Introduction
Vascular smooth muscle cell (VSMC) proliferation,
migration and cell-extracellular matrix (ECM) adhesion are all
known to contribute to the development of intimal hyperplasia in
certain vascular pathologies, including restenosis and
atherosclerosis (1,2). Interactions between VSMCs and the
ECM regulate these processes through binding of the integrin family
of cell adhesion receptors (2). A
key mediator of integrin signaling is kindlin-2, a recently
discovered family of evolutionarily conserved four point one
protein, ezrin, radixin and moesin (FERM) domain-containing
proteins that binds directly to the cytoplasmic tails of β1 and β3
integrins (3–5). Kindlin-2 is essential for integrin
clustering and activation, and regulates cell adhesion and directed
migration by guiding the formation and maturation of focal
adhesions (FA) and the organization of the cytoskeleton (6). Due to its essential role in integrin
activation, kindlin-2 is involved in many important physiological
processes, including heart development, cell migration and cancer
progression (4,7). In mice, the loss of kindlin-2 causes
peri-implantation lethality resulting from detachment of the
endoderm and epiblast from the basement membrane as a consequence
of diminished levels of β1-integrin and also of β1-integrin
activation (8). In zebrafish,
knockdown of the kindlin-2 gene resulted in severe abnormalities in
the development of the heart. Ultrastructural analysis has revealed
disrupted intercalated disc formation and that myofibrils failed to
attach to the membrane complexes (9). Even partial inactivation of the
kindlin-2 gene markedly impairs angiogenesis in mice and zebrafish,
which arises from defective activation of integrin αVβ3 (10). In in vitro experiments
using cells derived from kindlin-2 deficient mice or small
interfering RNA (siRNA)-knockdown mice, defects in integrin
activation despite the presence of talin were noted (10). Recently, Yu et al found
that kindlin-2 forms a transcriptional complex with β-catenin and
transcription factor 4 (TCF4) and enhances Wnt signaling (11,12).
Wnts are a family of secreted glycoproteins that
bind to transmembrane Frizzled receptors and initiate signaling
cascades, and it has been previously noted that they play
indispensable roles during cell proliferation, migration, adhesion
and survival (13–15). Activation of the Wnt/β-catenin
signaling pathway leads to β-catenin nuclear translocation and
complex formation with lymphoid enhancer-binding factor/T cell
factor (LEF/TCF) transcription factors, followed by transcriptional
activation of target genes in the nucleus (13–15). In previous studies using animal
models of intimal hyperplasia, increased β-catenin levels have been
noted, and the role of Wnt signaling in VSMCs has thus been
considered (16,17). Wnt signaling is a novel regulator
of VSMC proliferation and, thereby, intimal hyperplasia (17). These findings suggest that
kindlin-2 and Wnts have overlapping functions in regulating cell
behavior and physiological processes. However, kindlin-2-mediated
interactions are not yet fully understood, and the relationship
between kindlin-2 and Wnt signaling remains to be fully elucidated.
Moreover, little is known about the role kindlin-2 plays in VSMC
proliferation, migration and intimal hyperplasia.
In the present study, we used RNA interference
(RNAi) to examine the effects of kindlin-2 on recombinant
Wnt3a-induced VSMC proliferation and migration in vitro and
intimal hyperplasia following balloon catheter injury of the
carotid artery of rats in vivo.
Materials and methods
Construction and production of lentiviral
vectors
Three siRNA sequences targeting rat kindlin-2
(GenBank accession no., NM_001011915) and a negative control
sequence were constructed by Genechem (Shanghai, China). The
kindlin-2 siRNA sequence which performed best was
CAAACAGATAACAGCACGG, and that of negative control siRNA (NC siRNA)
was TTCTCCGAACGTGTCACGT (data not shown). Short hairpin RNAs
(shRNAs) were inserted into the lentiviral vector GV118 driven by
the U6 promoter and containing the green fluorescent protein (GFP)
reporter gene. All constructs were then verified by sequence
analysis. Lentivirus-encoded shRNA against kindlin-2 and the
control were produced by co-transfecting 293T cells (purchased from
GeneChem, Shanghai, China) with Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to standard protocols. The virus
titers, measured in 293T cells as transducing units/milliliter
(TU/ml), were approximately 1×109 TU/ml.
Rat model of carotid artery injury and
lentiviral transfection
All animal protocols complied strictly with the
Institutional Animal Care and Use Committee guidelines. The
procedure for balloon injury in rat carotid arteries has been
described previously (18).
Briefly, male Sprague-Dawley (SD) rats (n=45) (Wuhan University
Experimental Animal Center, Wuhan, China), 3–4 months old and
weighing 350–400 g were anesthetized by intraperitoneal injection
of pentobarbital (40 mg/kg). After intravenous injection of 100
U/kg of heparin sodium, the left common, external and internal
carotid arteries were exposed, and a balloon angioplasty catheter
(balloon diameter 1.25 mm, balloon length 15 mm; Medtronic,
Minneapolis, MN, USA) was introduced through the external carotid
arteriotomy incision, advanced to the aortic arch, inflated to
produce moderate resistance, and gradually withdrawn 3 times. For
lentiviral transfection, a 50 µl solution of kindlin-2
siRNA-GFP lentivirus (2×108 TU/ml) or NC siRNA-GFP
lentivirus (2×108 TU/ml) was infused into the injured
common carotid artery segment (approximately 20 mm in length)
isolated by two microvascular clips, and incubated for 30 min. The
external carotid artery was then ligated, and blood flow through
the common and internal carotid arteries was restored.
Hematoxylin and eosin (H&E)
staining
The rats were sacrificed by jugular vein blood
collection, which leads to massive loss of blood and death in rats,
4 weeks after balloon injury and lentiviral transfection, and the
left common carotid arteries were harvested, fixed in 4%
paraformaldehyde, and then embedded in paraffin. For harvesting the
arteries, the rats were fixed on a board after being anesthetized.
The left common carotid arteries were exposed by layer separation
after skin incision, and the left common carotid arteries were then
clipped after clamping the proximal and distal ends of the
arteries. For morphologic analysis of intimal hyperplasia, five
round cross-sections (4 µm thickness) were cut from the
approximate middle of the artery, stained with H&E,
photographed, and analyzed using Image-Pro Plus 6.0 professional
image analysis software (Media Cybernetics, Silver Spring, MD,
USA). The intimal and medial cross-sectional areas of the carotid
arteries were measured, and the intima/media ratios were also
calculated.
VSMC culture and transfection
Primary VSMCs were isolated from the thoracic aortas
of male SD rats (100–150 g, n=16). The rats were fixed on a board
after being anesthetized. The thoracic arteries were exposed and
clipped. The thoracic arteries were then placed in a dish with
Dulbecco's modified Eagle's medium (DMEM) and the endothelial cells
were removed by opthalmic tweezers. Finally, the remaining cells
were the VSMCs. The cells were then cultured in DMEM containing 10%
fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 U/ml
penicillin, and 100 µg/ml streptomycin. The cells were
incubated at 37°C in a humidified atmosphere of 95% air and 5%
CO2. The purity of the VSMCs was assessed at
approximately 90% through studying the cell morphology and
immunostaining with anti-α-actin antibody (A5228; Sigma, St. Louis,
MO, USA). All VSMCs were used for experiments between the 3rd and
6th passages. VSMCs (1×105) were plated in 6-well plates
and grown to approximately 50% confluence, then transfected with
kindlin-2 siRNA lentivirus or NC siRNA lentivirus at multiplicities
of transfection (MOI) of 100. Lentiviral transfection was validated
by visualization of enhanced GFP under a fluorescence microscope
(Nikon TE2000; Nikon, Tokyo, Japan).
Cell proliferation assay
Cell proliferation was measured by studying the
incorporation of bromodeoxyuridine (BrdU) during DNA synthesis in
proliferating cells. Untreated VSMCs were seeded at a density of
5,000 cells/well in 96-well culture plates in DMEM with 10% FBS and
cultured for 24 h. VSMCs were then starved in DMEM without FBS for
12 h to achieve synchronous growth, and transfected with kindlin-2
siRNA lentivirus or NC siRNA lentivirus at MOI of 100 for 12 h.
Wnt3a (final concentration, 100 ng/ml; R&D Systems,
Minneapolis, MN, USA) was added to each well after transfection,
and 48 h after transfection 20 µl BrdU was also added to
each well to label the cells during 24 h of incubation.
Quantification of BrdU incorporation was performed using a BrdU
cell proliferation assay kit (Millipore, Billerica, MA, USA)
according to the manufacturer's instructions. The absorbance was
read at a wavelength of 450 nm with a spectrophotometric plate
reader (Infinite M200 PRO; Tecan, Männedorf, Switzerland).
Cell migration assay
The VSMC migration assay was performed using
Transwell cell culture inserts (Corning, High Wycombe, UK) in
24-well plates. One hundred microliters of VSMCs, which were stably
transfected with kindlin-2 siRNA, NC siRNA or left untreated
(3×105 cells/ml), suspended in serum-free DMEM, were
added to the upper polycarbonate membrane insert (pore size, 8
µm). Wnt3a (final concentration, 100 ng/ml) was added to the
upper chamber, and 600 µl culture medium containing 10% FBS
was added to the lower chamber. The cells were allowed to migrate
for 24 h while the plates were incubated in a humidified incubator
in an atmosphere with 5% CO2 at 37°C. After 24 h, the
cells that remained on the upper surface of the membrane were
removed with a cotton swab. The membrane was fixed with anhydrous
methanol for 20 min at room temperature and then stained with 0.1%
crystal violet for 15 min. A microscope was used to determine the
number of migratory cells by counting the cells in 5 randomly
selected fields of view. All experiments were performed in
triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
After transfection and Wnt3a (100 ng/ml) stimulation
for 3 days, total RNA was isolated from VSMCs using TRIzol
(Invitrogen) reagent. For RT-qPCR, we performed reverse
transcription to produce cDNA from total RNA with oligo(dT), and
the fragments were then amplified with a SYBR-Green-based assay kit
(Invitrogen) according to the manufacturer's instructions. Thermal
cycling conditions comprised an initial denaturation step at 50°C
for 2 min, 95°C for 10 min, followed by 40 cycles (95°C for 30 sec;
60°C for 30 sec). β-actin was used for normalization, and the data
were analyzed using the 2−ΔΔCt method. The primers were
as follows: kindlin-2 forward, 5′-AGATCT GGCTTCGCTGTGAT-3′ and
reverse, 5′-CGGGATTGATGTCAGTTGTG-3′; c-myc forward,
5′-CGAGCTGAAGCGTAG CTTTT-3′ and reverse,
5′-CTCGCCGTTTCCTCAGTAAG-3′; cyclin D1 forward,
5′-GCGTACCCTGACACCAATCT-3′ and reverse, 5′-GGCTCCAGAGACAAGAAACG-3′;
β-actin forward, 5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse,
5′-TAAAGACCTCTATGCCAACACAGT-3′.
Western blot analysis
After transfection, VSMCs treated with Wnt3a (100
ng/ml) and PBS (control) for 3 days were harvested. The cells were
lysed with RIPA buffer supplemented with proteinase inhibitors for
30 min on ice. Protein concentration was measured with a BCA
protein assay (Pierce, Rockford, IL, USA). Proteins were then
separated by SDS-polyacrylamide gels and transferred to
nitrocellulose membranes. The membranes were blocked with 10%
non-fat dry milk, and then immunoblotted overnight at 4°C with
antibodies that recognize kindlin-2 (1:500; K3269; Sigma),
β1-integrin (1:500; 04-1109; Millipore), total β-catenin (1:1,000;
9562), phospho-β-catenin (Ser675, 1:1,000; 9567) (both from Cell
Signaling Technology, Beverly, MA, USA), total glycogen synthase
kinase-3β (GSK-3β, 1:400; Sc-9166), phospho-GSK-3β (Ser9, 1:500;
Sc-373800) and β-actin (1:1,000; Sc-32251) (all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). After three washes, the
blots were incubated with peroxidase-conjugated secondary
antibodies (Pierce) for 1 h at room temperature, and subsequently
analyzed by an ECL detection system (Beijing Liuyi Instrument
Factory, Beijing, China).
Flow cytometric analysis
VSMCs were transfected with lentiviruses of
kindlin-2 siRNA or NC siRNA and supplemented with Wnt3a (100 ng/ml)
for 3 days. β1-integrin expression on the VSMC surface was
evaluated by indirect immunofluorescence using flow cytometry.
After being rinsed in PBS, the cells were incubated with rabbit
anti-rat antibody against β1-integrin (1:70) and mouse anti-rat
antibody against active β1-integrin (1:200; MAB2079Z) (both from
Millipore) for 30 min at room temperature in the dark. The cells
were then washed again and incubated with phycoerythrin
(PE)-conjugated goat anti-rabbit secondary IgG (1:50; bs-0295G) and
Cy3-conjugated goat anti-mouse secondary IgG (1:50; bs-0296G) (both
from Bioss, Beijing, China) for 45 min and analyzed by flow
cytometry using Becton-Dickinson FACSCalibur and CellQuest
software.
Statistical analysis
All statistical analysis was performed with
Statistical Product and Service Solutions 17.0 software (SPSS
17.0). Data are presented as the means ± SEM. All values were
analyzed using the Student's t-test for comparisons between two
groups or one-way ANOVA for multiple comparisons. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Kindlin-2 RNAi suppresses intimal
hyperplasia
Four weeks after balloon injury and lentiviral
transfection, the degree of intimal hyperplasia was evaluated
morphologically and quantitatively (Fig. 1). Kindlin-2 siRNA lentivirus
treatment significantly reduced intimal hyperplasia (P<0.05),
and the intima/media ratio was also markedly lower in kindlin-2
siRNA lentivirus-transfected arteries (0.687±0.117) than in NC
siRNA lentivirus-transfected arteries (1.545±0.277)
(P<0.05).
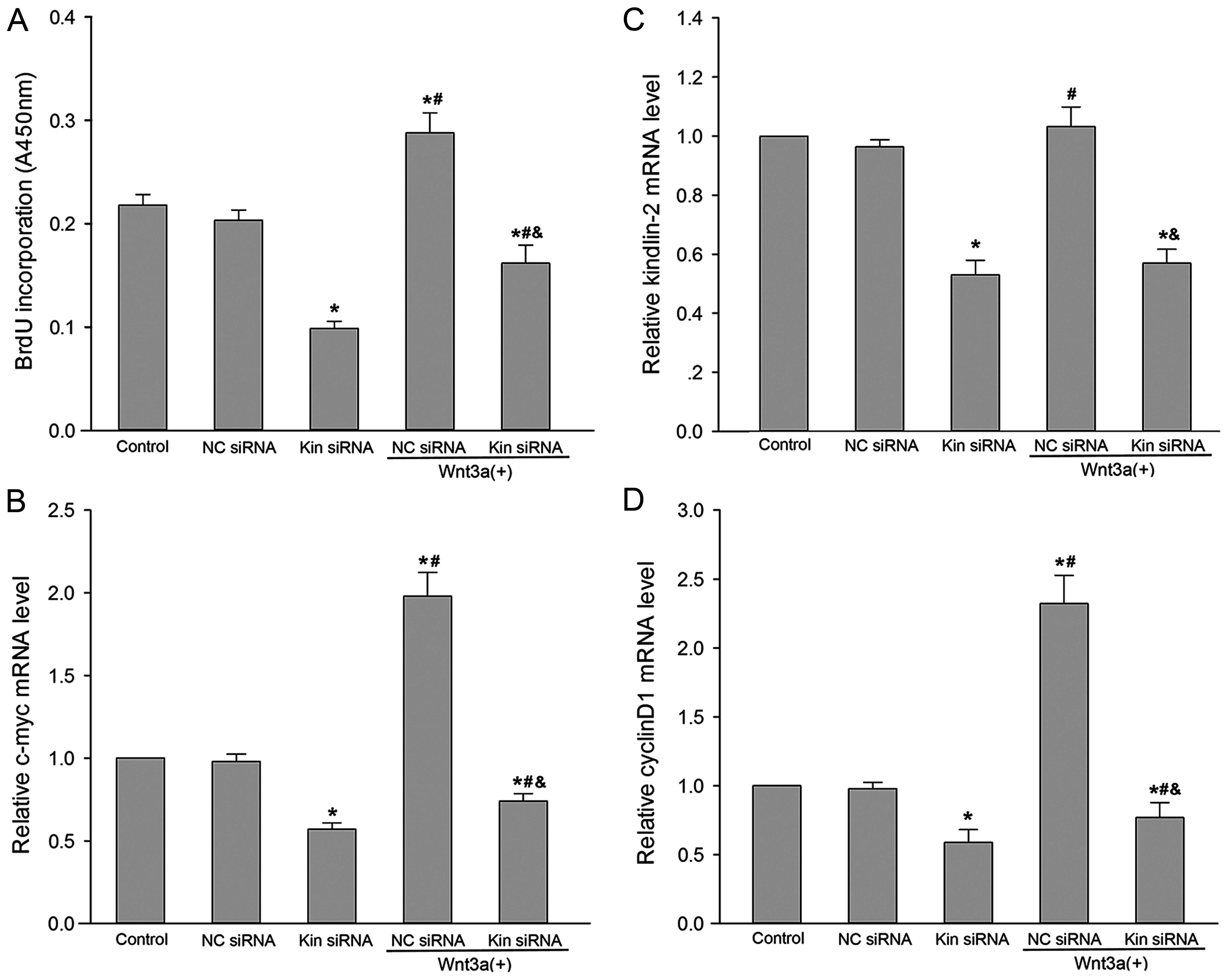
Kindlin-2 RNAi attenuates the VSMC
proliferation induced by Wnt3a
As shown in Fig.
2C, kindlin-2 mRNA expression was dramatically reduced in VSMCs
transfected with kindlin-2 siRNA lentivirus (P<0.05), but not in
VSMCs transfected with NC siRNA lentivirus (P>0.05). Compared
with the control group, a 47% reduction of kindlin-2 mRNA was
observed in VSMCs transfected with kindlin-2 siRNA lentivirus
(P<0.05), indicating that kindlin-2 RNAi is effective.
Subsequently, we examined the effect of kindlin-2 RNAi on
Wnt3a-induced VSMC proliferation by measuring the nuclear
incorporation of BrdU (DNA synthesis) and the mRNA expression
levels of c-myc and cyclin D1, which are critical genes involved in
cell cycle progression and cell proliferation; Wnt3a is a prominent
member of the Wnt family and can activate the canonical Wnt pathway
and induce cell proliferation (19,20). We observed that kindlin-2 RNAi
resulted in significant inhibition of BrdU incorporation compared
with the control group and the NC siRNA group with or without Wnt3a
stimulation (P<0.05; Fig. 2A).
The c-myc and cyclin D1 mRNA expression levels were significantly
suppressed by kindlin-2 siRNA lentivirus at MOI of 100 (P<0.05;
Fig. 2B and D). NC siRNA
lentivirus, which encodes for a non-homologous shRNA, did not
affect c-myc and cyclin D1 mRNA expression. After exposure of VSMCs
to Wnt3a at a concentration of 100 ng/ml for 3 days, the levels of
c-myc and cyclin D1 mRNA were significantly upregulated by 1.9- and
2.3-fold, respectively (P<0.05). By contrast, Wnt3a-induced
expression of c-myc and cyclin D1 was also markedly inhibited by
pretreatment with kindlin-2 siRNA lentivirus (P<0.05).
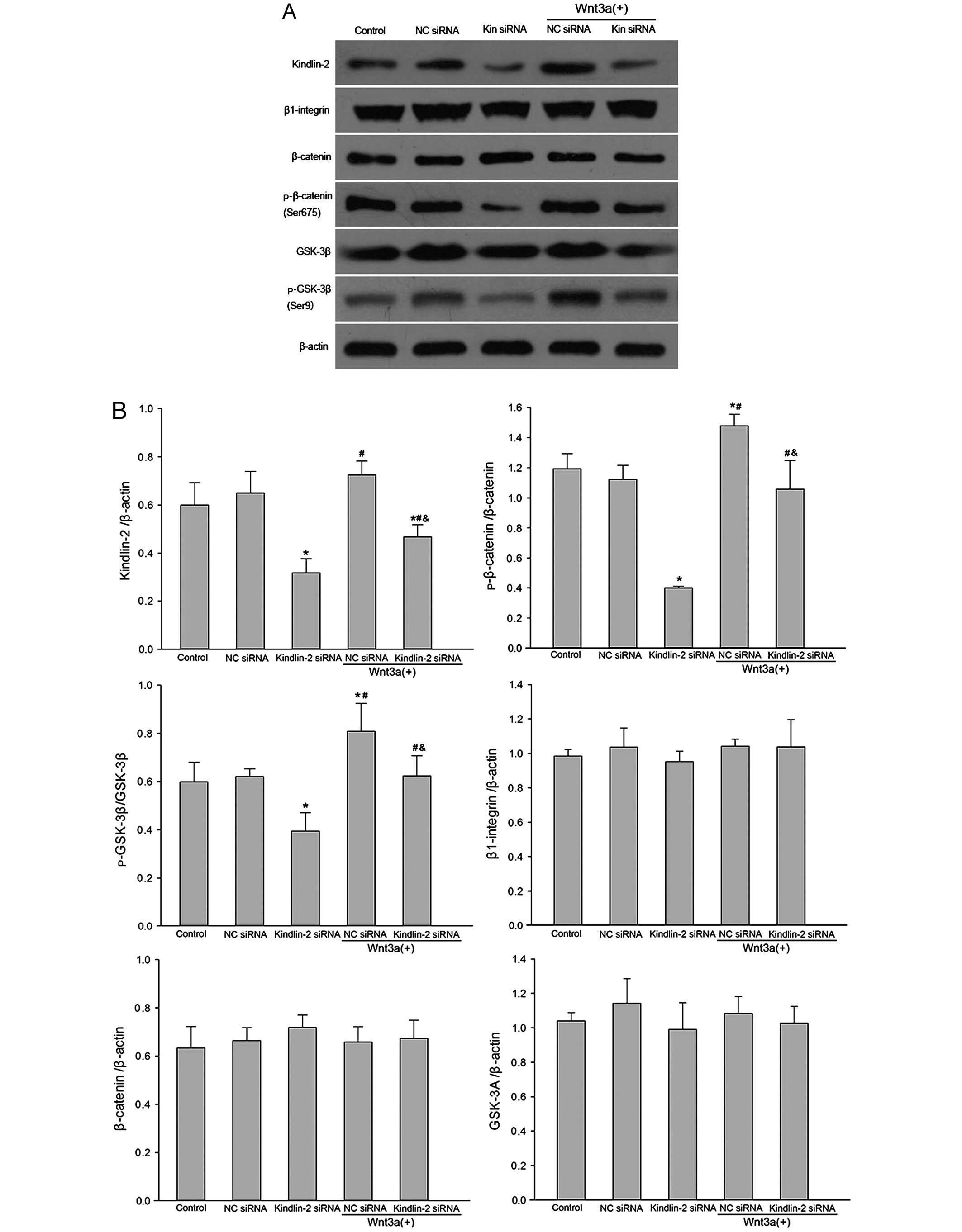
Kindlin-2 regulates cell proliferation by
Wnt/β-catenin signaling
β-catenin and GSK-3β were examined in this study, as
they are considered to be the major downstream Wnt signaling
molecules (13–15). Kindlin-2 has been found to be
coexpressed with β-catenin in the invasive front of tumors and in
tumor cells (11). To understand
the mechanisms of kindlin-2 knockdown on VSMC proliferation induced
by Wnt3a, we analyzed the protein expression of kindlin-2,
β1-integrin, β-catenin and GSK-3β (Fig. 3). Our results showed that
kindlin-2 knockdown significantly decreased protein expression
levels of kindlin-2, p-β-catenin (Ser675) and p-GSK-3β (Ser9)
(P<0.05). Treatment of VSMCs with Wnt3a upregulated the
expression of kindlin-2, p-β-catenin (Ser675) and p-GSK-3β (Ser9)
(P<0.05). However, the expression of β1-integrin, total
β-catenin and total GSK-3β did not differ significantly between
groups (P>0.05).
Kindlin-2 depletion impairs VSMC
migration
We performed a Transwell migration assay to
investigate the effect of kindlin-2 RNAi and Wnt3a treatment on
VSMC migration. The migratory ability of VSMCs transfected with
kindlin-2 siRNA lentivirus was significantly decreased (P<0.05;
Fig. 4). After treatment with
Wnt3a, the migratory ability of VSMCs was significantly increased
(P<0.05). The number of cells that migrated across the
polycarbonate membrane was higher in the NC siRNA group and Wnt3a
treatment group than in the kindlin-2 siRNA group (P<0.05).
Kindlin-2 and Wnt3a regulate integrin
activation
Since kindlin-2 knockdown and treatment with Wnt3a
did not markedly affect the protein expression of β1-integrin, we
quantified the expression of active β1-integrin on the surface of
VSMCs using flow cytometry. The anti-active-β1-integrin antibody is
specific for the active conformation of rat β1-integrin, and it can
also discriminate between the different activated states.
Therefore, it is exceptionally useful for studying how β1-integrin
activation is regulated. Our results showed that knockdown of
kindlin-2 significantly reduced the VSMC surface levels of
active-β1-integrin (P<0.05; Fig.
5). VSMCs that were stimulated with Wnt3a for 3 days after
transfection bound to more active-β1-integrin antibody than the
kindlin-2 siRNA group (P<0.05). However, kindlin-2 knockdown or
Wnt3a treatment did not have a marked effect on the total amount of
β1-integrin expression on the surface of VSMCs (P>0.05; Fig. 5). These results demonstrate that a
minimal level of kindlin-2 is required for optimal integrin
activation in VSMCs, and that Wnt3a treatment activates β1-integrin
without changing its expression levels.
Discussion
In the present study, to the best of our knowledge,
we have provided the first direct evidence that kindlin-2 regulates
VSMC proliferation and migration in vitro and intimal
hyperplasia in vivo. Additionally, we have shown that
Wnt/β-catenin signaling is involved in signal transduction and the
functional regulation of kindlin-2. Consequently, we propose that
modification of kindlin-2 or Wnt signaling is a potential target
for inhibition of VSMC proliferation, migration and intimal
hyperplasia.
Kindlin-2 belongs to the kindlin family of proteins;
the kindlins are emerging as a novel class of molecules which are
implicated in integrin activation, a critical process for cell
proliferation, migration, differentiation and adhesion as well as
for cell-ECM interactions (7).
They comprise three evolutionarily conserved members in
vertebrates, kindlin-1, kindlin-2 and kindlin-3, which share
considerable sequenctial and structural similarities (7,21–24). The kindlins have a FERM domain,
which is interrupted by a pleckstrin homology domain, and bind
directly to various classes of integrins as well as participating
in inside-out integrin activation (7,21–25). Loss-of-function mutations in
kindlin-1 and kindlin-3 cause Kindler syndrome and leukocyte
adhesion deficiency III syndrome, respectively, but no human
disease has yet been associated with the kindlin-2 gene (7,22–24).
At present, limited information exists on the
physiological functions of kindlin-2, and the data are mainly
derived from knockout animal models and in vitro studies
with cell lines. The essential role of kindlin-2 in development is
demonstrated by its peri-implantational lethality and the abnormal
heart development noted in kindlin-2 knockout mice and zebrafish
(8,9). At the cellular level,
kindlin-2−/− embryonic stem cells exhibited a normal
proliferation rate, but strongly reduced adhesion to laminins and
fibronectin was noted (8). In
C2C12 cells, kindlin-2 contributed to myocyte elongation and fusion
in multinucleated myotubes (26).
Knockdown of kindlin-2 in wild-type keratinocytes impaired cell
spreading (27). Kindlin-2 is
also important in the regulation of podocyte-matrix adhesion and
matrix deposition (28). Our
results revealed that kindlin-2 regulates VSMC proliferation and
migration. However, in previous studies, overexpression and
knockdown of kindlin-2 have yielded contradicting results regarding
specific functions: evidence for both suppression of cancer cell
invasion in leiomyosarcoma or colon carcinoma cell lines (3,29)
and stimulation of cell migration in fibroblasts, human umbilical
vein endothelial cells (HUVECs), malignant mesothelioma and gastric
cancer cells has been noted (6,30–32). These observations suggest that the
functions of kindlin-2 are cell type- or integrin-specific, and
that its role in cell motility differs depending on the biological
context. For example, over-expression of kindlin-2 in Chinese
hamster ovary (CHO) cells exogenously expressing αIIbβ3 integrin
enhances its activation (3,30).
However, overexpression of kindlin-2 in the same type of cells
inhibits endogenous α5β1-integrin activation (21). Taken together, these results
suggest that alterations of kindlin-2 expression affect
integrin-dependent functions. This study, and others, have observed
that kindlin-2 knockdown did not markedly affect the protein
expression of β1-integrin, but significantly reduced β1-integrin
activation (6,28).
To date, kindlin-2 is the only kindlin protein that
has not been implicated in disease pathophysiology, but it is
rapidly emerging as a key molecule in cardiac and muscle
development (7). Moreover, the
role of kindlin-2 in cell proliferation and migration is not yet
fully understood, and little is known about the signal transduction
pathways of kindlin-2; determining these will be important in
understanding the role of kindlin-2 in the pathophysiology of
disorders of integrin activation. To determine the effect of
kindlin-2 on cell proliferation and migration, in the present
study, RNA-mediated interference experiments were performed on
VSMCs. siRNAs targeting kindlin-2 or irrelevant RNA as a negative
control were constructed and introduced to VSMCs, and kindlin-2
expression levels were analyzed by RT-qPCR and western blot
analysis. Transfection with kindlin-2 siRNA but not NC siRNA
significantly inhibited the expression of kindlin-2. We detected
VSMC proliferation by measuring the nuclear incorporation of BrdU
and VSMC migration using a Transwell assay. The results showed that
kindlin-2 siRNA resulted in significant inhibition of BrdU
incorporation compared with the NC siRNA group, and the migratory
ability of VSMCs in the kindlin-2 siRNA group was significantly
decreased.
Although these previous studies have suggested that
kindlin-2 signaling is crucial to VSMC proliferation and migration,
the underlying mechanism is still unknown. Previous studies have
shown that kindlin-2 is important to the enhancement of
Wnt/β-catenin signaling, as it selectively binds to active
β-catenin and stabilizes it by preventing GSK-3β (a negative
regulator of Wnt signaling) from binding (11,12). It is well known that the major
downstream Wnt signaling target is β-catenin. Activation of the
β-catenin signaling pathway occurs by inhibiting GSK-3β from
phosphorylating the N-terminal part of β-catenin, which leads to
rapid degradation of β-catenin. However, β-catenin is also
activated by phosphorylation in the C-terminal at serine 675, which
facilitates the translocation of β-catenin to the nucleus and
enhances its transcriptional activity (20). In this study, we activated
Wnt/β-catenin signaling with recombinant Wnt3a to study the effect
of kindlin-2 siRNA on VSMCs. We noted increased β-catenin
phosphorylation at Ser675 and increased GSK-3β phosphorylation at
Ser9 (inactivation of GSK-3β) after Wnt3a stimulation. Kindlin-2
knockdown significantly decreased protein expression levels of
p-β-catenin (Ser675) and p-GSK-3β (Ser9). However, the expression
of total β-catenin and GSK-3β was not markedly affected by
kindlin-2 siRNA. Moreover, we noted that Wnt3a also elevated
β1-integrin activity and promoted VSMC migration.
Previous studies have revealed that Wnt/β-catenin
signaling is involved in the regulation of VSMC proliferation and
migration (14–17,33). Wang et al showed that
overexpression of constitutively active β-catenin increased cyclin
D1 promoter activity in a rat VSMC line (34). Cyclin D1 protein is important for
the regulation of the cell proliferation cycle. When cyclin D1 is
amplified or expressed, the G1/S transition is shortened and cell
proliferation is promoted; c-myc, which was investigated in the
present study, is known to act as a proto-oncogene and cell
proliferation-initiating factor (35). It has previously been pointed out
that c-myc and cyclin D1 are downstream target genes of the
Wnt/β-catenin signaling pathway (20,36). In the present study, we confirmed
that treatment with Wnt3a resulted in the activation of
Wnt/β-catenin signaling and increased the expression of β-catenin
responsive genes c-myc and cyclin D1. Taken together, these results
show that growth inhibition by kindlin-2 siRNA in VSMCs was
manifested partly through the dysregulated nuclear translocation of
β-catenin and the consequent downregulation of its transcriptional
targets c-myc and cyclin D1.
VSMC proliferation and migration play a critical
role in intimal hyperplasia through cellular expansion and ECM
deposition (1). Elucidating the
molecular mechanisms responsible for VSMC proliferation and
migration has led to the development of novel therapeutic
approaches. In this study, we have investigated how kindlin-2
silencing inhibits intimal hyperplasia. When we studied the
vascular morphology in our study, we noted that intimal hyperplasia
was clearly visible 4 weeks after balloon injury and that kindlin-2
siRNA lentivirus treatment significantly reduced intimal
hyperplasia. Moreover, the intima/media ratio was also markedly
reduced in arteries transfected with kindlin-2 siRNA lentivirus
compared with arteries transfected with NC siRNA lentivirus. The
possible reason for this result is that kindlin-2 RNAi inhibits
VSMC proliferation and migration to the intima. Our data indicate
that kindlin-2 knockdown plays dual roles in the treatment and
prevention of intimal hyperplasia. Kindlin-2 siRNA not only
inhibits VSMC proliferation and migration by Wnt signaling, but
also suppresses VSMC migration by regulating β-integrin activation.
Our results suggest that inhibition of kindlin-2 is an attractive
therapeutic approach for prevention of intimal hyperplasia.
Acknowledgments
This study was supported by the National Science
Foundation of China (NSFC) nos. 81170195 and 81200156. We thank all
teachers from the Renmin Hospital of Wuhan University for excellent
technical assistance.
References
|
1
|
Marx SO, Totary-Jain H and Marks AR:
Vascular smooth muscle cell proliferation in restenosis. Circ
Cardiovasc Interv. 4:104–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ho B, Hou G, Pickering JG, Hannigan G,
Langille BL and Bendeck MP: Integrin-linked kinase in the vascular
smooth muscle cell response to injury. Am J Pathol. 173:278–288.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda
K, Qin J, Plow EF and Wu C: The MIG-2/integrin interaction
strengthens cell-matrix adhesion and modulates cell motility. J
Biol Chem. 282:20455–20466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan AA, Shimokawa T, Strömblad S and
Zhang H: Functional characterization of human kindlin-2 core
promoter identifies a key role of SP1 in Kindlin-2 transcriptional
regulation. Cell Mol Biol Lett. 16:638–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Z, Gao J, Hong J and Ma YQ: Integrity
of kindlin-2 FERM subdomains is required for supporting integrin
activation. Biochem Biophys Res Commun. 434:382–387. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He Y, Esser P, Schacht V,
Bruckner-Tuderman L and Has C: Role of kindlin-2 in fibroblast
functions: implications for wound healing. J Invest Dermatol.
131:245–256. 2011. View Article : Google Scholar
|
|
7
|
Lai-Cheong JE, Parsons M and McGrath JA:
The role of kindlins in cell biology and relevance to human
disease. Int J Biochem Cell Biol. 42:595–603. 2010. View Article : Google Scholar
|
|
8
|
Montanez E, Ussar S, Schifferer M, Bösl M,
Zent R, Moser M and Fässler R: Kindlin-2 controls bidirectional
signaling of integrins. Genes Dev. 22:1325–1330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dowling JJ, Gibbs E, Russell M, Goldman D,
Minarcik J, Golden JA and Feldman EL: Kindlin-2 is an essential
component of intercalated discs and is required for vertebrate
cardiac structure and function. Circ Res. 102:423–431. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pluskota E, Dowling JJ, Gordon N, Golden
JA, Szpak D, West XZ, Nestor C, Ma YQ, Bialkowska K, Byzova T and
Plow EF: The integrin coactivator kindlin-2 plays a critical role
in angiogenesis in mice and zebrafish. Blood. 117:4978–4987. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Wu J, Wang Y, Zhao T, Ma B, Liu Y,
Fang W, Zhu WG and Zhang H: Kindlin 2 forms a transcriptional
complex with β-catenin and TCF4 to enhance Wnt signalling. EMBO
Rep. 13:750–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu Y, Qi L, Wu J, Wang Y, Fang W and Zhang
H: Kindlin 2 regulates myogenic related factor myogenin via a
canonical Wnt signaling in myogenic differentiation. PLoS One.
8:e634902013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van de Schans VA, Smits JF and
Blankesteijn WM: The Wnt/frizzled pathway in cardiovascular
development and disease: friend or foe? Eur J Pharmacol.
585:338–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao TP and Kühl M: An updated overview on
Wnt signaling pathways: a prelude for more. Circ Res.
106:1798–1806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsaousi A, Mill C and George SJ: The Wnt
pathways in vascular disease: lessons from vascular development.
Curr Opin Lipidol. 22:350–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mill C and George SJ: Wnt signalling in
smooth muscle cells and its role in cardiovascular disorders.
Cardiovasc Res. 95:233–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsaousi A, Williams H, Lyon CA, Taylor V,
Swain A, Johnson JL and George SJ: Wnt4/β-catenin signaling induces
VSMC proliferation and is associated with intimal thickening. Circ
Res. 108:427–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Natarajan R, Pei H, Gu JL, Sarma JM and
Nadler J: Evidence for 12-lipoxygenase induction in the vessel wall
following balloon injury. Cardiovasc Res. 41:489–499. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bao XL, Song H, Chen Z and Tang X: Wnt3a
promotes epithelial-mesenchymal transition, migration, and
proliferation of lens epithelial cells. Mol Vis. 18:1983–1990.
2012.PubMed/NCBI
|
|
20
|
Marchand A, Atassi F, Gaaya A, Leprince P,
Le Feuvre C, Soubrier F, Lompré AM and Nadaud S: The
Wnt/beta-catenin pathway is activated during advanced arterial
aging in humans. Aging Cell. 10:220–232. 2011. View Article : Google Scholar
|
|
21
|
Harburger DS, Bouaouina M and Calderwood
DA: Kindlin-1 and -2 directly bind the C-terminal region of beta
integrin cytoplasmic tails and exert integrin-specific activation
effects. J Biol Chem. 284:11485–11497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malinin NL, Plow EF and Byzova TV:
Kindlins in FERM adhesion. Blood. 115:4011–4017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meves A, Stremmel C, Gottschalk K and
Fässler R: The kindlin protein family: new members to the club of
focal adhesion proteins. Trends Cell Biol. 19:504–513. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ussar S, Wang HV, Linder S, Fässler R and
Moser M: The kindlins: subcellular localization and expression
during murine development. Exp Cell Res. 312:3142–3151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ali RH and Khan AA: Tracing the evolution
of FERM domain of Kindlins. Mol Phylogenet Evol. 80:193–204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dowling JJ, Vreede AP, Kim S, Golden J and
Feldman EL: Kindlin-2 is required for myocyte elongation and is
essential for myogenesis. BMC Cell Biol. 9:362008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bandyopadhyay A, Rothschild G, Kim S,
Calderwood DA and Raghavan S: Functional differences between
kindlin-1 and kindlin-2 in keratinocytes. J Cell Sci.
125:2172–2184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qu H, Tu Y, Shi X, Larjava H, Saleem MA,
Shattil SJ, Fukuda K, Qin J, Kretzler M and Wu C: Kindlin-2
regulates podocyte adhesion and fibronectin matrix deposition
through interactions with phosphoinositides and integrins. J Cell
Sci. 124:879–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi X and Wu C: A suppressive role of
mitogen inducible gene-2 in mesenchymal cancer cell invasion. Mol
Cancer Res. 6:715–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma YQ, Qin J, Wu C and Plow EF: Kindlin-2
(Mig-2): a co-activator of beta3 integrins. J Cell Biol.
181:439–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An Z, Dobra K, Lock JG, Stromblad S,
Hjerpe A and Zhang H: Kindlin-2 is expressed in malignant
mesothelioma and is required for tumor cell adhesion and migration.
Int J Cancer. 127:1999–2008. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen Z, Ye Y, Kauttu T, Seppänen H,
Vainionpää S, Wang S, Mustonen H and Puolakkainen P: Novel focal
adhesion protein kindlin-2 promotes the invasion of gastric cancer
cells through phosphorylation of integrin β1 and β3. J Surg Oncol.
108:106–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slater SC, Koutsouki E, Jackson CL, Bush
RC, Angelini GD, Newby AC and George SJ: R-cadherin:beta-catenin
complex and its association with vascular smooth muscle cell
proliferation. Arterioscler Thromb Vasc Biol. 24:1204–1210. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Xiao Y, Mou Y, Zhao Y,
Blankesteijn WM and Hall JL: A role for the beta-catenin/T-cell
factor signaling cascade in vascular remodeling. Circ Res.
90:340–347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamada N, Noguchi S, Mori T, Naoe T, Maruo
K and Akao Y: Tumor-suppressive microRNA-145 targets catenin δ-1 to
regulate Wnt/β-catenin signaling in human colon cancer cells.
Cancer Lett. 335:332–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Zhang J, Xu L, Xu C, Chen S, Yang
J and Jiang H: Inhibition of neointimal hyperplasia in the rat
carotid artery injury model by a HMGB1 inhibitor. Atherosclerosis.
224:332–339. 2012. View Article : Google Scholar : PubMed/NCBI
|