Introduction
Prostate cancer is one of the most common type of
malignant tumor which affects males and is the second leading cause
of cancer-related mortality among the male gender (1). However, currently available
treatments are unable to completely cure advanced prostate cancer,
as prostate cancer cells exhibit a high rate of proliferation, as
well as high invasion capacity and metastatic ability (2). The underlying mechanism of the
tumorigenesis of prostate cancer is not yet fully understood, and
as such the development of efficient therapies is impeded.
Therefore, the molecular mechanism of prostate cancer must be
studied in order to develop novel approaches and agents to prevent
and treat prostate cancer.
The nin one binding (NOB1) protein functions as an
oncogene which plays an important role in various human cancers
(3,4). The human NOB1 gene is located on
chromosome 16q22.1 encodes a 50-kDa protein and is widely expressed
in numerous organs, including the lungs, spleen, and liver
(5). NOB1 protein is mainly
distributed in the nucleus, and this protein is involved in the
biogenesis and function of the 20S proteasome (6). The important role which NOB1 plays
in numerous human cancers, including ovarian cancer (4), hepatocellular carcinoma (7), breast cancer (8), gliomas (9), and lung cancer (10), has been previously investigated.
Likewise, the involvement of NOB1 in prostate cancer has also been
previously explored: for example, prostate cancer tissues have been
shown to express NOB1 in the nucleus, and this expression has been
correlated with lymph node metastasis (11). NOB1 expression has been suggested
to function as a potential prognostic marker of prostate cancer
(12,13). Gene silencing of NOB1 has
previously been shown to suppress the malignant transformation of
prostate cancer cells (14).
Therefore, we posit that targeting NOB1 constitutes a promising
approach to preventing and treating prostate cancer.
It has previously been noted that a group of short,
non-coding RNAs termed microRNAs (miRs) act as novel regulatory
molecules of gene expression (15). miRs are known to negatively
regulate the expression of target genes at a post-transcriptional
level by binding the 3′-untranslated region (3′-UTR) of mRNA
(15,16). Thus, miRs play an important role
in the diagnosis, prognosis and treatment of various diseases
(17,18). miRs have also been implicated in
the pathogenesis of prostate cancer; these molecules serve as a
novel target in the treatment of prostate cancer (19).
The present study aimed to discover potential miRs
that target NOB1 and regulate NOB1 expression in prostate cancer.
miR-192 functions as a tumor suppressor in the development and
progression of certain human cancers, for example colon (20) and bladder (21) cancers. However, the role of
miR-192 in prostate cancer had not previously been examined, to the
best of our knowledge. In the present study, we revealed that
miR-192 expression was significantly decreased in prostate cancer
cells. miR-192 overexpression markedly impaired the tumorigenicity
of prostate cancer cells. NOB1 was predicted as a candidate target
gene of miR-192 through bioinformatics analysis; this result was
confirmed through a dual-luciferase reporter assay. We further
demonstrated that miR-192 directly regulated NOB1 expression, and
noted that miR-192 overexpression also inhibited the expression of
p38 mitogen-activated protein kinase (MAPK) by suppressing NOB1.
Thus, we suggest that miR-192 negatively regulated NOB1 expression
in prostate cancer. Targeting miR-192 and NOB1 may thus constitute
a novel strategy with which to develop new therapeutics for
prostate cancer.
Materials and methods
Cell cultures
The human prostate cancer lines PC-3 and DU145 were
both purchased from the The Cell Bank of Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). Human prostate
epithelial RWPE-1 cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). PC-3 and DU145 cells
were grown in RPMI-1640 medium with 10% fetal bovine serum (FBS)
and 1% streptomycin-penicillin (all from Invitrogen, Carlsbad, CA,
USA). The RWPE-1 cells were grown in keratinocyte serum-free media
containing 0.5% streptomycin-penicillin (both from Invitrogen). All
cells were cultured in a CO2 incubator (Heracell 2401;
Thermo Fisher Scientific, Waltham, MA, USA) containing 5%
CO2 at 37°C.
Cell transfection
The cells were grown in a 6-well tissue-culture
plate at a density of 1×106 cells/well. After the cells
reached 60–80% confluence, the miR-192 mimics and the non-specific
miR control (miR NC) synthesized by the Shanghai GenePharma Co.,
Ltd. (Shanghai, China) were transfected into the cells using
Lipofectamine® 2000 (Invitrogen) at a final
concentration of 50 nM, in accordance with the manufacturer's
recommendations. After 48 h of transfection, gene expression was
detected through reverse-transcription quantitative polymerase
chain reaction (RT-qPCR) and western blot analysis.
RT-qPCR analysis
Total RNA was obtained from the cells using an
miRNeasy mini kit (Qiagen, Dusseldorf, Germany) in accordance with
the manufacturer's instructions. In RT-qPCR mRNA analysis, total
RNA was reverse transcribed using M-MLV reverse transcriptase
(Clontech Laboratories, Palo Alto, CA, USA). Complementary DNA was
synthesized using an miScript reverse transcription kit (Qiagen).
qPCR experiments were conducted using an ABI 7500 Real-Time PCR
system (Applied Biosystems Life Technologies, Carlsbad, CA, USA)
with a SYBR Premix Ex Taq™ II commercial kit (Takara Bio, Dalian,
China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and U6
snRNA were used as the internal control for relative gene
expression quantification using the 2−ΔΔCt method.
Western blot analysis
Proteins were extracted from each sample of the
cells and quantified using a bicinchoninic acid kit (Beyotime
Institute of Biotechnology, Haimen, China). Equivalent amounts of
protein (25 µg) were loaded onto 12.5% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
nitrocellulose membranes (Miltenyi Biotec, Auburn, CA, USA). After
the membranes were blocked with 2.5% skim milk in Tris-buffered
saline (TBS) at 37°C for 1 h, the membranes were blotted with
primary antibodies, namely anti-NOB1 (sc-160594), anti-p38 MAPK
(sc-6176), and anti-GAPDH (sc-48166) antibodies (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight. After
the membranes were washed with TBS and Tween-20, horseradish
peroxidase-conjugated secondary antibodies (1:2,000; sc-2768; Santa
Cruz Biotechnology, Inc.) were applied for 1 h at room temperature.
The membranes were incubated for 1 h and subjected to an enhanced
chemiluminescence detection system (Amersham Biosciences, Little
Chalfont, UK). The blots were developed using an enhanced
chemiluminescence detection kit (Amersham Biosciences). The gray
value of each protein band was quantified using Image-Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Cell proliferation assay
Human prostate cancer cells were seeded into a
96-well plate at a density of 1×103 cells/well and
cultured for 24 h. The cells were transfected with 50 nM of miR-192
mimics or miR NC for 48 h. Cell proliferation was detected using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. In brief, 5 mg/ml MTT solution (Sigma-Aldrich, St. Louis,
MO, USA) was added at 20 µl/well to the cell cultures. After
4 h of incubation, the medium was discarded, and the formazan
product was dissolved with dimethyl sulfoxide (200 µl/well).
The optical density of each well was detected at 490 nm using an
enzyme-linked immunosorbent assay reader (ELx808; BioTek
Instruments, Inc., Winooski, VT, USA).
Colony formation assay
Human prostate cancer cells were cultured in a
6-well plate and transfected with the miR-192 mimics or miR NC.
After transfection for 48 h, the cells were re-plated into a 6-well
plate in growth medium containing 0.3% noble agar at 200 cells/well
to form natural colonies. After 14 days, the cells were washed with
phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde,
and stained with Giemsa (Sigma-Aldrich). The total number of
colonies was counted under a microscope (Leica AF6000; Leica,
Solms, Germany), and the results were then averaged.
Cell cycle analysis
In the present study, cell cycle distribution (G1, S
or G2/M) was detected through flow cytometry. Human prostate cancer
cells were transfected with the miR-192 mimics or miR NC for 48 h,
harvested, washed, and then fixed with 70% ethanol. Propidium
iodide (PI) (100 µg/ml; Sigma-Aldrich) in PBS containing 10
µg/ml of RNase A was added to the cells and incubated for 30
min in a dark place at 37°C. The percentage of cells in each cell
cycle phase was measured using a FACScan flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Cell migration assay
In the present study, human prostate cancer cells
were transfected with miR-192 mimics or miR NC for 48 h, and the
in vitro migration ability was determined using a Transwell
chamber (Corning Incorporated, Corning, NY, USA). In brief,
1.0×104 cells re-suspended in 200 µl serum-free
medium were seeded into the upper chamber. Subsequently, 500
µl medium containing 10% FBS was placed in the lower
chamber. After the cells were incubated at 37°C for 14 h, the cells
that remained on the upper chamber were removed using a cotton
swab. The cells that had migrated to the lower chamber were
subsequently fixed with 10% methanol for 30 sec and stained with
0.1% crystal violet (Abcam, Cambridge, UK) for 30 min. The number
of cells was then counted under the microscope, and the data
obtained in five random fields were averaged.
Dual-luciferase reporter assay
The 3′-UTR of NOB1 containing the predicted binding
sites of miR-192 was subcloned into the pGL3 luciferase promoter
vector (Promega Corp., Madison, WI, USA). The relevant mutant
containing the mutated binding sites of miR-192 was also
constructed. The 3′-UTR recombinants of pGL3-NOB1 (100 ng) were
cotransfected into the PC-3 cells with miR-192 mimics or miR NC
control using Lipofectamine® 2000 (Invitrogen) in
accordance with the manufacturer's instructions to detect the
luciferase reporter activity. pGL3 luciferase reporter vectors
contained either the 3′-UTR of wild-type NOB1 or its relevant
mutant and miR-192 mimics or non-specific miR control. After the
cells were transfected for 48 h, the cells were harvested and
lysed. Relative luciferase activity was evaluated using the
dual-luciferase reporter system detection method (Promega Corp.) in
accordance with standard protocols and the manufacturer's
instructions.
Statistical analysis
Data are expressed as the means ± standard
deviation. Statistical significance was calculated through one-way
ANOVA, followed by the Bonferroni post hoc test using SPSS version
11.5 (SPSS, Inc., Chicago, IL, USA). A p-value <0.05 was
considered to indicate a statistically significant difference
between groups.
Results
Expression of miR-192 in prostate cancer
cell lines
We evaluated the expression level of miR-192 in PC-3
and DU145 cells through RT-qPCR analysis to determine the potential
role of miR-192 in prostate cancer. We noted that miR-192
expression was significantly decreased in the prostate cancer cell
lines PC-3 and DU145 compared with that in the prostate epithelial
RWPE-1 cells (Fig. 1A). These
results indicate that miR-192 is involved in the tumorigenicity of
prostate cancer. To explore the potential role of miR-192 in
prostate cancer, chemosynthetic miR-192 mimics were used to
overexpress miR-192 in the PC-3 and DU145 cells. The results showed
that transfection with the miR-192 mimics significantly promoted
the expression level of miR-192 in PC-3 (Fig. 1B) and DU145 (Fig. 1C) cells.
Overexpression of miR-192 inhibits the
growth of prostate cancer cells
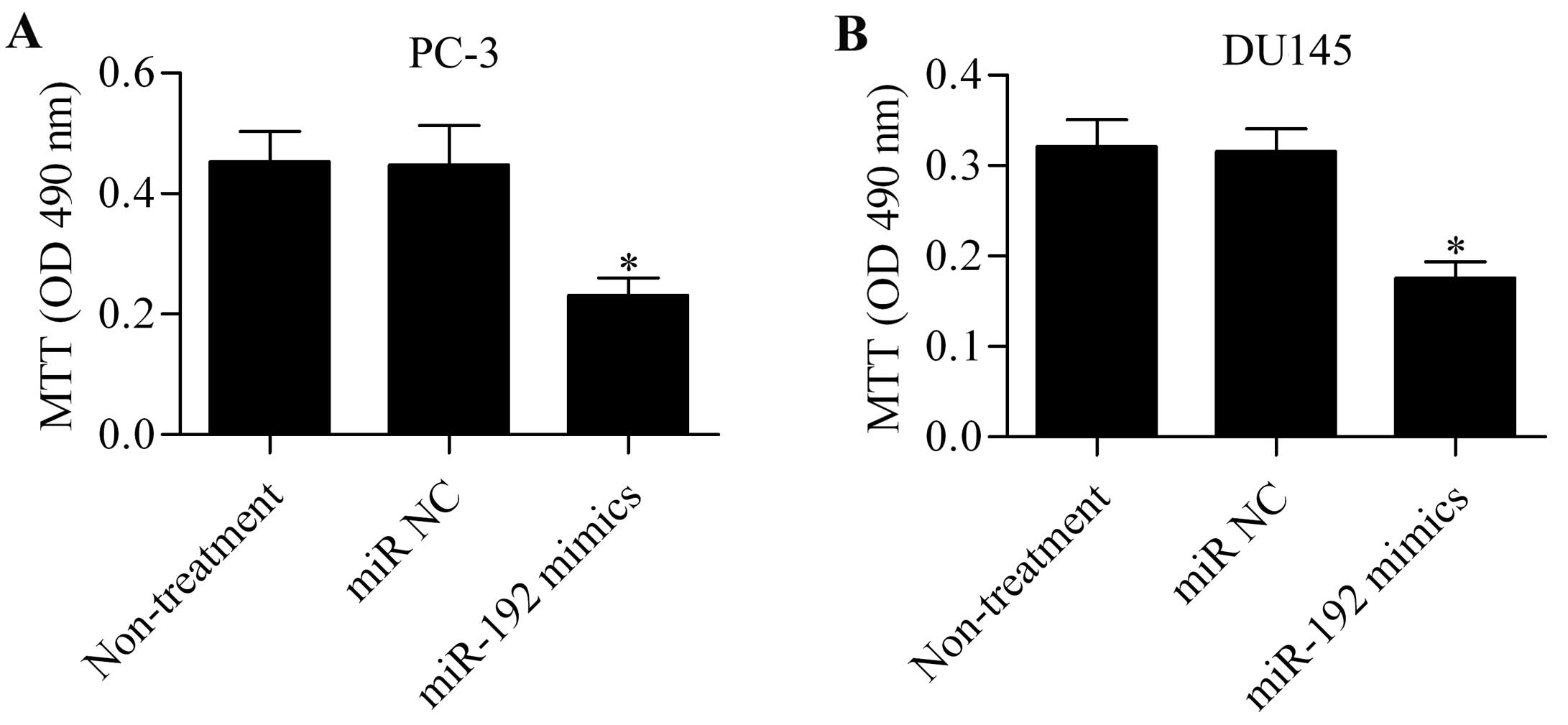
To investigate the effect of miR-192 overexpression
on prostate cancer cell proliferation, the PC-3 and DU145 cells
transfected with the miR-192 mimics were subjected to MTT assay.
miR-192 mimics significantly inhibited the proliferation of PC-3
(Fig. 2A) and DU145 (Fig. 2B) cells compared with the
untreated or miR NC-transfected cells. These data indicate that
miR-192 plays an important role in regulating the growth of
prostate cancer cells.
Overexpression of miR-192 suppresses the
colony-forming capacity of prostate cancer cells
To detect whether miR-192 plays an important role in
the colony-forming ability of prostate cancer cells, we
subsequently performed a colony-forming experiment using PC-3 and
DU145 cells which had been transfected with miR-192 mimics. The
results showed that miR-192 mimics significantly inhibited the
colony formation of the PC-3 (Fig.
3A) and DU145 (Fig. 3B)
cells.
Overexpression of miR-192 impairs cell
cycle progression
In order to further elucidate the role which miR-192
plays in the tumorigenicity of prostate cancer cells, we detected
the effect of miR-192 mimics on cell cycle progression. PC-3 and
DU145 cells transfected with miR-192 for 48 h were subjected to a
flow cytometry assay. The results indicated that miR-192
overexpression significantly increased the number of cells in the
G1 phase whereas miR-192 mimic transfection markedly decreased the
proportion of PC-3 (Fig. 4A) and
DU145 (Fig. 4B) cells in the
S-phase.
Overexpression of miR-192 reduces the
migration capacity of prostate cancer cells
To further detect whether miR-192 had an impact on
the cell migration of prostate cancer cells, a Transwell migration
assay was carried out. We found that miR-192 overexpression
considerably reduced the number of PC-3 (Fig. 5A) and DU145 (Fig. 5B) cells that migrated to the lower
chamber, implying that miR-192 overexpression reduced the migration
capacity of prostate cancer cells.
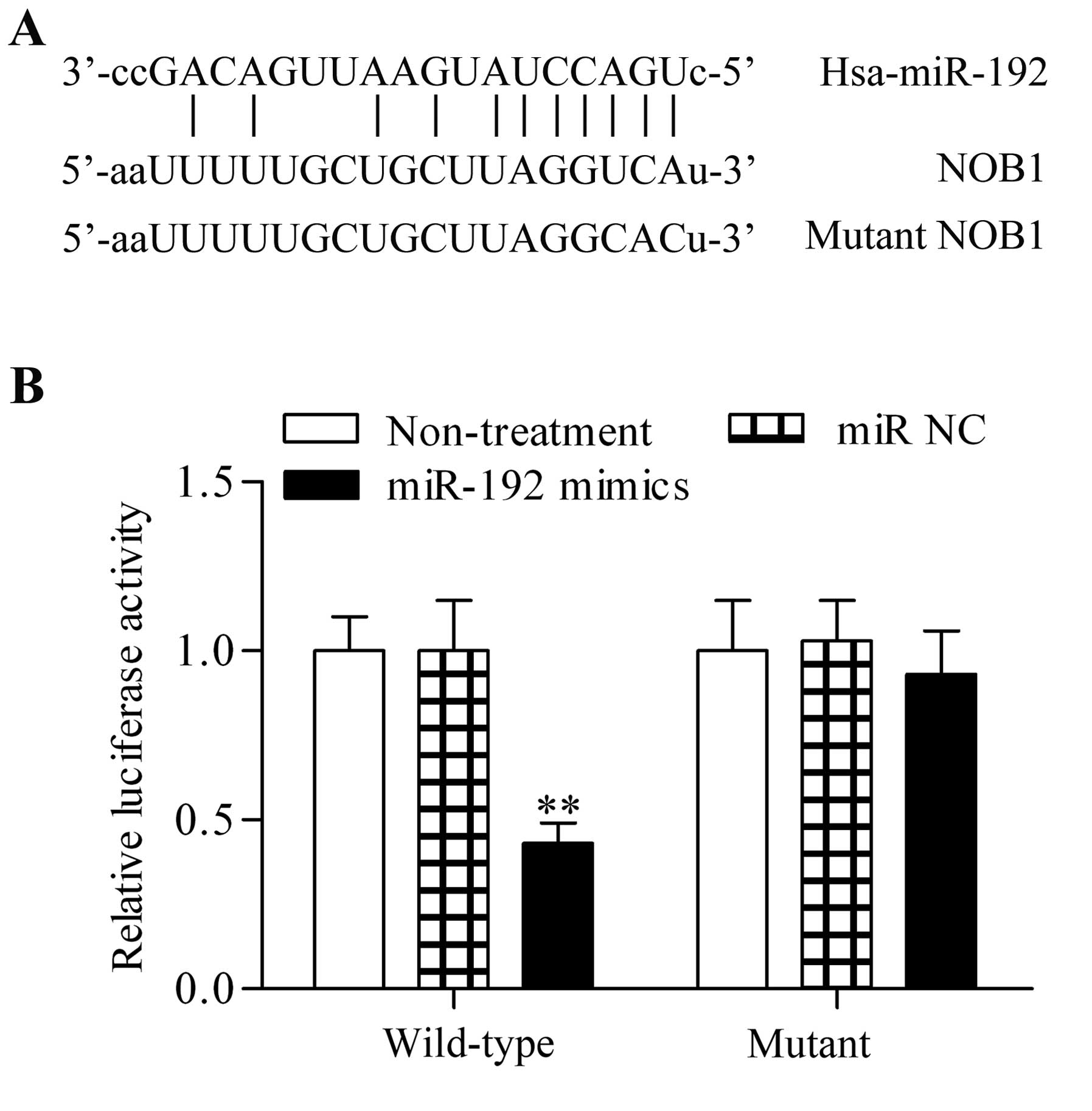
NOB1 is a direct target gene of
miR-192
Taking into consideration that miRs regulate
cellular processes through their target genes, we identified the
potential target gene of miR-192 through bioinformatics analysis.
Notably, we found that NOB1, an oncogene, contained the predicted
miR-192 targeting site in the 3′-UTR of NOB1 (Fig. 6A). To verify the direct
association between miR-192 and the 3′-UTR of NOB1, pGL3 luciferase
reporter vectors were constructed with the 3′-UTR of the wild-type
NOB1 or its relevant mutant. The miR-192 mimics were cotransfected
with the reporter vectors into the PC-3 cells. Transfection with
miR-192 mimics in the 3′-UTR of wild-type NOB1 significantly
reduced relative luciferase activity, whereas transfection with
miR-192 mimics had no obvious effect on the corresponding mutant
(Fig. 6B). These results showed
that NOB1 is a direct target gene of miR-192.
Overexpression of miR-192 reduces the
expression of NOB1
In the present study, in order to validate the
theory that miR-192 is a regulator of NOB1, we subsequently
detected the effect of miR-192 on mRNA and protein expression
levels of NOB1. The results of RT-qPCR analysis demonstrated that
miR-192 overexpression caused a marked decrease in the mRNA
expression level of NOB1 in PC-3 (Fig. 7A) and DU145 (Fig. 7B) cells. We also noted that the
protein expression of NOB1 was significantly reduced by miR-192
overexpression (Fig. 7C and D).
These results further confirmed that miR-192 targeted and modulated
NOB1 expression.
Overexpression of miR-192 suppresses p38
MAPK expression
To further explore the underlying mechanism of
miR-192 in prostate cancer, we detected the effect of miR-192
mimics on p38 MAPK. The results demonstrate that transfection with
miR-192 mimic significantly reduced the protein expression level of
p38 MAPK in PC-3 cells (Fig. 8A)
compared with the miR-NC-transfected group. Similar results were
observed in DU145 cells (Fig.
8B).
Discussion
Previous studies have suggested that abnormally
expressed miRs play an important role in the development and
progression of cancers (22,23). miR-192 has been reported to act as
a tumor suppressor in numerous human cancers (20,21). However, the role of miR-192 in
prostate cancers had not previously been documented: to the best of
our knowledge, this study is the first to describe the functional
significance of miR-192 in prostate cancers. We demonstrated that
miR-192 expression was significantly decreased in prostate cancer
cell lines. Our results further elucidated that miR-192
overexpression markedly impaired the tumorigenicity of prostate
cancer cells. The miR-192 overexpression induced by transfection
with miR-192 mimics significantly decreased the proliferation,
colony-forming capacity, and migration ability of prostate cancer
cells. Moreover, miR-192 overexpression induced cell cycle arrest
in the G1 phase. Thus, we suggest that miR-192 serves as a tumor
suppressor in prostate cancers.
The NOB1 protein was initially reported to play an
essential role in both 20S proteasome maturation and protein
degradation (6). NOB1 was noted
to be extensively involved in various human cancers (4,7,8).
NOB1 knockdown has been noted to markedly decrease the colony
forming- and proliferative abilities of ovarian cancer cells and
induce cell cycle arrest in the G0/G1 phase (4). Likewise, the gene silencing ability
of NOB1 inhibits the cell growth and tumor-formation ability of
human hepatocellular carcinoma cells (7). In breast cancer cells, NOB1
knockdown also suppresses the proliferation and growth of cancer
cells (8). Studies have also
reported that NOB1 was highly expressed in papillary thyroid
carcinoma tissues, and silencing NOB1 enhances the antitumor effect
of doxorubicin (9,24). NOB1 has also been implicated in
the development and progression of non-small-cell lung cancer; it
has been suggested that high NOB1 expression acts as a potential
indicator of poor prognosis (10,13,25,26). In other types of human cancers,
including gliomas (3),
osteosarcoma (27), colorectal
cancer (28,29), and renal cancer (30), NOB1 has also been found to
function as an oncogene. In line with these previous studies, NOB1
has also been studied as an oncogene in prostate cancers. We noted
that NOB1 is positively and highly expressed in prostate cancer
tissues, as has also previously noted (12). More recently, it has been reported
that high NOB1 expression in prostate cancer tissues is
significantly correlated with distant metastasis and Gleason score
(31). These studies also pointed
out that NOB1 serves as a potential prognostic indicator of
prostate cancer. Furthermore, the gene silencing of NOB1 was found
to significantly suppress the tumorigenicity of prostate cancer
cells (14). In accordance with
these findings, in the present study we found that the NOB1
downregulation induced by miR-192 overexpression also considerably
inhibited the tumorigenicity of prostate cancer cells. Our results
further confirmed the important role which NOB1 plays in regulating
prostate cancer; thus, we suggest that NOB1 serves as a promising
molecular target which will assist in the treatment of prostate
cancer.
The role which miR-192 plays in several types of
human cancer has been widely investigated (20–21,32–34). The tumorigenic or
tumor-suppressive function of miR-192 has also been reported in
various human cancers. Frequent, high miR-192 expression in gastric
cancer is likely involved in the progression of gastric cancer
(32). miR-192 overexpression
inhibits the proliferation and migration rates in neuroblastoma
cells by modulating dicer 1, ribonuclease type III (Dicer1)
expression (33). Geng et
al (20) have reported that
miR-192 overexpression inhibits the metastasis of colon cancer
cells. miR-192 overexpression also decreases cell proliferation and
increases cell apoptosis in lung cancer cells by regulating
retinoblastoma 1 expression (34). Furthermore, overexpression of
miR-192 inhibits the growth of human bladder cancer cells (21). However, whether miR-192 is
involved in the development of prostate cancer remains unknown. In
the present study, we demonstrated that miR-192 is downregulated in
prostate cancer cells, and we thus suggest that it serves as an
tumor suppressor in prostate cancer. We then further elucidated
that NOB1 is a direct target gene of miR-192 and that miR-192
overexpression inhibited the mRNA and protein expression of NOB1 in
prostate cancer cells. Our results indicate that downregulated
miR-192 expression in prostate cancer cells accounts for the
overexpressed NOB1 which promotes the development and progression
of prostate cancer.
miRs are known to be novel regulators of gene
expression (15,16). Several studies have evaluated the
possibility of decreasing NOB1 expression using specific miRs. For
example, it has previously been reported that miR-326 directly
targets and regulates NOB1, which inhibited the cell growth of
glioma cells associated with the MAPK pathway (35). Moreover, miR-326 has been found to
act as a tumor suppressor in colorectal cancer by modulating NOB1
expression (36). In a study on
clear cell renal carcinoma, it was noted that miR-646 negatively
modulates the expression of NOB1 and suppresses the proliferation
and migration of cancer cells; it has been suggested that the MAPK
pathway is involved in these processes (37). In the present study, we
demonstrated that miR-192 was a novel regulator of NOB1 expression.
We also demonstrated that overexpression of miR-192 inhibited the
expression of NOB1 and p38 MAPK, which affected the migratory and
invasive activity on tumor cells, as was also previously shown
(38). The results of our present
study are consistent with the reports of Che et al (11), who revealed that silencing of NOB1
inhibited p38 MAPK expression.
In conclusion, our data in the present study
demonstrated that miR-192 was downregulated in prostate cancer
cells, and also that the restoration of miR-192 inhibited the
proliferation, colony-forming capacity, and migratory ability of
prostate cancer cells. We also noted that miR-192 overexpression
induced cell cycle arrest in the G1 phase. The tumor-suppressive
role of miR-192 was likely mediated by regulating NOB1, an oncogene
in prostate cancer. Therefore, we suggest that miR-192 serves as a
promising therapeutic target to treat prostate cancer.
Abbreviations:
|
NOB1
|
nin one binding
|
|
miRs
|
microRNAs
|
|
miR-192
|
microRNA-192
|
|
MAPK
|
mitogen-activated protein kinase
|
|
3′-UTR
|
3′-untranslated region
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roy M, Kung HJ and Ghosh PM: Statins and
prostate cancer: role of cholesterol inhibition vs. prevention of
small GTP-binding proteins. Am J Cancer Res. 1:542–561.
2011.PubMed/NCBI
|
|
3
|
Wang H, Li P and Zhao B: Knockdown of NOB1
expression by RNAi inhibits cellular proliferation and migration in
human gliomas. Gene. 528:146–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin Y, Peng S, Yu H, Teng H and Cui M:
RNAi-mediated down-regulation of NOB1 suppresses the growth and
colony-formation ability of human ovarian cancer cells. Med Oncol.
29:311–317. 2012. View Article : Google Scholar
|
|
5
|
Zhang Y, Ni J, Zhou G, Yuan J, Ren W, Shan
Y, Tang W, Yu L and Zhao S: Cloning, expression and
characterization of the human NOB1 gene. Mol Biol Rep. 32:185–189.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veith T, Martin R, Wurm JP, Weis BL,
Duchardt-Ferner E, Safferthal C, Hennig R, Mirus O, Bohnsack MT,
Wöhnert J and Schleiff E: Structural and functional analysis of the
archaeal endonuclease Nob1. Nucleic Acids Res. 40:3259–3274. 2012.
View Article : Google Scholar :
|
|
7
|
Lu Z, Guo Q, Shi A, Xie F and Lu Q:
Downregulation of NIN/RPN12 binding protein inhibit the growth of
human hepatocellular carcinoma cells. Mol Biol Rep. 39:501–507.
2012. View Article : Google Scholar
|
|
8
|
Huang WY, Chen DH, Ning L and Wang LW:
siRNA mediated silencing of NIN1/RPN12 binding protein 1 homolog
inhibits proliferation and growth of breast cancer cells. Asian Pac
J Cancer Prev. 13:1823–1827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin S, Meng W, Zhang W, Liu J, Wang P, Xue
S and Chen G: Expression of the NOB1 gene and its clinical
significance in papillary thyroid carcinoma. J Int Med Res.
41:568–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Ma C, Qian M, Wen Z, Jing H and Qian
D: Downregulation of NOB1 suppresses the proliferation and tumor
growth of non-small cell lung cancer in vitro and in vivo. Oncol
Rep. 31:1271–1276. 2014.PubMed/NCBI
|
|
11
|
Che JP, Li W, Yan Y, Liu M, Wang GC, Li
QY, Yang B, Yao XD and Zheng JH: Expression and clinical
significance of the nin one binding protein and p38 MAPK in
prostate carcinoma. Int J Clin Exp Pathol. 6:2300–2311.
2013.PubMed/NCBI
|
|
12
|
Liu G, Shen D, Jiao L and Sun Y: Nin one
binding protein expression as a prognostic marker in prostate
carcinoma. Clin Transl Oncol. 16:843–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu K, Chen HL, Gu MM and You QS:
Relationship between NOB1 expression and prognosis of resected
non-small cell lung cancer. Int J Biol Markers. 30:e43–e48. 2015.
View Article : Google Scholar
|
|
14
|
Zhang X, Zhang D, Qu F, Hong Y, Cao J, Pan
X, Li L, Huang Y, Huang H, Yin L, et al: Knockdown of NOB1
expression inhibits the malignant transformation of human prostate
cancer cells. Mol Cell Biochem. 396:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ranganathan K and Sivasankar V: MicroRNAs
- Biology and clinical applications. J Oral Maxillofac Pathol.
18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YL, Wu S, Jiang B, Yin FF, Zheng SS
and Hou SC: Role of microRNAs in prostate cancer pathogenesis. Clin
Genitourin Cancer. 13:261–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geng L, Chaudhuri A, Talmon G, Wisecarver
JL, Are C, Brattain M and Wang J: MicroRNA-192 suppresses liver
metastasis of colon cancer. Oncogene. 33:5332–5340. 2014.
View Article : Google Scholar :
|
|
21
|
Jin Y, Lu J, Wen J, Shen Y and Wen X:
Regulation of growth of human bladder cancer by miR-192. Tumour
Biol. 36:3791–3797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Augello C, Vaira V, Caruso L, Destro A,
Maggioni M, Park YN, Montorsi M, Santambrogio R, Roncalli M and
Bosari S: MicroRNA profiling of hepatocarcinogenesis identifies
C19MC cluster as a novel prognostic biomarker in hepatocellular
carcinoma. Liver Int. 32:772–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Dong BF, Wang PS, Ren PY, Xue S,
Zhang X, Han Z and Chen G: Silencing NOB1 enhances doxorubicin
antitumor activity of the papillary thyroid carcinoma in vitro and
in vivo. Oncol Rep. 33:1551–1559. 2015.PubMed/NCBI
|
|
25
|
Liu K, Gu MM, Chen HL and You QS: NOB1 in
non-small-cell lung cancer: expression profile and clinical
significance. Pathol Oncol Res. 20:461–466. 2014. View Article : Google Scholar
|
|
26
|
Liu K, Chen HL, Gu MM and You QS: NOB1
expression predicts early response to Cisplatin-based chemotherapy
in patients with advanced non-small cell lung cancer. J Chemother.
May 13–2015.Epub ahead of print. View Article : Google Scholar
|
|
27
|
Chen B, Liu J, Wu D, Qin Y, Peng C, Li C
and Wang J: Gene silencing of NOB1 by lentivirus suppresses growth
and migration of human osteosarcoma cells. Mol Med Rep.
9:2173–2179. 2014.PubMed/NCBI
|
|
28
|
Liu Y, Huang H, Yuan B, Zhuang LY, Luo TP
and Zhang Q: Lentivirus-mediated knockdown of NOB1 suppresses the
proliferation of colon cancer cells. Z Gastroenterol. 52:429–435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He XW, Feng T, Yin QL, Jian YW and Liu T:
NOB1 is essential for the survival of RKO colorectal cancer cells.
World J Gastroenterol. 21:868–877. 2015.PubMed/NCBI
|
|
30
|
Jia JW, Liu AQ, Wang Y, Zhao F, Jiao LL
and Tan J: Evaluation of NIN/RPN12 binding protein inhibits
proliferation and growth in human renal cancer cells. Tumour Biol.
36:1803–1810. 2015. View Article : Google Scholar
|
|
31
|
Chen J, Wang J, Cui X, Liu Y, Yin L, Li Y,
Chen L, Xu D and Gao Y: Positive nin one binding protein expression
predicts poor outcome in prostate cancer. Mol Med Rep.
11:2671–2676. 2015.
|
|
32
|
Xu YJ and Fan Y: MiR-215/192 participates
in gastric cancer progression. Clin Transl Oncol. 17:34–40. 2015.
View Article : Google Scholar
|
|
33
|
Feinberg-Gorenshtein G, Guedj A, Shichrur
K, Jeison M, Luria D, Kodman Y, Ash S, Feinmesser M, Edry L,
Shomron N, et al: MiR-192 directly binds and regulates Dicer1
expression in neuroblastoma. PLoS One. 8:e787132013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng S, Cong S, Zhang X, Bao X, Wang W, Li
H, Wang Z, Wang G, Xu J, Du B, et al: MicroRNA-192 targeting
retinoblastoma 1 inhibits cell proliferation and induces cell
apoptosis in lung cancer cells. Nucleic Acids Res. 39:6669–6678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou J, Xu T, Yan Y, Qin R, Wang H, Zhang
X, Huang Y, Wang Y, Lu Y, Fu D and Chen J: MicroRNA-326 functions
as a tumor suppressor in glioma by targeting the Nin one binding
protein (NOB1). PLoS One. 8:e684692013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu L, Hui H, Wang LJ, Wang H, Liu QF and
Han SX: MicroRNA-326 functions as a tumor suppressor in colorectal
cancer by targeting the nin one binding protein. Oncol Rep.
33:2309–2318. 2015.PubMed/NCBI
|
|
37
|
Li W, Liu M, Feng Y, Xu YF, Huang YF, Che
JP, Wang GC, Yao XD and Zheng JH: Downregulated miR-646 in clear
cell renal carcinoma correlated with tumour metastasis by targeting
the nin one binding protein (NOB1). Br J Cancer. 111:1188–1200.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li XD, Liu ZY, Chang B, Liu DX, Chen B,
Guo C, Wang YG, Xu JK, Huang DY and Du SX: Panax notoginseng
saponins promote osteogenic differentiation of bone marrow stromal
cells through the ERK and P38 MAPK signaling pathways. Cell Physiol
Biochem. 28:367–376. 2011. View Article : Google Scholar : PubMed/NCBI
|