google Scholar
|
|
4
|
Chisolm GM and Steinberg D: The oxidative
modification hypothesis of atherogenesis: an overview. Free Radic
Biol Med. 28:1815–1826. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller NJ and Paganga G: Antioxidant
activity of low-density lipoprotein. Methods Mol Biol. 108:325–335.
1998.
|
|
6
|
Zhao W, Ma G and Chen X:
Lipopolysaccharide induced LOX-1 expression via TLR4/MyD88/ROS
activated p38MAPK-NF-κB pathway. Vascul Pharmacol. 63:162–172.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carnevale R, Bartimoccia S, Nocella C, Di
Santo S, Loffredo L, Illuminati G, Lombardi E, Boz V, Del Ben M, De
Marco L, et al: LDL oxidation by platelets propagates platelet
activation via an oxidative stress-mediated mechanism.
Atherosclerosis. 237:108–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cominacini L, Rigoni A, Pasini AF, Garbin
U, Davoli A, Campagnola M, Pastorino AM, Lo Cascio V and Sawamura
T: The binding of oxidized low density lipoprotein (ox-LDL) to
ox-LDL receptor-1 reduces the intracellular concentration of nitric
oxide in endothelial cells through an increased production of
superoxide. J Biol Chem. 276:13750–13755. 2001.PubMed/NCBI
|
|
9
|
Sakamoto N, Ishibashi T, Sugimoto K,
Sawamura T, Sakamoto T, Inoue N, Saitoh S, Kamioka M, Uekita H,
Ohkawara H, et al: Role of LOX-1 in monocyte adhesion-triggered
redox, Akt/eNOS and Ca2+ signaling pathways in
endothelial cells. J Cell Physiol. 220:706–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolfram S: Effects of green tea and EGCG
on cardiovascular and metabolic health. J Am Coll Nutr.
26:373S–388S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng Q, Kumagai T, Torii Y, Nakamura Y,
Osawa T and Uchida K: Anticarcinogenic antioxidants as inhibitors
against intracellular oxidative stress. Free Radic Res. 35:779–788.
2001. View Article : Google Scholar
|
|
12
|
Tipoe GL, Leung TM, Hung MW and Fung ML:
Green tea polyphenols as an anti-oxidant and anti-inflammatory
agent for cardiovascular protection. Cardiovasc Hematol Disord Drug
Targets. 7:135–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mukhtar H and Ahmad N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71:1698S-1704S-1702Sdiscussion 1703S-1694S. 2000.
|
|
14
|
Cao Y and Cao R: Angiogenesis inhibited by
drinking tea. Nature. 398:3811999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Y, Kurita-Ochiai T, Hashizume T and
Yamamoto M: Green tea epigallocatechin-3-gallate attenuates
Porphyromonas gingivalis-induced atherosclerosis. Pathog Dis.
67:76–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi YJ, Kang JS, Park JH, Lee YJ, Choi JS
and Kang YH: Polyphenolic flavonoids differ in their antiapoptotic
efficacy in hydrogen peroxide-treated human vascular endothelial
cells. J Nutr. 133:985–991. 2003.PubMed/NCBI
|
|
17
|
Choi JS, Choi YJ, Shin SY, Li J, Kang SW,
Bae JY, Kim DS, Ji GE, Kang JS and Kang YH: Dietary flavonoids
differentially reduce oxidized LDL-induced apoptosis in human
endothelial cells: Role of MAPK- and JAK/STAT-signaling. J Nutr.
138:983–990. 2008.PubMed/NCBI
|
|
18
|
Ahn HY and Kim CH:
Epigallocatechin-3-gallate regulates inducible nitric oxide
synthase expression in human umbilical vein endothelial cells. Lab
Anim Res. 27:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahn HY, Kim CH and Ha TS:
Epigallocatechin-3-gallate regulates NADPH oxidase expression in
human umbilical vein endothelial cells. Korean J Physiol Pharmacol.
14:325–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giniger E: Notch signaling and neural
connectivity. Curr Opin Genet Dev. 22:339–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Artavanis-Tsakonas S, Delidakis C and
Fehon RG: The Notch locus and the cell biology of neuroblast
segregation. Annu Rev Cell Biol. 7:427–452. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koch U, Lehal R and Radtke F: Stem cells
living with a Notch. Development. 140:689–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Sato C, Cerletti M and Wagers A:
Notch signaling in the regulation of stem cell self-renewal and
differentiation. Curr Top Dev Biol. 92:367–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tu J, Li Y and Hu Z: Notch1 and 4
signaling responds to an increasing vascular wall shear stress in a
rat model of arteriovenous malformations. Biomed Res Int.
2014:3680822014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guichet PO, Guelfi S, Teigell M, Hoppe L,
Bakalara N, Bauchet L, Duffau H, Lamszus K, Rothhut B and Hugnot
JP: Notch1 stimulation induces a vascularization switch with
pericyte-like cell differentiation of glioblastoma stem cells. Stem
Cells. 33:21–34. 2015. View Article : Google Scholar
|
|
26
|
Lee SH, Lee S, Yang H, Song S, Kim K,
Saunders TL, Yoon JK, Koh GY and Kim I: Notch pathway targets
proangiogenic regulator Sox17 to restrict angiogenesis. Circ Res.
115:215–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caolo V, Molin DG and Post MJ: Notch
regulation of hematopoiesis, endothelial precursor cells, and blood
vessel formation: orchestrating the vasculature. Stem Cells Int.
2012:8056022012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu B, Che W, Xue J, Zheng C, Tang K,
Zhang J, Wen J and Xu Y: SIRT4 prevents hypoxia-induced apoptosis
in H9c2 cardiomyoblast cells. Cell Physiol Biochem. 32:655–662.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Dai M and Jia W: Paeonol attenuates
high-fat-diet-induced atherosclerosis in rabbits by
anti-inflammatory activity. Planta Med. 75:7–11. 2009. View Article : Google Scholar
|
|
31
|
Liu S, Shen H, Xu M, Liu O, Zhao L, Liu S,
Guo Z and Du J: FRP inhibits ox-LDL-induced endothelial cell
apoptosis through an Akt-NF-(kappa)B-Bcl-2 pathway and inhibits
endothelial cell apoptosis in an apoE-knockout mouse model. Am J
Physiol Endocrinol Metab. 299:E351–E363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bai YP, Hu CP, Yuan Q, Peng J, Shi RZ,
Yang TL, Cao ZH, Li YJ, Cheng G and Zhang GG: Role of VPO1, a newly
identified heme-containing peroxidase, in ox-LDL induced
endothelial cell apoptosis. Free Radic Biol Med. 51:1492–1500.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jia SJ, Jiang DJ, Hu CP, Zhang XH, Deng HW
and Li YJ: Lysophosphatidylcholine-induced elevation of asymmetric
dimethylarginine level by the NADPH oxidase pathway in endothelial
cells. Vascul Pharmacol. 44:143–148. 2006. View Article : Google Scholar
|
|
34
|
Xu H, Duan J, Wang W, Dai S, Wu Y, Sun R
and Ren J: Reactive oxygen species mediate oxidized low-density
lipoprotein-induced endothelin-1 gene expression via extracellular
signal-regulated kinase in vascular endothelial cells. J Hypertens.
26:956–963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagle DG, Ferreira D and Zhou YD:
Epigallocatechin-3-gallate (EGCG): Chemical and biomedical
perspectives. Phytochemistry. 67:1849–1855. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Persson IA, Josefsson M, Persson K and
Andersson RG: Tea flavanols inhibit angiotensin-converting enzyme
activity and increase nitric oxide production in human endothelial
cells. J Pharm Pharmacol. 58:1139–1144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hishikawa K, Nakaki T and Fujita T: Oral
flavonoid supplementation attenuates atherosclerosis development in
apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol.
25:442–446. 2005. View Article : Google Scholar
|
|
38
|
Liu VW and Huang PL: Cardiovascular roles
of nitric oxide: a review of insights from nitric oxide synthase
gene disrupted mice. Cardiovasc Res. 77:19–29. 2008.
|
|
39
|
Huang JJ, Shi YQ, Li RL, Hu A, Lu ZY, Weng
L, Wang SQ, Han YP, Zhang L, Li B, et al: Angiogenesis effect of
therapeutic ultrasound on HUVECs through activation of the
PI3K-Akt-eNOS signal pathway. Am J Transl Res. 7:1106–1115.
2015.PubMed/NCBI
|
|
40
|
Rueckschloss U, Duerrschmidt N and
Morawietz H: NADPH oxidase in endothelial cells: impact on
atherosclerosis. Antioxid Redox Signal. 5:171–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen T, Margariti A, Kelaini S, Cochrane
A, Guha ST, Hu Y, Stitt AW, Zhang L and Xu Q: MicroRNA-199b
modulates vascular cell fate during iPS cell differentiation by
targeting the notch ligand jagged1 and enhancing VEGF signaling.
Stem Cells. 33:1405–1418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miura Y, Chiba T, Tomita I, Koizumi H,
Miura S, Umegaki K, Hara Y, Ikeda M and Tomita T: Tea catechins
prevent the development of atherosclerosis in apoprotein
E-deficient mice. J Nutr. 131:27–32. 2001.PubMed/NCBI
|