Introduction
Ginsenosides, or ginseng saponins, are the major
components of ginseng (Panax ginseng C.A. Mey.) and are
believed to be responsible for the majority of the beneficial
effects of ginseng (1). The
pharmacologically active ginsenosides reportedly have diverse
beneficial effects on the circulatory, endocrine, immune and
central nervous systems (2). For
example, the ginsenosides Rb1 and Rg1 have been shown to improve
the function of neurotransmitters, such as acetylcholine (3,4) as
well as to exert neuroprotective and neurite outgrowth-promoting
effects in cultured neurons (5).
In addition, cultured neurons treated with ginsenoside Rd
demonstrated a higher survival rate against excitotoxicity and
oxidative stress-induced injury (6–8).
Ginsenoside Rg3 has been shown to attenuate cell damage induced by
neurotoxicity and to inhibit the overproduction of nitric oxide and
malondialdehyde (MDA) formation induced by glutamate (9,10).
Of note, it has been demonstrated that ginsenosides prevent
neuronal cell death during ischemia and glutamate-induced
excitotoxicity (11), and enhance
neurological function recovery while reducing the infarct size
(12). In animals, the
application of ginsenosides has been shown to rescue neurons in the
forebrain from cell death and to prevent myocardial infarction. A
recent study suggested that the purified ginsenoside,
20(S)-protopanaxadiol (PPD), exerted protective effects against
human lung cancer cells and permanent focal cerebral ischemic
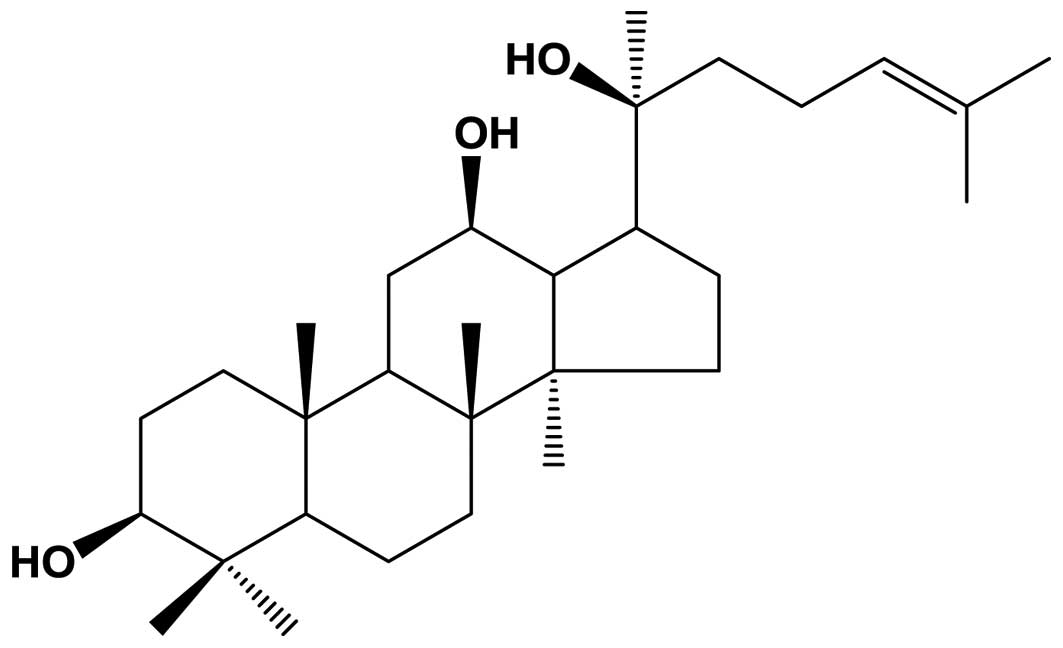
injury in rats (13,50). The molecular composition of PPD
(Fig. 1) has been suggested to
play a role in regulating Ca2+ levels, as well as the
activity of superoxide dismutase (SOD) and nitric oxide synthase
(NOS) (14–16). However, the precise mechanisms
responsible for the neuroprotective effects of PPD remain poorly
understood.
The mitochondria play a critical role in maintaining
cellular function (17). Studies
have shown that ginsenosides exert preventive effects against
mitochondrial dysfunction (18),
and that they enhance cell longevity (19,20), possibly by improving mitochondrial
quality control (21). The
regulation of reactive oxygen species (ROS) production by
mitochondria plays a critical role in neurons by affecting the
homeostasis of mitochondrial morphology and function. In addition,
ginsenoside Rg3 has been shown to exert protective effects on
mitochondrial function and energy status during
ischemia/reperfusion in the rat brain (22). Ischemia/reperfusion injury is
related to ROS production and mitochondrial damage, which
ultimately lead to cellular damage (23).
In the present study, we demonstrate that PPD exerts
anti-apoptotic effects through its antioxidant activity, and that
PPD prevents glutamate-induced excitotoxicity through mitochondrial
homeostasis in PC12 cells. Our data suggest that PPD may prove to
be a novel therapeutic agent which may provide neuroprotection and
prevent mitochondrial dysfunction.
Materials and methods
Materials
PPD (chemical structure shown in Fig. 1) was HPLC grade and purchased from
the Ambo Institute (Seoul, Korea). All chemicals and solvents used
were of the highest analytical grade available. Cell culture
supplies and media, fetal bovine serum (FBS), phosphate-buffered
saline (PBS) and penicillin-streptomycin were purchased from Thermo
Fisher Scientific (Waltham, MA, USA). Anti-cleaved capase-3
antibody (#9664) was purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and Hoechst 33258 were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Annexin V-FITC was purchased from BD Biosciences (Flanklin
Lakes, NJ, USA). Dihydroethidium (DHE) was purchased from
Calbiochem (EMD Millipore; Billerica, MA, USA).
Tetramethylrhodamine ethyl ester (TMRE), MitoSOX™ Red and
MitoTracker Green FM were purchased from Invitrogen (Waltham, MA,
USA).
Cell culture
The PC12 cell line was purchased from the American
Tissue Culture Collection (ATCC; Rockville, MD, USA). The cells
were cultured under sterile conditions at 37°C in a humid
environment with 5% of CO2 in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) (both from Thermo Fisher Scientific), 4 mM glutamine, 100
U/ml penicillin and 100 mg/ml streptomycin. The cultures were
regularly checked and trypsinized when the cell confluence reached
85%.
MTT assay
To determine the half maximal inhibitory
concentration (IC50 value) of glutamate on the PC12
cells, 5×103 cells in single cell suspensions were
seeded in individual wells of 96-well plates and incubated for 24 h
at 37°C prior to exposure to glutamate at the indicated
concentrations (1, 5 and 10 mM) for 24 h. After determining that
the glutamate IC50 value was 5 mM, the cells were
exposed to 5 mM glutamate for 24 h, 10 μM PPD, or a
combination of both (5 mM glutamate plus 10 μM PPD). MTT
solution was added to each well followed by incubation for 4 h at
37°C prior to removing the culture medium. DMSO was then added and
mixed for 30 min at room temperature. Cell viability was determined
by measuring the absorbance at 562 nm. The cell viability for each
group was calculated as a percentage of that of the control
group.
Morphological observation by
microscopy
The cells were seeded at a density of
1×105 cells/well into 24-well plates and were left to
grow exponentially at 37°C in a 5% CO2 atmosphere. The
PC12 cells were then treated with the different drugs [untreated
control (U), glutamine (Glut), PPD or Glut + PPD], and incubated
for 12 h. Subsequently, the cells were fixed with 4%
paraformaldehyde, washed with PBS 3 times and observed under an
inverted microscope (Olympus CKX41; Olympus Corp., Tokyo,
Japan).
Hoechst 33258 staining
To evaluate cell apoptosis, 1×106
cells/well were plated on a coverslip in a 12-well plate in
triplicate. The cells were treated with the different drugs [U,
Glut, PPD or Glut + PPD] and kept in a CO2 incubator at
37°C for 24 h. Following incubation and the addition of fresh
medium, the cells were incubated with 5 μl/well of Hoechst
33258 (Sigma-Aldrich) solution at 37°C for 10 min, followed by
observation under a laser confocal microscope (LSM-700; Carl Zeiss,
Oberkochen, Germany). Strong fluorescence with shrunken nuclei was
observed in the apoptotic cells, whereas weak fluorescence with
normal-sized nuclei was observed in the non-apoptotic cells. The
quantification of apoptotic cells was performed by capturing images
in random fields and counting at least 200 cells in 4 random fields
in each well.
Western blot analysis
Briefly, 30–50 μg of protein were resolved by
15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
then electro-blotted onto polyvinylidene difluoride membranes for
western blot analysis. The blots were probed with 1:1,000-diluted
primary antibodies [anti-cleaved caspase-3 (#9664; Cell Signaling
Technology, Inc.), actin (sc-7210; Santa Cruz Biotechnology,
Dallas, TX, USA)] overnight at 4°C, followed by horseradish
peroxidase-conjugated secondary antibodies (anti-rabbit IgG,
HRP-linked antibody, #7074; Cell Signaling Technology, Inc.). The
proteins were then visualized using enhanced chemiluminescent
reagent (ECL; Millipore Corp., Billerica, MA, USA) and exposure to
X-ray film.
Flow cytometric analysis
The cells were analyzed by flow cytometry using a BD
FACSCanto II flow cytometer as indicated by the manufacturer (BD
Biosciences). Following 2 washes with PBS, the cells were fixed in
4% paraformaldehyde for 10 min at 37°C and permeabilized with 0.25%
Triton X-100 in PBS for 10 min. The cells were stained with
individual dye for 1 to 2 h at 4°C and the secondary antibodies
(1:100) for 1 h on ice. Following 2 washes with PBS, the cells were
fixed in 4% para-formaldehyde and assayed immediately. Flow
cytometry data were collected using 10,000–30,000 cells and were
analyzed using FlowJo software (Tree Star, Inc., Ashland, OR,
USA).
Measurement of ROS production
The mitochondrial ROS levels were measured using the
mitochondrion-specific fluorescent hydroethidine-derivative dye,
MitoSOX Red (10 μM; Invitrogen), as previously described
(24). The cells were exposed to
glutamate with or without treatment with PPD and then incubated
with MitoSOX Red for 30 min at 37°C in 5% CO2. The cells
were washed twice with PBS, fixed with 4% paraformaldehyde, and
analyzed under a confocal microscope. To measure the intracellular
ROS levels, the cells were incubated for 30 min in Krebs-HEPES
buffer containing DHE (Calbiochem) washed twice, and analyzed using
a flow cytometer.
Immunofluorescence staining
The cells (1×106 cells/well) were
prepared on sterilized glass coverslips (BD Biosciences) in
triplicate. Following 24 h of treatment with the drugs (U, Glut,
PPD or Glut + PPD), the cells were fixed in 4% paraformaldehyde in
PBS for 10 min, permeabilized with 0.25% Triton X-100 in PBS for 10
min, and incubated with the primary antibody (Tomm20, ab56783;
Cambridge, MA, USA) for 2 h at room temperature. The cells were
washed to remove the excess primary antibody, and incubated with
the appropriate fluorescently-labeled secondary antibodies for 1 h
at room temperature. The nuclei were stained with DAPI and
incubated for 5 min. After mounting, fluorescence images were
captured using a confocal microscope (LSM 700; Carl Zeiss). To
quantify the immunoreacted cells, the fluorescence intensity was
measured in 10 randomly selected images.
Analysis of apoptosis
The determine cell apoptosis, monolayer cultures of
PC12 cells treated with the different drugs [U, Glut, PPD and Glut
+ PPD] were supplemented with nutrient medium. The cultures were
incubated for 24 h. The dead cells were analyzed prior to and
subsequent to exposure to glutamate and treatment with PPD using
the Muse™ Annexin V and Dead Cell Assay kit (Muse™Cell Analyzer;
Millipore Corp.) according to the manufacturer's instructions.
Transmission electron microscopy
(TEM)
For TEM analysis, the cells were collected and fixed
with 4% paraformaldehyde and 2% glutaraldehyde in 0.1 M sodium
cacodylate buffer (pH 7.4) for 2 h, post-fixed with 1% osmium
tetroxide for 1 h, washed, and stained for 1 h in 3% aqueous uranyl
acetate. The samples were then washed again, dehydrated with graded
alcohol, and embedded in Epon-Araldite resin (Canemco, Inc.,
Lakefield, QC, Canada). Ultrathin sections were cut on an
ultramicrotome (Reichert-Jung, Inc., Cambridge, UK), counterstained
with 0.3% lead citrate, and examined under a transmission electron
microscope (HT7700; Hitachi, Ltd., Tokyo, Japan).
Measurement of mitochondrial membrane
potential (MMP or ΔΨm)
The ΔΨm of intact cells was measured as previously
described (25), with some
modifications. Briefly, the cells were washed with PBS and
trypsinized. The protein concentration of the cells was adjusted to
0.2 mg/ml in DMEM without phenol red (Invitrogen Life
Technologies), FBS and antibiotics. TMRE (200 nM; T669;
Invitrogen-Molecular Probes) was added to the cell suspension. The
cells were incubated at 37°C for 30 min in the dark. ΔΨm was
measured by flow cytometry, and the data were analyzed using FlowJo
software. TMRE fluorescence was measured using the FL2 channel (582
nm).
Determination of mitochondrial mass and
visualization of mitochondria in living cells
Mitochondrial mass was measured using fluorescence
levels following staining with MitoTracker Green FM (100 nM; M7514;
Invitrogen) at 37°C for 30 min. Subsequently, the cells were washed
once in PBS and promptly used for flow cytometry. The percentage
and mean fluorescence intensity level of the mass were calculated
for each sample.
To visualize the mitochondria, the mitochondrial
marker, Tomm20, was used, and was added with DMEM at 37°C for 30
min. The cover glasses were then washed twice with PBS and images
were immediately captured using a confocal laser scanning
microscope (LSM 700; Carl Zeiss). The images were evaluated as
best-focus intensity projections.
Statistical analysis
Data obtained from independent experiments (means ±
SD) were analyzed using a two-tailed Student's t-test. Differences
were considered significant if P<0.05. 95% confidence intervals
were computed, 1.96 x standard error in each direction. Statistical
significance was evaluated using a log-rank (Mantel-Cox) test.
Results
Effect of PPD on cell viability
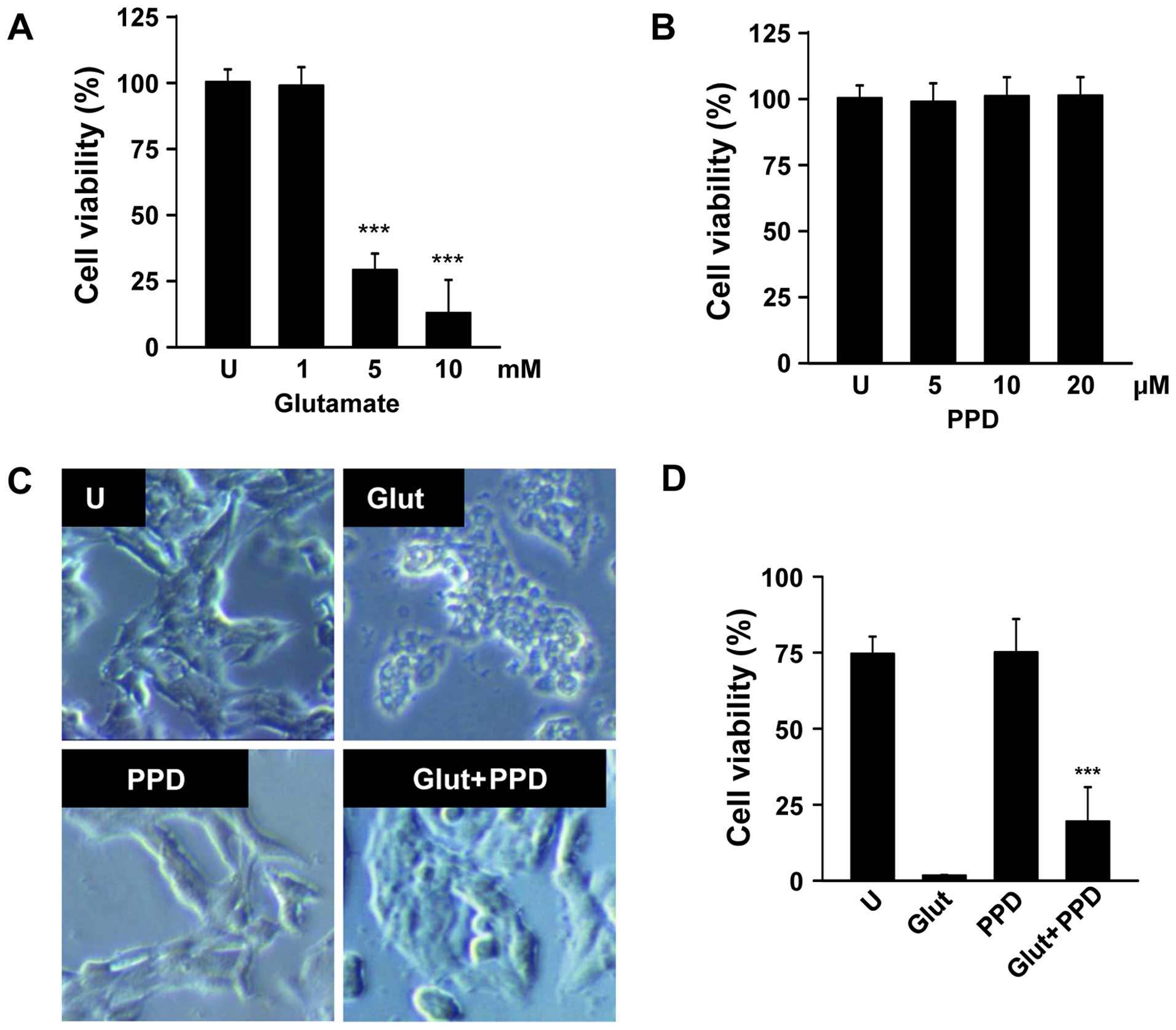
To determine the appropriate concentration of
glutamate, the PC12 cells were exposed to various concentrations of
glutamate for 24 h. Glutamate (1–10 mM) gradually reduced cell
viability in a dose-dependent manner, and the cell viability (as
shown by MTT assay) decreased by approximately 70% in the cells
incubated with 5 mM glutamate for 24 h (Fig. 2A). Hence, the concentration of 5
mM glutamate was selected for use in the subsequent assays. The
cells exposed to 5 mM glutamate were pre-treated with 5, 10 or 20
μM PPD (Fig. 2B). We
wished to examine the possible beneficial effects of PPD on the
viability of glutamate-exposed PC12 cells. The exposure of the
neuronal PC12 cells to glutamate for 24 h resulted in cell damage,
as shown by the changes in cell morphology, such as cell shrinkage
(Fig. 2C). As shown by MTT assay,
pre-treatment with PPD resulted in a higher cell viability compared
with the glutamate-exposed cells not treated with PPD (Fig. 2D). Thus, treatment with PPD
significantly improved the viability of the glutamate-exposed
cells.
PPD prevents glutamate-induced apoptosis
and cytotoxicity
We then wished to examine whether glutamate-induced
cytotoxicity is mediated by apoptotic processes, using Hoechst
33342 staining, Annexin V staining and caspase-3 antibody. Hoechst
33342 staining revealed that treatment with PPD significantly
decreased the amount of condensed chromatin compared with the
glutamate-exposed cells not treated with PPD (Fig. 3A). Pre-treatment with PPD also
significantly decreased the percentage of Annexin V-positive cells,
compared the glutamate-exposed cells not treated with PPD (Fig. 3B). The results of western blot
analysis also revealed that treatment with PPD prevented the
glutamate-induced cleavage of caspase-3 (Fig. 3C), consistent with the results
regarding the inhibition of apoptosis in the PPD pre-treated cells.
Thus, PPD plays a crucial role in reducing the cytotoxic effects of
glutamate by inhibiting apoptosis.
Effects of PPD on cytosolic and
mitochondrial ROS generation
Given that ginsenosides are able to reduce ROS
generation, and that the glutamate-induced apoptotic pathway is
linked to ROS of cytosolic and mitochondrial origin (26), in this study, we investigated the
effects of PPD on the accumulation of ROS in glutamate-exposed PC12
cells. We measured cytosolic and mitochondrial ROS formation by
flow cytometry and fluorescence microscopy using DHE and MitoSOX
Red, a fluoroprobe that selectively detects ROS formation in live
cells and mitochondria. The addition of PPD suppressed the
glutamate-induced production of ROS, including the DHE-reactive
superoxide anion (O2−) (Fig. 4A). Cytosolic ROS was measured by
flow cytometric analysis of the DHE-stained cells. In additional
experiments, mitochondrial ROS production was estimated using
MitoSOX Red, a cell-permeable probe that is non-fluorescent when
chemically reduced, but fluoresces following cellular oxidation and
the removal of acetate groups by cellular esterases (Fig. 4B). The mitochondrial ROS levels
were significantly decreased following treatment with PPD (Fig. 4C). PPD scavenged the mitochondrial
ROS produced by glutamate-induced mitochondrial damage. These
results suggest that PPD exerts major effects on cell survival
through its antioxidant effects.
PPD protects the mitochondria against
glutamate-induced damage
A recent study reported that glutamate is associated
with mitochondrial damage (27).
Thus, to determine whether glutamate induces mitochondrial damage,
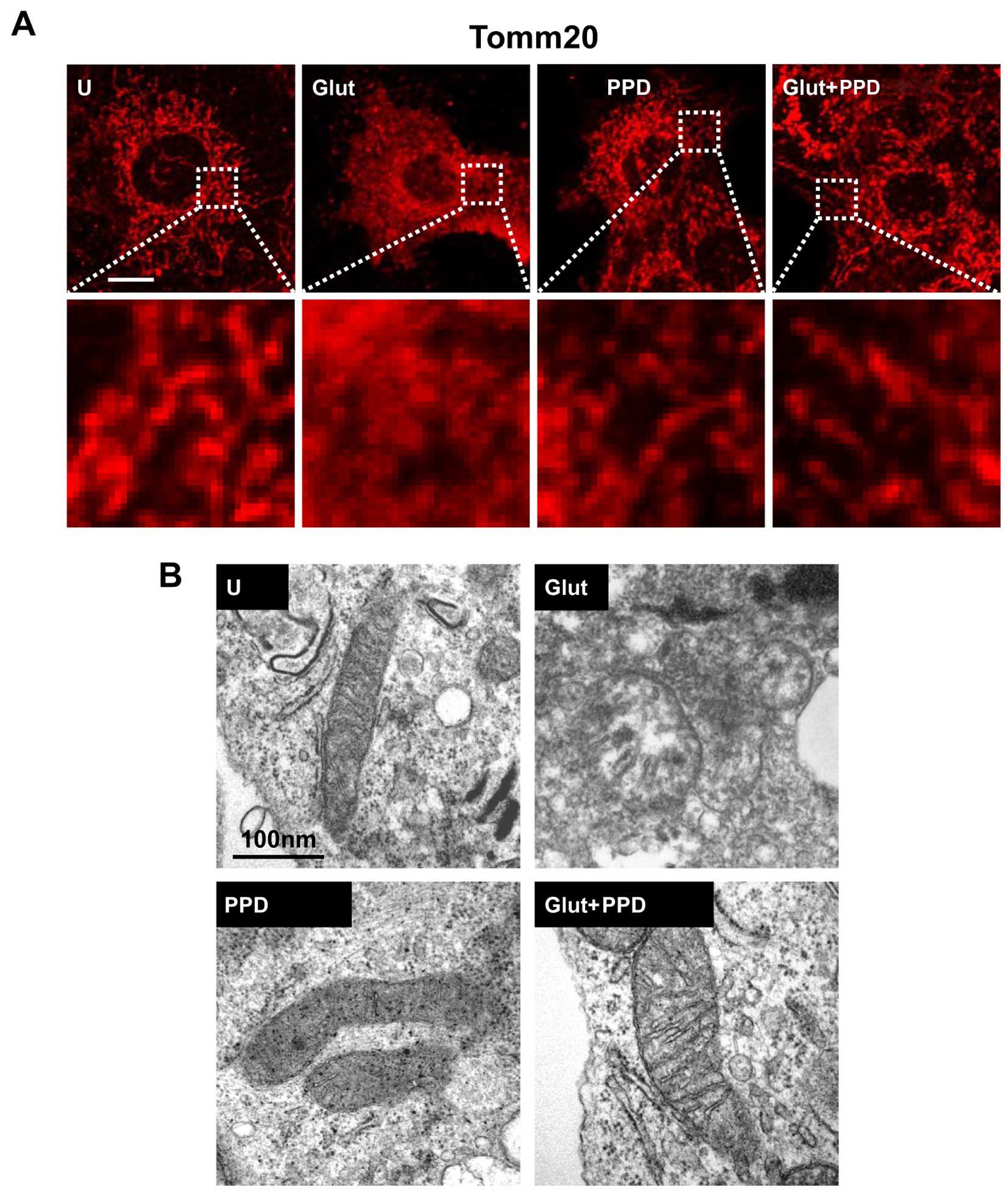
we first examined mitochondrial morphology. Mitochondrial
morphology was examined using Tomm20 (mitochondrial outer membrane)
staining following treatment with glutamate for 24 h. A significant
change in mitochondrial morphology was observed at 24 h in the
glutamate-exposed cells. Treatment of the glutamate-exposed cells
to PPD led to a substantial increase in the proportion of
mitochondria, compared to the cells exposed to glutamate and not
treated with PPD (Fig. 5A). We
also analyzed the changes in mitochondrial morphology using TEM
(Fig. 5B). Ultrastructural
imaging revealed that the rate of swollen mitochondria and broken
mitochondrial matrix was increased in the glutamate-exposed cells,
whereas treatment with PPD resulted in an apparent increase in the
number normal mitochondria compared with the glutamate-exposed
cells not treated with PPD. Thus, treatment with PPD may protect
the mitochondria against glutamate-induced damage.
Effects of PPD on mitochondrial
function
Treatment with ginsenosides has been shown to
improve mitochondrial function in various cells (28,29). However, in neuronal cells, it is
not known whether treatment with PPD is capable of controlling
mitochondrial function, density and morphology, particularly
following exposure to glutamate. Thus, in this study, to examine
the protective role of PPD in maintaining mitochondrial function,
we analyzed the MMP, as well as the changes in mitochondrial mass.
The MMP was measured by flow cytometry using a potentiometric
fluorescent probe (TRME) following exposure of the cells to
glutamate (Fig. 6A). Significant
depolarization of the mitochondria was observed at 24 h and
sustained until 24 h. The MMP was significantly higher in the cells
pre-treated with PPD than in the cells exposed to glutamate and not
treated with PPD. PPD protected the cells against glutamate-induced
mitochondrial damage by regulating the MMP.
To further elucidate the possible mechanisms
underlying the effects of PPD, we evaluated the mitochondrial mass.
MitoTracker green staining indicated that treatment with PPD
controlled mitochondrial mass following exposure to glutamate. The
mitochondrial mass decreased by approximately 70% in cells exposed
to glutamate and not treated with PPD (Fig. 6B). Thus, PPD improved
mitochondrial function, as indicated by changes in MMP and
mitochondrial mass, suggesting that PPD plays a role in maintaining
mitochondrial functional.
Discussion
Potential mechanisms, such as oxidative stress,
mitochondrial aberrations and inflammation, which lead to the
degeneration and death of neurons, are considered important factors
in the pathogenesis of a range of neurological disorders (30–32). Ameliorating therapeutic modulators
of these universal mechanisms may provide new insight into
therapeutic strategies for these fatal diseases by delaying the
disease onset or progression. The interactions of glutamate with
specific membrane receptors has been implicated in a number of
neurological functions regulated by neuronal cells in the central
nervous system (CNS), including synapse re-formation, perception
and muscle movement (33–35). However, it has been suggested that
glutamate participates in neuronal cell damage associated with
several neurological deficits in the CNS (36). Glutamate, one of the major
excitatory neurotransmitters in the brain (37), is the primary inducer of cell
death during cerebral ischemia and has a direct neurotoxic effect
by increasing ROS production (38). Glutamate from damaged cells is
delivered to adjacent cells and results in excessive ion influx
through the NMDA receptor, a specific glutamate receptor (39). Excessive glutamate stimulation
sequentially disrupts cellular ion homeostasis and induces
apoptosis. It has also been proposed that an overproduction of
oxygen free radicals plays a pivotal role in hypoxic-ischemic
injury (40,41).
Previous studies have demonstrated that extracts of
ginseng (the root of Panax ginseng) have various biological
effects, including anti-carcinogenic activities (42), as well as anticancer (43–45) and neuroprotective activities
(46). It has been demonstrated
that ginseng extracts enhance mitochondrial function and protect
against oxidative stress (47).
PPD is the most pharmacologically active ginsenoside that has been
evaluated. PPD possesses various biological properties, including
anti-inflammatory, antioxidant and antitumor properties (48,49). A recent study found that PPD
protected against cerebral ischemia in a model of middle cerebral
artery occlusion (MCAO) by reducing free radical formation, lipid
peroxidation and calcium overload, and increasing the expression of
anti-apoptotic proteins (50). In
the present study, we demonstrated the ability of PPD to rescue
PC12 cells from glutamate-induced excitotoxicity and examined the
cellular events that underlie this effect.
The mitochondria play a potentially important
homeostatic role in the molecular events surrounding cell death in
pathological situations, such as ischemia and neurodegenerative
diseases (51). Mitochondrial
death signal results in the opening of the mitochondrial
permeability transition pore and the collapse of ΔΨm, leading to
the release of various substances, such as cytochrome c,
which are associated with cell death. Previous studies have
demonstrated that exposure to glutamate is accompanied by a loss of
MMP (52), which may lead to ATP
depletion (53) and energetic
collapse (54–56), and ultimately contributes to cell
death. The inhibition of mitochondrial membrane rupture, induced as
a consequence of mitochondrial swelling caused by disturbed ionic
homeostasis, has been postulated as a cell survival mechanism that
follows the MMP (57). Thus,
protection of the mitochondria may be involved in PPD-induced
neuroprotection.
The present study found that the regulation of
mitochondrial homeostasis leads to increased cell viability. In
addition, it is speculated that the activation of mitochondrial
function with PPD treatment affects overall cell survival. Taken
together, the data from the current study strongly suggest that PPD
prevents glutamate-induced excitotoxicity through multiple effects
targeting mitochondria-dependent events, including the inhibition
of ROS generation, the dissipation of MMP and a reduction in
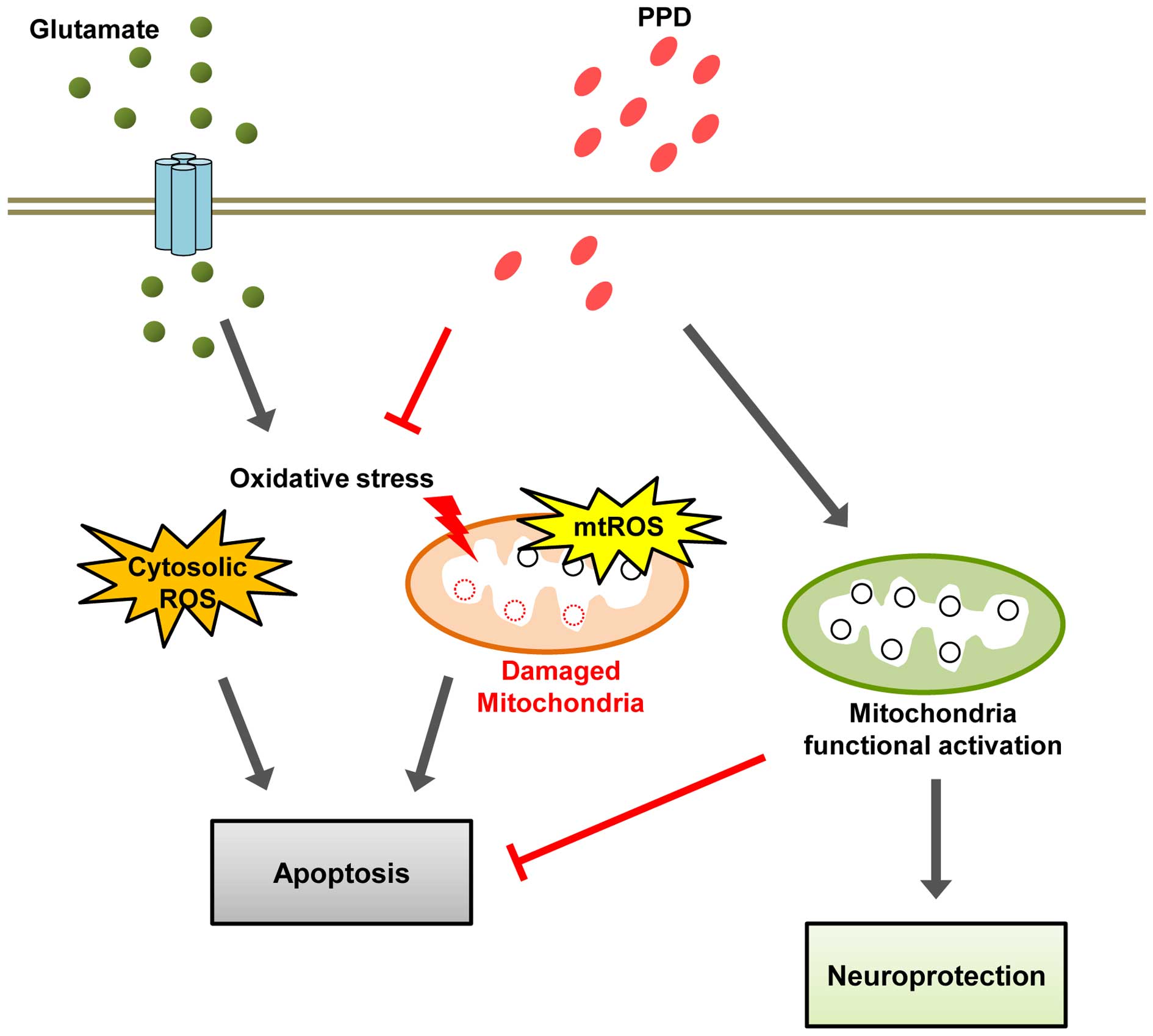
mitochondrial density. Our working hypothesis is that PPD functions
as a neurotrophic agent to ameliorate the sensory deficit caused by
glutamate-induced excitotoxicity through its antioxidant effects
and enhances mitochondrial function (Fig. 7). Thus, PPD may have therapeutic
value in the treatment of certain neurodegenerative diseases.
However, further studies are warranted in order to fully elucidate
the mechanisms responsible for its regulatory effects on the
function of mitochondria.
Acknowledgments
The present study was supported by a grant from the
Agenda Program (PJ008568), Rural Development Administration,
Republic of Korea.
References
|
1
|
Liu CX and Xiao PG: Recent advances on
ginseng research in China. J Ethnopharmacol. 36:27–38. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nah SY, Kim DH and Rhim H: Ginsenosides:
are any of them candidates for drugs acting on the central nervous
system? CNS Drug Rev. 13:381–404. 2007.PubMed/NCBI
|
|
3
|
Benishin CG: Actions of ginsenoside Rb1 on
choline uptake in central cholinergic nerve endings. Neurochem Int.
21:1–5. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benishin CG, Lee R, Wang LC and Liu HJ:
Effects of ginsenoside Rb1 on central cholinergic metabolism.
Pharmacology. 42:223–229. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim S, Ahn K, Oh TH, Nah SY and Rhim H:
Inhibitory effect of ginsenosides on NMDA receptor-mediated signals
in rat hippocampal neurons. Biochem Biophys Res Commun.
296:247–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang C, Du F, Shi M, Ye R, Cheng H, Han
J, Ma L, Cao R, Rao Z and Zhao G: Ginsenoside Rd protects neurons
against glutamate-induced excitotoxicity by inhibiting Ca(2+)
influx. Cell Mol Neurobiol. 32:121–128. 2012. View Article : Google Scholar
|
|
7
|
Ye R, Han J, Kong X, Zhao L, Cao R, Rao Z
and Zhao G: Protective effects of ginsenoside Rd on PC12 cells
against hydrogen peroxide. Biol Pharm Bull. 31:1923–1927. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye R, Li N, Han J, Kong X, Cao R, Rao Z
and Zhao G: Neuroprotective effects of ginsenoside Rd against
oxygen-glucose deprivation in cultured hippocampal neurons.
Neurosci Res. 64:306–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YC, Kim SR, Markelonis GJ and Oh TH:
Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from
glutamate-induced neurodegeneration. J Neurosci Res. 53:426–432.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nah SY: Ginseng ginsenoside pharmacology
in the nervous system: involvement in the regulation of ion
channels and receptors. Front Physiol. 5:982014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang S, Ryu JH, Kim DH and Oh S: Changes
of (3H)MK-801, (3H) muscimol and
(3H)flunitrazepam binding in rat brain by the prolonged
ventricular infusion of transformed ginsenosides. Neurochem Res.
29:2257–2266. 2004. View Article : Google Scholar
|
|
12
|
Zhang E, Shen J and So KF: Chinese
traditional medicine and adult neurogenesis in the hippocampus. J
Tradit Complement Med. 4:77–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang YL, Zhang R, Xu HL, Yu XF, Qu SC and
Sui DY: 20(S)-protopanaxadiol triggers mitochondrial-mediated
apoptosis in human lung adenocarcinoma A549 cells via inhibiting
the PI3K/Akt signaling pathway. Am J Chin Med. 41:1137–1152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee DC and Lau AS: Effects of Panax
ginseng on tumor necrosis factor-α-mediated inflammation: a
mini-review. Molecules. 16:2802–2816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang MS, Lee SG and Rho HM:
Transcriptional activation of Cu/Zn superoxide dismutase and
catalase genes by panaxadiol ginsenosides extracted from Panax
ginseng. Phytother Res. 13:641–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leung KW, Leung FP, Mak NK, Tombran-Tink
J, Huang Y and Wong RN: Protopanaxadiol and protopanaxatriol bind
to glucocorticoid and oestrogen receptors in endothelial cells. Br
J Pharmacol. 156:626–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gutierrez J, Ballinger SW, Darley-Usmar VM
and Landar A: Free radicals, mitochondria, and oxidized lipids: the
emerging role in signal transduction in vascular cells. Circ Res.
99:924–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu D, Zhang H, Gu W, Liu Y and Zhang M:
Neuroprotective effects of ginsenoside Rb1 on high glucose-induced
neurotoxicity in primary cultured rat hippocampal neurons. PLoS
One. 8:e793992013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: Multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu WK, Xu SX and Che CT:
Anti-proliferative effect of ginseng saponins on human prostate
cancer cell line. Life Sci. 67:1297–1306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun M, Huang C, Wang C, Zheng J, Zhang P,
Xu Y, Chen H and Shen W: Ginsenoside Rg3 improves cardiac
mitochondrial population quality: mimetic exercise training.
Biochem Biophys Res Commun. 441:169–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian J, Zhang S, Li G, Liu Z and Xu B:
20(S)-ginsenoside Rg3, a neuroprotective agent, inhibits
mitochondrial permeability transition pores in rat brain. Phytother
Res. 23:486–491. 2009. View
Article : Google Scholar
|
|
23
|
Perrelli MG, Pagliaro P and Penna C:
Ischemia/reperfusion injury and cardioprotective mechanisms: role
of mitochondria and reactive oxygen species. World J Cardiol.
3:186–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM,
Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, et al: Host cell autophagy
activated by antibiotics is required for their effective
antimycobacterial drug action. Cell Host Microbe. 11:457–468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bauerfeld CP, Rastogi R, Pirockinaite G,
Lee I, Hüttemann M, Monks B, Birnbaum MJ, Franchi L, Nuñez G and
Samavati L: TLR4-mediated AKT activation is MyD88/TRIF dependent
and critical for induction of oxidative phosphorylation and
mitochondrial transcription factor A in murine macrophages. J
Immunol. 188:2847–2857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
27
|
Kumari S, Mehta SL and Li PA: Glutamate
induces mitochondrial dynamic imbalance and autophagy activation:
preventive effects of selenium. PLoS One. 7:e393822012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Min JK, Kim JH, Cho YL, Maeng YS, Lee SJ,
Pyun BJ, Kim YM, Park JH and Kwon YG: 20(S)-Ginsenoside Rg3
prevents endothelial cell apoptosis via inhibition of a
mitochondrial caspase pathway. Biochem Biophys Res Commun.
349:987–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tamura T, Cui X, Sakaguchi N and Akashi M:
Ginsenoside Rd prevents and rescues rat intestinal epithelial cells
from irradiation-induced apoptosis. Food Chem Toxicol.
46:3080–3089. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Floyd RA: Antioxidants, oxidative stress,
and degenerative neurological disorders. Proc Soc Exp Biol Med.
222:236–245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tarnopolsky MA and Beal MF: Potential for
creatine and other therapies targeting cellular energy dysfunction
in neurological disorders. Ann Neurol. 49:561–574. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Minagar A, Shapshak P, Fujimura R, Ownby
R, Heyes M and Eisdorfer C: The role of macrophage/microglia and
astrocytes in the pathogenesis of three neurologic disorders:
HIV-associated dementia, Alzheimer disease, and multiple sclerosis.
J Neurol Sci. 202:13–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Headley PM and Grillner S: Excitatory
amino acids and synaptic transmission: the evidence for a
physiological function. Trends Pharmacol Sci. 11:205–211. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeng LH, Ouyang Y, Gazit V, Cirrito JR,
Jansen LA, Ess KC, Yamada KA, Wozniak DF, Holtzman DM, Gutmann DH,
et al: Abnormal glutamate homeostasis and impaired synaptic
plasticity and learning in a mouse model of tuberous sclerosis
complex. Neurobiol Dis. 28:184–196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Le Poul E, Boléa C, Girard F, Poli S,
Charvin D, Campo B, Bortoli J, Bessif A, Luo B, Koser AJ, et al: A
potent and selective metabotropic glutamate receptor 4 positive
allosteric modulator improves movement in rodent models of
Parkinson's disease. J Pharmacol Exp Ther. 343:167–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi DW, Maulucci-Gedde M and Kriegstein
AR: Glutamate neurotoxicity in cortical cell culture. J Neurosci.
7:357–368. 1987.PubMed/NCBI
|
|
37
|
van den Pol AN, Gao XB, Patrylo PR, Ghosh
PK and Obrietan K: Glutamate inhibits GABA excitatory activity in
developing neurons. J Neurosci. 18:10749–10761. 1998.PubMed/NCBI
|
|
38
|
Gliyazova NS, Huh EY and Ibeanu GC: A
novel phenoxy thiophene sulphonamide molecule protects against
glutamate evoked oxidative injury in a neuronal cell model. BMC
Neurosci. 14:932013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salińska E, Danysz W and Łazarewicz JW:
The role of excitotoxicity in neurodegeneration. Folia Neuropathol.
43:322–339. 2005.
|
|
40
|
Baker JE: Oxidative stress and adaptation
of the infant heart to hypoxia and ischemia. Antioxid Redox Signal.
6:423–429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peng YW, Buller CL and Charpie JR: Impact
of N-acetylcysteine on neonatal cardiomyocyte ischemia-reperfusion
injury. Pediatr Res. 70:61–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bi X, Xia X, Mou T, Jiang B, Fan D, Wang
P, Liu Y, Hou Y and Zhao Y: Anti-tumor activity of three
ginsenoside derivatives in lung cancer is associated with
Wnt/β-catenin signaling inhibition. Eur J Pharmacol. 742:145–152.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang W, Rayburn ER, Hao M, Zhao Y, Hill
DL, Zhang R and Wang H: Experimental therapy of prostate cancer
with novel natural product anti-cancer ginsenosides. Prostate.
68:809–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee JJ, Kwon HK, Jung IH, Cho YB, Kim KJ
and Kim JL: Anticancer activities of ginseng extract fermented with
Phellinus linteus. Mycobiology. 37:21–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Y, Xu Y, Zhu Y and Li X: Anti-cancer
effects of ginsenoside compound k on pediatric acute myeloid
leukemia cells. Cancer Cell Int. 13:242013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim S, Lee Y and Cho J: Korean red ginseng
extract exhibits neuroprotective effects through inhibition of
apoptotic cell death. Biol Pharm Bull. 37:938–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dong GZ, Jang EJ, Kang SH, Cho IJ, Park
SD, Kim SC and Kim YW: Red ginseng abrogates oxidative stress via
mitochondria protection mediated by LKB1-AMPK pathway. BMC
Complement Altern Med. 13:642013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lü JM, Yao Q and Chen C: Ginseng
compounds: an update on their molecular mechanisms and medical
applications. Curr Vasc Pharmacol. 7:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ren HC, Sun JG, Wang GJ, A JY, Xie HT, Zha
WB, Yan B, Sun FZ, Hao HP and Gu SH: Sensitive determination of
20(S)-protopanaxadiol in rat plasma using HPLC-APCI-MS: application
of pharmacokinetic study in rats. J Pharm Biomed Anal.
48:1476–1480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu H, Yu X, Qu S, Chen Y, Wang Z and Sui
D: Protective effect of 20(S)-protopanaxadiol saponins, isolated
from Pana quinquefolium, on permanent focal cerebral ischemic
injury in rats. Exp Ther Med. 7:165–170. 2014.
|
|
51
|
Fiskum G, Murphy AN and Beal MF:
Mitochondria in neurodegeneration: acute ischemia and chronic
neurodegenerative diseases. J Cereb Blood Flow Metab. 19:351–369.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tirosh O, Sen CK, Roy S and Packer L:
Cellular and mitochondrial changes in glutamate-induced HT4
neuronal cell death. Neuroscience. 97:531–541. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Almeida A and Bolaños JP: A transient
inhibition of mitochondrial ATP synthesis by nitric oxide synthase
activation triggered apoptosis in primary cortical neurons. J
Neurochem. 77:676–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Khodorov B, Pinelis V, Vergun O,
Storozhevykh T and Vinskaya N: Mitochondrial deenergization
underlies neuronal calcium overload following a prolonged glutamate
challenge. FEBS Lett. 397:230–234. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Stout AK, Raphael HM, Kanterewicz BI,
Klann E and Reynolds IJ: Glutamate-induced neuron death requires
mitochondrial calcium uptake. Nat Neurosci. 1:366–373. 1998.
View Article : Google Scholar
|
|
56
|
Vergun O, Keelan J, Khodorov BI and Duchen
MR: Glutamate-induced mitochondrial depolarisation and perturbation
of calcium homeostasis in cultured rat hippocampal neurones. J
Physiol. 519:451–466. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Marchi S, Giorgi C, Suski JM, Agnoletto C,
Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti
F, et al: Mitochondria-ros crosstalk in the control of cell death
and aging. J Signal Transduct. 2012:3296352012. View Article : Google Scholar
|