Introduction
Glucocorticoids (GCs) are widely used in the
treatment of various diseases, such as asthma, rheumatoid
arthritis, and systemic lupus erythematosus for its anti-immune and
anti-infectious effects (1,2).
However, GCs also have a number of adverse effects, including
osteoporosis. GC therapy is the most common cause of osteoporosis,
leading to osteonecrosis of the femoral head and fractures, which
may also be associated with fracture-related morbidity and a
decreased quality of life (3–5).
Osteoporosis guidelines recommend that patients who are
administered chronic GC therapy should also be treated for
osteoporosis (6–9). Therefore, the development of
compounds that attenuate GC-induced osteoporosis (GIOP) is of
clinical significance.
Curcumin is the main active ingredient of turmeric
(Curcuma Longa), which is a traditional Chinese medicine
with a long history of use as a treatment for inflammatory
conditions (10). Curcumin is a
highly pleiotropic molecule which is effective in treating a number
of chronic diseases, such as inflammatory bowel disease,
pancreatitis, arthritis and certain types of cancer (11). Moreover, previous studies have
found that curcumin also protects against ovariectomy-induced bone
loss and decreases osteoclastogenesis in rodent models (12–15). In addition, Yang et al
demonstrated that curcumin improved bone microarchitecture and
enhanced mineral density in APP/PS1 transgenic mice (16). In our previous study, we
demonstrated that curcumin attenuated GIOP by inhibiting osteocytic
apoptosis (17). In the present
study, we aimed to continue the investigation of the possible
mechanisms responsible for the protective effects of curcumin
against GIOP.
The Wnt/β-catenin signaling pathway is an important
pathway that is involved in the growth, development and maintenance
of a number of tissues, including bone tissue (18). Osteoblasts and osteoclasts are two
cell types that are critical for bone formation and maintenance.
Wnt/β-catenin signals in osteoblasts induce the expression of
osteoprotegerin (OPG) and thereby inhibit osteoclast
differentiation (19). In a study
on pre-osteoblast-specific β-catenin conditional knockout mice,
osteoblast differentiation was shown to be suppressed, whereas
adipocyte differentiation was enhanced in bone marrow stromal
cells, which indicates that the Wnt/β-catenin signal is the
determinant of the cell fate of pre-osteoblasts (20). In addition, loss-of-function
mutation of the low-density lipoprotein receptor-related protein 5
(LRP5), an important protein in the Wnt/β-catenin signaling pathway
(21,22), has been shown to correlate with a
decrease in bone mass (23), and
gain-of-function mutations in LRP5 have also been shown to cause
increased bone density at certain locations (24,25). Therefore, compounds that induce
the activation of Wnt/β-catenin signaling are beneficial for the
treatment of osteoporosis. Curcumin has been shown to activate the
Wnt/β-catenin signaling pathway in in vivo and in
vitro studies (26–28). However, other studies have
demonstrated that curcumin suppresses this pathway (29,30). In the present study, as a possible
pharmacological mechanism responsible for the bone-protective
effects of curcumin, the regulatory effects of curcumin on the
Wnt/β-catenin signaling pathway were investigated using in
vivo and in vitro models of dexamethasone (DXM)-induced
osteoporosis.
Materials and methods
Animals
Female 5-month-old Sprague-Dawley rats were obtained
from the Experimental Animal Centre of China Medical University
(Shenyang, China). The animals were housed under standard
laboratory conditions at a stable temperature (22–24°C) and a
12/12-h light/dark cycle. This study was approved by the Ethics
Committee of China Medical University (Shenyang, China).
Induction of osteoporosis and
treatments
The rats were randomly divided into 3 groups (n=6
per group) as follows: the control group, the DXM group and the DXM
+ curcumin group. The rats in the DXM and DXM + curcumin groups
received subcutaneous injections of dexamethasone (0.1 mg/kg/day,
Tianjin Pharmaceutical Group Xinzheng Co., Ltd., Zhengzhou, China)
for 60 days. The rats in the control group were injected
subcutaneously with the vehicle only. Bone mineral density (BMD)
was measured at the proximal tibia to confirm that the model had
been successfully established. The rats in the DXM + curcumin group
received an intragastric administration of curcumin (100 mg/kg/day,
Dalian Meilun Biology Technology Co., Ltd., Dalian, China) for a
further 60 days. The rats in the control and DXM groups received
the vehicle only [0.5% sodium carboxymethyl cellulose (CMC-Na)].
Following treatment, BMD was measured again, and the rats were
euthanatized by an overdose of pentobarbital. Blood was collected
from the inferior vena cava. The muscle around the femurs was
removed using surgical scissors and the femurs were collected.
Measurement of BMD
BMD was measured at the femurs by dual-energy X-ray
absorptiometry bone densitometry using a Hologic QDR 4500 machine
(Hologic, Bedford, MA, USA) and the accompanying experimental
animal assessment software. BMD was represented as
g/cm2. All samples were measured 3 times, and the mean
values were calculated. The coefficient of variation for the BMD
measurements was 1.02%.
Determination of the activities of serum
bone-specific alkaline phosphatase (ALP) and tartrate-resistant
acid phosphatase (TRAP)
The activities of bone-specific ALP and TRAP were
determined using commercial kits for ALP and TRAP activity assay
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
following the manufacturer's instructions. Briefly, the serum
samples were mixed with the test solutions and at 37°C for the
indicated periods of time. Finally, the absorbance at 520 nm (ALP)
or 530 nm (TRAP) was read using a microplate reader (ELX-800;
Bio-Tek Instruments, Winooski, VT, USA).
Determination of levels of serum
osteocalcin and serum collagen type I fragments (CTX)
The serum levels of osteocalcin and CTX were
determined using commercial ELISA kits specific to osteocalcin and
CTX (USCN Life Science, Wuhan, China) according to the
manufacturer's instructions. Briefly, the serum samples were
diluted according to the manufacturer's instructions and incubated
with antibody solutions specific for osteocalcin and CTX at 37°C
for 1 h. After washing, streptavidin-HRP was added followed by
incubation at 37°C for 30 min. The absorbance was measured
following the addition of stop solution by a microplate reader
(ELX-800; Bio-Tek Instruments) at 450 nm.
Histological examination
The femoral samples were fixed in 4%
paraformaldehyde for 24 h, dehydrated using a series of ethanol and
embedded in paraffin. Paraffin blocks were cut into
5-µm-thick slices, deparaffinized in xylene and hydrated
using a series of ethanol. The femoral tissue sections were then
stained with hematoxylin and eosin (H&E) solution (Solarbio
Science & Technology Co., Ltd., Beijing, China) and observed
under a light microscope (DP73; Olympus, Tokyo, Japan).
Cell culture
Calvarial bones from 40 24-h-old neonatal Sprague
Dawley rats (Experimental Animal Centre of China Medical
University, Shenyang, China) were used for primary osteoblast cell
culture. Following euthanasia, the calvarial bones were removed and
cut into 1–3-mm3 sections and digested in 1% trypsin and
0.2% collagenase type II at 37°C for 30 min. The digestion was then
terminated, and the mixture was filtered and centrifuged at 300 × g
for 10 min. The supernatant was discarded and the cells were washed
twice using Tris-buffered saline (PBS) and resuspended in
Dulbecco's modified essential medium (DMEM, Gibco Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; HyClone, Logan, UT, USA), 2 mM l-glutamine, 100 U/ml
penicillin and 100 µg/ml streptomycin. The cells were plated
into the indicated culture plates and maintained in a humidified
atmosphere of 5% CO2–95% air at 37°C.
MTT assay
The cells were seeded into a 96-well plate at
4×103 cells/well. The cells were exposed to DXM (0, 1,
10, 100 and 1,000 nM) for 24 h. Subsequently, the cells were seeded
in a 96-well plate at 4×103 cells/well and treated with
curcumin (0, 0.5, 1 and 2 µM) for 24 h. The cells were then
seeded in a 96-well plate at 4×103 cells/well and
pre-treated with curcumin (0.5, 1 and 2 µM) for 2 h, and
then exposed to DXM (100 nM) for 24 h. Finally, the cells were
seeded in a 96-well plate at 4×103 cells/well and
treated with curcumin (0.5, 1 and 2 µM and DXM 100 nM
simultaneously for 24 h.
Following treatment, MTT (0.2 mg/ml; Sigma-Aldrich,
St. Louis, MO, USA) was added to each well followed by incubation
at 37°C for 4 h. The supernatant was then removed, and 200
µl dimethyl sulfoxide (DMSO) were added to solve the
precipitate, and the absorbance at 570 nm was read using a
microplate reader (ELX-800, Bio-Tek Instruments).
mRNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total mRNA from the femoral tissues or primary rat
osteoblasts was isolated using an RNA Simple Total RNA kit (Tiangen
Biotech, Co., Ltd., Beijing, China) according to the manufacturer's
instructions. cDNA was reverse transcribed with oligonucleotide
primer using super Moloney Murine Leukemia Virus (M-MLV) (BioTeke,
Beijing, China) in a 20-µl system. The qPCR reactions were
performed in a 20-µl system containing 10 µl
SYBR-Green Master Mix (Tiangen Biotech Co., Ltd.), 0.5 µM of
forward and reverse primers, and 1 µl template cDNA on an
Exicycler™ 96 real-time quantitative thermal block (Bioneer,
Daejeon, Korea). The sequences of the primers are listed in
Table I. Data were normalized to
β-actin and analyzed using the comparative threshold cycle (CT)
method.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Primer sequences
|
|---|
| Upstream | Downstream |
|---|
| Wnt1 |
CGAAACCGCCGCTGGAACT |
GGAGGTGATTGCGAAGATAAACG |
| LRP5 |
GCAGAGCCACCATCCACAG |
TCTTGCCCATCCAATCCAC |
| SOST |
GTGCAAGTGCAAGCGCCTCA |
GCTCCGCCTGGTTGGCTTTG |
| Osteoclacin |
TAAGGTGGTGAATAGACTCCG |
GCCATAGATGCGCTTGTAG |
| Osterix |
GCCTACTTACCCGTCTGACTTTG |
ACTGCCTTGGGCTTATAGACATC |
| DKK1 |
TATGAGGGCGGGAACAAGTA |
AAATGGCTGTGGTCAGAGGG |
| Col1A1 |
CAAGGACTATGAAGTTGATGC |
ACCAGTAGAGAAATCGCAGT |
| Osteonectin |
GGGCAGACCAATACCTCACTA |
CCGACCATTCCTTCCGTTG |
| Runx2 |
CCATAACGGTCTTCACAAATC |
GAGGCGGTCAGAGAACAAACT |
| OPG |
GACCCCAGAGCGAAACACG |
GGCACAGCAAACCTGAAGAA |
| RANKL |
CATCGGGTTCCCATAAAG |
GAAGCAAATGTTGGCGTA |
| β-catenin |
AGTCCTTTATGAGTGGGAGCAA |
GTTTCAGCATCTGTGACGGTTC |
| β-actin |
GGAGATTACTGCCCTGGCTCCTAGC |
GGCCGGACTCATCGTACTCCTGCTT |
Western blot analysis
The bone tissues or cells were homogenized in
ice-cold radioimmunoprecipitation (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) supplemented with
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology). Following centrifugation at 12,000 × g at 4°C for
10 min, the supernatant was collected and the protein concentration
was determined using a bicinchoninic acid (BCA) protein assay kit
(Beyotime Institute of Biotechnology). Proteins were then separated
on 10% sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gels and
transferred electrophoretically onto polyvinylidene difluoride
(PVDF) membranes (Millipore, Bedford, MA, USA) using the wet
transfer method. The membranes with target proteins were blocked
with 5% non-fat milk at room temperature for 1 h, and then
incubated with the primary antibodies at 4°C overnight. After a
washing stage, the membranes were subsequently incubated with
horseradish peroxidase-labeled goat anti-rabbit or donkey anti-goat
IgG (1:5,000, Beyotime Institute of Biotechnology) at 37°C for 45
min. The protein blots were visualized using enhanced
chemiluminescence (7 Sea Pharmtech, Shanghai, China) and exposed to
Fuji Rx 100 X-ray film (Fuji Photo Film, Tokyo, Japan). The gray
values of the blots were analyzed using Gel-Pro-Analyzer (Media
Cybernetics, Bethesda, MD, USA). β-actin was used as a loading
control. The primary antibodies used in this study were as follows:
phosphorylated glycogen synthase kinase (p-GSK-3β antibody (1:200,
sc-11757; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), GSK-3β
antibody (1:200, sc-9166; Santa Cruz), OPG antibody (1:500,
bs-0431R; Bioss, Beijing, China) and receptor activator for nuclear
factor-kappa B ligand (RANKL) antibody (1:500, bs-0747R; Bioss,
Beijing, China).
Immunofluorescence staining
The cells were cultured on glass coverslips and
fixed in 4% paraformaldehyde for 15 min. Slips were washed in PBS 3
times and incubated with 0.1% Triton X-100 for 30 min at room
temperature. After a washing stage, the slips were blocked in goat
serum for 15 min at room temperature. The cells were then incubated
with anti-β-catenin antibody (1:200, BA0426; Boster, Wuhan, China)
at 4°C overnight. Subsequently, the cells were washed with PBS and
incubated with cy3-labeled goat anti-rabbit secondary antibody
(1:200, Beyotime Institute of Biotechnology) at room temperature
for 1 h. The cells were then co-stained with DAPI and then observed
under a fluorescence microscope (BX53; Olympus).
Statistical analysis
Statistical analyses were conducted with SPSS19
software (IBM, New York, NY, USA). Data are expressed as the means
± standard deviation (SD). Differences between groups were
evaluated by one-way ANOVA followed by Fisher's least significant
difference (LSD) test. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Effects of curcumin on BMD and
histological changes in the femurs of rats with DXM-induced
osteoporosis
We measured the BMD of rats before and after
treatment with curcumin. As illustrated in Fig. 1A, after 60 days of DXM
administration, the BMD of the rats was significantly decreased
compared with the rats in the control group (P<0.01). After a
further 60 days of treatment with curcumin, the BMD of the femurs
increased significantly (P<0.01 vs. DXM group and DXM + curcumin
group post-treatment), and the value was similar to that of the
control group (P>0.05).
Histological changes were examined by H&E
staining. In the control group, the femurs exhibited a complete
trabeculae structure and ordered arrangement of the trabeculae. In
the DXM group, significantly reduced and thinning trabeculae and
small numbers of empty bone lacunae were observed. Treatment with
curcumin markedly reversed these changes (Fig. 1B).
Effects of curcumin on the bone
metabolism of rats with DXM-induced osteoporosis
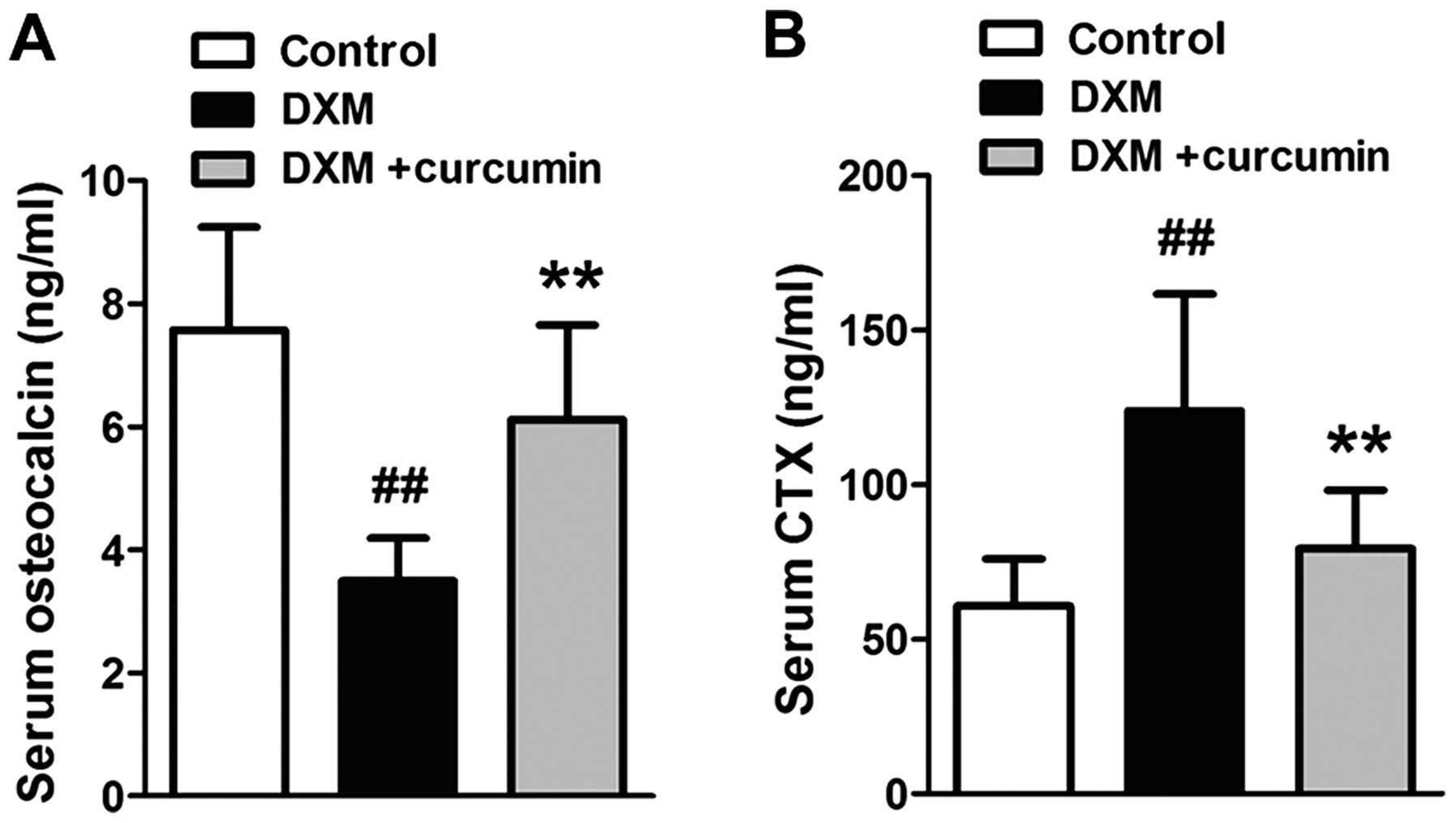
The osteocalcin content was examined as a bone
formation biomarker, and the CTX content was examined as a bone
resorption biomarker. As shown in Fig. 2, the osteocalcin levels were
markedly decreased and the CTX levels were markedly increased in
the serum following the administration of DXM. However, treatment
with curcumin significantly reversed these changes.
Effect of curcumin on the Wnt signaling
pathway
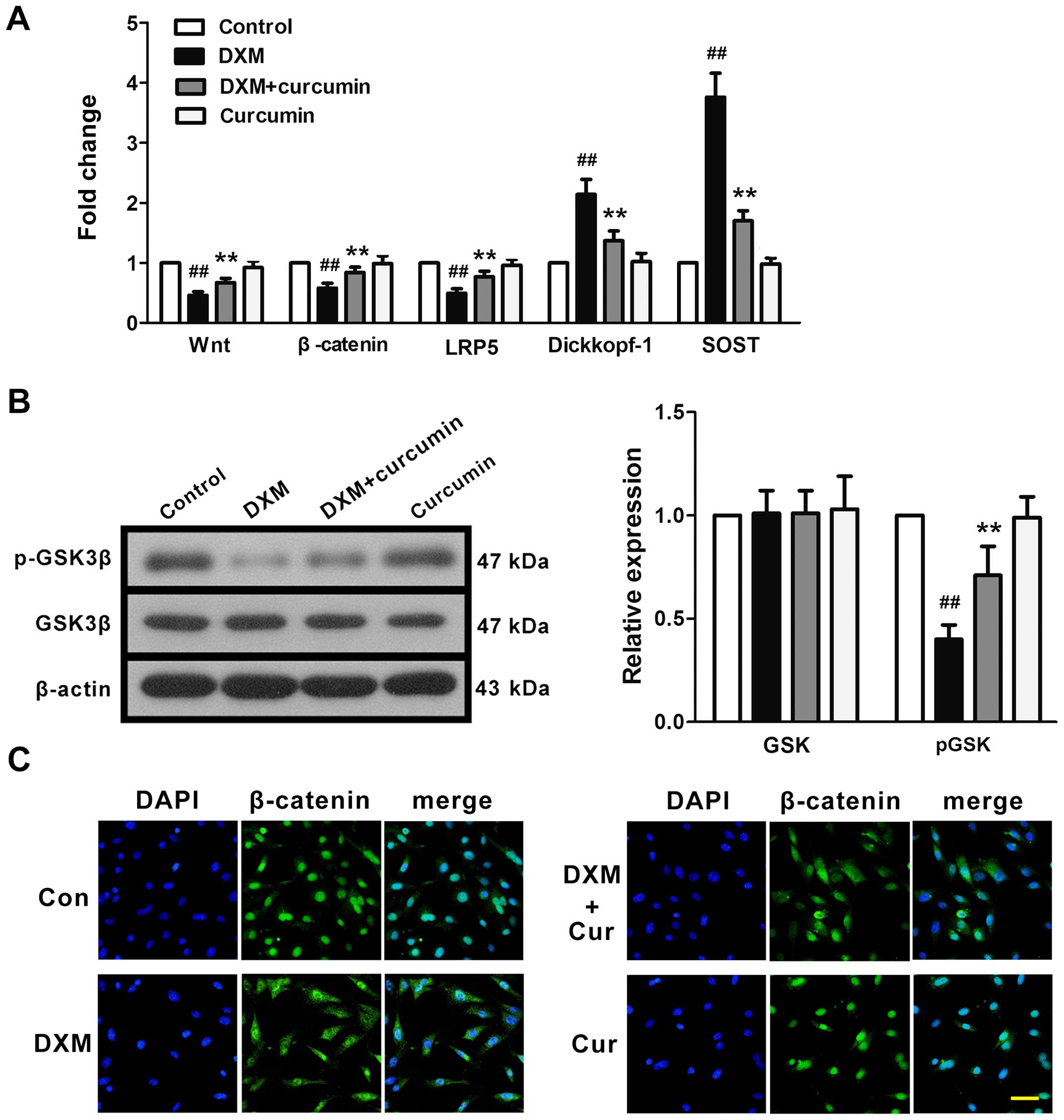
The mRNA expression levels of key proteins in the
Wnt signaling pathway were measured by RT-qPCR. Following exposure
to DXM, the mRNA expression levels of Wnt, β-catenin and LRP5 were
significantly downregulated, whereas the mRNA expression levels of
the two receptor inhibitors, sclerostin (SOST) and Dickkopf-1 were
upregulated (Fig. 3A). In
addition, the phosphorylation of GSK-3β, a cytosolic Wnt signaling
inhibitor, was inhibited by DXM (Fig.
3B). However, treatment with curcumin effectively reversed
these changes. These results indicated that DXM induced the
inhibition of Wnt signaling in the femurs of rats, and that
curcumin, at least partly, reversed this inhibition.
Effects of DXM and curcumin on the
viability of primary osteoblasts
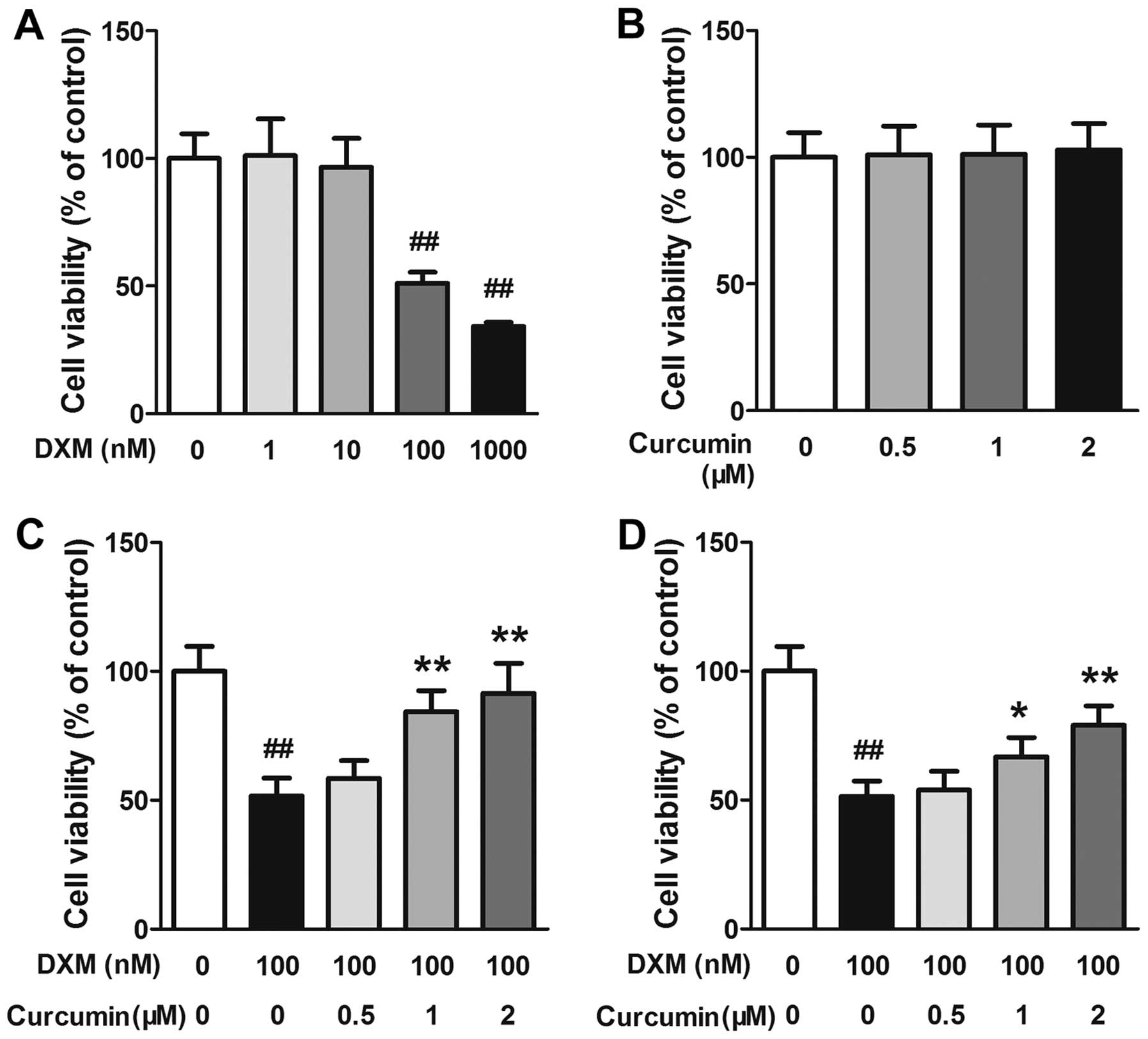
The effects of DXM and curcumin on the viability of
primary osteoblasts were evaluated by MTT assay. As shown in
Fig. 4A and B, exposure to DXM at
100 and 1,000 nM markedly decreased the viability of the
osteoblasts, whereas at all concentrations (0.5, 1 or 2 µM)
curcumin caused no toxicity to osteoblasts. Subsequently, the
effects of curcumin on the viability of DXM-stimulated osteoblasts
were evaluated. In the first experiment, the cells were pre-treated
using the indicated concentrations of curcumin for 2 h and then
exposed to 100 nM DXM for 24 h. In this experiment, curcumin at 1
and 2 µM significantly increased cell viability in a
dose-dependent manner (Fig. 4C).
In the second experiment, the cells were treated with the indicated
concentrations of curcumin and 100 nM DXM simultaneously. In this
experiment, curcumin also inhibited the toxic effects of DXM on
primary osteoblasts in a dose-dependent manner, although the values
were slightly lower than those in the first experiment (Fig. 4D).
Effects of curcumin on the
differentiation and maturation of primary osteoblasts
Based on the results of MTT assay, we treated the
cells with curcumin and DXM simultaneously in order to perform the
following experiments. As expected, DXM at 100 nM induced a marked
decrease in ALP activity in the primary osteoblasts. Curcumin at 2
µM significantly reversed this decrease, but exerted little
effect on normal cells (cells not stimulated with DXM; Fig. 5A). In addition, the mRNA
expression levels of osteoblast differentiation-associated proteins
were measured. As illustrated in Fig.
5B, the mRNA expression levels of osteoclacin, collagen, type
1, alpha 1 (Col1A1), osteonectin, runt-related transcription
factor-2 (Runx2) and osterix were markedly downregulated following
stimulation with DXM. We also examined the expression of two
crucial factors related to osteogenesis, OPG and RANKL. DXM induced
the marked downregulation of OPG and the upregulation of RANKL mRNA
and protein expression (Fig. 5C and
D). Curcumin at 2 µM partly, but still significantly,
reversed the effects of DXM on the expression of these genes.
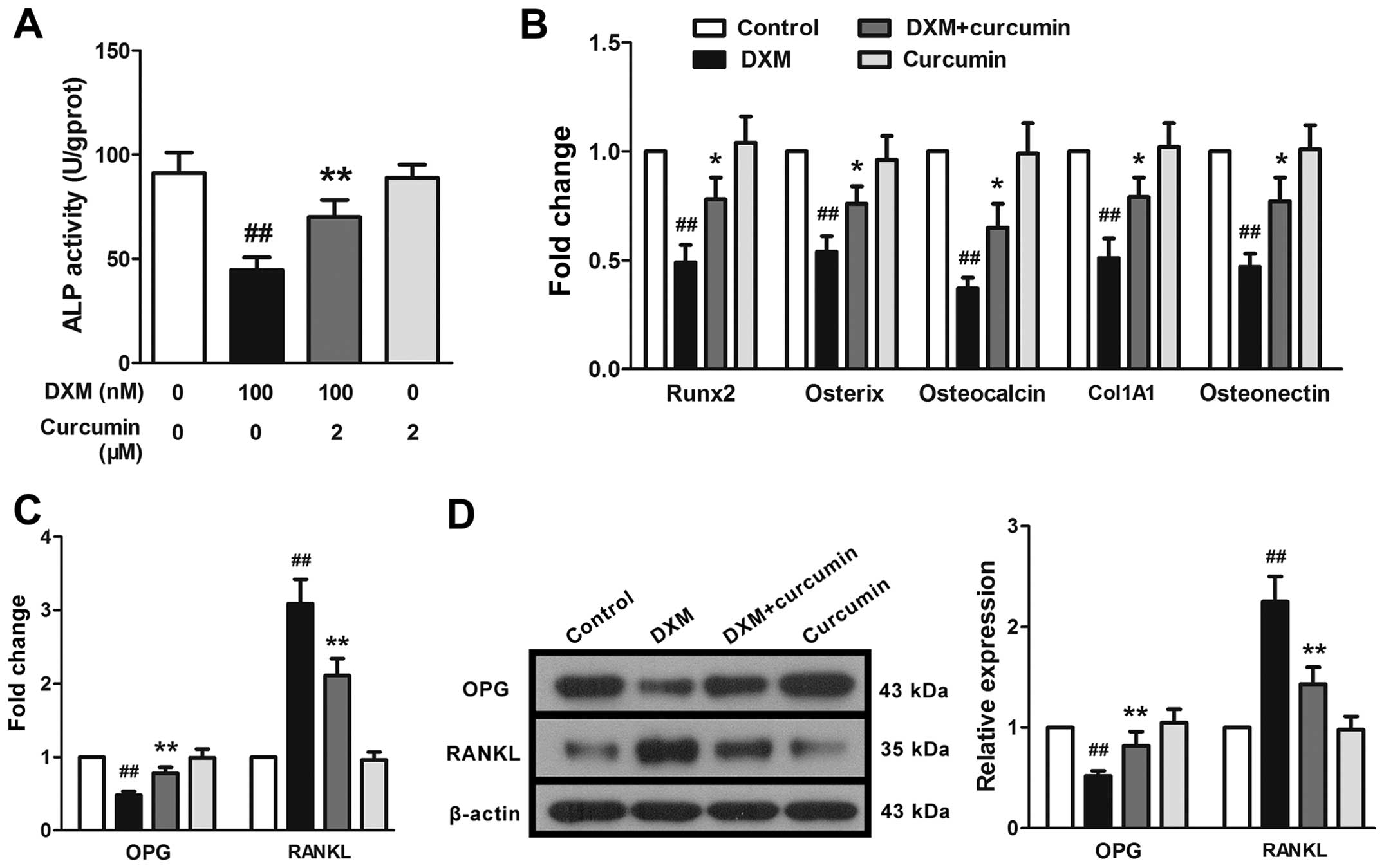 | Figure 5Effects of curcumin on the
differentiation of primary osteoblasts. Following exposure to
dexamethasone (DXM), (A) alkaline phosphatase (ALP) activity was
markedly reduced, and (B) the mRNA expression levels of
osteoclacin, collagen, type 1, alpha 1 (Col1A1), osteonectin,
runt-related transcription factor 2 (Runx2) and osterix were
significantly downregulated, and (C and D) osteoprotegerin (OPG)
expression was diminished and receptor activator for nuclear
factor-kappa B ligand (RANKL) expression was enhanced. Curcumin
reversed these changes. Data are presented as the means ± SD, n=3.
##P<0.01 vs. the control group,
*,**P<0.05 vs. the DXM (100 nM) group. |
Effects of curcumin on the Wnt signaling
pathway in primary osteoblasts
The effects of curcumin on the Wnt signaling pathway
were examined in vitro. In line with our in vivo
experiments, DXM downregulated the mRNA expression of Wnt,
β-catenin and LRP5, and upregulated the mRNA expression of
Dickkopf-1 and SOST (Fig. 6A). In
addition, GSK phosphorylation was inhibited by stimulation with DXM
(Fig. 6B). These changes were
partly reversed by treatment with curcumin. In addition, we used
immunofluorescence staining in order to examine β-catenin
trafficking in osteoblasts (Fig.
6C). In the control primary osteoblasts (no treatment), we
observed a strong intranuclear staining of β-catenin, reflecting
activated Wnt/β-catenin signaling. Exposure to DXM reduced the
intranuclear staining of β-catenin, representing the significant
inhibition of Wnt/β-catenin signaling. Treatment with curcumin
markedly, although not completely, restored the intranuclear
staining of β-catenin. Curcumin did not affect β-catenin nuclear
staining in the normal osteoblasts.
Discussion
In the present study, curcumin was found to be
effective at attenuating DXM-induced osteoporosis both in
vivo and in vitro, as evidenced through its restoration
of BMD and the serum levels of the bone metabolic biomarkers,
osteocalcin and CTX, in rats, and its regulation of bone
differentiation and mature-associated proteins in primary
osteoblasts. In addition, the Wnt/β-catenin signaling pathway was
found to be re-activated by curcumin, which may be related to the
bone-protective effects of curcumin. These results indicate that
curcumin exerts protective effects against GIOP.
GIOP is the most common cause of iatrogenic
osteoporosis (31). Osteoporosis
is characterized by reduced bone mass and deterioration of the bone
microarchitecture (32,33). In previous clinical studies, bone
loss was observed in proximal femurs following treatment with GC
(34,35). In previous studies, GC-treated
animals also exhibited decreased BMD and bone mineral content (BMC)
(36,37). In the present study, in line with
these findings, DXM caused a significant reduction in BMD. However,
following treatment with curcumint, this reduction in BMD was
reversed. Trabecular bone is one of the types of bone which is most
susceptible to the effects of GCs (38–40), and the proximal femur is rich in
trabecular bone. In the present study, the structure of femurs was
damaged upon DXM treatment, as represented by the occurrence of
bone lacunae and thinned trabeculae, and these injuries were
attenuated by treatment with curcumin. These results indicate the
therapeutic effects of curcumin against GIOP.
The disruption of the bone formation-resorption
balance plays a key role in osteoporosis (41). Serum osteocalcin is known as a
marker of bone formation, and CTX is known as a marker of bone
resorption (42). In the present
study, the reduced levels of osteocalcin and the increased levels
of CTX indicated the imbalance of bone formation-resorption in the
rats with DXM-induced osteoporosis. In addition, in our in
vitro experiments, DXM induced a marked reduction in ALP, a
marker of osteoblast differentiation, in primary rat osteoblasts.
Osteoblastic differentiation was also investigated at the gene
level. A significant downregulation in the mRNA expression levels
of Runx2, osterix, osteocalcin, Col1A1 and osteonectin was observed
in the DXM-stimulated osteoblasts. Runx2 and osterix are two major
transcription factors that play an essential role in the formation
of adult bones and the expression of osteoblast genes (43,44). Runx2 is the upstream controller of
osterix (45,46). A number of bone matrix protein
genes, such as Col1A1, osteoponin and osteocalcin, are regulated by
Runx2 (45). Osterix is a zinc
finger-containing transcription factor. The conditional deletion of
osterix postnatally severely disrupts the maturation, morphology
and function of osteocytes (47).
We found that curcumin recovered the serum osteocalcin and CTX
levels and significantly reversed the DXM-induced decrease in Runx2
and osterix genes, thereby upregulating downstream gene expression
and restoring ALP activity. These data suggest that the
bone-protective effects of curcumin are mediated through the
regulation of Runx2 and osterix expression.
Osteoblasts and osteoclasts are also important
participants in the regulation of adult bone remodeling. OPG and
RANKL are two key cytokines produced by osteoblasts that are
involved in this process. RANKL stimulates bone resorption by
binding with the receptor RANK on osteoclasts (48). OPG interacts with RANKL and
inhibits its binding with the receptor (49). Therefore, the balance of RANKL and
OPG production determines the rate of bone resorption and affects
bone maturation (50). In this
study, following the exposure of osteoblasts to DXM, we noted that
the expression of OPG decreased and that of RANKL increased,
indicating the abnormal enhancement of bone resorption. However,
this induction of bone resorption was abolished by treatment with
curcumin to some extent. This result indicates that the regulation
of OPG and RANKL also contributes to the bone-protective effects of
curcumin.
The canonical Wnt/β-catenin signaling pathway is
involved in a number of physiological processes throughout
development and adult life, including bone formation (51). Wnt binds with specific
cell-surface receptors Frizzled and LRP5/6, thereby leading to
binding with Axin, which in turn mediates the proteolysis of
β-catenin. LRP5 dysfunction leads to the development of diseases
associated with the loss of bone mass, such as osteoporosis
pseudoglioma syndrome (OPS) (23). By contrast, the hyperfunction of
LRP5 by mutations leads to the development of diseases associated
with increased bone mass, such as sclerosteosis and osteopetrosis.
In our study, we noted that Wnt, β-catenin and LRP5 gene expression
was significantly diminished following exposure to DXM both in
vivo and in vitro, which suggests that this signaling
pathway was inhibited. SOST and Dickkopf-1 are receptor inhibitors
which play a key role in the regulation of the Wnt signaling
pathway in bone formation (52).
SOST binds to LRP6 to inhibit its association with Wnt. Previous
studies have indicated that serum SOST is positively correlated
with BMD and BMC (53,54). The effects of GCs on SOST
expression appear complex and remain to be clarified in detail, as
SOST expression was found to be both decreased (55) and increased (56) in bone cells following exposure to
GCs. In our study, consistent with the findings of Yao et al
(56), DXM induced the marked
upregulation of SOST expression. Dickkopf (Dkk-1) is also known to
inhibit Wnt signaling by binding to LRP5/6 (57). The transient overexpression of
Dkk-1 has been shown to markedly decrease ALP activity induced by
Wnt3a and reduce the mineralizing capacity in mouse
pre-osteoblastic MC3T3-E1 cells (58). In agreement with the findings of
previous studies (59–61), we also noted a significant
increase in Dkk-1 mRNA expression following exposure to DXM.
GSK-3β is another negative regulator of the Wnt
signaling pathway at the β-catenin level (57). GSK-3β phosphorylates β-catenin in
the cytosol and targets it for proteolysis, thereby inhibiting its
nuclear translocation. The induction of the phosphorylation of
GSK-3β inhibits its activity and allows β-catenin to translocate to
the nucleus and interact with T-cell factor/lymphocyte
enhancer-binding factor (TCF/LEF) to regulate the transcription of
target genes (62). Previous
studies have demonstrated that the GC-induced activation of GSK-3β
in osteoblasts and the inhibition of GSK-3β attenuated bone loss
(63–65). In the present study, the
phosphorylation of GSK-3β was reduced following exposure to DXM,
which was accompanied by the decreased nuclear expression of
β-catenin. These data demonstrate that the inhibition of the entire
Wnt signaling pathway, from the upstream cell surface receptor to
the downstream β-catenin nuclear translocator, was inhibited by
exposure to DXM. As expected, treatment with curcumin recovered the
activity of this signaling pathway. These results suggest that the
Wnt/β-catenin signaling pathway is involved in the bone-protective
effects of curcumin against DXM-induced osteoporosis.
In conclusion, in this study, we demonstrated that
curcumin attenuated DXM-induced osteoporosis in vivo and
osteoblast differentiation dysfunction in vitro.
Furthermore, the mechanisms underlying the bone-protective effects
of curcumin against DXM-induced osteoporosis involved the
activation of the Wnt/β-catenin pathway. In future, curcumin may
become a potential bone-protective therapeutic agent for the
treatment of GIOP.
Acknowledgments
This study was supported by grants from the National
Nature Science Foundation of China (nos. 81370981 and 31201053) and
the Outstanding Scientific Fund of Shengjing Hospital.
References
|
1
|
Buttgereit F, Burmester GR and Lipworth
BJ: Optimised glucocorticoid therapy: the sharpening of an old
spear. Lancet. 365:801–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
den Uyl D, Bultink IE and Lems WF:
Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol
Rep. 13:233–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hallberg I, Bachrach-Lindström M, Hammerby
S, Toss G and Ek AC: Health-related quality of life after vertebral
or hip fracture: a seven-year follow-up study. BMC Musculoskelet
Disord. 10:1352009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cauley JA, Thompson DE, Ensrud KC, Scott
JC and Black D: Risk of mortality following clinical fractures.
Osteoporos Int. 11:556–561. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weinstein RS: Glucocorticoid-induced
osteoporosis. Rev Endocr Metab Disord. 2:65–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Recommendations for the prevention and
treatment of glucocorticoid-induced osteoporosis: 2001 update.
American College of Rheumatology Ad Hoc Committee on
Glucocorticoid-Induced Osteoporosis. Arthritis Rheum. 44:1496–1503.
2001. View Article : Google Scholar
|
|
7
|
Adler RA and Hochberg MC: Suggested
guidelines for evaluation and treatment of glucocorticoid-induced
osteoporosis for the Department of Veterans Affairs. Arch Intern
Med. 163:2619–2624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nawata H, Soen S, Takayanagi R, Tanaka I,
Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S,
et al: Guidelines on the management and treatment of
glucocorticoid-induced osteoporosis of the Japanese Society for
Bone and Mineral Research (2004). J Bone Miner Metab. 23:105–109.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sambrook PN, Diamond T, Ferris L,
Fiatarone-Singh M, Flicker L, MacLennan A, Nowson C, O'Neill S and
Greville H; Osteoporosis Australia; National Asthma Campaign:
Corticosteroid induced osteoporosis. Guidelines for treatment. Aust
Fam Physician. 30:793–796. 2001.PubMed/NCBI
|
|
10
|
Lestari ML and Indrayanto G: Curcumin.
Profiles Drug Subst Excip Relat Methodol. 39:113–204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jurenka JS: Anti-inflammatory properties
of curcumin, a major constituent of Curcuma longa: a review of
preclinical and clinical research. Altern Med Rev. 14:141–153.
2009.PubMed/NCBI
|
|
12
|
Kim WK, Ke K, Sul OJ, Kim HJ, Kim SH, Lee
MH, Kim HJ, Kim SY, Chung HT and Choi HS: Curcumin protects against
ovariectomy-induced bone loss and decreases osteoclastogenesis. J
Cell Biochem. 112:3159–3166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folwarczna J, Zych M and Trzeciak HI:
Effects of curcumin on the skeletal system in rats. Pharmacol Rep.
62:900–909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hussan F, Ibraheem NG, Kamarudin TA, Shuid
AN, Soelaiman IN and Othman F: Curcumin protects against
ovariectomy-induced bone changes in rat model. Evid Based
Complement Alternat Med. 2012:1749162012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho DC, Jung HS, Kim KT, Jeon Y, Sung JK
and Hwang JH: Therapeutic advantages of treatment of high-dose
curcumin in the ovariectomized rat. J Korean Neurosurg Soc.
54:461–466. 2013. View Article : Google Scholar
|
|
16
|
Yang MW, Wang TH, Yan PP, Chu LW, Yu J,
Gao ZD, Li YZ and Guo BL: Curcumin improves bone microarchitecture
and enhances mineral density in APP/PS1 transgenic mice.
Phytomedicine. 18:205–213. 2011. View Article : Google Scholar
|
|
17
|
Chen Z, Xue J, Shen T, Ba G, Yu D and Fu
Q: Curcumin alleviates glucocorticoid-induced osteoporosis by
protecting osteoblasts from apoptosis in vivo and in vitro. Clin
Exp Pharmacol Physiol. Oct 30–2015.Epub ahead of print. View Article : Google Scholar
|
|
18
|
Cadigan KM and Nusse R: Wnt signaling: a
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar
|
|
19
|
Glass DA II, Bialek P, Ahn JD, Starbuck M,
Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA and
Karsenty G: Canonical Wnt signaling in differentiated osteoblasts
controls osteoclast differentiation. Dev Cell. 8:751–764. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song L, Liu M, Ono N, Bringhurst FR,
Kronenberg HM and Guo J: Loss of wnt/β-catenin signaling causes
cell fate shift of preosteoblasts from osteoblasts to adipocytes. J
Bone Miner Res. 27:2344–2358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pinson KI, Brennan J, Monkley S, Avery BJ
and Skarnes WC: An LDL-receptor-related protein mediates Wnt
signalling in mice. Nature. 407:535–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tamai K, Semenov M, Kato Y, Spokony R, Liu
C, Katsuyama Y, Hess F, Saint-Jeannet JP and He X:
LDL-receptor-related proteins in Wnt signal transduction. Nature.
407:530–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong Y, Slee RB, Fukai N, Rawadi G,
Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et
al: Osteoporosis-Pseudoglioma Syndrome Collaborative Group: LDL
receptor-related protein 5 (LRP5) affects bone accrual and eye
development. Cell. 107:513–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Little RD, Carulli JP, Del Mastro RG,
Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace
B, et al: A mutation in the LDL receptor-related protein 5 gene
results in the autosomal dominant high-bone-mass trait. Am J Hum
Genet. 70:11–19. 2002. View
Article : Google Scholar
|
|
25
|
Boyden LM, Mao J, Belsky J, Mitzner L,
Farhi A, Mitnick MA, Wu D, Insogna K and Lifton RP: High bone
density due to a mutation in LDL-receptor-related protein 5. N Engl
J Med. 346:1513–1521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tiwari SK, Agarwal S, Seth B, Yadav A,
Nair S, Bhatnagar P, Karmakar M, Kumari M, Chauhan LK, Patel DK, et
al: Curcumin-loaded nanoparticles potently induce adult
neurogenesis and reverse cognitive deficits in Alzheimer's disease
model via canonical Wnt/β-catenin pathway. ACS Nano. 8:76–103.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tiwari SK, Agarwal S, Tripathi A and
Chaturvedi RK: Bisphenol-A mediated inhibition of hippocampal
neurogenesis attenuated by curcumin via canonical Wnt pathway. Mol
Neurobiol. May 12–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen F, Wang H, Xiang X, Yuan J, Chu W,
Xue X, Zhu H, Ge H, Zou M, Feng H and Lin J: Curcumin increased the
differentiation rate of neurons in neural stem cells via wnt
signaling in vitro study. J Surg Res. 192:298–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He M, Li Y, Zhang L, Li L, Shen Y, Lin L,
Zheng W, Chen L, Bian X, Ng HK and Tang L: Curcumin suppresses cell
proliferation through inhibition of the Wnt/β-catenin signaling
pathway in medulloblastoma. Oncol Rep. 32:173–180. 2014.PubMed/NCBI
|
|
30
|
Cui L, Jia X, Zhou Q, Zhai X, Zhou Y and
Zhu H: Curcumin affects β-catenin pathway in hepatic stellate cell
in vitro and in vivo. J Pharm Pharmacol. 66:1615–1622. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Albaum JM, Levesque LE, Gershon AS, Liu G
and Cadarette SM: Glucocorticoid-induced osteoporosis management
among seniors, by year, sex, and indication, 1996–2012. Osteoporos
Int. 26:2845–2852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baofeng L, Zhi Y, Bei C, Guolin M,
Qingshui Y and Jian L: Characterization of a rabbit osteoporosis
model induced by ovariectomy and glucocorticoid. Acta Orthop.
81:396–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Castañeda S, Calvo E, Largo R,
González-González R, de la Piedra C, Díaz-Curiel M and
Herrero-Beaumont G: Characterization of a new experimental model of
osteoporosis in rabbits. J Bone Miner Metab. 26:53–59. 2008.
View Article : Google Scholar
|
|
34
|
Reid IR, Evans MC, Wattie DJ, Ames R and
Cundy TF: Bone mineral density of the proximal femur and lumbar
spine in glucocorticoid-treated asthmatic patients. Osteoporos Int.
2:103–105. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sambrook P, Birmingham J, Kempler S, Kelly
P, Eberl S, Pocock N, Yeates M and Eisman J: Corticosteroid effects
on proximal femur bone loss. J Bone Miner Res. 5:1211–1216. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng M, Zhang R, Gong F, Yang P, Fan L, Ni
J, Bi W, Zhang Y, Wang C and Wang K: Protective effects of
necrostatin-1 on glucocorticoid-induced osteoporosis in rats. J
Steroid Biochem Mol Biol. 144B:455–462. 2014. View Article : Google Scholar
|
|
37
|
Samir SM and Malek HA: Effect of
cannabinoid receptors 1 modulation on osteoporosis in a rat model
of different ages. J Physiol Pharmacol. 65:687–694. 2014.PubMed/NCBI
|
|
38
|
Ehrlich PJ and Lanyon LE: Mechanical
strain and bone cell function: a review. Osteoporos Int.
13:688–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Laan RF, Buijs WC, van Erning LJ, Lemmens
JA, Corstens FH, Ruijs SH, van de Putte LB and van Riel PL:
Differential effects of glucocorticoids on cortical appendicular
and cortical vertebral bone mineral content. Calcif Tissue Int.
52:5–9. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reid IR and Heap SW: Determinants of
vertebral mineral density in patients receiving long-term
glucocorticoid therapy. Arch Intern Med. 150:2545–2548. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim HJ, Zhao H, Kitaura H, Bhattacharyya
S, Brewer JA, Muglia LJ, Ross FP and Teitelbaum SL: Glucocorticoids
suppress bone formation via the osteoclast. J Clin Invest.
116:2152–2160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miyazaki T, Matsunaga T, Miyazaki S,
Hokari S and Komoda T: Changes in receptor activator of nuclear
factor-kappaB, and its ligand, osteoprotegerin, bone-type alkaline
phosphatase, and tartrate-resistant acid phosphatase in
ovariectomized rats. J Cell Biochem. 93:503–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wysokinski D, Pawlowska E and Blasiak J:
RUNX2: A master bone growth regulator that may be involved in the
DNA damage response. DNA Cell Biol. 34:305–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sinha KM and Zhou X: Genetic and molecular
control of osterix in skeletal formation. J Cell Biochem.
114:975–984. 2013. View Article : Google Scholar :
|
|
45
|
Komori T: Regulation of skeletal
development by the Runx family of transcription factors. J Cell
Biochem. 95:445–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou X, Zhang Z, Feng JQ, Dusevich VM,
Sinha K, Zhang H, Darnay BG and de Crombrugghe B: Multiple
functions of Osterix are required for bone growth and homeostasis
in postnatal mice. Proc Natl Acad Sci USA. 107:12919–12924. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa
N, et al: A novel molecular mechanism modulating osteoclast
differentiation and function. Bone. 25:109–113. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsuda E, Goto M, Mochizuki S, Yano K,
Kobayashi F, Morinaga T and Higashio K: Isolation of a novel
cytokine from human fibroblasts that specifically inhibits
osteoclastogenesis. Biochem Biophys Res Commun. 234:137–142. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Buckley KA and Fraser WD: Receptor
activator for nuclear factor kappaB ligand and osteoprotegerin:
regulators of bone physiology and immune responses/potential
therapeutic agents and biochemical markers. Ann Clin Biochem.
39:551–556. 2002. View Article : Google Scholar
|
|
51
|
Kim JH, Liu X, Wang J, Chen X, Zhang H,
Kim SH, Cui J, Li R, Zhang W, Kong Y, et al: Wnt signaling in bone
formation and its therapeutic potential for bone diseases. Ther Adv
Musculoskelet Dis. 5:13–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rossini M, Gatti D and Adami S:
Involvement of WNT/β-catenin signaling in the treatment of
osteoporosis. Calcif Tissue Int. 93:121–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Amrein K, Amrein S, Drexler C, Dimai HP,
Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR and Fahrleitner-Pammer
A: Sclerostin and its association with physical activity, age,
gender, body composition, and bone mineral content in healthy
adults. J Clin Endocrinol Metab. 97:148–154. 2012. View Article : Google Scholar
|
|
54
|
Sheng Z, Tong D, Ou Y, Zhang H, Zhang Z,
Li S, Zhou J, Zhang J and Liao E: Serum sclerostin levels were
positively correlated with fat mass and bone mineral density in
central south Chinese postmenopausal women. Clin Endocrinol (Oxf).
76:797–801. 2012. View Article : Google Scholar
|
|
55
|
Sutherland MK, Geoghegan JC, Yu C, Winkler
DG and Latham JA: Unique regulation of SOST, the sclerosteosis
gene, by BMPs and steroid hormones in human osteoblasts. Bone.
35:448–454. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yao W, Cheng Z, Pham A, Busse C,
Zimmermann EA, Ritchie RO and Lane NE: Glucocorticoid-induced bone
loss in mice can be reversed by the actions of parathyroid hormone
and risedronate on different pathways for bone formation and
mineralization. Arthritis Rheum. 58:3485–3497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bafico A, Liu G, Yaniv A, Gazit A and
Aaronson SA: Novel mechanism of Wnt signalling inhibition mediated
by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol.
3:683–686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rawadi G, Vayssière B, Dunn F, Baron R and
Roman-Roman S: BMP-2 controls alkaline phosphatase expression and
osteoblast mineralization by a Wnt autocrine loop. J Bone Miner
Res. 18:1842–1853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mak W, Shao X, Dunstan CR, Seibel MJ and
Zhou H: Biphasic glucocorticoid-dependent regulation of Wnt
expression and its inhibitors in mature osteoblastic cells. Calcif
Tissue Int. 85:538–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ohnaka K, Taniguchi H, Kawate H, Nawata H
and Takayanagi R: Glucocorticoid enhances the expression of
dickkopf-1 in human osteoblasts: novel mechanism of
glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun.
318:259–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang FS, Ko JY, Yeh DW, Ke HC and Wu HL:
Modulation of Dickkopf-1 attenuates glucocorticoid induction of
osteoblast apoptosis, adipocytic differentiation, and bone mass
loss. Endocrinology. 149:1793–1801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lin X, Farooqi AA and Ismail M: Recent
progress in fungus-derived bioactive agents for targeting of
signaling machinery in cancer cells. Drug Des Devel Ther.
9:1797–1804. 2015.PubMed/NCBI
|
|
63
|
Wang FS, Chuang PC, Lin CL, Chen MW, Ke
HJ, Chang YH, Chen YS, Wu SL and Ko JY: MicroRNA-29a protects
against glucocorticoid-induced bone loss and fragility in rats by
orchestrating bone acquisition and resorption. Arthritis Rheum.
65:1530–1540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Smith E and Frenkel B: Glucocorticoids
inhibit the transcriptional activity of LEF/TCF in differentiating
osteoblasts in a glycogen synthase kinase-3beta-dependent and
-independent manner. J Biol Chem. 280:2388–2394. 2005. View Article : Google Scholar
|
|
65
|
Wang FS, Ko JY, Weng LH, Yeh DW, Ke HJ and
Wu SL: Inhibition of glycogen synthase kinase-3beta attenuates
glucocorticoid-induced bone loss. Life Sci. 85:685–692. 2009.
View Article : Google Scholar : PubMed/NCBI
|